Abstract
Patchouli alcohol (PA) is a widely used pharmaceutical ingredient in various Chinese traditional herbal medicine (THM) formulations, known for its modulatory effects on the gut microbiota. The present study investigated PA's anti-inflammatory and regulatory effects on gut microbiota and its mode of action (MOA). Based on the assessments of ulcerative colitis (UC) symptoms, PA exhibited promising preventions against inflammatory response. In accordance, the expressions of pro-inflammatory factors, including interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and chemokine ligand 5 were significantly attenuated under PA treatment. Furthermore, PA enhanced the intestinal barrier damage caused by dextran sodium sulfate (DSS). Interestingly, PA exhibited negligible inventions on DSS-induced gut microbiota dysbiosis. PA did not affect the diversity of the DSS gut microbiota, it did alter the composition, as evidenced by a significant increase in the Firmicutes-Bacteroidetes (F/B) ratio. Finally, the MOA of PA against inflammation in DSS-treated mice was addressed by suppressing the expressions of heme oxygenase-1 (HO-1) and inducible nitric oxide synthase (iNOS). In conclusion, PA prevented inflammatory response in the DSS-induced UC mice model via directly suppressing HO-1 and iNOS-associated antioxidant signal pathways, independent of its effects on gut microbiota composition.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory condition that affects the entire body, mainly including crohn's disease (CD) and ulcerative colitis (UC). The investigation of the pathogenesis and treatment of IBD is of increasing importance and rising concern in recent years due to its increasing prevalence worldwide1,2. The pathogenesis of IBD is not fully understood, but an increasing number of studies have evidenced that inflammation plays an essential role in the development of the disease3,4,5. The overexpression of cytokines in response to stress, and the damage to the intestinal barrier caused by simultaneous cell death signals, jointly lead to significant intestinal inflammations6,7,8,9. Systemic symptoms of IBD include weight loss, blood in the stool, and colonic ulcers, which also act as risk factors for further progression to colon cancer10,11. Meanwhile, damage to the intestinal barrier was a critical process for the development of IBD. Recently Anoop Kumar et al. discovered that the Down Regulated in Adenoma (DAR) encoded by an intestinal chloride SLC26A3, played an important function in maintaining the integrity of the intestinal barrier in a dextran sodium sulfate (DSS)-induced IBD model12.
Patchouli alcohol (PA, C15H26O) is the main active ingredient in Pogostemon cablin (Blanco) Benth, which is one of the main components of the traditional Chinese medicine Huoxiang Zhengqi (HXZQ) Oral Liquid13. Available studies show diverse health-beneficial effects of PA in immunomodulation, anti-inflammatory, antioxidant, anti-mucositis, and anti-tumor have been found14,15,16. Recent studies have extensively demonstrated the anti-inflammatory effects of PA, a key pharmacological component in traditional herbal medicine (THM) administered orally, in gastrointestinal diseases and its modulatory effects on the gut microbiota17,18,19. For instance, PA intervention effectively suppressed the expression level of inflammatory factors in LPS-stimulated RAW264.7 cells, and promisingly modulated the gut microbiota imbalance in normal and colorectal cancer mice models20,21,22,23. In gastrointestinal diseases, PA was able to improve the excessive contraction of smooth muscle in the digestive organs through the calcium ion pathway, achieving further antiemetic benefits24.
As reported in our previous works, the modulatory effects on the gut microbiota and intestinal immune-microenvironment, as well as the anti-inflammatory effects of HXZQ oral liquid, were investigated in both antibiotic and Lipopolysaccharide(LPS)-induced gut dysbiosis mice models25. However, these modulatory effects of PA on the IBD model have never been investigated. Investigations of the modulatory effects on the gut microbiota of PA used alone will promote our understanding of the intricate MOAs of THM, and further confer the application of PA or THM formulas (which contain PA) in clinical use and the food industry. In this project, a DSS-induced UC mice model was constructed to investigate the existence of the modulatory capacity on gut microbiota imbalance, and its role in the anti-inflammatory effects of PA against IBD.
Materials and methods
Chemical and reagents
PA was purchased from Topscience Co., Ltd. (Cat. #T2916, TargetMol, Shanghai, China). DSS was purchased from Yeasen Biotechnology Co., Ltd. (Cat. #60316ES60, Yeasen, Shanghai, China). Toluidine blue O (TBO) solution and Hematoxylin–eosin and alcian blue periodic acid schiff (AB-PAS) staining kit were gained from Solarbio Science & Technology Co., Ltd. (Cat. #G3661 and Cat. #G1285, Solarbio, Beijing, China). The antibodies used in this study are as follows: anti-HO-1 (Cat. #10701-1-AP, Proteintech, Chicago, USA, 1:1 000); anti-ZO-1(Cat. #21773-1-AP, Proteintech, Chicago, USA, 1:200); anti-Occludin (Cat. #27260-1-AP, Proteintech, Chicago, USA, 1:200); anti-β-actin (Cat. #AC026, Abclonal, Wuhan, China, 1:40 000); goat anti-rabbit antibody conjugated with HRP (Cat. #E030120-01, EarthOx, CA, USA, 1:10 000).
Animals
Animal protocols were reviewed and approved by the Animal Administration and Ethics Committee of Chongqing Medical University (June 2020), in line with the Guide for ARRIVE to decrease animal number and suffering as much as possible. All methods were carried out in accordance with relevant regulations and ARRIVE guidelines. A total of 60 healthy male C57BL/6J mice, aged 7 weeks, were purchased from the Experimental Animal Center of Chongqing Medical University [Chongqing, China, license numbers: SCXK (Yu) 2018-0003]. Five mice were housed in a single home cage, and mice were placed in a regulated environment (temperature 22 ± 2 °C and humidity 50 ± 10%, light, and darkness were maintained for 12 h each) with free access to food and water. Some studies have shown that PA is safe and the maximum dose in animal studies can reach 100 mg/kg via oral administration26. We converted the dose of HXZQ on LPS-induced inflammatory response based on the previous studies of our team27. And a study has shown that PA (40 mg/kg) intervention was discovered to modulate the immune response and gut microbiota in mice23. Therefore, after one week of adaptive feeding in the animal experiment center, mice were randomly divided into six groups (n = 10 in each group): vehicle, PA20, (low dose, 20 mg/kg of PA), PA40 (high dose, 40 mg/kg of PA), DSS, DSS + PA20 and DSS + PA40. The vehicle group was treated orally with solution (5% DMSO, 15% PEG300, 2.5% Tween 80, 77.5% SPSS). PA20 and PA40 were gavaged with PA (20 mg/kg or 40 mg/kg) for 14 days. After PA treatment for 7 days, the mice in the DSS-treated groups were orally exposed to drinking water containing 4% DSS for 7 days (Fig. 1A). Mice were euthanized on day 15 under tribromoethanol anesthesia and then fecal, blood and colon tissue samples were collected.
