Abstract
Citrullus lanatus var. Colocynthoide “Gurum” is an unconventional crop that can be utilized as a new source of edible oil and has the ability to grow in a variety of harsh conditions. To mitigate the adverse effects of salinity on seed germination and plant performance of C. lanatus, seeds were primed in the aqueous extracts of the seaweed Ulva lactuca before planting under greenhouse conditions. The aqueous extract of U. lactuca at 8% w/v led to maximal seed germination percentage and seedling growth of C. lanatus. Moreover, U. lactuca extract counteracted the negative effects of salt stress on the plant by significantly increasing the activity of SOD, CAT, and POD. The bioactive components of U. lactuca, e.g. glycine betaine and phenolic compounds can account for such beneficial role of algal extract on C. lanatus. Thus, priming of C. lanatus seeds in U. lactuca extract with various concentrations of U. lactuca extract can be employed as an effective practice for successful seed germination, improved plant growth and enhanced salt resistance, probably as a result of increased antioxidant enzymes activity and photosynthetic pigments.
Similar content being viewed by others
Introduction
Salinity is one of the widely spread agricultural problems all over the world that can severely limit crop production1,2. It is estimated that up to 20% of the irrigated lands in worldwide are affected different extents by salinity3, while 2.1% of the arid lands are suffering from salt stress, and the problem is aggravating4. Salinity stress decreases plant growth by adversely affecting various physiological and biochemical processes5. Salinity stress decreases photosynthesis rate with a significant reduction in the chlorophyll content which eventually resulted in reduced sunflower yield6. One of the physiological changes occurring when plants are exposed to stress conditions is the enhanced production of reactive oxygen species (ROS)7. Excessive salt ions can exert dangerous effects like ion toxicity, osmotic stress, and nutritional imbalance8,9, which eventually lead to decreased growth and yield10. To cope with salinity stress, plants experience increased activity of antioxidant enzymes11.
Seed germination is a critical developmental phase in the life cycle of plants12 and is a major limiting factor for establishing plants under salinity conditions13. Salt stress affects percentage and rate of germination, as well as the subsequent seedling growth in different ways depending on plant species14. The impact of stress arising from more than one salt on seed germination is less severe than that arising from a single salt15,16. To overcome the impact of salt stress on plant growth and performance, seed priming can be an efficient and economical technique during the early stage of plant life17,18. Seed priming is a process in which seeds are hydrated in different solutions of natural or synthetic compounds17. Algal extracts have priming effects that can increase seed germination and plant growth under the impact of salinity and other abiotic stresses19,20.
Algae are rich in biologically active components, that have the potential to be used as soil cleansing agents, biofertilizers, plant growth promoters and can help in soil cleansing and; fertilization, and plant protectants agents from biotic and abiotic stress factors20,21,22. Due to their plentiful content of biostimulants, algae have been employed to boost plant performance and resistance to environmental stresses due to their plentiful biostimulant chemicals, plant performance, and tolerance to environmental stresses23. Bioactive compounds derived from marine algae have recently been used as biofertilizers for crops to increase the magnitude and yield of agricultural and horticultural crops and quality while minimizing environmental impacts24. Seaweed extracts contain a variety of growth hormones, polysaccharides, and macro- and micronutrients25. The extract of the seaweed Ulva rigida improved salt stress resistance and protect plants from oxidative damage arising from abiotic stress23,26.
Citrullus lanatus var. Colocynthoides (Gurum), family Cucurbitaceae, locally known as seed watermelon or “Gorma” is a promising fodder and oil crop in Egypt. The foliage of the plant is used as animal feed and its seeds have been investigated as an alternative source of vegetable oil27 and as a new source of high-quality pectin for commercial utilization28. However, seed germination and plant growth of C. lanatus are affected adversely by salinity. Meanwhile, the use of synthetic chemicals as priming agents for seeds under stress conditions has major negative environmental concerns. Therefore, this work is conducted to overcome the negative effects of salinity on seed germination and growth of C. lanatus through seed priming in algal extracts. The study hypothesized that priming of C. lanatus in extracts of the seaweed Ulva lactuca could improve seed germination and plant growth under the impact of salinity, probably via increasing the content of photosynthetic pigments, antioxidants and other bioactive constituents.
Materials and methods
Plant materials
Seaweed collection
Fronds of U. lactuca L. were collected from the coastal area of the Red Sea coast, near Hurghada, Egypt, in January 2021, and washed with tap water to remove any salt residues or other impurities. Then, the samples were dried and ground into fine powder with a Wiley mill, and kept in paper bags at room temperature until used. Citrullus lanatus var. Colocynthoides seeds were kindly provided by the Agricultural Research Center (ARC), Giza, Egypt.
Seaweed analysis
Assay of carbohydrates, lipids, proteins and glycine betaine
Soluble sugars were extracted from U. lactuca fronds according to the method adopted by Upmeyer and Koller29. To estimate insoluble sugars, the residue left after the extraction of soluble sugars was hydrolyzed by reflux in 0.2 N H2SO4 in a boiling water bath for 1 h30. Carbohydrate fractions were determined by the anthrone method31. The lipid content of seaweed was estimated by extracting an aliquot of 3 g of the powdered fronds in petroleum ether for 6 h in the Soxhlet system according to AOAC40, and the extraction was continued using as a solvent32. The soluble protein content of the alga was determined according to the method of Lowry et al.33, while total protein content was calculated by multiplying the Kjeldahl nitrogen content34 by 6.25. Assay of glycine betaine (GB) was carried out according to the method of Gorham35. Briefly, leaf extract was prepared by chopping 0.5 g of leaves in 5 mL of toluene-water mixture (0.05% toluene). The contents were shaken for 24 h at 25 °C. After filtration, 0.5 mL of the extract was mixed with 1 mL of 2 N HCl and 0.1 mL of potassium tri-iodide solution (containing 7.5 g iodine and 10 g potassium iodide in 100 mL of 1 N HCl) and the mixture was shaken in an ice-water bath for 90 min, and then 2 mL of ice-cooled water was added. After gentle shaking, 10 mL of dichloromethane (chilled at − 10 °C) was poured into the above mixture. By passing a continuous stream of air for 1–2 min, two layers were separated, the upper aqueous layer was discarded, and the absorbance of the organic layer was recorded at 365 nm. The concentration of GB was estimated by using a standard curve of GB in the range of 0–15 mg.
