Abstract
COVID-19 is a highly contagious virus that uses Angiotensin-converting enzyme 2 (ACE2) as a receptor to enter human cells. The virus leads to an increase in inflammatory cytokines (i.e. IL-6) and an impaired coagulation system, which can cause serious complications during and after the disease. Physical exercise has been shown to improve COVID-19 complications through various mechanisms, such as modulation of the immune and coagulation systems. Therefore, this study investigated the effects of 8 weeks of training on inflammatory, coagulation, and physical factors in patients with COVID-19 during the recovery phase. Twenty-seven male and female volunteers (age 20–45 years) who recently recovered from COVID-19 were assigned to the control (n = 13) or the training group (n = 14). Blood samples, aerobic capacity and muscle endurance were collected 24 h before the start of the interventions and 24 h after the final training session in week 4 and 48 h after the final training session in week 8. IL-6, ACE2, fibrinogen, and D-dimer were measured using ELISA. The training group showed a significant increase in muscle endurance (p = 0.004) and aerobic capacity (p = 0.009) compared to the control group. Serum levels of IL-6 and fibrinogen decreased in the training group but this decrease was not statistically significant (p > 0.05). Despite a slight increase in the quality of life and sleep in the training group, no statistically significant difference was observed between the training and the control group. It appears that physical training has beneficial effects on the coagulation system, inflammatory factors, and sleep quality and can facilitate the recovery of COVID-19 patients.
Similar content being viewed by others
Introduction
Coronavirus is classified in the Orthocoronavirinae subfamily of the Coronaviridae family1. In late 2019, a new coronavirus (SARS-CoV-2) was discovered in Wuhan, Hubei, China, which caused respiratory illness. This disease was named Coronavirus Disease 2019 or COVID-19. Infection by SARS-CoV-2 changes the binding of host cells to the angiotensin-converting enzyme-2 (ACE2), which is the virus's receptor2. The spike protein of SARS-CoV-2 identifies ACE2 receptors on host cells, resulting in the virus fusing with them3. The ACE2 protein is expressed in multiple organs, such as the heart, kidneys, adrenal glands, pancreas, and skeletal muscle, as well as arterial and venous endothelial cells and arterial smooth muscle cells4,5. Recent studies have that when ACE2 expression was decreased, the virus was less likely to infect mice6,7. Accordingly, ACE2 may contribute to COVID-19 pathophysiology8.
COVID-19 is a self-limiting infection in which the host's immune system plays an important role in the defense response9. To control viral replication and inflammation, effective antiviral responses from the host's innate and adaptive defenses, including the generation of numerous pro-inflammatory cytokines and the activation of T cells, are necessary10. Following exposure to COVID-19 and the occurrence of cytokine storms, the blood levels of pro-inflammatory cytokines increase11,12. Among the excessive cytokines generated by activated macrophages, IL-6 is one of the main cytokines13. IL-6 is a cytokine with multiple functions, involved in both pro- and anti-inflammatory processes14,15,16. Elevated levels of IL-6 were consistently found in several studies, suggesting that it could be used as a biomarker to predict disease severity17,18,19.COVID-19 can also be associated with thrombogenic coagulopathy, with a variety of manifestations. SARS-CoV-2 directly invades the endothelial cells with ACE2 receptors, constituting the main pathway through which the virus enters these cells. This invasion causes angiotensin II accumulation and downregulation of the ACE2 receptors, resulting in prothrombotic effects such as hemostatic imbalance via activation of the coagulation cascade, impaired fibrinolysis, thrombin generation, vasoconstriction, endothelial and platelet activation, and pro-inflammatory cytokine release.20 Thus, coagulopathy is associated with a higher death rate in COVID-19 patients21,22. While clinical biomarkers are still required to predict disease severity, hematological markers such as D-dimer and fibrinogen have been shown to correlate with the presence of coagulopathy in COVID-1923,24. D-dimer is widely used in clinical settings as a biomarker of endogenous fibrin clot formation and a recognized laboratory indicator of hypercoagulability25,26. COVID-19 patients with higher D-dimer levels are at higher risk of intensive care unit admission or even death27,28. One of the most precise tests for diagnosing disseminated intravascular coagulation (DIC) is fibrinogen concentration29. The mean fibrinogen concentrations in COVID-19 patients were found to be above the normal range30. Thus, these two hematological factors have the potential to be a promising marker for monitoring COVID-19 patients during and after treatment.
