Abstract
A comprehensive body of scientific evidence indicates that rhizobial bacteria and melatonin enhance salt tolerance of crop plants. The overall goal of this research was to evaluate the ability of Rhizobium leguminoserum bv phaseoli to suppress salinity stress impacts in common bean treated with melatonin. Treatments included bacterial inoculations (inoculated (RI) and non-inoculated (NI)), different salinity levels (non-saline (NS), 4 (S1) and 8 (S2) dS m−1 of NaCl) and priming (dry (PD), melatonin (PM100) and hydro (PH) priming) with six replications in growing media containing sterile sand and perlite (1:1). The results showed that the bacterial strain had the ability to produce indole acetic acid (IAA), ACC deaminase and siderophore. Plants exposed to salinity stress indicated a significant decline in growth, yield, yield components, nitrogen fixation and selective transport (ST), while showed a significant increase in sodium uptake. However, the combination of PM100 and RI treatments by improving growth, photosynthesis rate and nitrogen fixation positively influenced plant performance in saline conditions. The combined treatment declined the negative impacts of salinity by improving the potassium translocation, potassium to sodium ratio in the shoot and root and ST. In conclusion, the combination of melatonin and ACC deaminase producing rhizobium mitigated the negative effects of salinity. This result is attributed to the increased ST and decreased sodium uptake, which significantly reduced the accumulation of sodium ions in shoot.
Similar content being viewed by others
Introduction
Common bean (Phaseolus vulgaris L.) is a main grain legume belonging to the Fabaceae family, which includes about 20,000 species1. This plant is one of the oldest domesticated crops widely cultivated in cropping systems of many parts of the world due to its ability to fix atmospheric nitrogen2,3. The N2-fixing legumes have been used as cheaper and more sustainable alternatives to chemical N fertilizers in small-scale farming systems4. However, N2 fixation can be greatly affected by salinity stress, especially at the initial phases of growth5. Hence, N2 fixation is usually used as a surrogate to identify salinity tolerance of common bean6.
Salinity stress is a major factor limiting crop production worldwide, and is a serious risk to the growth and yield of legume plants in specific7,8. In addition, salinity inhibits the uptake and transportation of potassium in plants which in turn causes ion imbalance and toxicity9. Among the suggested strategies to combat ionic stress imposed by salinity, combined application of ACC deaminase rhizobium and melatonin has been proposed as a biostimulation strategy10.
PGPR’s especially rhizobium bacteria are one of the most important microorganisms in the soil contributing to plant productivity under normal and stress conditions through siderophore production, nitrogen fixation activity, phytohormone biosynthesis, and the biosynthesis of ACC-deaminase6,11,12. PGPR’s can enhance the growth of root by producing ACC deaminase and IAA, and by reducing ethylene production in the roots13. The potential of Rhizobium to enhance growth of plant under stress conditions has been shown in various legume plants5,14. Also, symbiotic beneficial rhizobacteria through influencing the bio-synthesis of the proteins having role in cellular defense mechanism, protect the host plants against salt toxicity6.
Melatonin (N-acetyl-5-methoxytryptamine), an effective biostimulant derived from the amino acid tryptophan, enhances the stress tolerance of plants and bacteria15,16,17,18. The positive effects of melatonin in alleviating salt stress through scavenging the reactive oxygen species (ROS), maintaining sodium/potassium homeostasis, increasing potassium/sodium ratio, promoting the retention of potassium in roots, and enhancing potassium uptake transporters in the root tips have been well documented19,20,21. In addition, melatonin priming not only improves plant growth and yield but also enhances seed quality22. ElSayed et al.23 stated that an exogenous application of melatonin regulated the redox homeostasis by its ability to induce either enzymatic or non-enzymatic antioxidant systems in peanut. The impacts of PGPR’s on the development and yield of legumes have been investigated under different environmental stresses, but there is no information on their application with melatonin on legumes in saline conditions20,21.
We hypothesized in this study that the cumulative effects of PGPR’s and melatonin will enhance the stress tolerance of common bean grown under saline conditions, probably by modulating the stress-responsive pathways. Hence, the research specific objectives were: (1) to investigate some PGP traits of Rhizobium leguminosarum b.v. phaseoli; and (2) explore the interactive effect of melatonin priming and PGPR to suppress salinity stress by enhancing ion homeostasis and nitrogen fixation in common bean. Such knowledge enables us to use the potential of PGPR in combination with melatonin priming to improve plant tolerance to salinity.
