Abstract
Carotenoids play a role in preventing and impeding the progression of atherosclerotic cardiovascular diseases (ASCVDs) through their anti-oxidative effects. This study evaluated associations between ASCVD risk and skin carotenoid (SC) levels, reflecting dietary carotenoid intake. Participants’ ASCVD risk was assessed using the Hisayama ASCVD risk prediction model, and SC levels were measured through a reflection spectroscope (Veggie Meter). The associations between high ASCVD risk and SC levels were analyzed using logistic regression analysis and a restricted cubic spline (RCS) model. A total of 1130 men and women (mean age: 56 years) from participants who underwent a health examination in Seirei Center for Health Promotion and Prevention Medicine in 2019 and 2022 were analyzed. Of these, 4.6% had moderate or high ASCVD risk. Mean SC values were 236, 315, 376, 447, and 606 in quintile Q1 to Q5, respectively. The adjusted odds ratios (95% confidence intervals) of SC quintile for moderate- or high-risk ASCVD was 0.24 (0.12–0.51) in Q5 (495 ≤), 0.42 (0.23–0.77) in Q4, 0.50 (0.29–0.88) in Q3, and 0.68 (0.41–1.12) in Q2 compared to Q1 (< 281). High SC values continuously showed non-linear inverse association with moderate- or high-risk for ASCVD in Japanese adults. Non-invasive SC measurements may be a good indicator for recommending carotenoids to prevent cardiovascular disease.
Similar content being viewed by others
Introduction
Carotenoids are phytonutrients comprising eight isoprene molecules and contain 40 carbon atoms. Their 9–11 conjugated double bonds provide an anti-oxidative function by quenching singlet oxygen and scavenging free radicals1,2. Carotenoids also possess anti-inflammatory effects in organisms3,4. Among more than 750 carotenoids, approximately 40 types are detected in the human body, and several studies elucidated the association between blood carotenoid concentrations and numerous diseases and mortality5,6,7,8. Bohn et al. reported that a serum carotenoid concentration of > 1725 nmol/L was required for maintaining health9, and Donaldson reported that a serum carotenoid concentration of > 2500 nmol/L decreases the risk of cardiovascular disease (CVD), cancer, and other chronic diseases whereas a concentration of < 1000 nmol/L increases the risk of the diseases mentioned above10.
Atherosclerotic cardiovascular diseases (ASCVD) are disorders caused by cholesterol plaque formation in the arterial walls and its disruption by thrombosis. ASCVD is a single pathologic entity that affects different vasculatures in the body, which encompasses coronary heart disease and atherothrombotic brain infarction11. Atherosclerosis is induced in part by low-density lipoprotein oxidation12, and oxidative stress considerably contributes to atherosclerosis and CVD onset13. Since high serum carotenoid concentrations and their antioxidant capacity have been reported to suppress early-stage atherosclerosis14 and CVD15, carotenoids play a role in the prevention and impeding the progression of ASCVD16,17.
In previous studies, carotenoids in the body were quantified by blood (serum or plasma) carotenoid concentrations. Although blood carotenoid concentrations can be accurately measured by high performance liquid chromatography, the measurement is costly, and taking blood samples is invasive. Conversely, skin carotenoid (SC) level measurement is known to be a non-invasive rapid screening alternative that can be used in large-scale studies and dietary education. SC measurements can be conducted using resonance Raman spectroscopy (RRS) or reflection spectroscopy (RS) methods18,19,20,21,22,23. SC levels measured using RRS and RS are strongly correlated with blood carotenoid concentrations24. RRS requires laser excitation and highly sensitive detection schemes to measure the Raman signals of carotenoid vibration which are highly expensive. Conversely, a pressure-mediated RS can measure SC levels with less complexity and cost than RRS. Therefore, RS is advantageous for its use in public health studies and studies with preschool and school children25,26,27,28,29.
The association between SC and CVD risk was observed in Singapore30, and we reported the association between SC measured using RS and metabolic syndrome in the Japanese population during a health examination previously31; in this study, the dose–response association between ASCVD risk and SC levels in the same population of participants were analyzed. The results obtained in this study enhance the relevance of SC measurements, providing valuable data for using SC measurements in nutrition education aimed at improving vegetable intake and health promotion.