While millions of individuals were quarantined during the COVID-19 outbreak, the sedentary behaviour and increase in health disorders caused by the quarantine might have impacted their overall health and quality of life31,32. Studies investigating the association between physical activity (PA) and the risk of COVID-19 infection are currently scarce. However, several studies have reported a positive impact of regular physical activity on comorbidities associated with severe COVID-1933,34. Emerging evidence suggests that an active lifestyle is the best choice for good health and that physical exercise is an effective non-pharmacological intervention for many chronic diseases, including COVID-1935. Studies have shown that those who are physically active have a reduced incidence, severity of symptoms, and morbidity from viral infections36. Additionally, it has been reported that exercise can improve risk factors for severe COVID-1937.
Regular exercise improves cardiorespiratory health, strengthens muscles, and reduces the risk of systemic inflammation, which are the processes by which it reduces the symptoms of various acute and chronic morbidities38. Physical exercise appears to have a significant impact on the immune and coagulation systems in patients diagnosed with COVID-1936,39,40. In this regard, it has been documented that long-duration or high-intensity exercises (above 80% VO2max) result in immunosuppression markers, which are accompanied by an elevation in pro-inflammatory cytokines (e.g., IL-6 and TNF-α). As a result, individuals with infectious diseases like COVID-19 are at a greater risk of infection41,42. Regular physical activity has decreased by 9–48% in response to self-isolating instructions to slow COVID-19 outbreak40. COVID-19 patients often experience decreased physical activity due to venous thromboembolism, which can involve pulmonary embolism or deep vein thrombosis43,44,45,46,47.
With the initial activation of the immune system upon infection with SARS-CoV-2, the clotting pathway also becomes activated, which has been termed the thrombi-inflammatory state48. The coronavirus decreases ACE2 activity by deregulating the renin–angiotensin–aldosterone system (RAAS). As a consequence, angiotensin II is not converted and remains active in prothrombotic and inflammatory processes49. In response to abnormal tissue factors, primarily caused by IL-6 and also by the complement factor C5a, vessel walls are disrupted, resulting in thrombin, fibrinogen, and fibrin production. In addition to activating fibrin and fibrinogen, thrombin and coagulation factor X boosts the immune response by producing more inflammatory cytokines. COVID-19 has a thrombi-inflammation state, which can be confirmed by the presence of IL-6, D-dimer, and fibrinogen. The thrombosis leads to both microvascular thrombosis and multiple organ dysfunction syndromes48,50,51.
Considering the severe impact of COVID-19 and the limitations in accessing information about the recovery rate of COVID-19 patients, a detailed and comprehensive rehabilitation program with individual exercise protocols will be useful for patients with long-term COVID-19 to restore a level of physical fitness comparable to the initial level before the disease52. This study aimed to evaluate the effects of training on IL-6, ACE-2, D-dimer, and fibrinogen in COVID-19 survivors. We hypothesized that 8 weeks of exercise during the recovery period can positively affect the coagulation parameters, interleukin-6, and angiotensin-converting enzyme-2 who have recovered from COVID-19.
Methods
Subjects
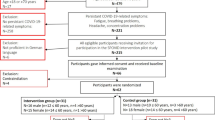
This study was a semi-quasi study with a pre and post-test design. The sample size required for this study was calculated using G*Power version 3.1.9.653. With a repeated measures analysis of variance (ANOVA), a rejection criterion of 0.05, a power of 0.8, and a large effect size (f = 0.6), at least twenty participants were required to achieve the above criterion.
The participants of the current study, twenty-seven individuals (age 20–45 years) who had recovered from COVID-19, were matched based on the severity of their condition and randomly assigned to the training (6 men and 8 women) or the control group (8 men and 5 women). This project was conducted at the Nabie-Akram Clinical Institution, in Bushehr, Iran. Patients with COVID-19, diagnosed according to WHO interim guidance, and 10 to 30 days after a positive COVID-19 PCR test, were eligible to participate in this study. The supervising physician evaluated the severity of their condition and only participants with low to moderate levels of illness were eligible to participate. The extent of lung involvement at the start of the study was very mild or mild, as determined by clinical examinations and recorded by the supervising physician.
Patients who had a history of diabetes, heart disease, cancer, drug misuse or dependency, or high blood pressure were not eligible to participate in the study. To comply with ethical considerations, ensure the confidence of the participants, and observe safety precautions, all sessions were conducted in the presence of a supervising physician.