Materials and methods
Rhizobium tests
All methods were performed in accordance with the relevant guidelines and legislations. The bacteria Rhizobium leguminosarum b.v. phaseoli was obtained from the Department of Soil Sciences, Shiraz University. This bacterium was selected based on its traits of PGP such as IAA production, siderophore production and ACC deaminase activity. IAA production, siderophore production and ACC deaminase activity were calculated by following the methods of Dell’Amico et al.24, Glickmann and Dessauxm25 and Alexander and Zuberer26, respectively.
Plant tests
Experimental details
A greenhouse pot experiment was undertaken at Shiraz University, Shiraz from May to September of 2019. This experiment was carried out in a factorial arrangement based on a completely randomized design (CRD) with six replicates to test the effects of three factors i.e. : (1) salinity stress (non-saline (NS), 4 dS m−1 (S1), and 8 dS m−1 (S2)) (2) priming treatments (PH (hydro priming), PM100 (melatonin priming (100 µM)) and PD (no priming)) and (3) bacterial inoculations (non-inoculated (NI), and inoculated (RI)).
Soil characteristics
The sandy soil (0–20 cm in depth) was collected from the Bakhtegan Lake in Fars, Iran (29° 16′ N; 53° 52′ E). The sandy soil had organic matter content of 1400 mg kg−1, available P of 5.5 mg kg−1, available K of 65.56 mg kg−1, total nitrogen of 530 mg kg−1 and available NO3–1 of 4.53 mg kg−1. Its electrical conductivity (EC) and the hydrogen potential (pH) were 0.89 dS m−1 and 7.62, respectively. The sand was sterilized by autoclaving, and around 6 kg of sand and perlite (1:1, v/v) mixture were mixed into PVC pots (diameter = 15 cm, height = 60 cm).
Priming treatments
Common bean seed priming was done at different concentrations (PD, PH, 20, 100, and 500 µM melatonin) as previously reported27. Based on the results, 100 µM melatonin for a duration of 10 h had the highest effect on germination indices compared to the other concentrations27.
Preparation of rhizobium
Bacterial inoculum was prepared by cultivating the bacterial strain in Yeast Mannitol Broth (YMB) medium28, followed by incubation at 27 °C on a rotary shaker (105 rpm) for two days. The bacterial cells were centrifuged at 5000 rpm for 5 min and the pellet was washed with sterile phosphate buffered saline and repeating centrifugation steps. The bacterial pellets diluted with PBS solution to make a final concentration of 108 cells/mL. Then, 2 ml of the bacterial suspension was pipetted into the soil surrounding the seed; 2 ml of sterile PSB solution was added to the NI.
Growth conditions and treatments
Common bean seeds (cv. Dorsa) were obtained from Markazi Agricultural and Natural Resources Research and Education Center, Iran. The seeds were surface-sterilized using NaClO solution (3%) and then washed twice by distilled water. Sterilized seeds were primed and sown in Petri-dishes and were misted with distilled water. Seeds started germination one day after sowing, and reached about 80% germination two days later. Thereafter, four uniform seedlings were inoculated and transferred to 15-cm diameter disinfected PVC pots containing autoclave-sterilized substrate (50% perlite and 50% sand). Pots were watered daily with a nitrogen-free nutrient solution according to Broughton and Dilworth29. Upon emergence of the first trifoliolate leaf, NaCl was added to the irrigation water to apply salinity treatments: 4 and 8 dS m−1 (using 2.6, and 5.1 g L−1 NaCl in tap water, respectively)30. Plants were thinned to two healthy plants at the four-leaf stage.
Measurement of growth, yield and yield components
At the physiological maturity stage, plants were harvested i.e. shoots, nodules, height, number of pod, grain number, pod yield, thousand-grain weight, grain yield, root length and total number of nodules were measured on both plants in each pot. The shoot and root biomass were determined after drying plants at 70ºC until constant weight.
Total chlorophyll and net photosynthesis rate measurement
The chlorophyll contents of common bean leaves were measured by the Arnon31 method. In short, fresh leaves (0.1 g) were extracted in 80% acetone (10 mL), and then the extract was centrifuged for five minutes at 300 rpm. The supernatant was separated and used for the measurement of chlorophyll contents at 646, and 663 nm using an ultraviolet–visible spectrophotometer (7315 UV/visible Spectrophotometer, Jenway, UK)32.
Forty days after sowing, net photosynthesis rate (Pn) of fully developed leaves (six replicates) was recorded using a portable LCi photosynthesis system (ADC Bioscientific Ltd., UK) between 9:00 and 11:00 AM. The system was calibrated before data recording.