Methods
Participants
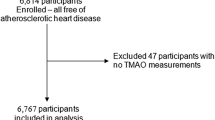
Among the 3115 participants who underwent a health examination in Seirei Center for Health Promotion and Prevention Medicine from September 2019 to December 2020, 2540 participants who underwent SC measurement for the first time and without a history of myocardial infarction (n = 53), stroke (n = 63), and cancer (n = 279) were included in the study. A participant flowchart is shown in Fig. 1. Some of the participants were the same respondents investigated in our previous study31. The participants were interviewed by trained medical staff regarding their lifestyle, disease history, use of antihypertensive agents, antihyperlipidemic agents, oral diabetes drugs, and insulin, smoking status, alcohol drinking status, and regular exercise. The participants underwent physiological examinations, including body mass index (BMI), systolic and diastolic blood pressure, SC level measurement, and blood tests.
The current cross-sectional study complied with the tenets of the Declaration of Helsinki. The Institutional Review Boards of Seirei Hamamatsu General Hospital and Seirei Center for Health Promotion and Prevention Medicine approved the research (IRB No. 3030, 31–02). All participants provided written informed consent for inclusion in the study.
ASCVD risk assessment
ASCVD risk was assessed using the Hisayama ASCVD risk prediction model32, which was adopted from the Japanese Arteriosclerosis Society Guidelines for the Prevention of Arteriosclerotic Cardiovascular Disease 2022 (https://www.j-athero.org/jp/jas_gl2022/). In this model, a 10-year probability of developing ASCVD events was calculated in each participant using a multivariable formula with age (in years), sex, systolic blood pressure (in mmHg), diabetes, serum high-density lipoprotein (HDL) cholesterol (in mg/dL), serum low-density lipoprotein (LDL) cholesterol (in mg/dL), protein urea, current smoking, and regular exercise habit. Additionally, a Hisayama ASCVD risk score was calculated by simply adding the scores corresponding to the categories of each of the above variables except age, that is, sex (women: 0; men: 7 points), systolic blood pressure (< 120 mmHg: 0; 120–129 mmHg: 1; 130–139 mmHg: 2; 140–159 mmHg: 3; 160 mmHg: 4 points), diabetes (no: 0; yes: 3 points), serum HDL cholesterol (60 mg/dL: 0; 40–59 mg/dL: 1; < 40 mg/dL: 2 points), serum LDL cholesterol (< 120 mg/dL: 0; 120–139 mg/dL: 1; 140–159 mg/dL: 2; 160 mg/dL: 3 points), proteinuria (no: 0; yes: 4 points), current smoker (no: 0; yes: 2 points), and regular exercise (yes: 0; no: 2 points)32. The classification was based on the presence or absence of proteinuria and diabetes, the distinction of smoking habit into those who smoke one or more cigarettes per day or those who do not, and the categorization of regular exercise to individuals engaging in sports or other forms of activity at least three times a week or not32. However, in our study, regular exercise was defined as light exercise of 30 min or more, performed at least twice a week for at least 1 year. In the Hisayama ASCVD risk prediction model, a 10-year probability of developing an ASCVD event is defined as “moderate risk” if the probability is more than 2% and “high risk” if the probability is more than 10%, considering the incidence of ASCVD in the Japanese population. In this study, “moderate or high risk” was defined as a Hisayama ASCVD risk score of 15 or higher, with a 10-year probability of developing an ASCVD event of at least 2% among participants aged 40–49 years.
When calculating the Hisayama score and the ASCVD probability, there were missing values for blood tests (n = 1), urine tests (n = 1410), and exercise habits (n = 39), resulting in a total of 1130 participants for complete case analysis without missing values. Additionally, owing to the large number of missing values, a multiple imputation analysis was also conducted, with the variables used for the Hisayama score and probability calculations to complement the missing values (20 sets were created; n = 2540).
SC level measurement
The SC levels were measured using Veggie Meter (VM) (version 2.0., serial number, 618W0091, Longevity Link Corporation, Salt Lake City, Utah) as VM scores (range: 0–1200, arbitrary unit), according to the manufacturer’s instructions. Calibration was conducted with the manufacturer-provided reference materials before daily measurements twice daily (before the morning and afternoon sessions)33. All participants washed their hands with soap and disinfected their fingers with a disinfectant. The researcher then assessed the hands of the participants, ascertaining the absence of contamination. The participants then inserted their left middle finger into the device’s finger cradle and had the tip pushed against the convex contact lens surface with the aid of a spring-loaded lid. The modest pressure applied to the fingertip reduced the blood perfusion of the measured tissue volume, preventing the strongly absorbed blood from interfering with SC level measurements. The VM scores were determined as the average of three consecutive measurements. The obtained VM scores were converted to values with our standard VM (version 1.0., serial number, 415W0156-1) using the regression equation of our previous study34, and compensated values were used for analyses.