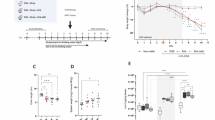
Effect of PA on DSS-induced UC mice. (A) It is an experimental flow chart. (B) The body weight changes were calculated using initial body weight as a datum line, n = 5. (C) Histopathological scores of each group were evaluated. Score 1 indicating no disease; 4 indicating the most severe disease) of average scores based on the combination of clinical symptoms body weight, diarrhea, and fecal blood, n = 5. (D) This image shows the length of the colonic tissue and the size of the spleen in each group of mice. (E) The lengths of colons from each group of mice, n = 3. (F–I) The mRNA expression of four inflammatory indicators in the colorectal tissues of mice was detected using qRT-PCR. The results showed that the mRNA expression level of Il-1β, Il-6, Tnf-α, and Ccl5 was significantly decreased by PA treatment, n = 3. It is worth noting that (B, C) using Statistical analysis was performed by two-way ANOVA. Other experiments still assessed the significance of differences by between-group analysis of variance. *p < 0.05; **p < 0.01; ***p < 0.001 versus the Vehicle group; #p < 0.05; ##p < 0.01; ###p < 0.001 versus the DSS-treated group. Data were presented as mean ± S.E.M.
Observation of IBD signs and symptoms
For determining and verifying the score, all mice were observed daily for body weight, fecal consistency, and the presence of total blood in the fecal and anal regions. Calculation of disease activity index (DAI) for each group by referring to the Tohru method11. On this basis, weight loss score (S1), stool status score (S2), and blood stool score (S3) were recorded daily. DAI score is calculated as (S1 + S2 + S3)/3.
H&E staining
The tissues from the colonic tissue that were taken were preserved in recently made 4% paraformaldehyde. At least five slides were prepared from each tissue. The Seemann et al.28 standard study was followed by experimental modifications. The paraffin slices were baked in a 65 °C oven for at least 2 h to ensure adequate melting of the paraffin wax on the tissue sections. After that, the slices were dehydrated in gradient ethanol and thrice submerged in xylene to remove the melted paraffin. Hematoxylin and eosin were applied to the sections in order, and ethanol was used to dehydrate them thereafter. Following staining, the sections were fixed with neutral resin and thrice cleared with xylene. Ultimately, an optical microscope (Olympus, IX53, Tokyo, Japan) was used to examine the sections. Two separate experimenters who were not informed of the experimental treatments separately rated the pathological alterations based on the following indicators: destruction of mucosal structures, proliferative changes in epithelial cells, and infiltration of inflammatory cells29. The following criteria were used to award the grades based on the level and extent of damage to the tissue section: 0, none; 1, mild; 2, moderate; 3, substantial; and 4 severe30. Using ImageJ software (National Institutes of Health, USA), the smooth muscle thickness and mucosal depth of intestinal tissue were measured.
TBO staining
Freshly collected colonic tissue was placed in 4% paraformaldehyde. Each sample was prepared with at least 5 sections. TBO staining was executed as previously described31. Briefly, the pathological tissue of paraffin sections was heated in a 65 °C oven for at least 2 h. After dewaxed in xylene for 30 min and repeated once, the sections were performed gradient absolute ethanol hydration, then were stained with toluidine blue dye for 15 min, dehydrated with ethanol, transparent with xylene, and fixed with neutral resin. The sections were observed under an optical/fluorescence microscope.
AB-PAS staining
As previously described, the AB-PAS staining was performed32. Briefly, colon tissues were collected and fixed in a 4% freshly prepared paraformaldehyde for at least 24 h. Each tissue was prepared in at least 5 sections. After baking for more than 2 h, colon tissue sections were dewaxed in xylene, hydrated in gradient ethanol, and washed with distilled water. Slices were stained sequentially with alcian blue, Schiff reagent, and hematoxylin. Gradient absolute ethanol hydration was performed followed by xylene transparency. The sections were mounted with neutral resin sealing and observed under an optical/fluorescence microscope.
Immunofluorescence analysis
The immunofluorescence analysis was carried out as previously reported33. Briefly, the colon tissue slices were incubated with antigens repaired with sodium citrate, and washed three times with PBS. Then, the tissue sections were permeabilization with 0.3% Triton-X-100 in PBS for 15 min and blocked with 1% BSA for 20 min. Then, the tissue sections were incubated with anti-ZO-1 and anti-Occludin overnight at 4 °C. Subsequently, the tissue sections were incubated with Alexa Fluor 488-conjugated anti-rabbit IgG antibody for 2 h at room temperature and then stained with 5 μg/mL of DAPI for 5 min. Each step was washed with PBS three times. The image was obtained using an optical/fluorescence microscope. During the image, the gain (voltage), laser intensity setting, and imaging parameter were kept constant between all the samples.
Tissue total RNA extraction and qRT-PCR
The gene expression by using qRT-PCR was conducted as previously described32. In brief, the total RNA of the fresh colonic tissue was extracted by using TRizol (Cat. #9109, TaKaRa, Beijing, China), and then reverse-transcribed by using the Perfect Real Time Prime Script™ RT Master Mix. The mRNA levels of related genes were determined by quantitative RT-PCR, carried out with the TB Green Premix Ex Taq™ II (TliRNaseH Plus) on CFX Connect™ Real-Time System (Bio-Rad, Hercules, CA, USA). The primer sequences of the target genes were synthesized by Sangon Biotech, Co., Ltd. (Shanghai, China). The Primer sequences of the target genes are shown in Supplementary Table 1.