Measurements of amount of fixed nitrogen and protein content
After harvesting, oven-dried (48 h at 70 °C) shoot and root samples—from each treatment were powdered using an electric mill. The shoot and root nitrogen concentration was determined using titration after distillation by the Kjeldahl method33. Total nitrogen (N) content per plant, the N fixed and the crude protein were calculated following the methods of Yaman and Cinsoy34 and Egan et al.35, respectively.
Tissue ion accumulation
Dry material (0.5 g) was taken and ashed at 500 °C for 5 h. After complete dissolution in 2 N HCl, the mixture was filtered using filter paper. The final volume of 50 mL in the flask was reached by adding deionized water. The ionic concentrations were determined using a flame photometer (Sherwood Science, United Kingdom)36.
The ionic translocation, sodium uptake, and selective transport (ST) were determined using the equations given by Wang et al.37, Shahzad38 and Yan et al.39, respectively.
Determination of electrical conductivity (EC) and potential of hydrogen (pH)
At the end of the experiment, 100 g of sand were collected, dried and mixed with deionized water (100 mL) at 25 °C for 24 h. Soil pH (1:2 soil/water suspension) was measured using a pre-calibrated pH-meter (HI 2211 pH/ORP Meter, HANNA Instruments, Woonsocket, RL, USA). EC in soil saturation extracts was determined using a pre-calibrated EC-meter (Hanna HI-2315 Bench Top Conductivity Meter, Hanna Instruments Ltd, USA).
Statistical analysis
One-way analysis of variance (ANOVA) for the factorial experiment based on a CRD was performed in six replicates, using SAS v. 9.1 software40. Mean comparisons were made between treatments through the LSD test at 5% probability level. In addition, regression analysis was performed using Excel software to estimate the relations between traits.
Results
Rhizobium tests
The bacterial strain was investigated for the production of PGP traits i.e. IAA, ACC deaminase and siderophore which are indicated in Table 1 and Fig. 1. The IAA production by the bacteria increased to 8.45 μg ml−1 in the presence of L-tryptophan in the media.
Rhizobium leguminoserum bv phaseoli was able to grow on DF minimal medium containing ACC as the sole N source, indicating that they have the ACC deaminase activity. Optical densities of Rhizobium leguminosarum suspensions in minimal medium without nitrogen source (negative control), amended with 2 g of (NH4)2SO4 L−1 (positive control), or with ACC (3 mmol L−1) are listed in Table 1.
Plant tests
Growth parameters
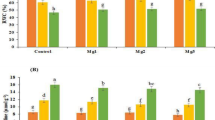
With enhancing salinity levels, height, shoot and root biomass, and root length decreased. However, salinity appears to affect shoots more than roots (Table 2). The melatonin application, regardless of salinity level and RI, improved height, shoot and root biomass, root length, and number of nodules compared to their respective PD treatments by 68.2%, 43.7%, 38.2%, 5.5%, and 8.9 times, respectively (Table 2). However, application of S2, regardless of the priming and inoculation treatments, led to a decrease of 79.5% in the height, 27.1% in shoot biomass, 24.4% in root biomass, and 70.5% in the number of nodules compared to NS (Table 2). Height, shoot and root biomass, and length of root, improved when PM100 was applied with RI (Table 2). The combination of PM100 and RI treatments increased height, shoot biomass, root length and root biomass by 11.6%, 13.5%, 8.4% and 7.5%, respectively (Table 2). Of the bacterial inoculated plants, those primed with melatonin achieved the highest total number of nodules (332.25) under NS level (Table 2; Fig. 2a–d).
Total chlorophyll and net photosynthesis rate (Pn) measurement
The regular exposure of plants to salinity stress decreased the total chlorophyll concentration and Pn. The results showed that the PM100 plus RI treatments significantly improved the total chlorophyll as compared to the PD plus NI treatments (Fig. 3a,b). Under NS condition, the combination of RI and PM100 treatments increased total chlorophyll and Pn compared to the PD plus NI treatments by 3.04 and 4.65 times, respectively (Fig. 3a,b).
Effects of salinity stress, ACC deaminase-producing rhizobial bacterium and melatonin on chlorophyll (a) and Pn (b) of common bean. PM100, melatonin priming; PH, hydro priming; PD, no priming; NI, non-inoculated; RI, Rhizobium inoculated; NS, S1 and S2 are non-saline, 4 and 8 dSm−1 of salinity stress, respectively. The vertical bars followed the different letters were significantly differences according to the LSD test at P < 0.05.
