Abstract
Cancer and diabetes mellitus (DM) are diagnosed within the same individual more frequently and share common risk factors. Although diabetes among cancer patients may result in more aggressive clinical courses of cancer, there is limited evidence about its burden and associated factors. Hence, this study aimed to assess the burden of diabetes and prediabetes among cancer patients and its associated factors. Institution-based cross-sectional study was conducted at the University of Gondar comprehensive specialized hospital from 10 January to 10 March 2021. A systematic random sampling technique was used to select 423 cancer patients. The data was collected using a structured interviewer-administered questionnaire. Prediabetes and diabetes diagnosis was made based on World Health Organization (WHO) criteria. Bi-variable and multivariable binary logistic regression models were fitted to identify factors associated with the outcome. Adjusted Odds Ratio (AOR) with a 95% confidence interval was estimated to show the direction and strength of associations. Variables with a p-value less than 0.05 in the multivariable model were considered significantly associated with the outcome. The final analysis was based on 384 patients with cancer. The proportion of prediabetes and diabetes was 56.8% (95% CI 51.7, 61.7) and 16.7% (95% CI 13.3, 20.8), respectively. Alcohol consumption was found to increase the odds of elevated blood sugar among cancer patients (AOR: 1.96; 95%CI: 1.11, 3.46). The burden of prediabetes and diabetes is alarmingly high among cancer patients. Besides, alcohol consumption was found to increase the odds of having elevated blood sugar among cancer patients. Hence, it is essential to recognize cancer patients are at high risk of having elevated blood sugar and design strategies to integrate diabetes and cancer care.
Similar content being viewed by others
Introduction
Noncommunicable diseases (NCDs) are the leading causes of death globally, killing more people each year than all other causes combined1. The combined burden of these diseases is rising fastest among lower-income countries, including Ethiopia2. Of these diseases, cancer and Diabetes Mellitus (DM) are challenging the health system in Ethiopia. Although there is limited data, the Federal Ministry of Health (FMOH) estimated that there could be more than 150,000 cancer cases in Ethiopia each year3. Besides, of total national mortality in the country, 5.8% is caused by cancer4. On the other hand, it is estimated that developing countries, including Ethiopia, bear 77% of the global burden of the DM epidemic in the twenty-first century5.
Cancer and DM are diagnosed within the same individual more frequently than expected by chance, suggesting a common underlying mechanism6. About 26.9% of all people over 65 have diabetes and 60% have cancer worldwide. Overall, 8 to18% of cancer patients have DM7.
Diabetes and cancer share common risk factors such as increased age, obesity, physical inactivity, poor diet, alcohol, and smoking6. Although most evidence shows DM to be a risk factor for different types of cancer8,9,10, some findings suggest that the relationship is bidirectional11. Unfortunately, many cancer-fighting treatments are linked to diabetes and the incidence of diabetes and prediabetes is significantly higher among people with cancer compared with those who don’t have cancer12,13,14.
Some epidemiological studies suggest that diabetes significantly increases mortality in patients with cancer8,15,16,17 and is also the common cause of non-cancer mortality18. Diabetes may result in a more aggressive clinical course of cancer, strengthening its metastatic potential and favoring cancer growth by making the host organism less resistant to cancer progression, possibly by known impaired immune function in diabetes7,17,19. It is also associated with physiologic distress and decreased quality of life20 among cancer patients. On the other hand, DM significantly negatively impacts cancer patients' treatment outcomes21.
NCDs, including cancer and DM, are among the health targets of the Sustainable Development Goals22. Despite the devastating consequence of diabetes among cancer patients, there is limited evidence about its burden and associated factors. Besides, available evidence focuses on specific types of cancer. Hence, this study aimed to assess the burden of diabetes and prediabetes among cancer patients and its associated factors.
Methods
Study design and setting
An institution-based cross-sectional study was conducted from 10 January to 10 March 2021 among cancer patients on treatment at the University of Gondar Comprehensive Specialized Hospital (UoGCSH). The hospital was established in 1954 and is in the Central Gondar administrative zone, Amhara National Regional State, about 750 km Northwest of Addis Ababa, the capital city of Ethiopia. According to the 2015 population projection of major cities in Ethiopia, the total population of Gondar town was estimated to be 323,900. Currently, Gondar town has one Referral Hospital and eight government Health Centers. The University of Gondar Referral Hospital is a teaching Hospital that serves more than five million people in the North Gondar zone and people of the neighboring zones. The hospital has one Oncology ward. The ward serves more than 1000 cancer patients per year. The hospital's oncology unit currently has ten beds for managing cancer patients.