Western blot
The western blot analysis was performed as previously described34. Fresh colon tissue was weighed about 50 mg for subsequent experiments. Each colon tissue was immersed in RIPA-buffer (Cat. #P0013B, Beyotime, Shanghai, China), mixed with 1 mmol/L PMSF (Cat. #ST506, Beyotime, Shanghai, China) and protease inhibitor cocktail (Cat. #HY-K0010, MCE, NJ, USA) for 10 min on ice. Colon tissue specimens were homogenized (shaking on the rotator for 1 h on ice), and centrifuged at 13,300 g for 20 min at 4 °C twice. Protein levels were measured by using an enhanced bicinchoninic acid (BCA) protein assay kit (Cat. #P0009, Beyotime, Shanghai, China). The proteins were concentrated (80 V for 20 min) and loaded (120 V for 65 min) on 8% SDS–polyacrylamide gel electrophoresis (SDS-PAGE), then electrophoretically (300 mA for 100 min) and transferred to a PVDF film (Cat. #ISEQ00010, Millipore, Billerica, MA, USA). The non-specific integration on PVDF film was blocked with 5% no-fat milk in TBST for 1 h at room temperature. And then, the membrane was incubated overnight (4 °C) with primary antibody against anti-β-actin, and anti-HO-1. The next day, the membrane was washed with TBST three times for 10 min, then immersed in HRP‐bounded goat anti-rabbit for 1 h at room temperature. Relative protein expression was normalized to the expression of β-actin. The expression of HO-1 was detected by using Clarity western ECL substrate. Relative protein abundance was measured by densitometry in the ImageJ software (NIH, Bethesda, MD). Data were presented as the mean ± S.E.M of at least three separate tests.
16S ribosomal RNA gene sequencing
The 16S rRNA gene sequencing was performed according to the previous description35. Briefly, at the end of treatment (Day 14), the fecal samples were collected under sterile conditions and stored at -80 °C before using. Total genomic DNA was extracted and purified by 1% agarose gel electrophoresis. The PCR purified by the reaction was performed on the ABI GeneAmp 9700 (Thermal cyclers from Applied Biosystems, CA, USA) with TransStart Fastpfu DNA Polymerase. The PCR products were recovered from the agarose and purified by AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, CA, USA). Subsequently, the PCR products were quantified by the QuantiFluor™-ST Blue Fluorescence Quantification System (Promega Co., WI, USA). Library construction and 16s rRNA gene sequencing based on an Illumina MiSeq platform (Illumina, SanDiego, USA) were performed by Majorbio Bio-Tech Co. Ltd (Shanghai, China), with primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) (Mori, et al., 36). The sequencing data were analyzed on a free online platform of the Majorbio Cloud Platform (www.majorbio.com). Reads are typically clustered into operational taxa (OTUs) based on a 97% similarity of 16S rRNA genes. All sequencing data in this study have been deposited at GenBank under BioProject accession number PRJNA889194.
Statistics
The experimental data was analyzed by using GraphPad Prism 8.0 (CA, USA) and shown as mean ± standard error of mean (S.E.M). The significance of difference was assessed by the analysis of variance (ANOVA) among groups, except where noted in figure legends, p < 0.05 indicated the statistical significance.
Ethics statement
The animal study was reviewed and approved by the Chongqing Medical University Animal Care and Use Committee.
Results
PA significantly attenuates DSS-induced UC and inhibits the expression of inflammatory factors in mice
Mice treated with DSS showed apparent signs of colitis, including weight loss, diarrhea, and fecal occult blood, showing that the UC mice model was successfully constructed. Overall, all indices including the body weight change, DAI, and colorectal length demonstrated that PA significantly alleviated the symptoms in DSS-treated mice. Compared to group DSS, the weight loss of DSS-treated mice administrated with PA (group DSS + PA20 and DSS + PA40) was significantly relieved (Fig. 1B), and the DAI scores were gradually decreased (Fig. 1C). Meanwhile, the colorectal length of mice in groups DSS + PA20 and DSS + PA40 was gradually restored (Fig. 1D,E). These results revealed that PA could alleviate DSS-induced UC inflammation in a dose-dependent manner. Moreover, the spleen is essential for immunity, and its size is an indicator of the severity of inflammation. Thus, we further assessed the level of splenomegaly (which occurs during the progression of colitis) by observing the spleen sizes in each group. As shown in Fig. 1D, the spleen size of DSS mice treated with PA was smaller than that in the DSS group, indicating PA could suppress the inflammation of UC mice. These results combined preliminarily indicated the potential of PA against the inflammation response of DSS-induced UC in mice models, which were by studies previously reported19,21, 23, 37.
To further test the ameliorative effect of PA on the DSS-induced UC in mice, the inflammatory factors were next examined in this study. As shown in Fig. 1F–I, the expressions of all four pro-inflammatory factors in the colon tissue of mice in groups PA20 and PA40 were maintained at the normal level, compared with the vehicle group. At the same time, the expressions were significantly upregulated in group DSS. In groups, DSS + PA20 and DSS + PA40, PA remarkably suppressed the expressions of these factors, with a concentration-dependent manner in suppression of interleukin (Il)-1β, Il-6, tumor necrosis factor-α (Tnf-α), and chemokine ligand 5(Ccl5). These findings indicated that PA could effectively suppress the inflammatory response in the colon of DSS-induced colitis.