Nitrogen concentration and content, protein content and amount of fixed nitrogen
The results presented in Table 3 show that nitrogen concentration, nitrogen content and shoots and roots protein content under salinity stress conditions were lower than those under normal conditions. The plants inoculated with RI or primed with melatonin showed a significant increase in all parameters compared to NI or PD plants, irrespective of the salinity treatment. The interaction of RIS and P treatments resulted in the maximum increase of all tested parameters in plants primed with melatonin and inoculated with the bacterial isolate, compared to those tested alone in NS, S1 and S2 (Table 3). Under salinity condition (S1), nitrogen concentration and content in the shoots and roots enhanced significantly (2.1, 1.6, 2.8, and 2.1 times, respectively) in RI + PM100 treatment compared to NI + PM100 (Table 3). The shoots and roots protein content of treated with RI and PM100 significantly increased by 2.1, 1.6, 1.3 and 1.3 times, respectively, compared to the NI under salinity conditions (S1 and S2) (Table 3). On the other hand, the stressed and non-stressed plants primed with melatonin and inoculated with Rhizobium had significantly higher values of biochemical parameters; nitrogen concentration and content, and protein content in shoots and roost, compared to non-treated ones.
Significant increases in the amount of nitrogen fixation was observed in the PM100 plus NS treatments (33.6 g pot−1), followed by the PM100 plus S1 (22.6 g pot−1) (Fig. 4). In contrast, the amount of nitrogen fixation decreased by 87.4 and 79.9% in plants that germinated from PD-treated seeds compared to PM100 and PH in the S2 treatment (Fig. 4).
Effects of salinity stress, and melatonin on fixed nitrogen amount in shoot of common bean. PM100, melatonin priming; PH, hydro priming; PD, no priming; NS, S1 and S2 are non-saline, 4 and 8 dSm−1 of salinity stress, respectively. The vertical bars followed the different letters were significantly differences according to the LSD test at P < 0.05.
Potassium (K+), sodium (Na+), K+/Na+ ratio
As shown in Table 4, the concentration of sodium increased significantly in both shoots and roots in the P plus RIS treatments under salinity stress. However, the Na+ concentration in shoots was 1.8 times lower in the combination of RI, S2 and PM100 treatments than in the combination of NI, S2 and PD, whereas in roots, the Na+ concentrations was 1.1 times higher. In contrast, under salinity stress, K+ concentration declined in shoots and roots (Table 4). In shoots, the K+ concentration increased by 1.4 times in combined treatment of RI, S2 and PM100 compared to the combination of NI, S2 and PM100 treatments. In roots, the K+ concentration increased more (1.5 times) in the combination of RI, S2 and PM100 treatments than combination of NI, S2 and PM100.
The K+/Na+ ratio increased significantly in both shoots and roots when seeds primed with melatonin and inoculated with RI compared to the combination of NI and PM100 under NS, S1 and S2 salinity levels (Table 4). The increase in potassium concentration and potassium to sodium ratio, and the reduction of sodium concentration in both shoots and roots, resulting from melatonin priming and Rhizobium inoculation, were significant under all salinity levels.
Ion translocation and Na+ uptake and ST measurement
The results shown in Fig. 5a–d presents the changes in potassium, sodium translocation, sodium uptake and ST in the stressed common bean after applying the PM100 plus RI treatments. The common bean seedlings exposed to 4 and 8 dS m−1 levels showed that the translocation of sodium and potassium was increased by 90.2 and 77%, and 30.9 and 63.5% in the combined application of PD and NI treatments (Fig. 5a,b), while they increased by 40.1 and 50%, and 38.1 and 24.6% in the combination of PH and NI treatments respectively, as compared to the NS, PD and NI combination treatments (Fig. 5a,b). Conversely, S2 treatment decreased ST by 9%, whereas Na+ uptake in roots was also enhanced by 7.98 times in the combination of PD and NI, when compared to the NS level. The combination of PM100 and RI treatments under severe salinity conditions (S2) improved ST (2.2%), while Na+ translocation and Na+ uptake were reduced by 1.22 and 2.88 times, respectively, compared to combined NS, PD and NI (Fig. 5).
Effects of salinity levels, BI, and priming on Na+ and K+ translocation factors (a and b) and selective transport (c) of common bean. PM100, priming treatment with 100 µM melatonin; PH, priming treatment with distilled water; PD, dry seed without priming; NI, non- Rhizobium inoculation; RI, Rhizobium inoculation; NS, S1 and S2 are non-saline, 4 and 8 dSm−1 of salinity stress, respectively. Data are presented as the means of six replicates; The vertical bars indicate standard deviations. Different letters indicate significant differences according to the LSD test at P < 0.05.