Proximate analysis of the seaweed
The detection of secondary metabolites (flavonoids, phenolics, alkaloids, saponins, tannins, and terpenoids) in U. lactuca aqueous extract was performed using the method of Sofowora36.
Quantitative determination of phenolic compounds
The phenolic content of U. latuca was determined using an Agilent-1100 HPLC system equipped with a quaternary gradient pump unit, an ultraviolet (UV) detector at 320 nm and a Zorbax Eclipse XDB-C18 analytical column (Agilent, USA) of 150 × 406 mm, 5 µm particle size. Elution was carried out at a flow rate of 0.075 mL min−1 at 23 °C. The mobile phase consisted of 8% acetonitrile, 22% isopropyl alcohol, and 70% formic acid solution (1%). All dissolved standards and samples were filtered through a 0.22 μm syringe filter before HPLC analysis. Seaweed aqueous extracts were frozen at − 20 °C for 24 h before drying in a frieze dryer, and the residues were dissolved in methanol (HPLC grade) before injecting into the HPLC; the injection volume was 20 μl. Identification of phenolic compounds was made by comparing the relative retention times of the sample peaks with those of the reference standards.
Preparation of U. lactuca seaweed aqueous extract
One hundred grams of U. lactuca powder were extracted by soaking in 1000 mL distilled water for 24 h, with shaking using an orbital shaker at 25 °C; the mixture was then centrifuged at 5000 rpm for 20 min to remove the debris. The extract was filtered through Whatman No. 4 filter paper, and the final volume was completed to 1 L with distilled water to obtain the stock extract (10% w/v). The resulting extract was stored at − 4 °C until used.
Effect of seaweed extracts on seed germination and plant growth of C. lanatus
The experiment was conducted during 2021/2022 at the Faculty of Science, Al-Azhar University (Girl Branch), Egypt. Plastic pots (20 cm in diameter and 20 cm in height) were filled with 3 kg of a clay/sand mixture (1:1) with the addition of the recommended doses of ammonium sulphate, ammonium nitrate and potassium sulphate fertilizers before sowing. The chemical analysis of the experimental soil mix was conducted at the beginning and the end of the experiment. Soil–water extracts were prepared by soaking 100 g of soil in 500 mL of distilled water for 1:5, soil extract and the filtrate was used for mineral analysis37. Soil chemical analysis revealed the following characteristics: pH (7.82–8.27), EC (0.36–0.56 dS m−1), and the mineral content (meq L−1): Ca2+ (12.455–13.23), Mg2+ (4.165–13.27), Na+ (26.63–36.44), K+ (2.32–2.62), CO32− (98.86–68.48), HCO3− (779–752), S (2.21–2.42) and Cl− (12.16–15.25) for the pre-and post-cultivation soil samples, respectively; the water holding capacity was up to 13.0%38.
Twenty seeds of C. lanatus were sown in each pot and planted in April after soaking for 6 h in U. lactuca extracts at four concentrations (0, 3, 5, and 8%), The pots were watered with four salinity levels (0,100, 200, and 300 mM NaCl) every 7 days until the end of the experiment. After complete emergence (10 days), number of seedlings was counted39, and seedlings were sequentially thinned to five per pot across the following five days. Pots were arranged in a randomized complete block design with three replicates in the greenhouse at 34 °C on average with 16 h of light and 8 h of darkness.
Plant harvesting and analysis
Assay of plant growth and photosynthetic pigments
Three representative plant samples were taken from each treatment for measuring of growth traits after 45 days of growth: shoot length (cm), root length (cm), and the number of branches were measured; leaf area (cm2), was measured using leaf area meter (SYSTRONICS Leaf Area Meter-211). Photosynthetic pigments (chlorophyll a [Chl a], chlorophyll b [Chl b] and carotenoids [C(x + c)] were quantitatively determined spectrophotometrically according to the procedure adopted by Metzner et al.40 and, Abbas41. An aliquot of the fresh leaf tissue (0.5 g) was homogenized in a mortar with 10 mL of 80% acetone; the homogenate was centrifuged at 3000 rpm for 15 min at room temperature, and the supernatant was stored at 4 °C. The absorbance of the extract was measured at 645, 663 and 480 nm using a spectrophotometer (VEB Carl Zeiss). Chlorophyll a, chlorophyll b, and carotenoids were determined as µg g−1 leaf fresh weight using the following equations:
The concentrations of chlorophylls and carotenoids were expressed as mg g−1 fresh weight (FW) of plant material.
Estimation of C. lanatus antioxidant enzyme activities
An aliquot of the fresh leaves (0.5 g) was homogenized in liquid nitrogen with the addition of 5 mL of 0.2 mol L−1 of sodium phosphate buffer solution (pH 7.8). Homogenates were centrifuged for 20 min at 4 °C, and supernatants were immediately used to determine enzyme activity. Total soluble protein content of the leaves was determined according to the procedure of Bradford and Williams42. Superoxide dismutase (SOD) activity was assayed in terms of the extent of inhibition of the photochemical reduction of p-nitro blue tetrazolium chloride (NBT)43. Catalase (CAT) activity was determined based on the rate of disappearance of H2O2, as measured by the decline in absorbance at 240 nm44. Peroxidase activity (POD) was calculated from the rate of formation of guaiacol dehydrogenation product and was expressed as mmol GDHP min−1 mg−1 protein45.