PA slightly modulates DSS-induced UC gut microbiota dysbiosis in mice
The fecal samples of mice in the vehicle group, the DSS group, the PA40 group, and the DSS + PA40 group were collected, and 16S rRNA gene sequencing was conducted. The fecal microbial community profiles were clustered into OTUs at a threshold of 97% of 16s rRNA gene sequence similarity for further bacterial diversity analyses. Overall, the intestinal microbial community structure revealed by α-diversity (Ace, Chao, Sobs, Simpson, Shannon, Coverage index on OTUs level) (Fig. 2A–F), showed no significant difference between group vehicle and group PA40, which was consistent with the results of DAI scores, body weight, and colorectal length. While DSS-treated mice (group DSS) showed a significantly deficient microbial diversity compared to those in the vehicle group (p < 0.05). Although without statistical significance, there were increasing trends in the Shannon index in both OTUs and genus levels in group DSS + PA40 compared to those of group DSS. The result showed apparent separation of each group in terms of microbial β-diversity (PCA, PCoA, and NMDS analysis on OTUs level) (Fig. 2G). However, samples from the PA40 and vehicle groups clustered together, and samples from the DSS + PA40 group were separated from samples from the DSS group, close to the vehicle and PA40 groups on the longitudinal axis (second principal component). These results suggest that PA alone has little modulatory effect on the structure of the gut microbiota, even as a dominant ingredient in the HXZQ.
Effect of PA on DSS-induced gut microbiota in mice (n = 6 in each group). (A–F) α-diversity based on observed OTUs level (Ace, Chao, Sobs Shannon, Simpson, Coverage). (G) β-diversity based on observed OTUs level (PCA, PCoA, NMDS). (H) Visual representation of p-values on phylum. Statistical analysis was performed by Kruskal–Wallis H test. (I) PA increased the Firmicutes-Bacteroidetes ratio on the phylum level. (J) At the species level, differential SCFAs-producing strains of Phylum Firmicutes and Phylum Bacteroidetes. *p < 0.05; **p < 0.01; ***p < 0.001 versus the Vehicle group; #p < 0.05; ##p < 0.01; ###p < 0.001 versus the DSS-treated group. Data were presented as mean ± S.E.M.
At the phylum level, PA was able to significantly elevate the abundance of Proteobacteria (p < 0.05) and also reduced the abundance of Verrucomicrobiota in the DSS group (p < 0.05) (Fig. 2H). Whereas there was an increasing trend of Bacteroidetes in the DSS group and a decreasing trend of Bacteroidetes in the DSS + PA group compared to the vehicle group. Compared with the vehicle group, there is a decreasing trend of Firmicutes in the DSS group and an increasing trend of Firmicutes in the DSS + PA group (Fig. 2H). Firmicutes-Bacteroidetes (F/B) ratio showed a very important role in many diseases, like hypertension38, and obesity39, and then we did the F/B ratio. Unfortunately, there was no significant difference in F/B in the four groups of Vehicle, PA40, DSS, and DSS + PA40, but there was a tendency to improve after PA intervention (Fig. 2I). Short-chain fatty acids (SCFAs)-producing gut microbiota play an essential role in the homeostasis and immune-microenvironment. At the species level, we analyzed the main bacterial communities of Firmicutes and Bacteroidetes that produce SCFAs (Fig. 2J). The results indicated five bacterial species in the Bacteroidetes, which were differentiated into Bacteroides thetaiotaomicron and Bacteroides_acidifaciens (p < 0.05). Clostridium_leptum_g__norank was found in the Firmicutes was different (p < 0.05). Of these, only Bacteroides thetaiotaomicron had the most statistically significant differences. In summary, our results indicated that PA exhibited faintly modulatory effects on gut microbial community structure in DSS-induced UC mice.
PA upgrades morphophonological changes in the colon of DSS-induced UC mice
To further confirm the anti-inflammatory effect and to study the MOA, H&E staining of the colon tissue was performed. Results in Fig. 3A showed an intact and integrated outer membrane structure in the vehicle group's mucosa, submucosa, and muscle layer. While DSS-induced UC mice suffered severe mucosal damages, such as crypt loss (arrow 1), necrosis (arrow 2), the focal influx of inflammatory cells (arrow 3), and abnormal shortening of the villus, accompanied by irregular or destructions of the villus tip. Under the administration of PA, DSS-treated mice showed significantly improved colon tissue damage in a dose-dependent manner, with more complete crypts (arrows 4 and 5) and less inflammatory cell influx (arrows 6 and 7). The total histopathological scores exhibited that the DSS group had higher than the vehicle group and DSS + PA40 group were close to those of the vehicle group (Fig. 3B). The morphological analysis demonstrated that there was a significant reduction in colonic mucosal depth and smooth muscle thickness in the DSS group, and the DSS + PA20 and DSS + PA40 groups were able to increase in those (Fig. 3C,D). In addition, the AB-PAS staining assay observed an increase in the number and size of goblet cells in the colon tissue of DSS-treated mice (Fig. 3E). The number of goblet cells decreased significantly after PA treatment. TBO staining mainly detects the immune barrier of the intestines, and mast cells are the main detection index of TBO staining. Mast cells are widely distributed around the microvessels under the skin and visceral mucosa, and secrete a variety of cytokines, which are involved in immune regulation40,40,42. Therefore, the number of mast cells was next detected by TBO assay. Compared with the carrier, TBO staining showed an increase in the number of mast cell positivity in DSS-treated mice and a gradual decrease in the number of mast cells in PA-treated mice with DSS-induced colitis (Fig. 3F). The above data suggest that PA ameliorates the abnormal colonic changes in DSS-induced UC mice.
Morphological effects of PA on the colon of DSS-induced UC mice (n = 3 in each group). (A) H&E staining was used to detect morphological changes in the structure of colorectal tissues. The results showed DSS-induced crypt loss (arrow 1), necrosis (arrow 2), and the focal influx of inflammatory cells (arrow 3), after PA treatment showed more complete crypts (arrows 4 and 5) and less inflammatory cell influx (arrows 6 and 7). (B) Scoring of colonic histopathological changes in each treatment group. The following criteria were used to award the grades based on the level and extent of damage to the tissue section: 0, none; 1, mild; 2, moderate; 3, substantial; and 4 severe. (C, D) Quantitative results of pathological changes in villus length and mucosal thickness of colonic tissue in each treatment group. (E) AB-PAS staining assay was used to evaluate the number and size of goblet cells of each group in this study. (F) The number of TBO mast cells was evaluated by TBO staining. Scale bar, 100 μm or 50 μm. *p < 0.05; **p < 0.01; ***p < 0.001 versus the Vehicle group; #p < 0.05; ##p < 0.01; ###p < 0.001 versus the DSS-treated group. Data were presented as mean ± S.E.M.