Population and sample
The source population for this study is all cancer patients who came to the outpatient and inpatient oncology department at UoGCSH. All cancer patients who visited the oncology department during the study were included. However, cancer patients who cannot communicate and have severe psychiatric problems were excluded from the study. The sample size for the study was determined using single population proportion formula considering a 95% confidence level, 5% margin of error, 50% expected proportion of diabetes, and 10% non-response rate. Then, the final sample size was found to be 423. The sample was selected using a systematic random sampling technique with a skip interval of two.
Variables and data collection procedure
The outcome variable for this study was elevated blood sugar level, which is defined as a patient who is in a prediabetic or diabetic state and it was extracted from the patient’s medical file. The blood sugar of every cancer patient was measured using a CareSenseN glucometer. DM was diagnosed based on WHO and American Diabetes Association(ADA) guidelines which is, fasting blood sugar (FBS) ≥ 126 mg/dl or 2-h plasma glucose(PG) during Oral Glucose Tolerance Test(OGTT) ≥ 200 mg/dl23,24 or self-report of previous diagnosis of DM by health professional or currently taking treatment for diabetes. Besides, prediabetes was defined as a patient with FBS 100–125 mg/dl or 2-h PG during OGTT 140–199 mg/dl23,24. Socio-demographic characteristics of the patients, such as age, sex, residence, religion, occupation, and income, were assessed. In addition, behavioral characteristics such as cigarette smoking, alcohol consumption, Khat chewing, physical activity, and type of oil were assessed. Furthermore, cancer-related characteristics such as stage of cancer, duration of cancer, duration of treatment, treatment type, and metastasis were extracted from patient’s medical file. Physical measurements such as weight, height, waist circumference (WC) and blood pressure (BP) were measured. BP was measured three times in a sitting position using standard mercury sphygmomanometer. BP cuff with the appropriate cuff size that covers two-thirds of the upper arm was used. The measurement was taken after the participant rested for at least five minutes and assuring no smoking or caffeine 30 min before measurement. The second and the third measurements were taken five-to-ten minutes after the first and the second measurement, respectively. Finally, the average of the three BP measurements was calculated to determine the BP status of the participant. An individual was diagnosed as hypertensive if the systolic blood pressure (SBP) is ≥ 140 mg/dl or the diastolic blood pressure (DBP) is ≥ 90 mg/dl, or previous diagnosis of hypertension or current use of anti-hypertensive drug25. WC was measured at an approximate mid-point between the lower margin of the lowest palpable rib and the top of the iliac crest using flexible plastic tape without heavy outdoor closing. WC of ≥ 94 for males and ≥ 80 for females was considered high risk26. Weight and height to calculate BMI and were measured using calibrated equipment and BMI was calculated by dividing weight in kg by height in meters square. BMI < 18.5 kg/m2 was considered underweight, 18.5–24.9 kg/m2 as normal, 25–29.9 kg/m2 as overweight, and ≥ 30 kg/m2 as obese27.
A structured interviewer-administered questionnaire was used for data collection, and it was collected by four general practitioners working in the oncology department under the supervision of the principal investigator and one oncology specialist doctor. First, the patients were interviewed about their socio-demographic and behavioral characteristics. Then, all physical measurements were done, and finally, blood sugar levels and cancer-related characteristics were extracted from the medical file of patients. Both the interview and measures were completed at a single time point. The questionnaire was prepared in English, then translated to Amharic, then back-translated to English to maintain consistency. All the data collectors and supervisors took a one-day training on how to collect data. The principal investigator and the supervisor checked the collected data for its completeness and consistency daily.
Data processing and analysis
The survey data were entered into Epidata version 3.1 and analyzed by STATA 14 software. Descriptive statistics were used to describe the study population in relation to different variables, and it is presented using texts, graphs, and tables. The chi-square assumption was checked for all categorical independent variables. A binary logistic regression model was used to identify factors associated with elevated blood sugar. Both bi-variable and multivariable logistic regression models were carried out. Variables with a p-value of less than 0.2 in the bi-variable analysis were entered into the multivariable analysis. Both Crude Odds Ratio (COR) and AOR with a 95% confidence interval were estimated to show the strength of associations. Finally, p-value < 0.05 in the multivariable logistic regression analysis was used to declare a statistically significant association. Hosmer and Lemeshow goodness of fit test was used to check the goodness of fit of the model. All methods were performed in accordance with the relevant guidelines and regulations.