Data analysis
The data were subjected to two-way ANOVA according to Snedecor and Cochran46 using SPSS ver. 26.0, and considering p < 0.05 as a significant level. Tukey's HSD is used to test differences among sample means for significance. Spearman correlation was done by Sigmaplot 12.5, to assess the effects of seaweed and salinity on germination, seedling growth and plant performance.
Ethical approval
All the steps of experimentation on C. lanatus var. Colocynthoide plants, including the collection of plant material, are in compliance with relevant Institutional, National, and International guidelines. The greenhouse studies were conducted in accordance with local legislation and with permissions from our institutes and complied with the IUCN Policy Statement.
Results and discussions
Chemical composition of Ulva lactuca
Results of the biochemical analyses of U. lactuca fronds indicate that the algal biomass contained (one dry weight basis): 0.93 mg g−1 total carbohydrates, 3.01 mg g−1 of lipids, and 3.21 mg g−1 protein, in addition to appreciable content of glycine betaine (5.13 mg g−1) and total phenolics (0.0188 mg g−1). This result is in agreement with Latique et al.47 who found that Ulva rigida extract is rich in soluble sugars, polyphenols, and proteins which might be necessary for the stimulation of antioxidant enzymes. Glycine betaine is an osmoregulatory molecule that enables plant cells to cope with salt stress48.
The aqueous extract of U. lactuca contained appreciable content of saponins, alkaloids, and tannins along with high content of flavonoids and phenolics but lacks terpenes. Hence, the high phenolic and flavonoid content of U. lactuca extract may account for its high efficiency in the enhancement of C. lanatus and alleviation of the impact of salt stress.
Nine free compounds were identified in the aqueous extract of U. lactuca extract (Table 1) by reverse-phase high-performance liquid chromatography (HPLC). These compounds were identified based on their relative retention times. Ascorbic acid and coumaric acid amounted to about 48.59 and 36.23 µg g−1 DW, respectively, and were the two main components in U. lactuca extract. Caffeic, ferulic, protocatechuic and pyrogallic acids, and resorcinol all recorded moderate amounts of 29.3, 19.67, 25.92, 19.72, and 25.3 µg g−1 DW, respectively. But, chlorogenic and salicylic acids were detected with relatively low quantities of 15.33 and 16.97% μg g−1 DW, respectively. U. lactuca methanolic extract has been reported to contain phenolics, flavonoids, alkaloids, and tannins49. Phenolics are identified as the largest group that can cause a stabilized electrolytic effect, as the substances are soluble in water50. Phytohormones, amino acids, polyphenols, carbohydrates, alginates, essential macro- and micronutrients, betaines, and vitamins are among the physiologically active compounds found in seaweed extracts that stimulate plant growth and development28. Klejdus et al.51 and Kovacik et al.52 who found a variety of phenolic compounds in algae. Algae are rich in biologically active components such as phenolic compounds, polysaccharides, hormone-like substances, and proteins53.
Effect of U. lactuca an aqueous extract on C. lanatus seed germination
Figure 1 shows that priming of C. lanatus seeds in U. lactuca extracts significantly improved germination of seeds, and the increase was most evident at high salinity, Increasing concentration of algal extract during priming from 0 to 0.8% increased. Germination percentage of C. lanatus seeds by 24.5%, 53.9%, 61.3%and 66.7% at 0.0, 100, 200 and 300 mM NaCl salinity, respectively. Increasing NaCl salinity reduced seed germination and the magnitude of reduction differed according to the concentration of the priming algal extract. Whereas increasing salinity from 0 to 300 mM NaCl led to progressive 20.3% reduction in seed germination in absence of algal extract, the reductions were relatively mild and amounted to 80.3%, 65.0%, 60.0 and 60.0% at 3%, 5% and 8% algal extract, respectively. The germination in C. lanatus was affected significantly by algal concentrations (F = 44.73, p < 0.000) and salt concentrations (F = 14.07, p < 0.000), while, there was interaction within algal concentrations and salt concentrations (F = 6.25, p < 0.02) (Table 2). Salt stress presents a threat to C. lanatus germination, growth parameters, and photo/biosynthetic materials. Therefore, Algal extracts from U. lactuca can be used as priming practices to promote C. lanatus cultivation under salinity stress. Seeds are usually soaked in an osmotic solution that allows them to imbibe water and go through the first stages of germination54. Seed pretreatment with algal extract that contains bioactive substances can stimulate the metabolic process in C. lanatus seeds under the impact of salinity stress. Seed presoaking can stimulated the antioxidant enzyme activities that play a vital role in C. lanatus resistance against salt stress. The beneficial et of algal extract in improvement of seed germination percentage and the subsequent plant growth of C. lanatus under the impact of salinity can be attributed to its effect on seed surface permeability to salts, and its content of bioactive components in U. lactuca extract of glycine betaine and phenolics and ascorbic acid could potentially participate in the alleviation of salinity stress. Thus, priming of C. lanatus seeds with U. lactuca extract could induce many physiological and biochemical changes that can stimulate seeds to germinate faster and increase the germination percentage of C. lanatus. Algal extracts increase seed germination and seedling growth in several plants spices with alleviation of the impact of a biotic stress55,56,57,58 and fruit production59.