PA enhances DSS-induced intestinal barrier damage and oxidative damage in UC mice
To further confirm the role of PA on inflammation, immunofluorescence was employed to detect tight junction protein expressions in the intestinal tissue to assess the integrity of the intestinal barrier. As shown in Fig. 4A,B, the fluorescence intensity of both Zona occludens 1 (ZO-1) and Occludin was significantly reduced in DSS-treated mice compared to the control group, which was restored under PA treatment. Taken together, these results suggested that the protective effects of PA on DSS-induced colitis are associated with the maintenance of the epithelial and mucosal barrier.
Effect of PA on the intestinal barrier and oxidative damage in DSS-induced UC mice. (A, B) IF imaging showed PA treatment partly rescued the changes in the expressions of Occludin and ZO-1 resulting from DSS treatment. Scale bar, 100 μm or 50 μm, n = 3. (C) Effects of PA on protein expression of HO-1. The bands for HO-1 and β-actin were cut before incubation with different primary antibodies, and both bands are on the same membrane and continuous. (D) The protein expression quantification of HO-1, n = 4. (E) The result showed that PA treatment could effectively decrease iNOS mRNA, n = 3. *p < 0.05; **p < 0.01; ***p < 0.001 versus the Vehicle group; #p < 0.05; ##p < 0.01; ###p < 0.001 versus the DSS-treated group. Data were presented as mean ± S.E.M.
The results above defined the MOA of PA against DSS-induced UC since it is directly related to the cellular level of the intestinal tissue. An increasing number of evidence suggests that oxidative stress is a major causal factor in the development of IBD43. Therefore, we detected the protein expression of Heme oxygenase-1(HO-1, a significant protein in protecting against inflammatory damage and oxidative tissue injury44) and inducible nitric oxide synthase (iNOS, which contributes to the initiation and progression of inflammation and tissue injury associated with oxidative stress). As shown in Fig. 4C,D, it was found that the expression of HO-1 protein was significantly increased in the colonic tissue of DSS-induced colitis mice compared to vehicle. While PA treatment under 40 mg/kg significantly down-regulated the expression of HO-1. Similarly, the overexpressed iNOS in group DSS was attenuated under PA treatment. Combined with the expression of the pro-inflammatory factors, these results revealed that PA suppressed the anti-inflammatory responses via targeting HO-1 and iNOS-associated anti-oxidant signaling pathways (Fig. 4E).
Discussion
THM has been used worldwide to treat various diseases in both historical and modern medical science, possessing anti-inflammation, anti-cancer activity, and antioxidant activity. Under the circumstances of the COVID-19 pandemic, the THM exhibited conspicuously anti-influenza and anti-virus activities in clinical or animal experiments45. It is widely accepted that over 80% of drugs or substances are derived from or developed based on natural products46. Herbs and plants are the most important sources of drug development. However, the development and application of herbal medicine confront the dilemma of effective THM formula but with complex ingredients and intricated molecular mechanisms, while single compounds with clear principles but fewer pharmaceutical effects. Fortunately, the blooming development of commensal microbiome sequencing provides a standpoint to investigate the relationship between the body/immune system and the THM ingredients from an overall perspective. Integrated with the straightforward MOA of each component, we finally have the opportunity to depict the intricated MOA of THM.
In our previous works, the HXZQ oral liquid, a traditional Chinese THM formula targeting gastrointestinal disorders associated with disease (i.e. gastrointestinal cold and acute gastroenteritis), exhibited the modulatory effects on gut microbiota homeostasis against antibiotic-induced gut microbiota dysbiosis and LPS-induced inflammatory response in mice model. As the predominant pharmaceutical component in the HXZQ formula, PA has been reported to possess anti-inflammatory activity, anti-cancer activity, antioxidant activity, prevention of gastritis, intestinal protection, anti-depressant activity, anti-metabolic diseases, and vasorelaxation activity47. The antioxidant activity is the main MOA of the anti-inflammatory activity against IBD. However, the gut microbiota modulatory capacity of PA and the dose it contributes to PA’s anti-inflammatory activity in the DSS-induced ulcerative colitis model have never been investigated.
Inflammation in the intestinal mucosa contains a complex array of inflammatory mediators, the inflamed intestines of patients with IBD are massively infiltrated by inflammatory cells that release a large number of pro-inflammatory mediators, such as cytokines like IL-6, IL-1β, TNF-α and CCL548. IL-6 has been identified as a crucial regulator of IBD pathogenesis, and patients with IBD have also shown augmented IL-6 serum levels49. Several studies have proved that the blockade of IL-6 signaling in chronic intestinal inflammation caused significant suppression of colitis activity50,51. Chemokines also play an essential role in inflammation and immune response. CCL5 is a typical chemotaxis-active cytokine secreted by normal T cells. It belongs to a family member of CC-type chemokines and regulates the activity and secretion capacity of T lymphocytes. It can mediate the chemotaxis of monocytes and lymphocytes, and participate in the wound-healing process. Our results showed that PA remarkably suppressed the expressions of these pro-inflammatory factors, which led to relatively attenuated UC symptoms.