Ethics approval and consent to participate
The study protocol was approved by the ethical review committee of the college of Medicine and Health Sciences, University of Gondar. A letter of permission was also obtained from the oncology department. Informed consent was obtained from all subjects and/or their legal guardian. Respondents’ names and other personal identifiers were not included to keep confidentiality. The collected data was password protected.
Results
Socio-demographic and behavioral characteristics
A total of 384 cancer patients were included in the analysis after a response rate of 90.8%. More than half, 197(51.3%) of the participants were female, and 215(55.1%) were in the age group 41–60 years. More than two-thirds, 246(64.4%) of the participants were from rural areas, and 42(17%) had diabetes. Of the patients, 144(37.5%) were farmers and 87(60.4%) had prediabetes. Regarding alcohol consumption, 179(46.6%) are current alcohol consumers, and most 115(64.2%), have prediabetes. More than half, 202 (52.6%) of the participants had poor physical activity, and 172(45.6%) used liquid oil for cooking (Table 1).
Cancer-related and clinical characteristics
Of the total participants, the majority, 233(58.4%), were in stage I cancer and 264(68.8%) had no metastasis. Regarding cancer treatment, 317(82.6) have already started treatment; of these, more than one-third, 123(38.8%), had surgery. The majority, 178(46.4%) of the cancer patients, knew their diagnosis before 3–7 months, and 28(15.7%) of them were diagnosed to have diabetes. Besides, 230(59.9%) of participants have cancer pain. Regarding BMI, only 47(12.2%) were underweight (Table 2).
Prevalence of diabetes among cancer patients
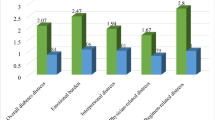
The proportion of elevated blood sugar among cancer patients was 73.4% (95% CI 68.8, 77.6). Of these, the proportion of prediabetes was 56.8% (95% CI 51.7, 61.7), and diabetes was 16.7% (95% CI 13.3, 20.8). Of the total DM cases identified, 26(40.6) were newly diagnosed, and the remaining were known DM patients on medication. The proportion of elevated blood sugar was highest (75.5%) among stage IV cancer patients and lowest (71.7%) among stage I (Fig. 1).
Factors associated with elevated blood sugar level
Of the nine independent variables included in the multivariable model, only alcohol consumption was found to have a statistically significant association with elevated blood sugar among cancer patients. The odds of elevated blood sugar increased by 96% among cancer patients who consume alcohol compared to their counterparts keeping other variables constant (AOR: 1.96; 95%CI: 1.11, 3.46) (Table 3).
Discussion
This study mainly investigated the prevalence of prediabetes and diabetes and its associated factors. The study found the proportion of diabetes and prediabetes among cancer patients to be 56.8% and 16.7%, respectively. Besides, alcohol consumption was found to be the independent risk factor for elevated blood sugar levels among cancer patients.
The proportion of prediabetes and diabetes in this study is higher than prospective cohort study conducted among cancer patients in the United States of America(USA), which reported the burden of prediabetes and diabetes at the end of the follow-up to be 21.2% and 32.6%, respectively28. The possible reason for this difference could be due to the kind of laboratory test used to assess the patient's blood sugar because the USA study uses hemoglobin A1c to diagnose prediabetes and diabetes in addition to FBS and RBS. Nonetheless, our study only uses FBS and OGTT to diagnose those diseases, which might increase our study's burden. The proportion of prediabetes and diabetes in this study is also much higher than other community-based studies conducted in Ethiopia which reported the burden of prediabetes and diabetes respectively to be 15.7% and 6.8%29, 9.3% and 6.3%30, 12% and 2.3%31, 15.9% and 6.5%32. This could be due to the difference in the study population since our study participants are only cancer patients, while the others are conducted in the general community. Both cancer and diabetes mellitus share common risk factors like age6. The risk of cancer increase with age due to the sequential accumulation of oncogenic mutations in a single clone and cell DNA damage accumulating over time33. Ageing also contributes to the pathogenesis of T2DM through the decreased β-cell function that accentuates the lack of insulin secretion34. In addition, cancer and DM share other risk factors such as obesity, physical inactivity, poor diet, alcohol consumption, and smoking6, which may increase the burden of elevated blood sugar among cancer patients. Insulin-like growth factor (IGF) is also a possible mechanism linking diabetes and cancer, as hyperinsulinemia causes a rise in the level of free and bioactive IGF-1, which increases the underlying risk of cancer in type 2 diabetic patients35,36,37. On the other hand, some studies suggest that cancer types such as pancreatic, colorectal and breast cancer increase the risk of diabetes11,38,39,40. Another reason for the higher prevalence in this study could be due to increased blood sugar caused by the cancer treatment. Corticosteroids are widely used for various purposes in patients with cancer, including for the prevention of chemotherapy-related hypersensitivity, management of brain metastasis11, and control of hematologic cancers41. These drugs are associated with an increased risk of hyperglycemia and diabetes since it causes reduced insulin sensitivity12, and this might increase the burden of diabetes among cancer patients. In addition, chemotherapies like L-asparaginase42, total body irradiation therapy14, and immunosuppressive agents such as Calcineurin inhibitors13 may also increase insulin resistance and the risk of elevated blood sugar. On the other hand, antidiabetic drugs such as pioglitazone, GLP-1 receptor antagonists, and insulin analogues are reported to be associated with bladder, medullary thyroid, and breast cancers, respectively, although there are some conflicting results43. Furthermore, cancer patients are more likely to lose weight and appetite, leading them to develop cancer cachexia syndrome11. This syndrome is linked to impaired glucose tolerance and diabetes due to various mechanisms44,45,46.