Effect of U. lactuca extract on the growth of C. lanatus
Shoot length, root length, number of branches and leaf area of C. lanatus significantly increased with priming in U. lactuca extract under various levels of sodium chloride as compared to its controls (no salt-no extract). Figure 2 shows that priming of C. lanatus seeds in U. lactuca extracts at 0.8% significantly improved C. lanatus by 42.25%, 53.14%, 70.80% and 100.0% (shoot length), 143.90%, 169.16%, 210.0% and 265.38% (root length), 100.0%, 75.0%, 133.33% and 300.0% (number of branches), 87.50%, 111.11%, 22.8% and 140.0% (leaf area) at 0.0, 100, 200 at 0.0, 100, 200 and 300 mM NaCl salinity, respectively. Whereas increasing salinity led to progressive 30.28%, 36.58%, 50.0% and 41.56% reduction in shoot length, root length, number of branches and leaf area from 0 to 300 mM NaCl in absence of algal extract. The reductions were relatively mild and amounted to 15.85%, 20.42%, and 30.2% (shoot length), 18.53%, 26.82%, and 36.58% (root length), 0.0%, 25.0%, and 50.0% (number of branches), 29.68%, 30.78% and 41.56% (leaf area) at 3%, 5% and 8% algal extract, respectively. The traits in C. lanatus of shoot length were affected by algal concentrations significantly (F = 27.27, p < 0.000) and salt concentrations (F = 16.05, p < 0.000), root length by salt concentrations (F = 18.015, p < 0.000), number of branches by algal concentrations (F = 6.06.575, p < 0.02) and by salt concentrations (F = 5.7, p < 0.00) and leaf areas by algal concentrations (F = 17.54, p < 0.0000) and salt concentrations (F = 11.64, p < 0.0000) (Fig. 2, Table 2). The highest increment in shoot length was obtained from U. lactuca extract at 8% concentration to reach about 42.25, 53.14, 70.80 and 100.0%, of 0, 100, 200, and 300 mM NaCl, respectively. The greatest increment was achieved at 8% of root length reaching about 143.9, 169.16, 210.0 and 265.38%, of 0, 100, 200, and 300 mM NaCl, respectively. The use of U. lactuca extract at 8% increased the total number of branches per plant significantly to give about 100.0, 75.0, 133.3 and 300.0%, of 0, 100, 200, and 300 mM NaCl, respectively. The leaf area increased significantly with the application of U. lactuca extract, and the highest increment was attained at 8% algal extract to give about 87.5, 111.1, 122.8 and 186.1%, with all NaCl levels of 0,100, 200, and 300 mM, respectively.
The priming of C. lanatus seeds in U. lactuca aqueous extract improved plant growth and photosynthetic pigments significantly under the impact of salinity. Shoot length, root length and leaf area as well as the content of photosynthetic pigments have been reported to increase in the salinized plants in response to presoaking of seeds in Ulva fasciata extract60,61. The beneficial effect of primed cherry tomato seeds in Ulva flexuosa extract on could be attributed to the high concentration of glycine betaine in the algal extract62. In support to this claim, exogenous application of glycine betaine significantly increased plant height and the number of leaves of Dalbergia odorifera seedlings under mild and severe salinity63.
Effect of U. lactuca extracts on photosynthetic pigments of C. lanatus
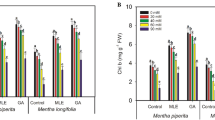
The effects of salinity and algal priming on leaf photosynthetic pigments of C. lanatus were substantial (Table 3), with relatively stronger effect of salinity than algal priming and very highly significant interaction, except for the non-significant interaction on carotenoid content. The content of chlorophyll a, chlorophyll b, and carotenoids increased progressively by increasing the concentration of U. lactuca extract. The increase in chlorophyll a, chlorophyll b, and carotenoids at 8% aqueous algal extract amounted to 469%, 918%, and 961% respectively above the non-treated control (Fig. 3). Our results are in agreement with Ahmed et al.64 who found that the content of chlorophylls a and b and carotenoids in cotton leaves was significantly improved by algal extract as compared to the control. The application of U. rigida extract to wheat plants under salt stress conditions led to a significant increase in Total Chl (T-Chl), Chl-a, and Chl-b65,78. Leaf pigments are a major physiological attribute that is directly related to the photosynthesis process under various environmental conditions66. This increase in chlorophyll content could be a result of the protection of chlorophyll from degradation that might be attributable to betaines of the seaweed extract67. Seaweed extracts by virtue of their high mineral content, particularly, magnesium and can increase leaf chlorophyll and carotenoid concentration68. Application of extracts of Chlorella liposies and Spirolina maxima to wheat plants under salt-stress caused a significant increase in the content of Chl-a and Chl-b69,70,71. The application of Ulva fasciata extracts significantly increased the production of Vigna sinensis and Zea mays growth and photosynthetic pigments under salt stress conditions72.
Effect of U. lactuca extracts on superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) activities of C. lanatus
The results in Fig. 4a–c revealed that the algal extract at 3%, 5%, and 8% significantly induced SOD, CAT, and POD activities under the tested salt levels. The maximum activity of SOD, CAT, and POD was recorded at 8% algal extract with 10.23, 8.08, and 22.5 µg−1 protein, respectively. The increasing values at 8% algal extract were 98.51, 84.63, 86.59 and 78.85% (SOD), 191.64, 131.01, 58.43, and 39.39% (CAT) and 68.62, 51.0, 36.94 and 34.71% (POD), in NaCl levels of 0,100, 200, and 300 mM respectively compared with it’s the control. The SOD activity of C. lanatus was affected significantly by algal concentrations (F = 6.22, p < 0.02) and salt concentrations (F = 11.51, p < 0.000), while, there was an interaction between algal concentrations and salt concentrations (F = 5.83, p < 0.000). The CAT was affected significantly by algal concentrations (F = 66.99, p < 0.00) and salt concentrations (F = 61.30, p < 0.000), and there was a significant interaction between algal concentrations and salt concentrations (F = 22.28, p < 0.000). The POX was affected significantly by algal concentrations (F = 185.04, p < 0.00) and salt concentrations (F = 64.63, p < 0.000), and there was a significant interaction between algal and salt concentrations (F = 36.29, p < 0.000) (Table 3). The algal extract could enhance the activity of SOD, CAT, and POD in C. lanatus along with improving salt tolerance that boosts the plant development under stress conditions. Salinity tolerance is strongly associated with enhanced antioxidant enzyme activities as well as high content of non-enzymatic antioxidants73. The role of antioxidant enzymes in salt-resistance has been reported in many plant species (Arabidopsis, barley, pea, and tobacco)74,75,76. Macroalgae have been recognized as a rich source of antioxidants77. Algal extract of U. rigida antagonizes the harmful effects of abiotic stress by increasing the activity of antioxidant enzymes78. Ulva rigida extract led to a significant promotion in antioxidant enzyme activity in salt-stressed wheat plants79. Superoxide dismutase (SOD) is the first enzyme that acts against reactive oxygen species80. A significant enhancement in catalase (CAT) and superoxide dismutase (SOD) activities was observed in wheat plants treated with 25% of U. rigida extract under salt stress conditions81. Superoxide dismutase, catalase and peroxidases as well as ascorbate peroxidase have been claimed to protect plants against the harmful effects of reactive oxygen species82,83. The content of soluble sugars, polyphenols, and proteins in Fucus spiralis extract may account for the increased salt resistance of durum wheat, probably via stimulation of antioxidant enzymes47.