In this work, our results revealed that PA possessed a satisfactory anti-inflammatory activity in DSS-induced colitis mice, according to previous reports19,37. However, our results showed a negligibly modulatory effect of PA on gut microbiota diversity in neither healthy C57BL/6 mice nor DSS-induced gut microbiota dysbiosis. Noteworthy, PA significantly elevated the F/B ratio in DSS-treated mice. Unfortunately, there is no significant difference. The F/B ratio has been widely accepted as an essential hallmark of maintaining intestinal homeostasis. A decreased F/B ratio is usually observed with IBD52. In the presence of PA intervention, there was a tendency for the F/B ratio of the DSS + PA40 group to recover, suggesting that PA can, to some extent, promote gut homeostasis in the DSS-induced colitis model in mice. The F/B ratio is intensely associated with the concentrations and components of SCFAs, the main metabolic products of gut microbiota. SCFAs are mainly composed of acetic acid, propionic acid, and butyric acid, which account for more than 95% of the total SCFAs with a ratio of 60:20:2053. These three acids can regulate the host immune response and repair the intestinal barrier function by maintaining the intestinal environment and inhibiting the growth of pathogenic bacteria. As is well known, Bifidobacteriaceae and Prevotellaceae are bacterial communities that produce SCFAs in the Bacteroidetes. In the Firmicutes, the Trichospiridae family and the Ruminococcaceae family, as producers of SCFAs, are responsible for the degradation of various polysaccharides and fibers54. Therefore, we identified 15 SCFAs-producing strains from the above Vehicle, DSS, and DSS + PA40, and found the most statistically significant difference in Bacteroides thetaiotaomicron. Bacteroides thetaiotaomicron, a Gram-negative anaerobic bacterium, is a significant component of the human cecum and colon microbiota55. The use of Bacteroides thetaiotaomicron in IBD mouse models could improve inflammation. It follows that PA may restore the F/B ratio by decreasing Bacteroides thetaiotaomicron thus restoring the F/B ratio. Unfortunately, this modulation by PA was weak and made no difference in the overall bacterial population. This is also a limitation of our present work. To interpret the MOA of THM or single-component against IBD, screening out more novel gut microbiota isolates by optimized culture conditions will facilitate more precisely addressing the species of amplicon sequences . Secondly, the metabonomics should assist in depicting the way PA restores the F/B ratio and the impacts of its anti-inflammatory effects, which could be conducted in our future work.
Meanwhile, intestinal barrier integrity is an important indicator of the inflammatory level of IBD, which was maintained by tight junction proteins such as ZO-1 and Occludin. It has been shown that tight junction proteins are severely downregulated under IBD conditions, leading to increased intestinal permeability to microbial ligands, harmful metabolites, and systemic inflammatory response56,57. In our DSS-induced colitis mice model, PA significantly ameliorated intestinal barrier damage. These results revealed the PA protected the intestinal barrier in both hands by suppressing pro-inflammatory factors and preventing colorectal tissue damage.
For the discussion about the MOA of PA, we focused on the direct interaction of PA and colorectal tissue on a cellular level, eliminating the possibility of gut microbiota manipulation in DSS-treated mice. Cellular oxidative stress is present during the initiation and progression of inflammation and dramatically influences the inflammatory environment. HO-1 is a member of the heat shock protein family, which is associated with cellular antioxidant defenses and anti-apoptotic functions. Moreover, it was reported that the expression of iNOS is an important marker of cellular oxidation status, and the overexpression of iNOS is related to the severity of IBD58. Our results revealed that PA directly alleviated the expression of HO-1 and iNOS. These results also gave us evidence about the therapeutic advantages of the THM formula in clinical. Like PA, a single component with effective pharmaceutical activity could synergize with other components with modulatory effects on gut homeostasis, to enhance the overall therapeutic effect of polypharmaceutical THM. Taken together, the underlying MOA of PA against inflammation in DSS-induced UC might be directly suppressing the HO-1 and iNOS-associated antioxidant signal pathways, irrelated with the indirect interactions via modulating the gut microbiota diversity.
Conclusion
PA, a widely used pharmaceutical component of various Chinese THM formulas, prevents the inflammatory response in DSS-induced IBD mice model, via directly suppressing HO-1 and iNOS-associated antioxidant signal pathways, which are irrelevant to the modulation of gut microbiota composition.
References
Burisch, J. & Munkholm, P. Inflammatory bowel disease epidemiology. Curr. Opin. Gastroenterol. 29, 357–362. https://doi.org/10.1097/MOG.0b013e32836229fb (2013).
Kaplan, G. G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12, 720–727. https://doi.org/10.1038/nrgastro.2015.150 (2015).
Biesiada, G. et al. Expression and release of leptin and proinflammatory cytokines in patients with ulcerative colitis and infectious diarrhea. J. Physiol. Pharmacol. 63, 471–481 (2012).
Monteleone, G., Caruso, R. & Pallone, F. Targets for new immunomodulation strategies in inflammatory bowel disease. Autoimmun. Rev. 13, 11–14. https://doi.org/10.1016/j.autrev.2013.06.003 (2014).
Sun, Y., Shi, X., Zheng, X., Nie, S. & Xu, X. Inhibition of dextran sodium sulfate-induced colitis in mice by baker’s yeast polysaccharides. Carbohydr. Polym. 207, 371–381. https://doi.org/10.1016/j.carbpol.2018.11.087 (2019).
Gordon, J. N. et al. Matrix metalloproteinase-3 production by gut IgG plasma cells in chronic inflammatory bowel disease. Inflamm. Bowel Dis. 14, 195–203. https://doi.org/10.1002/ibd.20302 (2008).
Laukens, D. et al. Tauroursodeoxycholic acid inhibits experimental colitis by preventing early intestinal epithelial cell death. Lab. Investig. 94, 1419–1430. https://doi.org/10.1038/labinvest.2014.117 (2014).
Han, F. et al. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 194, 1882–1893. https://doi.org/10.4049/jimmunol.1402300 (2015).
Yadav, V. et al. Inflammatory bowel disease: Exploring gut pathophysiology for novel therapeutic targets. Transl. Res. 176, 38–68. https://doi.org/10.1016/j.trsl.2016.04.009 (2016).
Pile, J. E., Navalta, J. W., Davis, C. D. & Sharma, N. C. Interventional effects of plumbagin on experimental ulcerative colitis in mice. J. Nat. Prod. 76, 1001–1006. https://doi.org/10.1021/np3008792 (2013).
Argollo, M. et al. Comorbidities in inflammatory bowel disease: A call for action. Lancet Gastroenterol. Hepatol. 4, 643–654. https://doi.org/10.1016/s2468-1253(19)30173-6 (2019).