Statistical analysis
Participant characteristics by SC quintiles were examined using the Chi-square test for categorical variables and analysis of covariance for continuous variables. The association between moderate or high risk for ASCVD (15 or more of the Hisayama ASCVD risk score) and SC quintiles (Q1 to Q5, Q1: reference) was estimated using logistic regression analysis adjusted for age and BMI, which were excluded from the calculation of the Hisayama ASCVD risk score. Trend-p values were obtained with SC quintiles as continuous variables. Additionally, the non-linear association between moderate or high risk for ASCVD and SC levels (arbitrary unit) was estimated using logistic regression analysis adjusting for age and BMI in a restricted cubic spline (RCS) model with three knots (0.10. 0.50, 0.90) in complete case analysis.
Data were analyzed using IBM SPSS Statistics for Windows, version 28.0 (IBM Corp., Armonk, NY, USA) and R software, version 4.1.3. All tests were two-tailed, and statistical significance was set at P < 0.05.
Results
A total of 499 men and 631 women were analyzed in a complete case analysis. The mean (standard deviation, SD) of age was 56.4 (11.0) years (men: 57.0 [10.2]; women: 56.0 [11.5]), and the mean (SD) of the SC values expressed as VM score was 395.5 (135.0) (men: 371.1 [129.5]; women 414.7 [136.2]). The characteristics of the participants according to SC quintile are shown in Table 1. The mean SC values (range) were 236 (< 282), 315 (282 ≤ and < 345), 376 (345 ≤ and < 407), 447 (407 ≤ and < 495), and 606 (495 ≤) in quintiles Q1 to Q5, respectively. Male sex, young age, and current smokers were more likely to belong in the lower quintile of the SC, whereas people who do regular exercise were less likely to belong in the lower quintile of the SC. Diastolic blood pressure, serum triglycerides, and BMI were higher, while serum HDL cholesterol was lower in the lower quintile of the SC.
The distribution of the Hisayama ASCVD Risk Score and the mean 10-year probability of developing an ASCVD event using the score for the complete case are shown in Fig. 2. 4.6% of participants had a Hisayama ASCVD Risk Score of 15 or more and were identified as moderate or high risk for ASCVD.
Distribution of Hisayama ASCVD Risk Score and mean 10-year probability of developing an ASCVD event using the score. Left axis is the frequency of the Hisayama ASCVD Risk Score. Right axis is the mean 10-year probability of developing an ASCVD event obtained using multivariable formula of Hisayama ASCVD Risk Prediction Model32.
The association between moderate or high risk for ASCVD and SC quintile using logistic regression analysis is shown in Table 2. The adjusted odds ratios of SC for moderate- or high-risk ASCVD was 0.25 (95% confidence interval [CI], 0.08–0.77) in Q5, 0.42 (95% CI, 0.17–1.03) in Q4, 0.46 (95% CI, 0.20–1.06) in Q3, and 0.46 (95% CI, 0.21–1.02) in Q2 compared to Q1 as reference in the complete case analysis. The results were almost similar in the multiple imputation analysis; however, the association was significant in Q3 and Q4 in addition in Q5. The RCS model demonstrated a non-linear inverse dose–response relationship between moderate- or high-risk ASCVD and SC values after adjusting for age and BMI (Fig. 3).
Association between moderate or high risk for ASCVD and skin carotenoid values. OR odds ratio, CI confidence interval. Skin carotenoid was estimated via refraction spectroscopy, Veggie Meter. Moderate or high risk for ASCVD was defined as 15 or more points on the Hisayama ASCVD Risk Score32.
Discussion
In this cross-sectional study, SC was estimated using a commercially available RS (VM), and ASCVD risk was evaluated using the Hisayama ASCVD risk prediction model. We found that the mean SC values (VM scores) were 395.5, and 4.6% of the participants had moderate- or high-ASCVD risk. The OR for moderate- or high-ASCVD risk was statistically low in the highest quintile of SC compared to the lowest quintile in the complete case analysis. Additionally, the RCS model showed a non-linear inverse dose–response relationship between the moderate- or high-risk ASCVD and SC values, and the ASCVD risk increased more rapidly, when the SC value was low.