The exercise protocol was implemented following health guidelines, which included wearing masks and maintaining social distancing. The exercise protocol implementation site and used equipment were thoroughly disinfected before and after each session. Additionally, the training facility was well-ventilated. All blood sampling procedures were performed in a research laboratory by a qualified phlebotomist under all legal regulations. The risks and benefits of the study were explained to all participants and they signed a written informed consent form in accordance with the Declaration of Helsinki. Participants were informed about the voluntary nature of the study and were allowed to withdraw from the study at any time. In addition, the Ethics Committee of the Research Institute of Physical Education and Sports Sciences reviewed and approved the study.
Training protocol
Training was performed three times per week for 60 min in a well-equipped gymnasium in Bushehr. A summary of the training protocol from week 0 to week 8 is shown in Tables 1 and 2. Table 1 details the exercises for weeks 0–4, and Table 2 details the exercises for weeks 5–8. To prevent training boredom among participants and to offer training variations, each period consisted of two training plans that were applied alternately. These programs were developed based on two pilot sessions in which 5 participants attended. The intensity of the exercises was established based on the feedback from the pilot group and deemed appropriate for the patients in this study. Participants received between 60 and 90 s of rest between each set which appeared to be adequate recovery. A physician examined the subjects before each training session and assessed their vital signs to ensure that the training intensity did not surpass their capacity and did not cause any adverse effects.
Blood collection and analysis
Blood samples were collected after 8–10 h of overnight fasting from the median cubital vein 24 h before the beginning of the intervention, and 24 h after the last training session in week 4 and 48 h after the last training session in week 8. The samples were centrifuged for 10 min at 3000 RPM. The serum was separated and kept at -80°C until further analysis. Blood variables were measured using ELISA kits (Zell Bio GmbH, Germany). The analyses were performed by following the instruction of the manufacturer of the following kits: Fibrinogen (cat no:ZB-12006C-H9648), IL-6 (cat no:ZB-10090C-H9648), ACE-2 (cat no:ZB-13169C-H9648), and D-dimer (cat no:ZB-09049C-H9648). The sensitivity ranges of the kits were as follows: fibrinogen (0.08 mg/ml), IL-6 (3–5 pg/ml), ACE2 (0.5 ng/ml), and D-Dimer (1–3 ng/ml) were measured in blood serum using an ELISA kit (Zell Bio GmbH, Germany).
Aerobic capacity
All performance tests were administered 24 h before the first training session, 24 h after the last training session in week 4, and 48 h after the last training session in week 8. The Queens College Step Test was used to estimate the subjects' aerobic capacity. The participants stepped on and off a 41.3 cm box at a rate of 24 steps per minute (men) and 22 steps per minute (women) for 3 min. Afterward, the participants stood until their heart rate was recorded for 15 s between the 5th and 20th seconds of the return to baseline. The following VO2max formula was used to estimate their aerobic capacity54:
Muscle endurance
A push-up test was performed to assess muscle endurance. Participants positioned their hands and toes on the ground in a plank position. Female participants performed modified push-ups, placing their knees on the ground instead of their toes. While performing a controlled contraction, participants lowered their chest close to the ground, then extended their elbows to return to the starting position. The number of push-ups performed until failure was recorded.
Assessment of sleep quality
The Petersburg Sleep Quality Index (PSQI) was used to assess sleep quality55. PSQI is a validated tool consisting of 19 questions in seven sections: subjective sleep quality (C1), sleep latency (C2), sleep duration (C3), habitual sleep efficiency (C4), sleep disturbance (C5), use of sedative medications (C6), and daytime dysfunction C7). Each component is given a score that ranges from 0 to 3. Scores of 0, 1, 2, and 3 on each scale indicate normal, slight problems, average problems, and severe problems, respectively. The overall score is then calculated totaling seven component scores, where lower scores denote better sleep quality.
The quality of life
To measure the quality of life among participants in this study, the WHOQOL-BREF questionnaire was employed. The WHOQOL-BREF questionnaire consists of 26 items and assesses four domains: physical health (7 questions), psychological health (6 questions), social relationships (3 questions), and the environment (8 questions)56. The first two questions are not relevant to these domains and are only intended to evaluate health and quality of life in general. Thus, there were 26 questions in this survey. The score in each domain ranges from 4 to 20, with 4 and 20 representing the worst and best conditions in each respective domain. Finally, the subjects received a score between 0 and 10057.