Kumar, A. et al. A novel role of SLC26A3 in the maintenance of intestinal epithelial barrier integrity. Gastroenterology 160, 1240-1255.e1243. https://doi.org/10.1053/j.gastro.2020.11.008 (2021).
Bhatia, S. P., Letizia, C. S. & Api, A. M. Fragrance material review on patchouli alcohol. Food Chem. Toxicol. 46(Suppl 11), S255-256. https://doi.org/10.1016/j.fct.2008.06.069 (2008).
Jeong, J. B., Choi, J., Lou, Z., Jiang, X. & Lee, S. H. Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits anti-tumorigenic activity in human colorectal cancer cells. Int. Immunopharmacol. 16, 184–190. https://doi.org/10.1016/j.intimp.2013.04.006 (2013).
Wu, X. L. et al. Immunologic mechanism of Patchouli alcohol anti-H1N1 influenza virus may through regulation of the RLH signal pathway in vitro. Curr. Microbiol. 67, 431–436. https://doi.org/10.1007/s00284-013-0381-y (2013).
Hu, G., Peng, C., Xie, X., Zhang, S. & Cao, X. Availability, pharmaceutics, security, pharmacokinetics, and pharmacological activities of patchouli alcohol. Evid. Based Complement. Alternat. Med. 2017, 4850612. https://doi.org/10.1155/2017/4850612 (2017).
Zheng, Y. F. et al. Gastroprotective effect and mechanism of patchouli alcohol against ethanol, indomethacin and stress-induced ulcer in rats. Chem. Biol. Interact. 222, 27–36. https://doi.org/10.1016/j.cbi.2014.08.008 (2014).
Chen, X. Y. et al. The gastroprotective effect of pogostone from Pogostemonis Herba against indomethacin-induced gastric ulcer in rats. Exp. Biol. Med. (Maywood) 241, 193–204. https://doi.org/10.1177/1535370215600099 (2016).
Qu, C. et al. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacol. Res. 121, 70–82. https://doi.org/10.1016/j.phrs.2017.04.017 (2017).
Xian, Y. F. et al. Anti-inflammatory effect of patchouli alcohol isolated from Pogostemonis Herba in LPS-stimulated RAW264.7 macrophages. Exp. Ther. Med. 2, 545–550. https://doi.org/10.3892/etm.2011.233 (2011).
Leong, W. et al. Patchouli essential oil and its derived compounds revealed prebiotic-like effects in C57BL/6J mice. Front. Pharmacol. 10, 1229. https://doi.org/10.3389/fphar.2019.01229 (2019).
Wu, J. et al. Patchouli alcohol attenuates 5-fluorouracil-induced intestinal mucositis via TLR2/MyD88/NF-kB pathway and regulation of microbiota. Biomed. Pharmacother. 124, 109883. https://doi.org/10.1016/j.biopha.2020.109883 (2020).
Leong, W. et al. Traditional Patchouli essential oil modulates the host’s immune responses and gut microbiota and exhibits potent anti-cancer effects in Apc(Min /+) mice. Pharmacol. Res. 176, 106082. https://doi.org/10.1016/j.phrs.2022.106082 (2022).
Ichikawa, K., Kinoshita, T. & Sankawa, U. The screening of Chinese crude drugs for Ca2+ antagonist activity: Identification of active principles from the aerial part of Pogostemon cablin and the fruits of Prunus mume. Chem. Pharm. Bull. (Tokyo) 37, 345–348. https://doi.org/10.1248/cpb.37.345 (1989).
Gao, M. et al. Modulatory effects of Huoxiang Zhengqi oral liquid on gut microbiome homeostasis based on healthy adults and antibiotic-induced gut microbial dysbiosis mice model. Front. Pharmacol. 13, 841990. https://doi.org/10.3389/fphar.2022.841990 (2022).
Zhang, R. et al. A pharmacokinetic study of patchouli alcohol after a single oral administration of patchouli alcohol or patchouli oil in rats. Eur. J. Drug Metab. Pharmacokinet. 41, 441–448. https://doi.org/10.1007/s13318-015-0272-7 (2016).
Gao, M. et al. Preventive effects of traditional Chinese medicine formula Huoxiangzhengqi against lipopolysaccharide-induced inflammatory response. Phytomedicine 99, 153968. https://doi.org/10.1016/j.phymed.2022.153968 (2022).
Seemann, S., Zohles, F. & Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 24, 60. https://doi.org/10.1186/s12929-017-0370-8 (2017).
Erben, U. et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 7, 4557–4576 (2014).
Ma, E. L. et al. Bidirectional brain–gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav. Immun. 66, 56–69. https://doi.org/10.1016/j.bbi.2017.06.018 (2017).
Yu, W. et al. Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat. Commun. 8, 14716. https://doi.org/10.1038/ncomms14716 (2017).
Wong, H. L. X. et al. Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling. Nat. Commun. 10, 1745. https://doi.org/10.1038/s41467-019-09744-3 (2019).
Li, H. L. et al. Isoastragaloside I suppresses LPS-induced tight junction disruption and monocyte adhesion on bEnd.3 cells via an activating Nrf2 antioxidant defense system. RSC Adv. 8, 464–471 (2018).
Feng, R. et al. Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. J. Extracell. Vesicles 9, 1783869. https://doi.org/10.1080/20013078.2020.1783869 (2020).
Diao, J. et al. Silicon dioxide nanoparticles induced neurobehavioral impairments by disrupting microbiota-gut-brain axis. J. Nanobiotechnol. 19, 174. https://doi.org/10.1186/s12951-021-00916-2 (2021).
Mori H, et al. Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes. DNA Res. 21(2), 217-27 https://doi.org/10.1093/dnares/dst052 (2014).
Wu, Z. et al. Patchouli alcohol: A natural sesquiterpene against both inflammation and intestinal barrier damage of ulcerative colitis. Inflammation 43, 1423–1435. https://doi.org/10.1007/s10753-020-01219-8 (2020).
Yang, T. et al. Gut dysbiosis is linked to hypertension. Hypertension 65, 1331–1340. https://doi.org/10.1161/hypertensionaha.115.05315 (2015).