In this study, cancer patients who consume alcohol were found to have increased odds of having elevated blood sugar compared to their counterparts. This aligns with other studies conducted in Ethiopia47 and elsewhere48,49,50. This could be due to the effect of alcohol consumption on decreasing the function of pancreatic β-cells and increasing insulin resistance which may lead to hyperglycemia50. Alcohol beverages may also directly increase the blood sugar level51,52.
Although this study is the first in Ethiopia, it has some limitations. Due to the study design's cross-sectional nature, there is a problem of a chicken or egg dilemma. In addition, the study does not use Hemoglobin Alc to diagnose prediabetes and diabetes, which may overestimate the burden of those diseases. The study included all types of cancers, and the relation between each cancer type with blood glucose is not assessed. Besides, important factors such as the dietary habits of patients and paraneoplastic phenomena were not assessed. As a hospital-based study with a small sample size, it is impossible to generalize the findings to the general community.
Conclusion
The burden of prediabetes and diabetes is alarmingly high among cancer patients. Alcohol consumption was found to increase the odds of having elevated blood sugar among cancer patients. Hence, it is essential to recognize cancer patients are at high risk of having elevated blood sugar and design strategies to prevent this disease. It is also good to link cancer and diabetic clinics to give coordinated care that can improve the treatment outcome of both diseases. It is also recommended to advise cancer patients to avoid alcohol consumption. It is also important to do further prospective cohort studies to assess the temporal relationship between cancer and diabetes.
Availability of data and material
Data will be available from the corresponding author upon request.
Abbreviations
- AOR:
-
Adjusted odds ratio
- BMI:
-
Body Mass Index
- BP:
-
Blood pressure
- CI:
-
Confidence interval
- COR:
-
Crude odds ratio
- CSA:
-
Central statistical agency
- DBP:
-
Diastolic blood pressure
- DM:
-
Diabetes mellitus
- FBS:
-
Fasting blood sugar
- FMOH:
-
Federal ministry of health
- IDF:
-
International diabetes federation
- IGF:
-
Insulin-like growth factor
- IQR:
-
Inter quartile range
- NCD:
-
Noncommunicable disease
- RBS:
-
Random blood sugar
- SBP:
-
Systolic blood pressure
- UoGCSH:
-
University of Gondar comprehensive specialized hospital
- USA:
-
United States of America
- WC:
-
Waist circumference
- WHO:
-
World Health Organization
References
Misganaw, A., Mariam, D. H., Ali, A. & Araya, T. Epidemiology of major non-communicable diseases in Ethiopia: A systematic review. J. Health Popul. Nutr. 32(1), 1 (2014).
Solomon, S. & Mulugeta, W. Diagnosis and risk factors of advanced cancers in Ethiopia. J. Cancer Prevent. 24(3), 163 (2019).
Wurjine, T. H. & Goyteom, M. H. Prevalence of cancer pain, anxiety and associated factors among patients admitted to oncology ward, Tikur Anbessa Specialized Hospital, Ethiopia, 2019. Open J. Pain Med. 4(1), 009–017 (2020).
Bona, L. G. et al. Economic burden of cancer on cancer patients treated at Hawassa University comprehensive specialized hospital. Cancer Control 28, 10732748211009252 (2021).