Furthermore, germination and growth traits were positively correlated to enzyme activity and pigments (Table 4). Shoot length, root length, and germination were associated and positively correlated with chlorophyll (b) by 0.984, 0.984, and 0.943, respectively. The number of branches and leaf area were correlated positively with carotenoids by 0.985, and 0.996, respectively. Shoot length, root length, Number of branches and leaf area recorded the strongest correlation with CAT enzyme by 0.924, 0.895, 0.881, and 0.887, respectively. Germination recorded a positive correlation with SOD, CAT, POD enzymes by 0.697, 0.826, and 0.780, respectively. Seed priming boosts pre-germination metabolic activities, boosts antioxidant system activity, and speeds up membrane mending84. The promoting priming effects of algal extracts in plant growth can contributed to the supply of plant nutrients from the soils85. Some of these elements are directly related to leaf pigments or catalyze some physiological process that leads to promoting the production of biochemical substitutes86. Seaweed extracts enhanced the biochemical constituents in crops87. Algal extracts can enforce different physiological and biochemical mechanisms to improve the salinity tolerance of plants60. Seaweed extract of U. rigida can increase salt stress tolerance and protect wheat plants against oxidative deterioration88. Macroalgae could enhance plant salt tolerance through the increasing antioxidant compounds, compounds such as ascorbic acid and polyphenols, which might be necessary for the stimulation of antioxidant enzymes47. Algal extracts can relieve the effects of salt stress through the activation of metabolic pathways that contribute to promoting plant growth and yield89. The enhancement of plant salt resistance was associated with an increase in water use efficiency, photosynthetic activity, phenolic compounds, and ROS scavenging activity. Glycine betaine is involved in mitigating salt stress in plants through osmotic adjustment and ROS scavenging90. Ulva rigida extract is rich in glycine betaine, which delays the loss of photosynthetic activity by inhibiting chlorophyll degradation67. Exogenous application of glycine betaine significantly increased plant height and number of leaves of Dalbergia odorifera under mild and severe salinity stress conditions63. It could be concluded that the aqueous extract of the seaweed U. lactuca can boost seed germination percentage, plant growth, photosynthetic pigments and performance of C. lanatus under salt stress conditions by virtue of their contents of bioactive components and biochemical parameters, they could stimulate the tolerance response of C. lanatus toward biotic stress of salts. Therefore, this research advised breeders to use seaweed extract as an effective seed priming technique to promote C. lanatus germination and growth is an effective under salt stress conditions for increasing plant productivity.
Data availability
The datasets used during the current study are available from the corresponding author upon reasonable request.
References
Liu, J., Xia, J., Fang, Y., Li, T. & Liu, L. Effects of salt drought stress on growth and physiobiochemical characteristics of Tamarix chinensis seedlings. Sci. World J. 2014, 1–7. https://doi.org/10.1155/2014/532713 (2014).
Li, W. et al. A Salt tolerance evaluation method for sunflower (Helianthus annuus L.) at the seed germination stage. Sci. Rep. 10, 10626. https://doi.org/10.1038/s41598-020-67210-3 (2020).
Mostafazadeh-Fard, B., Heidarpour, M., Aghakhani, Q. A. & Feizi, M. Effect of irrigation water salinity and leaching on soil chemical properties in an arid region. Int. J. Agric. Biol. 3, 166–469 (2007).
Rajabi Dehnavi, A., Zahedi, M., Ludwiczak, A., Cardenas, P. S. & Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 10, 859. https://doi.org/10.3390/agronomy10060859 (2020).
Wu, G. et al. Comparative ecophysiological study of salt stress for wild and cultivated soybean species from the Yellow River Delta, China. Sci. World J. 20, 1–13. https://doi.org/10.1155/2014/651745 (2014).
Amin, M. A. et al. The potency of fungal-fabricated selenium nanoparticles to improve the growth performance of Helianthus annuus L. and control of cutworm Agrotis ipsilon. Catalysts 11, 1551. https://doi.org/10.3390/catal11121551 (2021).
Dioniso-Sese, M. L. & Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 135, 1–9. https://doi.org/10.1016/S0168-9452(98)00025-9 (1998).
Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 25, 239–250 (2002).
Poustini, K. & Siosemardeh, A. Ion distribution in wheat cultivars in response to salinity stress. Field Crop Res. 85(2), 125–133 (2004).
Ashraf, M. & Ali, Q. Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ. Exp. Bot. 63, 266–273. https://doi.org/10.1016/j.envexpbot.2007.11.008 (2008).