Magne, F. et al. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients?. Nutrients https://doi.org/10.3390/nu12051474 (2020).
Zhang, S. et al. Chang-Kang-Fang alleviates diarrhea predominant irritable bowel syndrome (IBS-D) through inhibiting TLR4/NF-κB/NLRP3 pathway. J. Ethnopharmacol. 330, 118236. https://doi.org/10.1016/j.jep.2024.118236 (2024).
Ortiz-Cerda, T. et al. Effects of polyphenolic maqui (Aristotelia chilensis) extract on the inhibition of NLRP3 inflammasome and activation of mast cells in a mouse model of Crohn’s disease-like colitis. Front. Immunol. 14, 1229767. https://doi.org/10.3389/fimmu.2023.1229767 (2023).
Shao, M., Yuan, F., Liu, J. & Luo, H. Mast cell specific receptor Mrgprb2 regulating experimental colitis is associated with the microbiota-gut-brain axis. J. Inflamm. Res. 15, 6137–6151. https://doi.org/10.2147/jir.S383812 (2022).
Lal, R., Dhaliwal, J., Dhaliwal, N., Dharavath, R. N. & Chopra, K. Activation of the Nrf2/HO-1 signaling pathway by dimethyl fumarate ameliorates complete Freund’s adjuvant-induced arthritis in rats. Eur. J. Pharmacol. 899, 174044. https://doi.org/10.1016/j.ejphar.2021.174044 (2021).
Takahashi, T. et al. Heme oxygenase-1 is an essential cytoprotective component in oxidative tissue injury induced by hemorrhagic shock. J. Clin. Biochem. Nutr. 44, 28–40. https://doi.org/10.3164/jcbn.08-210-HO (2009).
Zhao, Z. et al. Prevention and treatment of COVID-19 using Traditional Chinese Medicine: A review. Phytomedicine 85, 153308. https://doi.org/10.1016/j.phymed.2020.153308 (2021).
Maridass, M. & Britto, A. J. D. Origins of plant derived medicines. Ethnobotanical Leaflets (2008).
Lee, H. S., Lee, J., Smolensky, D. & Lee, S. H. Potential benefits of patchouli alcohol in prevention of human diseases: A mechanistic review. Int. Immunopharmacol. 89, 107056. https://doi.org/10.1016/j.intimp.2020.107056 (2020).
Soufli, I., Toumi, R., Rafa, H. & Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 7, 353–360. https://doi.org/10.4292/wjgpt.v7.i3.353 (2016).
Neurath, M. F. & Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 22, 83–89. https://doi.org/10.1016/j.cytogfr.2011.02.003 (2011).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588. https://doi.org/10.1038/75068 (2000).
Yamamoto, M., Yoshizaki, K., Kishimoto, T. & Ito, H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J. Immunol. 164, 4878–4882. https://doi.org/10.4049/jimmunol.164.9.4878 (2000).
Stojanov, S., Berlec, A. & Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms https://doi.org/10.3390/microorganisms8111715 (2020).
Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P. & Macfarlane, G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227. https://doi.org/10.1136/gut.28.10.1221 (1987).
Sagheddu, V., Patrone, V., Miragoli, F., Puglisi, E. & Morelli, L. Infant early gut colonization by lachnospiraceae: High frequency of Ruminococcus gnavus. Front. Pediatr. 4, 57. https://doi.org/10.3389/fped.2016.00057 (2016).
Gul, L. et al. Extracellular vesicles produced by the human commensal gut bacterium Bacteroides thetaiotaomicron affect host immune pathways in a cell-type specific manner that are altered in inflammatory bowel disease. J. Extracell. Vesicles 11, e12189. https://doi.org/10.1002/jev2.12189 (2022).
Tang, X. et al. Gut Epithelial Barrier Function is Impacted by Hyperglycemia and Secondary Bile Acids in Vitro: Possible Rescuing Effects of Specific Pectins. Mol. Nutr. Food Res. 68, 2300910 https://doi.org/10.1002/mnfr.202300910 (2024).
Han, M. et al. Bifidobacterium bifidum Ameliorates DSS-Induced Colitis in Mice by Regulating Microbial Metabolome and Targeting Gut Microbiota. J. Agric. Food Chem. 72(24), 13593-13609 https://doi.org/10.1021/acs.jafc.4c00365 (2024).
Avdagić, N. et al. Nitric oxide as a potential biomarker in inflammatory bowel disease. Bosn. J. Basic. Med. Sci. 13, 5–9. https://doi.org/10.17305/bjbms.2013.2402 (2013).
Acknowledgements
This study was supported by the key project of the Natural Science Foundation of Chongqing (cstc2020jcyj-zdxmX0029), the Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN202100412), the Postdoctoral Fellowship Program of CPSF (GZC20233340), and the Chongqing's Special Funding for Postdoctoral Research Projects (2023CQBSHTB3082).
Author information
Authors and Affiliations
Contributions
H.H.: methodology, formal analysis, validation, and software; M.G.: methodology, formal analysis, validation, and software; F.W.: methodology, formal analysis, validation, and software; Z.L.: methodology, conceptualization, supervision, and validation; X.J.: methodology, conceptualization, supervision, and validation; Y.Q.: methodology, conceptualization, supervision, and validation; J.S.: methodology, supervision, data curation; X.D.: methodology and formal analysis; S.L.: methodology and formal analysis; S.T.: methodology and formal analysis; A.K.: writing-review editing; Z.Z.: methodology and formal analysis; C.C.: Project administration, conceptualization, supervision, and validation; writing-review editing; Q.Y.: project administration, conceptualization, supervision, and validation, writing the original draft manuscript, writing-review editing and funding acquisition; J.Q.: conceptualization, supervision, resources, and funding acquisition; H.Z.: project administration, conceptualization, supervision, and validation, writing the original draft manuscript, funding acquisition, and writing-review editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, H., Gao, M., Wang, F. et al. Protective effects of patchouli alcohol against DSS-induced ulcerative colitis. Sci Rep 14, 16745 (2024). https://doi.org/10.1038/s41598-024-66259-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-66259-8