Nandeshwar, S., Jamra, V. & Pal, D. Indian diabetes risk score for screening of undiagnosed diabetic subjects of Bhopal city. Natl. J. Commun. Med. 1(2), 176–177 (2010).
Giovannucci, E. et al. Diabetes and cancer: a consensus report. CA Cancer J. Clin. 60(4), 207–21 (2010).
Habib SL, Rojna M. Diabetes and risk of cancer. International Scholarly Research Notices. 2013;2013.
Noto, H., Tsujimoto, T., Sasazuki, T. & Noda, M. Significantly increased risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Endocr. Pract. 17(4), 616–628 (2011).
Noto, H., Osame, K., Sasazuki, T. & Noda, M. Substantially increased risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis of epidemiologic evidence in Japan. J. Diabetes Compl. 24(5), 345–353 (2010).
Wang, C. et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int. J. Cancer 130(7), 1639–1648 (2012).
Hwangbo, Y. et al. Incidence of diabetes after cancer development: A Korean national cohort study. JAMA Oncol. 4(8), 1099–1105 (2018).
Clore, J. N. & Thurby-Hay, L. Glucocorticoid-induced hyperglycemia. Endocr. Pract. 15(5), 469–474 (2009).
Davidson, J., Wilkinson, A., Dantal, J., Dotta, F., Haller, H., & Hernandez, D., et al. New-onset diabetes after transplantation: 2003 international consensus GUIDELINES1. Transplantation. 2003;75(10):SS3-SS24.
Meacham, L. R. et al. Diabetes mellitus in long-term survivors of childhood cancer: Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch. Intern. Med. 169(15), 1381–1388 (2009).
Barone, B. B. et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA 300(23), 2754–2764 (2008).
Lipscombe, L. L., Goodwin, P. J., Zinman, B., McLaughlin, J. R. & Hux, J. E. The impact of diabetes on survival following breast cancer. Breast Cancer Res. Treat. 109(2), 389–395 (2008).
Arreskov, A. B. et al. The impact of cancer on diabetes outcomes. BMC Endocr. Disord. 19(1), 1–9 (2019).
Shin, D. W. et al. Non-cancer mortality among long-term survivors of adult cancer in Korea: National cancer registry study. Cancer Causes Control 21(6), 919–929 (2010).
Karlin, N.J., Kosiorek. H.E., Castro, J.C., & Cook, C.B. Risk of hospitalization in patients with diabetes mellitus who have solid-organ malignancy. Fut. Sci. OA. 2016;2(3):FSO129.
Thong, M. S. et al. Diabetes mellitus and health-related quality of life in prostate cancer: 5-year results from the Prostate Cancer Outcomes Study. BJU Int. 107(8), 1223 (2011).
Lega, I. C. et al. The impact of diabetes on breast cancer treatments and outcomes: A population-based study. Diabetes Care 41(4), 755–761 (2018).
Judd A. Sustainable development goal 3: ensure healthy lives and promote well-being for all at all ages. 2020.
Riley, L. et al. The World Health Organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. Am. J. Public Health 106(1), 74–78 (2016).
Association AD. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes care. 2023;46:S19-S40.
Parati, G. et al. European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 32(7), 1359–1366 (2014).
Lau, D. C. et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ 176(8), S1–S13 (2007).
Organization WH. Obesity: preventing and managing the global epidemic. 2000.
Ose DJ, Viskochil R, Holowatyj AN, Larson M, Wilson D, Dunson WA, et al. Understanding the prevalence of prediabetes and diabetes in patients with cancer in clinical practice: A real-world cohort study. J. Natl. Compreh. Cancer Netw. 2021;1(aop):1–10.
Endris, T., Worede, A. & Asmelash, D. Prevalence of diabetes mellitus, prediabetes and its associated factors in Dessie Town, Northeast Ethiopia: A community-based study. Diabetes, Metabolic Syndrome Obes. Targ. Therapy. 12, 2799 (2019).
Wolde, H.F., Derso, T., Biks, G.A., Yitayal, M., Ayele, T.A., & Gelaye, K.A., et al. High Hidden burden of diabetes mellitus among adults aged 18 years and above in urban northwest Ethiopia. J. Diabetes Res.. 2020;2020.
Worede, A., Alemu, S., Gelaw, Y. A. & Abebe, M. The prevalence of impaired fasting glucose and undiagnosed diabetes mellitus and associated risk factors among adults living in a rural Koladiba town, northwest Ethiopia. BMC. Res. Notes 10(1), 1–7 (2017).