The inverse association between SC and CVD risk observed in this study corroborates with the results of a study conducted in Singapore by Toh et al.30 despite differences in SC measurement and CVD risk assessment methods. That is, they measured SC using RRS, and we measured it using the simpler RS (VM), and they evaluated CVD risk using the Framingham Risk Score, and we assessed it using the Hisayama ASCVD risk score developed for the Japanese population. The inverse association between SC and CVD risk, commonly observed regardless of the measurement or assessment method, infers that the association is robust. East Asian countries, such as Japan, Korea, and China, have lower coronary heart disease (CHD) mortality rates than Western countries, including the United States and the United Kingdom; in contrast, stroke is generally more common in East Asian countries than in Western countries35. Major risk factors for CVD, such as hypertension, hypercholesterolemia, diabetes, and smoking, are common in both Western and East Asian countries. Many models for predicting CVD risk, such as the Framingham Risk Score, have been developed worldwide36, and numerous risk prediction models have also been developed in Japan32,37,38,39,40,41. Of these, the Hisayama ASCVD risk prediction model32 was adopted in this study because it predicts atherothrombotic brain infarction in addition to CHD and provides both a 10-year probability of developing ASCVD events using a multivariable formula and a simple risk score that can be evaluated by age group.
As shown in Fig. 3, the risk for ASCVD decreases with higher SC levels; SC values in the highest quintile, specifically a VM score of 495 or higher, were potentially effective in CVD prevention. Evidence on the benefits of SC in preventing CVD or maintaining health is currently lacking. However, several reports examined the association between blood carotenoid concentrations and CVD and other diseases5,6,7,8,9,10,42,43,44,45. To estimate the VM score from plasma carotenoid concentrations, Rush et al.46 created a regression equation. According to this regression equation, the serum carotenoid level of 2500 nmol/L, which was reported as effective in preventing CVD and other diseases by Donaldson10, equivalent to 310. Rush et al. proposed VM scores of 530 which were desirable for 2011 #196}, might be equivalent to 451, and 1725 nmol/L, which was reported effective for health maintenance by Bohn et al.9, might be health maintenance46. In this study, the result of a favorable VM score of 495 or higher is broadly consistent with the favorable VM score predicted by the estimation equation from serum carotenoids. The validity of this value needs to be verified in the future by other populations and by cohort and intervention studies.
VM has been widely used in the field of nutrition because VM can measure SC levels non-invasively in a short time. Several studies27,47,48,49,50,51 clarified the association between fruit and vegetable intake and SC levels measured by VM and some interventional studies52,53 revealed that VM accurately detects differences in carotenoid intake through diet. In our previous study54, informing participants of their VM scores has proven effective in motivating fruit and vegetable intake, and repeated measurements have increased SC levels. In addition to these nutritional approaches, SC level identification as an effective measure for preventing various diseases would serve as a valuable indicator in nutritional guidance towards a diet beneficial for disease prevention.
The major limitation of this study was the cross-sectional observational nature of the study; thus, we cannot infer the causal relationship between SC and ASCVD risk. Furthermore, the study population may be more health-conscious, as they were selected from those who had undergone a health check-up. Since the variables used to calculate the Hisayama ASCVD risk score and probability had many missing values, we added a multiple imputation analysis. The results demonstrated a more evident association between SC and ASCVD risk.
In conclusion, high SC levels, estimated by VM, continuously showed a non-linear inverse association with moderate- or high-risk ASCVD in Japanese adults. The highest quintile of SC (495 ≤ in VM score) was associated with low risk of ASCVD and the ASCVD risk increased more rapidly, when the SC value was low. Whether CVD occurrence can be predicted from skin carotenoids requires further research. However, given the numerous reports on the CVD preventive effects of fruit and vegetable consumption, non-invasive measurement of SC may be an optimal indicator for recommending adequate fruit and vegetable intake for CVD prevention.
Data availability
Data described in the manuscript were substituted as supplement data.
References
Miller, N. J., Sampson, J., Candeias, L. P., Bramley, P. M. & Rice-Evans, C. A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 384, 240–242. https://doi.org/10.1016/0014-5793(96)00323-7 (1996).