Motylevam, S. E., Vlasova, N. K., Gins, M. & Gins, V. Morphological and anatomical characterization of Actinidia kolomikta Rupr. & Maxim. Maxim. (C3) and Amaranthus tricolor L. (C4) leaves. Agron. Res. 20(2), 341–356 (2022).
Saberali, S. F. & Moradi, M. Effect of salinity on germination and seedling growth of Trigonella foenum-graecum, Dracocephalum moldavica, Satureja hortensis and Anethum graveolens. J. Saudi Soc. Agric. Sci. 18(3), 316–323 (2019).
Besma, B. D. & Mounir, D. Biochemical and mineral responses of okra seeds (Abelmoschus esculentus L. variety Marsaouia) to salt and thermal stresses. J. Agron. 9(2), 29–37. https://doi.org/10.3923/ja.2010.29.37 (2010).
Tian, Y., Guan, B., Zhou, D., Yu, L. J. & Lou, Y. Responses of seed germination, seedling growth, and seed yield traits to seed pretreatment in maize (Zea mays L.). Sci. World J. https://doi.org/10.1155/2014/834630 (2014).
Tobe, K., Li, X. & Omasa, K. Effects of five different salts on seed germination and seedling growth of Haloxylon ammodendron (Chenopodiaceae). Seed Sci. Res. 14, 345–353. https://doi.org/10.1079/SSR2004188 (2004).
Orlovsky, N. S., Japakova, U. N., Shulgina, I. & Volis, S. Comparative study of seed germination and growth of Kochia prostrata and Kochia scoparia (Chenopodiaceae) under salinity. J. Arid Environ. 75, 532–537. https://doi.org/10.1016/j.jaridenv.2011.01.014 (2011).
Jisha, K. C., Vijayakumari, K. & Puthur, J. T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 35, 1381–1396. https://doi.org/10.1007/s11738-012-1186-5 (2013).
Ibrahim, E. A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 192, 38–46. https://doi.org/10.1016/j.jplph.2015.12.011 (2016).
El-Sheekh, M. M. & El -Saied A.,. Effect of crude seaweed extracts on seed germination, seedling growth and some metabolic processes of Vicia faba L.. Cytobios 101, 23–33 (2000).
Górka, B., Korzeniowska, K., Lipok, J. & Wieczorek, P. P. The biomass of algae and algal extracts in agricultural production. In Algae Biomass: Characteristics and Applications 103–114 (Springer, 2018).
Ronga, D. et al. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 9, 192 (2019).
Jafarlou, M. B., Pilehvar, B., Modarresi, M. & Mohammadi, M. Performance of algae extracts priming for enhancing seed germination indices and salt tolerance in Calotropis procera (Aiton) WT.. Iran J. Sci. Technol. Trans. Sci. https://doi.org/10.1007/s40995-021-01071-x (2021).
Sharma, H. S., Fleming, C., Selby, C., Rao, J. R. & Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 26, 465–490 (2014).
Reddy, C. A. & Saravana, R. S. Polymicrobial multi-functional approach for enhancement of crop productivity. In Advances in Applied Microbiology, Vol***82 (eds Sariaslani, S. & Gadd, G. M.) 53–113 (Academic Press, 2013).
Craigie, J. S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 23, 371–393. https://doi.org/10.1007/s10811-010-9560-4 (2011).
Gharib, F., Zeid, I. M., Salem, O. & Ahmed, E. Z. Effects of Sargassum latifolium extract on growth, oil content and enzymatic activities of rosemary plants under salinity stress. J. Life Sci. 11, 933–994 (2014).
Ziyada, A. K. & Elhussien, S. A. Physical and chemical characteristics of CitrulluslanatusVar Colocynthoide seed oil. J. Phys. Sci. 19(2), 69–75 (2008).
Mahmoud, S. H., Salama, D. M., El-Tanahy, A. M. M. & Abd El-Samad, E. H. Utilization of seaweed (Sargassum vulgare) extract to enhance growth, yield and nutritional quality of red radish plants. Ann. Agric. Sci. 64, 167–175. https://doi.org/10.1016/j.aoas.2019.11.002 (2019).
Upmeyer, D. J. & Koller, H. R. Diurnal trends in net photosynthetic rate and carbohydrate levels of soybean leaves. Plant Physiol. 51, 871–874. https://doi.org/10.1104/pp.51.5.871 (1973).
Streeter, J. G. & Jeffers, D. L. Distribution of total nonstructural carbohydrates in soybean plants having increased reproductive load. Crop Sci. 19, 729–734 (1979).
Hedge, J. E. & Hofreiter, B. T. Methods. In Carbohydrate Chemistry, ***Vol 17 (eds Whistler, R. L. & BeMiller, J. N.) 420 (Academic Press, 1962).
A.O.A.C. Official Methods of Analysis. Association of Official Analytical Chemists 16th edn. (K Hlrich, 1995).
Cunniff, P. & Washington, D. C. Official methods of analysis of AOAC international. J. AOAC Int. 80, 127A (1997).
Lowry, O. H., Rosenbrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
James, C. S. Analytical Chemistry of Foods 178 (Blackie Academie, 1995).
Gorham, J. Separation of plant betaines and their sulphur analogues by cation-exchange high-performance liquid chromatography. J. Chromatogr. A 287, 345–351 (1984).
Sofowora, A. Medicinal Plants and Traditional Medicinal in Africa. Screening Plants for Bioactive Agents 2nd edn, 134–156 (Spectrum Books Ltd., 1993).
ISTA. International rules for seed testing. Seed Sci. Technol. 27, 1–333 (1999).
Mohamed, K. Potential utilization of Citrullus lanatus var. Colocynthoides waste as a novel source of pectin. J. Food Sci. Technol. 52, 2401–2407 (2015).
Metzner, H., Rau, H. & Senger, H. Untersuchungen zur Synchronisierbakeit einzelner Pigmentmangel-Mutanten von Chlorella. Planta 65, 186–194 (1965).