Aynalem, S.B., & Zeleke. A.J. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 years and above in Mizan-Aman town, Southwest Ethiopia, 2016: A cross sectional study. Int. J. Endocrinol. 2018;2018.
Laconi, E., Marongiu, F. & DeGregori, J. Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br. J. Cancer 122(7), 943–952 (2020).
Bellary, S., Kyrou, I., Brown, J. E. & Bailey, C. J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 17(9), 534–548 (2021).
Key, T. J. Diet, insulin-like growth factor-1 and cancer risk. Proc. Nutrit. Soc. 70(3), 385–388 (2011).
Larsson, O., Girnita, A. & Girnita, L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br. J. Cancer 92(12), 2097–2101 (2005).
Hua, F., Yu, J.-J. & Hu, Z.-W. Diabetes and cancer, common threads and missing links. Cancer Lett. 374(1), 54–61 (2016).
Lipscombe, L. et al. Incidence of diabetes among postmenopausal breast cancer survivors. Diabetologia 56(3), 476–483 (2013).
De Bruijn, K. M. & van Eijck, C. H. New-onset diabetes after distal pancreatectomy: a systematic review. Ann. Surg. 261(5), 854–861 (2015).
Singh S, Earle CC, Bae SJ, Fischer HD, Yun L, Austin PC, et al. Incidence of diabetes in colorectal cancer survivors. JNCI 2016;108(6).
Greenstein, S., Ghias, K., Krett, N. L. & Rosen, S. T. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin. Cancer Res. 8(6), 1681–1694 (2002).
Yoshida, H., Imamura, T., Saito, A.M., Takahashi, Y., Suenobu, S.-I., & Hasegawa, D., et al. Protracted administration of L-asparaginase in maintenance phase is the risk factor for hyperglycemia in older patients with pediatric acute lymphoblastic leukemia. PloS one. 2015;10(8):e0136428.
Laskar, J., Bhattacharjee, K., Sengupta, M. & Choudhury, Y. Anti-diabetic drugs: cure or risk factors for cancer?. Pathol. Oncol. Res. 24, 745–755 (2018).
Honors, M. A. & Kinzig, K. P. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J. Cachexia. Sarcopenia Muscle 3(1), 5–11 (2012).
Fearon, K. C., Glass, D. J. & Guttridge, D. C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 16(2), 153–166 (2012).
Porporato, P. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5(2):e200-e.
Dereje, N., Earsido, A., Temam, L., & Abebe A. Prevalence and associated factors of diabetes mellitus in Hosanna Town, Southern Ethiopia. Ann. Glob. Health. 2020;86(1).
Lee, D.-Y. et al. Association between alcohol consumption pattern and the incidence risk of type 2 diabetes in Korean men: a 12-years follow-up study. Sci. Rep. 7(1), 1–7 (2017).
Cullmann, M., Hilding, A. & Östenson, C. G. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a Swedish population. Diabet. Med. 29(4), 441–452 (2012).
Kim, S.-J. & Kim, D.-J. Alcoholism and diabetes mellitus. Diabetes Metab. J. 36(2), 108–115 (2012).
Teferra, S. et al. Hazardous alcohol use and associated factors in a rural Ethiopian district: a cross-sectional community survey. BMC Public Health 16(1), 1–7 (2016).
Getachew, T. et al. Magnitude and predictors of excessive alcohol use in Ethiopia: Findings from the 2015 national non-communicable diseases STEPS survey. Ethiop. J. Health Dev. 31(1), 312–319 (2017).
Acknowledgements
Firstly, we would like to forward our kindest regards to our study participants. We extend our thanks to the data collectors and supervisors without them, the report will not be materialized.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
A.A.K., H.F.W., M.D.M., A.H., E.B.M., Y.A.A., E.T.T., H.K., D.G.A., Y.B.W., and D.G.B. conceived and designed the study, acquired, analyzed, and interpreted data, prepared the manuscript, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wolde, H.F., Molla, M.D., Aragie, H. et al. High burden of diabetes and prediabetes among cancer patients at University of Gondar comprehensive specialized hospital, Northwest Ethiopia. Sci Rep 13, 9431 (2023). https://doi.org/10.1038/s41598-023-36472-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-36472-y
This article is cited by
-
Association of diabetes mellitus and breast cancer in adult men and women: a cross-sectional survey
BMC Cancer (2025)
-
Carcinogenic Link to Diabetes, Obesity and Atherosclerosis—A Focus on Differently Programmed Cholesterol Homeostasis
Indian Journal of Clinical Biochemistry (2025)