Stahl, W. & Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 24, 345–351 (2003).
Tian, Y., Kijlstra, A., Webers, C. A. B. & Berendschot, T. Lutein and Factor D: Two intriguing players in the field of age-related macular degeneration. Arch. Biochem. Biophys. 572, 49–53. https://doi.org/10.1016/j.abb.2015.01.019 (2015).
Nakamura, M. & Sugiura, M. Serum lutein and zeaxanthin are inversely associated with high-sensitivity C-reactive protein in non-smokers: The Mikkabi study. Antioxidants https://doi.org/10.3390/antiox11020259 (2022).
Buijsse, B. et al. Plasma carotene and alpha-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: The Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA). Am. J. Clin. Nutr. 82, 879–886. https://doi.org/10.1093/ajcn/82.4.879 (2005).
Shardell, M. D. et al. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: The Third National Health and Nutrition Examination Survey. Nutr. Res. 31, 178–189. https://doi.org/10.1016/j.nutres.2011.03.003 (2011).
Huang, J., Weinstein, S. J., Yu, K., Mannisto, S. & Albanes, D. Serum beta carotene and overall and cause-specific mortality. Circ. Res. 123, 1339–1349. https://doi.org/10.1161/CIRCRESAHA.118.313409 (2018).
Fujii, R., Tsuboi, Y., Maeda, K., Ishihara, Y. & Suzuki, K. Analysis of repeated measurements of serum carotenoid levels and all-cause and cause-specific mortality in Japan. JAMA Netw. Open 4, e2113369. https://doi.org/10.1001/jamanetworkopen.2021.13369 (2021).
Bohn, T. et al. Mechanistic aspects of carotenoid health benefits—Where are we now?. Nutr. Res. Rev. 34, 276–302. https://doi.org/10.1017/S0954422421000147 (2021).
Donaldson, M. S. A carotenoid health index based on plasma carotenoids and health outcomes. Nutrients 3, 1003–1022. https://doi.org/10.3390/nu3121003 (2011).
Viles-Gonzalez, J. F., Fuster, V. & Badimon, J. J. Atherothrombosis: A widespread disease with unpredictable and life-threatening consequences. Eur. Heart J. 25, 1197–1207. https://doi.org/10.1016/j.ehj.2004.03.011 (2004).
Steinberg, D., Parthasarathy, S., Carew, T. E., Khoo, J. C. & Witztum, J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 320, 915–924. https://doi.org/10.1056/nejm198904063201407 (1989).
Madamanchi, N. R., Vendrov, A. & Runge, M. S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 25, 29–38. https://doi.org/10.1161/01.ATV.0000150649.39934.13 (2005).
Karppi, J., Kurl, S., Ronkainen, K., Kauhanen, J. & Laukkanen, J. A. Serum carotenoids reduce progression of early atherosclerosis in the carotid artery wall among Eastern Finnish men. PLoS ONE 8, e64107. https://doi.org/10.1371/journal.pone.0064107 (2013).
Cervantes Gracia, K., Llanas-Cornejo, D. & Husi, H. CVD and oxidative stress. J. Clin. Med. https://doi.org/10.3390/jcm6020022 (2017).
Ciccone, M. M. et al. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 782137. https://doi.org/10.1155/2013/782137 (2013).
Milani, A., Basirnejad, M., Shahbazi, S. & Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 174, 1290–1324. https://doi.org/10.1111/bph.13625 (2017).
Hata, T. R. et al. Non-invasive Raman spectroscopic detection of carotenoids in human skin. J. Investig. Dermatol. 115, 441–448. https://doi.org/10.1046/j.1523-1747.2000.00060.x (2000).
Ermakov, I. V., Ermakova, M. R., McClane, R. W. & Gellermann, W. Resonance Raman detection of carotenoid antioxidants in living human tissues. Opt. Lett. 26, 1179–1181 (2001).
Ermakov, I. V., Sharifzadeh, M., Ermakova, M. & Gellermann, W. Resonance Raman detection of carotenoid antioxidants in living human tissue. J. Biomed. Opt. 10, 064028. https://doi.org/10.1117/1.2139974 (2005).
Ermakov, I. V. & Gellermann, W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J. Biophotonics 5, 559–570. https://doi.org/10.1002/jbio.201100122 (2012).