Statistical analysis
The statistical package for the social sciences (SPSS) was used to analyze the data. The central tendency and dispersion of the data are reported as the mean and standard deviation (SD). A Shapiro–Wilk test was used to determine the normal distribution of the data. A series of mixed model repeated measures analysis of variance (ANOVA) were executed to determine the effects of condition and time on dependent variables. The level of statistical significance was set at 0.05 for all analyses.
Ethics approval and consent to participate
All procedures in this study were carried out under the Helsinki Statement regarding human research. The proposal for this project was presented to the Ethics Committee of the Research Institute of Physical Education and Sports Sciences of Iran and approved (IR.SSRC.REC.1400.041). Also, participants signed an informed consent form prior to participation in the study.
Results
The hematological indices at week 0, week 4, and week 8 are presented in Table 3. A statistically significant time and group interaction was observed in white blood cells, Eosinophil and Eosinophil % (p < 0.05). Additionally, there was a significant time effect on red blood cells, hemoglobin, hematocrit, Mean Corpuscular Volume, Mean Corpuscular Hemoglobin, Red Cell Distribution Width standard deviation, platelets, plateletcrit, monocyte, Eosinophil%, Monocyte, and Eosinophil (p < 0.05). The only group effect was observed in Eosinophil% (p < 0.05). The changes observed in all other variables did not reach the level of statistical significance (p > 0.05).
Anthropometric indices are presented in Table 4. There was a significant interaction effect on WHR, and similarly, there was a significant time effect on BMI (p < 0.05). The changes in all other variables did not reach levels of statistical significance (p > 0.05).
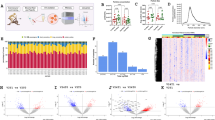
The D-Dimer levels for both groups are presented in Fig. 1. There was no significant interaction effect F (2, 50) = 2.545, p = 0.089, group F (1, 25) = 1.700, p = 0.204, or time F (2, 50) = 0.629, p = 0.537.
The circulatory levels of fibrinogen are presented in Fig. 2. There was a significant effect of time F (2, 50) = 11.130, p < 0.001. However, there was no significant interaction effect F (2, 50) = 0.705, p = 0.499, or group F (1, 25) = 0.987, p = 0.330.
The plasma levels of IL-6 are presented in Fig. 3. There was a significant effect of group F (1, 25) = 6.496, p = 0.017 and time F (2, 50) = 8.733, p = 0.001. However, the interaction effect did not reach statistical significance F (2, 50) = 2.433, p = 0.098.
The levels of ACE2 for the training and the control group are presented in Fig. 4. There was a significant effect of group F (1, 25) = 8.830, p = 0.006. However, the interaction effect F (2, 50) = 0.231, p = 0.794 and time effect F (2, 50) = 0.075, p = 0.928 were not statistically significant.
The quality of life and the sleep quality were measured in week 0, week 4 and week 8 and the results are presented in Table 5. The Quality of Life was not statistically different between the two groups across time. However, the sleep quality improved significantly across time F (2, 50) = 4.705, p = 0.013. The interaction effect F (2, 50) = 0.730, p = 0.487, and the group effect F (1, 25) = 0.024, p = 0.878 on sleep quality was not statistically significant.
Discussion
This study investigated the effects of 8 weeks of physical training on coagulation parameters, IL-6, and ACE2 in patients who had recovered from COVID-19. The findings indicate that performing low-intensity training during recovery from COVID-19 improves aerobic capacity and muscle endurance, reduces serum levels of IL-6 and fibrinogen and, increases D-dimer. Therefore, our hypothesis was confirmed that physical training can mitigate the negative effects of COVID-19, lower inflammatory and fibrinogen indices, and improve the quality of life for patients by enhancing the recovery process.
After infection, ACE2 plays a regulatory role in the immune response and cytokine secretion58. COVID-19 is a highly infectious virus that uses ACE2 as a receptor to enter cells. After entering the body, the virus increases inflammatory cytokines. IL-6, an inflammatory factor, has been reported to remain elevated in the body for a long time after infection, causing physical dysfunction even in patients who have recovered59,60. Therefore, reducing inflammatory markers such as IL-6 is considered an appropriate therapeutic target for the treatment of viral infections, including COVID-19. Our results showed that the serum level of IL-6 decreased significantly in the training group (47%) compared to the control group (15%). This inverse relationship between physical training and IL-6 levels has already been established and regular exercise reduces the circulatory level of IL-661. Additionally, the body fat percentage and waist-to-hip ratio in the training group showed a significant decrease compared to the control group, indicating the effect of exercises on reducing fat tissue. An increase in white adipose tissue can increase serum IL-662; therefore, exercise may lower inflammatory indicators by reducing fat tissue.