Soil EC and pH
Soil EC and pH were highly affected by the combination of RI and PM100 treatments under salinity stress (Fig. 6a,b). The results showed that the PM100 plus RI treatments were significantly decreased EC by 57.6, 20, and 37.4% and pH by 10.6, 7.4, and 8.4% compared to the PD plus NI treatments under NS, S1 and S2 levels (Fig. 6a,b).
Effects of salinity stress, ACC deaminase-producing rhizobial bacterium and melatonin on EC and pH of soil (a) EC of the soil within 0–50 cm depth, (b) pH of the soil within 0–50 cm depth. PM100, melatonin priming; PH, hydro priming; PD, no priming; NI, Rhizobium non-inoculation; RI, Rhizobium inoculation; NS, S1 and S2 are non-saline, 4 and 8 dS m−1 of salinity stress, respectively. The vertical bars followed the different letters were significantly differences according to the LSD test at P < 0.05.
Yield and yield components
Both P and RI treatments caused significant increases in all yield components (Table S1). The combination of PM100 and RI treatments produced the highest number of pods (9 plant−1), pod yield (42.9 g pot−1), number of grains (5 pod−1), thousand-grain weight (299.2 g) and grain yield (13.8 g pot−1), an increase of 71.4, 26.6, 25, 4.55 and 23.4% compared to the PM100 plus NI treatments under NS, respectively. Additionally, under saline conditions, the maximum number of pods (plant−1), pod yield, number of grains (pod−1), thousand-grain weight and grain yield were found in the combined PM100, RI and S1 treatments, with an average of 7, 17.84 g pot−1, 4, 279.2 g, and 12.4 g pot−1, representing 2.2, 2.0, 1.3, 1.0 and 1.2 times increases compared to the combined PM100, NI and S1 treatments, respectively. The lowest number of pods (plant−1), pod yield, number of grains (pod−1), thousand-seed weight and grain yield were recorded for the combined of PD, RI and S2 treatments, where no pod was produced (Table S1).
Relationships between amounts of fixed nitrogen, ST and growth parameters
Significant positive polynomial relationships were observed between the amount of nitrogen fixation and the total number of nodules, potassium concentration of root, the potassium to sodium ratio of shoots and roots and ST across the salinity-priming treatments (Supplementary Fig. S1). The K+ concentration in both shoots and roots showed significant positive exponential relationships with the amount of fixed nitrogen (Figure S1).
As shown in Figure S2, across all treatments, significant positive linear relationships were observed in the number of pods (plant−1), pod yield, number of grains (pod−1), grain yield with ST, while significant linear and logarithmic relationships were found in ST for length of root, root biomass and number of nodules (Figure S3).
Furthermore, the relationships between the enhancement rates for the functional traits, such as amount of fixed nitrogen, chlorophyll, and Pn were investigated. As shown in Figure S4, the amount of fixed nitrogen enhancement rate had positive power and exponential relationships with chlorophyll and Pn.
Discussion
Salinity stress negatively affects plant attributes by influencing ion toxicity and ethylene signaling41. Although melatonin and ACC deaminase-producing rhizobium have been implicated in the amelioration of the plant development and protection against salinity in various crops13,20, there is no sufficient information on the influence of both in saline conditions. The results showed that the combination of RI and PM100 significantly improved shoot and root growth, and yield in salinity treatments by maintaining ion homeostasis and increasing chlorophyll content and photosynthesis. A significant increase in growth of shoots and roots showed the RI efficacy along with melatonin compared to the RI plus PD treatments in saline conditions.
The negative impact of increased salinity on grain yield and biomass of common bean was significant in the present study. Growth, yield and yield components of the common bean grown under high-salinity level (8 dS m−1) were significantly reduced compared to NS condition. In addition, a substantial increase was observed in the growth and yield of plants exposed to RI and PM100. Salinity stress reduced plant biomass where no RI and PM100 were applied due to the reduction of chlorophyll content and photosynthesis. High production of chlorophyllase and degradation of chlorophyll in salinity treatments leads to accelerate leaf senescence42,43. The combination of RI and PM100 increased the photosynthetic efficiency by inhibiting the expression of chlorophyllase gene resulting in protecting the photosynthetic pigments from degradation10,18. Furthermore, the important role of melatonin as an antioxidant is directly linked with the improvement of plants’ growth parameters and yield. Siddiqui et al.44 noted that the inhibition of chlorophyll degradation and improvement in photosynthetic capacity by applying melatonin played a crucial role in the enhancement of growth and yield under stress.