Darvin, M. E., Meinke, M. C., Sterry, W. & Lademann, J. Optical methods for noninvasive determination of carotenoids in human and animal skin. J. Biomed. Opt. 18, 61230. https://doi.org/10.1117/1.jbo.18.6.061230 (2013).
Scherr, R. E. et al. Innovative techniques for evaluating behavioral nutrition interventions. Adv. Nutr. 8, 113–125. https://doi.org/10.3945/an.116.013862 (2017).
Ermakov, I. V. et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 646, 46–54. https://doi.org/10.1016/j.abb.2018.03.033 (2018).
<Evaluating the impact of a high school garden in El Paso Texas f.pdf>.
Bakirci-Taylor, A. L., Reed, D. B., McCool, B. & Dawson, J. A. mHealth improved fruit and vegetable accessibility and intake in young children. J. Nutr. Educ. Behav. 51, 556–566. https://doi.org/10.1016/j.jneb.2018.11.008 (2019).
Martinelli, S., Acciai, F., Tasevska, N. & Ohri-Vachaspati, P. Using the Veggie meter in elementary schools to objectively measure fruit and vegetable intake: A pilot study. Methods Protoc. https://doi.org/10.3390/mps4020033 (2021).
May, K. et al. Use of the Veggie Meter(R) as a tool to objectively approximate fruit and vegetable intake among youth for evaluation of preschool and school-based interventions. J. Hum. Nutr. Diet. 33, 869–875. https://doi.org/10.1111/jhn.12755 (2020).
Jones, A. M. et al. Measuring skin carotenoids using reflection spectroscopy in a low-income school setting. Nutrients https://doi.org/10.3390/nu13113796 (2021).
Toh, D. W. K. et al. Skin carotenoids status as a potential surrogate marker for cardiovascular disease risk determination in middle-aged and older adults. Nutr. Metab. Cardiovasc. Dis. 31, 592–601. https://doi.org/10.1016/j.numecd.2020.10.016 (2021).
Takayanagi, Y. et al. Relationships between skin carotenoid levels and metabolic syndrome. Antioxidants https://doi.org/10.3390/antiox11010014 (2021).
Honda, T. et al. Development and validation of a risk prediction model for atherosclerotic cardiovascular disease in Japanese adults: The Hisayama study. J. Atheroscler. Thromb. 29, 345–361. https://doi.org/10.5551/jat.61960 (2022).
Radtke, M. D. et al. Recommendations for the use of the veggie meter(R) for spectroscopy-based skin carotenoid measurements in the research setting. Curr. Dev. Nutr. 5, nzab104. https://doi.org/10.1093/cdn/nzab104 (2021).
Obana, A., Asaoka, R., Takayanagi, Y. & Gohto, Y. Inter-device concordance of Veggie Meter, a reflection spectroscopy to measure skin carotenoids. J. Biophotonics https://doi.org/10.1002/jbio.202300071 (2023).
Ueshima, H. et al. Cardiovascular disease and risk factors in Asia: A selected review. Circulation 118, 2702–2709. https://doi.org/10.1161/CIRCULATIONAHA.108.790048 (2008).
Damen, J. A. et al. Prediction models for cardiovascular disease risk in the general population: Systematic review. BMJ 353, i2416. https://doi.org/10.1136/bmj.i2416 (2016).
<Risk Assessment Chart for Death From Cardiovascular Disease Based on a 19-Year Follow-up Study of a Japanese Representative Population.pdf>.
<Predicting Coronary Heart Disease Using Risk Factor Categories for a Japanese Urban Population, and Comparison with the Framingham Risk Score_The Suita Study.pdf>.
Arima, H. et al. Development and validation of a cardiovascular risk prediction model for Japanese: The Hisayama study. Hypertens. Res. 32, 1119–1122. https://doi.org/10.1038/hr.2009.161 (2009).
Tanabe, N. et al. Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events: The JALS-ECC. Circ. J. 74, 1346–1356. https://doi.org/10.1253/circj.cj-09-0861 (2010).
Matsumoto, M. et al. Risk charts illustrating the 10-year risk of myocardial infarction among residents of Japanese rural communities: The JMS Cohort Study. J. Epidemiol. 19, 94–100. https://doi.org/10.2188/jea.je20080081 (2009).