Abbas, S. M. Effects of low temperature and selenium application on growth and the physiological changes in sorghum seedlings. J. Stress Physiol. Biochem. 8, 269–286 (2012).
Bradford, M. M. & Williams, W. L. New, rapid, sensitive method for protein determination. Fed. Proc. 35, 274 (1976).
Zhang, S., Gan, Y. & Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 7, 1405. https://doi.org/10.3389/fpls.2016.01405 (2016).
Li, Y. et al. Mechanisms of tolerance differences in cucumber seedlings grafted on rootstocks with different tolerance to low temperature and weak light stresses. Turk. J. Bot. 39, 1–9. https://doi.org/10.3906/bot-1404-115 (2015).
Bałabusta, M., Szafranska, K. & Posmyk, M. M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 7, 575. https://doi.org/10.3389/fpls.2016.00575 (2016).
Snedecor, G. W. & Cochran, W. G. Statistical Methods, 2nd Printing 7th edn. (Iowa State University Press, 1982).
Latique, S., Mohamed, A. E., Halima, C., Chérif, H. & Mimoun, E. K. Alleviation of salt stress in durum wheat (Triticum durum L.) seedlings through the application of liquid seaweed extracts of Fucus spiralis. Commun. Soil Sci. Plant Anal. 48(21), 2582–2593 (2017).
Sakamoto, A. & Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 25(2), 163–171. https://doi.org/10.1046/j.0016-8025.2001.00790.x (2002).
Morsy, G. M. T., Elsayed, K., Entesar, A. M. & Rawhya, A. Phytochemical screening for antibacterial compounds of some seaweeds from coastal area of Abo-Qir, Alexandria, Egypt. Egypt. J. Phycol. 19, 47–57 (2018).
Ahmad, P., Azooz, M. M. & Prasad, M. N. V. Ecophysiology and Responses of Plants Under Salt Stress 283–314 (Springer, 2013).
Klejdus, B., Lojková, L., Plaza, M., Snóblová, M. & Stěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 1217(51), 7956–7965. https://doi.org/10.1016/j.chroma.2010.07.020 (2010).
Kovacik, J., Gruz, J., Backor, M., Strnad, M. & Repcak, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 28, 135–143. https://doi.org/10.1007/s00299-008-0627-5 (2009).
Chiaiese, P., Corrado, G., Colla, G., Kyriacou, M. C. & Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 9(3), 1782. https://doi.org/10.3389/fpls.2018.01782 (2018).
Heydecker, W. Germination of an idea: The priming of seeds. University of Nottingham, School of Agriculture Rep. 1973/74 http://www.jstor.org/stable/4034044 (1973).
Mohan, V. R., Venkataraman, K., Murgeswari, R. & Muthusamy, S. Effect of crude and commercial seaweed extract on seed germination and seedling growth in Cajanus Cajan L.. Phykos 33, 47–51 (1994).
Gencer, O. A. H. Effect of seaweed Ascophyllum nodosum extract and seaweed Durvilla potatorum suspension on morphological and technological properties of cotton Gossypium hirsutum L. Proc FAO-IRCRNC; cotton nutrition and growth regulators, pp. 177–182, Cairo, Egypt (1997).
Calvo, P., Nelson, L. & Kloepper, J. W. Agricultural uses of plant biostimulants. Plant Soil 383, 3–41. https://doi.org/10.1007/s11104-014-2131-8 (2014).
Mansori, M. et al. Effect of seaweed extract (Ulva rigida) on the water deficit tolerance of Salvia officinalis L.. J. Appl. Phycol. 28, 1363–1370 (2016).
Mehta, P. M., Mini, S. N. & Gajaria, S. C. Impact of extracts of higher plants and algae on germination, seedling growth and oxidizing enzymes of rice seedlings. Adv. Plant Sci. 12, 567–572 (1999).
Khan, W. et al. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 28, 386–399. https://doi.org/10.1007/s00344-009-9103-x (2009).
Salah El Din, R. A., Elbakry, A. A., Ghazi, S. M. & Abdel Hamid, O. M. Effect of seaweed extract on the growth and yield of faba bean (Vicia faba L.). Egypt. J. Phycol. 9(1), 25–38 (2008).
Chanthini, K. M. P. et al. Sustainable agronomic strategies for enhancing the yield and nutritional quality of wild tomato, Solanum lycopersicum (l) var Cerasiforme Mill. Agronomy 9, 1–22. https://doi.org/10.3390/agronomy9060311 (2019).
Cisse, E. H. M. et al. Glycine betaine surpasses melatonin to improve salt tolerance in Dalbergia odorifera. Front. Plant Sci. 12, 1–12. https://doi.org/10.3389/fpls.2021.588847 (2021).
Ahmed, H. S. A., Hussein, M. H. A., El-Said, G. A. E. & El-Syed, H. A. M. Effect of growing area on yield traits, fiber and yarn properties in Egyptian cotton genotypes by using biofertilizer inoculation, vermicompost and algae extract. J. Plant Prod. Mansoura Univ. 12, 1087–1100. https://doi.org/10.21608/JPP.2021.98337.1065 (2021).
Iyengar, E. R. & Reddy, M. P. Photosynthesis in highly salt tolerant plants. In Handbook of Photosynthesis (ed. Pesserkali, M.) 897–909 (Marshal Dekar, 1996).
Badawy, A. A., Abdelfattah, N. A. H., Salem, S. S., Awad, M. F. & Fouda, A. Efficacy assessment of biosynthesized copper oxide nanoparticles (Cuo-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.). Plant Biol. (Basel) 10, 233. https://doi.org/10.3390/biology10030233 (2021).
Whapham, C. A., Blunden, G., Jenkins, T. & Hankins, S. D. Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J. Appl. Phycol. 5, 231 (1993).