Yan et al.45 pointed out that the rate of sodium and potassium uptake was controlled by application of melatonin. Greater amounts of potassium in the shoots of common bean at S1 and S2 levels in RI + PM100 treatment might be another responsible factor for mitigating salinity stress. According to Jiang et al.20, high ion homeostasis and decreasing ion toxicity by melatonin contribute to the increased concentration of potassium and potassium/sodium ratio in plants. Better ST can maintain ion homeostasis by compartmentalization of ions into vacuoles and decrease the amount of the ions in cytoplasm46,47. Similarly, an increase in potassium concentration in shoots and ST as well as a decreased sodium uptake and translocation were observed in the RI plus melatonin treatments. The increase in potassium concentration, potassium/sodium ratio and ST might also contribute to the higher salt tolerance of RI in the presence of melatonin which in turn finally increases transporters of potassium under S1 and S2. Our findings are in agreement with previous findings of Abd El-Ghany and Attia10, who showed that melatonin improves the abiotic stress tolerance of PGPR.
The improvement in the growth of shoots and roots might be also due to a reduction in ethylene levels by Rhizobium leguminosarum b.v. phaseoli. Production of ACC-deaminase by this bacterium may be the crucial cause of plant better tolerance than NI in saline conditions. Dubois et al.47 found an increase in the production of ethylene in roots and shoots in saline conditions by increasing the ACC level in plants. The released ACC from roots into the rhizosphere is hydrolyzed by ACC-deaminase into ammonia and α-ketobutyrate which ultimately reduces ethylene level48. Reduction in ethylene will lead to a greater growth of roots which will increase plant nutrients uptake by increasing root surfaces46. Rhizobium leguminosarum b.v. phaseoli reduced the negative impacts of salinity in common bean in terms of enhancement in net photosynthesis rate and total chlorophyll content.
In addition to the ACC-deaminase, the improvement in common bean growth might also be favored by the production of siderphore and IAA by Rhizobium leguminosarum as compared to NI. Benková et al.49 indicated enhancement in the control of cell division, differentiation and elongation induced by IAA synthesis by PGPR. These plant physiological processes are crucial for organ initiation and differentiation such as nodule development. The IAA produced by PGPR increases free amino acids in host plants that play an important role in photosynthetic products supplied to nodules8,50. In addition, increases the expression of the genes involved in carbon transport to bacteroids50,51. Additionally, siderphores synthesis and organic acids production by PGPR increase nutrient availability via the enhancement of chelate potassium ions, and change the EC and pH of the rhizosphere52,53.
Conclusion
Salinity stress is one of the most serious limiting factors of common bean production that causes changes in transportability of sodium and potassium ions and nitrogen fixation. It is concluded that both melatonin and RI effectively decline the severity of stress in common bean by reducing the effects of stress ethylene and increasing selective transportability for sodium and potassium ions and nitrogen fixation activity. The combination of melatonin and RI is a better approach than individual application of melatonin and RI under salinity stress for improving growth, yield and yield components and mitigating salinity toxicity in common bean. More studies are required to introduce the halotolerant PGPR combined with melatonin as an efficient treatment to alleviate salinity in common bean and subsequently improve salt tolerance of this crop under field conditions.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Graham, P. H. & Vance, C. P. Legumes: Importance and constraints to greater use. Plant Physiol. 131(3), 872–877 (2003).
Nwokolo, E. & Smartt, J. Food and Feed from Legumes and Oilseeds (Springer, 1996).
Hayat, R., Ali, S., Siddique, M. T. & Chatha, T. H. Biological nitrogen fixation of summer legumes and their residual effects on subsequent rainfed wheat yield. Pak. J. Bot. 40, 711–722 (2008).
Samago, T. Y., Anniye, E. W. & Dakora, F. D. Grain yield of common bean (Phaseolus vulgaris L.) varieties is markedly increased by rhizobial inoculation and phosphorus application in Ethiopia. Symbiosis 75(3), 245–255 (2018).
Sulieman, S. & Tran, L. S. Legume Nitrogen Fixation in a Changing Environment (Springer, 2016).
Viscardi, S. et al. Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. J. Soil Sci. Plant Nutr. 16, 848–863 (2016).
Keshavarz, H. & Moghadam, R. S. G. Seed priming with cobalamin (vitamin B12) provides significant protection against salinity stress in the common bean. Rhizosphere 3, 143–149 (2017).
Gupta, S. & Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 10, 1506 (2019).
Qiao-li, M. A. et al. Proteomic analysis of salt and osmotic-drought stress in alfalfa seedlings. J. Integr. Agric. 15(10), 2266–2278 (2016).