Aune, D. et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality: A systematic review and dose–response meta-analysis of prospective studies. Int. J. Epidemiol. 46, 1029–1056. https://doi.org/10.1093/ije/dyw319 (2017).
Aune, D. et al. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose–response meta-analysis of prospective studies. Am. J. Clin. Nutr. 108, 1069–1091. https://doi.org/10.1093/ajcn/nqy097 (2018).
Lee, H. A., Lim, D., Oh, K., Kim, E. J. & Park, H. Mediating effects of metabolic factors on the association between fruit or vegetable intake and cardiovascular disease: The Korean National Health and Nutrition Examination Survey. BMJ Open 8, e019620. https://doi.org/10.1136/bmjopen-2017-019620 (2018).
Li, N. et al. Green leafy vegetable and lutein intake and multiple health outcomes. Food Chem. 360, 130145. https://doi.org/10.1016/j.foodchem.2021.130145 (2021).
Rush, E., Amoah, I., Diep, T. & Jalili-Moghaddam, S. Determinants and suitability of carotenoid reflection score as a measure of carotenoid status. Nutrients https://doi.org/10.3390/nu12010113 (2020).
Jilcott Pitts, S. B. et al. A non-invasive assessment of skin carotenoid status through reflection spectroscopy is a feasible, reliable and potentially valid measure of fruit and vegetable consumption in a diverse community sample. Public Health Nutr. 21, 1664–1670. https://doi.org/10.1017/s136898001700430x (2018).
Hasnin, S. et al. Systematic review of reflection spectroscopy-based skin carotenoid assessment in children. Nutrients https://doi.org/10.3390/nu15061315 (2023).
Liu, R. et al. Weight status and visceral adiposity mediate the relation between exclusive breastfeeding duration and skin carotenoids in later childhood. Curr. Dev. Nutr. 5, nzab010. https://doi.org/10.1093/cdn/nzab010 (2021).
Nagao-Sato, S. et al. Skin carotenoid scores assessed with reflection spectroscopy are associated with self-reported fruit and vegetable intake among Latino early adolescents. J. Acad. Nutr. Diet. 121, 1507–1514. https://doi.org/10.1016/j.jand.2021.02.019 (2021).
Caparello, G. et al. Association between skin carotenoid score measured with veggie meter((R)) and adherence to the mediterranean diet among adolescents from Southern Italy. Nutrients https://doi.org/10.3390/nu15234920 (2023).
Casperson, S. L. et al. Sensitivity of pressure-mediated reflection spectroscopy to detect changes in skin carotenoids in adults without obesity in response to increased carotenoid intake: A randomized controlled trial. J. Nutr. 153, 588–597. https://doi.org/10.1016/j.tjnut.2023.01.002 (2023).
Jilcott Pitts, S. et al. Reflection spectroscopy-assessed skin carotenoids are sensitive to change in carotenoid intake in a 6-week randomized controlled feeding trial in a racially/ethnically diverse sample. J. Nutr. 153, 1133–1142. https://doi.org/10.1016/j.tjnut.2023.02.017 (2023).
Obana, A. et al. Improving skin carotenoid levels in young students through brief dietary education using the veggie meter. Antioxidants https://doi.org/10.3390/antiox11081570 (2022).
Acknowledgements
The authors would like to thank Sunao Okano, Seirei Center for Health Promotion and Prevention Medicine, Seirei Social Welfare Community for the data collection.
Funding
Asaoka R received Grants (19H01114, 18KK0253, and 20K09784) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Translational Research program, and the Strategic Promotion for Practical Application of Innovative Medical Technology (TR-SPRINT) from the Japan Agency for Medical Research and Development (AMED). Nakamura M received JSPS KAKENHI (23K09691). Miura A received JSPS KAKENHI (23K12695). Nozue M received JSPS KAKENHI (23K02694). The other authors received no external funding.
Author information
Authors and Affiliations
Contributions
A.O., A.M., M.Nozue, and R.A. designed the research; S.M. conducted the research; M.Nakamura and RA analyzed the data; A.O. and M.Nakamura wrote the paper; A.O. had the primary responsibility for the final content. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obana, A., Nakamura, M., Miura, A. et al. Association between atherosclerotic cardiovascular disease score and skin carotenoid levels estimated via refraction spectroscopy in the Japanese population: a cross-sectional study. Sci Rep 14, 12173 (2024). https://doi.org/10.1038/s41598-024-62772-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-62772-y