Mohy El-Din, S. M. Utilization of seaweed extracts as bio-fertilizers to stimulate the growth of wheat seedlings. Egypt J. Exp. Biol. 11, 31–39 (2015).
Raupp, J. & Oltmanns, M. Farmyard manure, plant based organic fertilizers, Inorganic fertilizer-which sustains soil organic matter best. Asp. Appl. Biol. 79, 273–276 (2006).
Yassen, A. A., Badran, N. M. & Zaghloul, S. M. Role of some organic residues as tools for reducing metals hazard in plant. World J. Agric. Sci. 3(2), 204–209 (2007).
Abd El-Baky, A., Hussein, M. M. & Game, S. E. Algal extracts improve antioxidant defense abilities and salt tolerance of wheat plant irrigated with sea water. Afr. J. Biochem. Res. 2, 151–164. https://doi.org/10.5897/AJBR.9000014 (2008).
Hussein, M. H., Eltanahy, E., Al Bakry, A. F., Elsafty, N. & Elshamy, M. M. Seaweed extracts as prospective plant growth bio-stimulant and salinity stress alleviator for Vigna sinensis and Zea mays. J. Appl. Phycol. 33, 1273–1291 (2021).
Gupta, K. J., Stoimenova, M. & Kaiser, W. M. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J. Exp. Bot. 56, 2601–2609 (2005).
Zeng, L. et al. Evaluation of salt tolerance in rice genotypes by physiological characters. Euphytica 129, 281–292 (2003).
Lehener, A. et al. Changes in soluble carbohydrates, lipid peroxidation and antioxidant enzyme activities in the embryo during ageing in wheat grains. J. Cereal Sci. 47, 555–565 (2008).
Liu, H., Xin, Z. & V.Z.,. Changes in activities of antioxidant-related enzymes in leaves of resistant and susceptible wheat inoculated with Rhizoctonia cerealis. Agric. Sci. China 10(13), 526–533 (2011).
Maadanea, A. B. et al. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J. Biotechnol. 215, 13–19 (2015).
Kasim, W. A., Hamada, E. A. M., El-Din, N. G. S. & Eskander, S. B. Influence of seaweed extracts on the growth, some metabolic activities and yield of wheat grown under drought stress. Int. J. Agron. Agric. Res. 7(2), 173–189 (2015).
Salma, L. et al. Foliar application of Ulva rigida water extracts Improves salinity tolerance in wheat (Triticum durum L.). Agronomy 11, 265 (2021).
Alscher, R. G., Erturk, N. & Heath, L. S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 53, 1331–1341. https://doi.org/10.1093/jexbot/53.372.1331 (2002).
Hemida, A. K., Refaat, M. A. & Wael, M. I. Enhance salt stress tolerance in wheat (Triticum aestivum L.) plant using exogenous β-carotene or algal extract. Int. J. Biomed. Sci. Bioinform. 2, 26–32. https://doi.org/10.1186/s40529-022-00352-x (2015).
Asada, K. Ascorbate peroxidase-a hydrogen scavenging enzyme in plants. Physiol. Plant. 85, 235–241. https://doi.org/10.1111/j.1399-3054.1992.tb04728.x (1992).
Noctor, G. & Foyer, C. H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 249–279 (1998).
Masondo, N. A., Kulkarni, M. G., Finnie, J. F. & Van Staden, J. Influence of biostimulants-seed-priming on Ceratotheca triloba germination and seedling growth under low temperatures, low osmoticpotential and salinity stress. Ecotoxicol. Environ. Saf. 147, 43–48. https://doi.org/10.1016/j.ecoenv.2017.08.017 (2018).
Yusuf, R., Kristiansen, P. & Warwick, N. Potential effect of plant growth regulators in two seaweed products. Acta Hortic. 958, 133–138. https://doi.org/10.17660/ActaHortic.2012.958.15 (2012).
Hamouda, M. M., Saad-Allah, K. M. & Gad, D. Potential of seaweed extract on growth, physiological, cytological and biochemical parameters of wheat (Triticum aestivum L.) seedlings. J. Soil Sci. Plant Nutr. 22, 1818–1831. https://doi.org/10.1007/s42729-022-00774-3 (2022).
Sivasankari, S., Venkatesalu, V., Anantharaj, M. & Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 97(14), 1745–1751. https://doi.org/10.1016/j.biortech.2005.06.016 (2006).
Chernane, H., Latique, S., Mansori, M. & El Kaoua, M. Salt stress tolerance and antioxidative mechanisms in wheat plants (Triticum durum L.) by seaweed extracts application. J. Agric. Vet. Sci. 8, 36–44 (2015).
Di Stasio, E. et al. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 30, 2675–2686. https://doi.org/10.1007/s10811-018-1439-9 (2018).
Acosta, M. J. R. et al. Towards a sustainable agriculture: Strategies involving phytoprotectants against salt stress. Agronomy 10, 194. https://doi.org/10.3390/agronomy10020194 (2020).
Acknowledgements
The authors express their appreciation to the Water and Soil Analysis lab at the Desert Research Center, Cairo, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Investigation and resources; A.M.R., E.A.A., A.E.M. Phytochemical analysis and writing—original draft; A.E.R.D., data analysis and review the manuscript; M.A.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Radwan, A.M., Ahmed, E.A., Donia, A.M. et al. Priming of Citrullus lanatus var. Colocynthoides seeds in seaweed extract improved seed germination, plant growth and performance under salinity conditions. Sci Rep 13, 11884 (2023). https://doi.org/10.1038/s41598-023-38711-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-38711-8
This article is cited by
-
Future Directions of Seaweed-based Bioeffectors and Biofertilizer
Journal of Soil Science and Plant Nutrition (2026)
-
Growth and physiological responses of Grammosciadium platycarpum seed treated with seaweed bio-stimulant under salinity stress
BMC Plant Biology (2025)