Abd El-Ghany, M. F. & Attia, M. Effect of exopolysaccharide-producing bacteria and melatonin on faba bean production in saline and non-saline soil. Agronomy 10(3), 316–332 (2020).
JahandidehMahjen Abadi, V. A. et al. Role of dominant phyllosphere bacteria with plant growth-promoting characteristics on growth and nutrition of maize (Zea mays L.). Soil Sci. Plant Nutr. 20(4), 2348–2363 (2020).
JahandidehMahjen Abadi, V. A., Sepehri, M., Khatabi, B. & Rezaei, M. Alleviation of zinc deficiency in wheat inoculated with root endophytic fungus Piriformospora indica and rhizobacterium Pseudomonas putida. Rhizosphere 17, 100311 (2021).
Ali, S., Charles, T. & Glick, B. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 80, 160–167 (2014).
Naseri, B. Legume root rot control through soil management for sustainable agriculture. In Sustainable Management of Soil and Environment (eds Meena, R. S. et al.) 217–258 (Springer, 2019).
Hardeland, R. et al. Melatonin-A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93(3), 350–384 (2011).
Nawaz, M. A. et al. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 220, 115–127 (2018).
Tiwari, R. K. et al. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 272, 109592 (2020).
Alinia, M., Kazemeini, S. A., Sepehri, M. & Dadkhodaie, A. Simultaneous application of rhizobium strain and melatonin improves the photosynthetic capacity and induces antioxidant defense system in common bean (Phaseolus vulgaris L.) under salinity stress. J. Plant Growth Regul. 41, 1367–1381 (2021).
Li, X., Yu, B., Cui, Y. & Yin, Y. Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl− accumulation in rice. Plant Growth Regul. 83(3), 441–454 (2017).
Jiang, C. et al. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant 38(4), 82 (2016).
Liu, J. et al. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell. Environ. 43(11), 2591–2605 (2020).
Szafrańska, K., Reiter, R. J. & Posmyk, M. M. Melatonin application to Pisum sativum L. seeds positively influences the function of the photosynthetic apparatus in growing seedlings during paraquat-induced oxidative stress. Front. Plant Sci. 7, 1663 (2016).
ElSayed, A. I. et al. Melatonin regulatory mechanisms and phylogenetic analyses of melatonin biosynthesis related genes extracted from peanut under salinity stress. Plants 9(7), 854 (2020).
Dell’Amico, E., Cavalca, L. & Andreoni, V. Analysis of rhizobacterial communities in perennial Graminaceae from polluted water meadow soil, and screening of metal-resistant, potentially plant growth-promoting bacteria. FEMS Microbiol. Ecol. 52, 153–162 (2005).
Glickmann, E. & Dessauxm, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 61, 793–796 (1995).
Alexander, D. B. & Zuberer, D. A. Use of chrome Azurol S reagent to evaluate sidrophore by rhizosphere bacteria. Biol. Fertil. Soil 12, 39–45 (1991).
Alinia, M., Kazemeini, S. A., Dadkhodaie, A., Sepehri, M. & Pessarakli, M. Improving salt tolerance threshold in common bean cultivars using melatonin priming: a possible mission?. J. Plant Nutr. 44(18), 2691–2714 (2021).
Fred, E. B. & Waksman, S. A. Laboratory Manual of General Microbiology (McGraw Hill, 1928).
Broughton, W. J. & Dilworth, M. J. Control of leghemoglobin synthesis in snake beans. Biochem. J. 125, 1075–1080 (1971).
Rhoades, J. D., Chanduvi, F. & Lesch, S. M. Soil Salinity Assessment: Methods and Interpretation of Electrical Conductivity Measurements (Food and Agriculture Organization, 1999).
Arnon, D. I. Copper enzymes in isolated chloroplasts, polyphenoloxidase in Beta vulgaris L. Plant Physiol. 24, 1–15 (1949).
Wellburn, A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144(3), 307–313 (1994).
Okalebo, J. R., Gathua, K. W. & Woomer, P. L. Laboratory Methods of Soil and Plant Analysis: A Working Manual. Sacred Africa, Nairobi (2002).
Yaman, M. & Cinsoy, A. S. Determination of the most effective Rhizobium strain (Rhizobium japonicum L.) in soybean. AARI 6, 84–96 (1996).
Egan, H., Kirk, R. S. & Sawyer, R. Pearson’s Chemical Analysis of Food (Churchill Livingstone, 1981).
Horneck, D. A. & Hanson, D. Handbook of reference methods for plant analysis. In Determination of Potassium and Sodium by Flame Emission Spectrophotometry (ed. Kalra, P. Y.) 153–157 (CRC Press, 1998).
Wang, S., Zhao, G., Gao, Y., Tang, Z. & Zhang, C. Puccinellia Tenuiflora exhibits stronger selectivity for K+ over Na+ than wheat. J. Plant Nutr. 27, 1841–1857 (2005).
Shahzad, A. N. The role of Jasmonic Acid (JA) and Abscisic Acid (ABA) in salt resistance of maize (Zea mays L). VVB Laufersweiler (2011).
Yan, K., Wu, C., Zhang, L. & Chen, X. Contrasting photosynthesis and photoinhibition in tetraploid and its autodiploid honeysuckle (Lonicera Japonica Thunb.) under salt stress. Front. Plant Sci. 6, 227 (2015).
Institute, S. A. S. SAS Users’ Guide (SAS Institute, 2003).
Tao, J. J. et al. The role of ethylene in plants under salinity stress. Front. Plant Sci. 6, 1059 (2015).
Stoeva, N. & Kaymakanova, M. Effect of salt stress on the growth and photosynthesis rate of bean plants (Phaseolus vulgaris L.). JCEA. 9, 385–391 (2008).
Rady, M. M., Bhavya Varma, C. & Howladar, S. M. Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci. Hortic. 162, 63–70 (2013).
Siddiqui, M. H. et al. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 20(2), 353 (2019).
Yan, F. et al. Melatonin enhances Na+/K+ homeostasis in rice seedlings under salt stress through increasing the root H+-pump activity and Na+/K+ transporters sensitivity to ROS/RNS. Environ. Exp. Bot. 182, 104328 (2021).
Ilangumaran, G. & Smith, D. L. Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front. Plant Sci. 8, 1768 (2017).
Dubois, M., Van den Broeck, L. & Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 23(4), 311–323 (2018).
Glick, B. R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 169(1), 30–39 (2014).
Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115(5), 591–602 (2003).
Defez, R. et al. Bacterial IAA-delivery into medicago root nodules triggers a balanced stimulation of C and N metabolism leading to a biomass increase. Microorganisms 7(10), 403 (2003).
Figueiredo, M. V. B., Martinez, C. R., Burity, H. A. & Chanway, C. P. Plant growth-promoting rhizobacteria for improving nodulation and nitrogen fixation in the common bean (Phaseolus vulgaris L.). World J. Microbiol. Biotechnol. 24(7), 1187–1193 (2008).
Li, X., Sun, P., Zhang, Y., Jin, C. & Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 174, 104023 (2020).
Zafar-ul-Hye, M. et al. Compost mixed fruits and vegetable waste biochar with ACC deaminase rhizobacteria can minimize lead stress in mint plants. Sci. Rep. 11(1), 6606 (2021).
Acknowledgements
Thanks for Shiraz University for the support of this research.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M.A. Conceptualization, M.A., S.A.K., A.D. and A.A.A. Data curation, M.A. and M.S. Formal analysis, V.A. J.M., S.F.A. and P.P. Investigation, M.A., S.A. K. and A. D. Methodology, M.A. Project administration, S.A.K., A.A.A. and S.F.A. Supervision: M.A., S.A.K., V.A.J.M., S.F.A., P.P. and M.A.B. Writing-original draft, A.A.A., S.F.A. and S.A.K and D.E. Writing-review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alinia, M., Kazemeini, S.A., Dadkhodaie, A. et al. Co-application of ACC deaminase-producing rhizobial bacteria and melatonin improves salt tolerance in common bean (Phaseolus vulgaris L.) through ion homeostasis. Sci Rep 12, 22105 (2022). https://doi.org/10.1038/s41598-022-26084-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-26084-3
This article is cited by
-
Harnessing phyllosphere and rhizobium bacteria for salt stress alleviation in common bean (Phaseolus vulgaris)
BMC Plant Biology (2026)
-
Co-inoculation of Stenotrophomonas maltophilia and Rhizobium leguminosarum phaseoli improves salinity tolerance in common bean cultivars
Scientific Reports (2026)
-
Effect of Silicon and Melatonin Co-application on the Physiological and Biochemical Responses of Cyamopsis Tetragonoloba L. Under Salinity Conditions
Journal of Plant Growth Regulation (2026)
-
Plant growth-promoting native rhizobia isolated from red clover (Trifolium pratense L.) nodules: potential for metal-phytostabilization
BMC Microbiology (2025)
-
Functional characterization of a novel plant growth-promoting rhizobacterium enhancing root growth and salt stress tolerance
Scientific Reports (2025)