Our data also indicated that serum levels of ACE2 in the training group did not show a significant difference compared to the control group. Increased serum ACE2 level in COVID patients could indicate poor prognosis and is linked to various diseases that increase angiotensin-renin system activity62. Coronavirus decreases membrane activity by damaging membrane ACE2, resulting in thrombosis by increasing vascular permeability and expressing tissue factors in leukocytes and platelets63,64.
Numerous studies have examined the impact of physical activity on inflammatory factors and the immune system. The results demonstrated that regular exercise may have an anti-inflammatory effect on the body in certain diseases, such as COVID-1965,66. Given the benefits of physical activity in reducing the risks of various diseases, it can be assumed that active individuals have better control over high-risk comorbidities that increase their susceptibility to severe COVID-19 compared to sedentary people. However, some studies have reported different roles of physical activity in COVID-19 patients. While moderate-intensity exercise is beneficial for the immune system, prolonged and high intensity exercise can suppress the immune system by disrupting the balance of cytokines type I and II, which may increase the risk of infection. The effects of physical activity on ACE2 receptor modulation have been established, but clinical implications for angiotensin-related pathways in humans are still unclear67,68.
The accumulation of evidence indicates that impaired blood coagulation, particularly in serum D-dimer and fibrinogen levels, is a significant complication in patients with COVID-19 that could last for several months. Improving these coagulation indices through training can lead to better and faster recovery in these individuals69. Because of this, the full recovery period for individuals with very severe symptoms can take months, and some of them may have experience the disorder for a long time after the infection. Accordingly, physicians believe that the post-recovery period is as important as the period of the disease.40,70 Blood clots and their complications are concerning for patients with COVID-19. The results indicated a non-significant increase in serum levels of D-dimer and a decrease in fibrinogen during the research period. Mean serum fibrinogen decreased in the training group by 7.9% and 23.1% after 4 and 8 weeks, respectively, while the control group experienced a change of 0.6% and 13.5%. Numerous studies indicated that physical activity does not significantly impact serum fibrinogen levels71,72,73. However, some reports have shown a positive correlation between physical activity and fibrinogen levels in various studies involving middle-aged to elderly active and inactive individuals of both genders74,75,76,77. Studies have indicated that the levels of D-dimer can decrease78 or increase79,80 during exercise. In addition, hematological findings from our data showed that the level of WBC and eosinophil in COVID-19 patients were significantly different from those in the control group.
Lowering inflammation levels can enhance sleep quality81. Hence, regular exercise has the potential to improve both the sleep quality and quality of life. Indeed, many studies have shown that exercise improves sleep quality82. After 8 weeks of exercise, the sleep quality of the training group showed a greater improvement compared to the control group, but these changes were not statistically significant. The PSQI in the training group decreased by 33.9% after eight weeks, while the control group experienced a 17.2% decrease. A decrease in PSQI indicates an increase in sleep quality. These findings are supported by studies showing a lack of significant association between exercise and sleep quality in different patients, such as those with lymphoma and cancer83,84,85. Additionally, the findings indicate a non-significant increase in the quality of life in the training group. Various studies have demonstrated that exercise has a positive impact on quality of life82,86. The present study demonstrated that short-term exercise has no significant effect on individuals in the recovery period.
One significant issues with COVID-19 is the decrease in aerobic endurance and cardiorespiratory fitness caused by lung infection, which can result in reduced physical function. Physical dysfunction, fatigue, and muscle weakness are common complaints among patients with COVID-19. Our results demonstrated that aerobic fitness and muscle endurance of patients can increase significantly in response to exercise. The positive effect of physical activity on muscle endurance is highlighted by previous studies87,88. Therefor the negative effects of COVID-19 on physical fitness may be mitigated by regular training.
Researchers of the current study encountered several limitations during this project. The most challenging task was the eligibility assessment and continues monitoring of participants’ progress throughout the study. The lack of access to a CT scanner limited the accurate assessment of participants’ conditions. With CT scans, the results of this study could be better discussed in relation to different stages of the disease.
Early diagnosis of COVID-19 in crucial in reducing the morbidity and mortality rates and preventing further spread of the disease. It has been reported that CT scans have higher sensitivity in diagnosing COVID-19 than other methods such as PCR89. With a lung CT scan, physicians can accurately determine the extent of lung involvement at various stages of COVID-19 infection, providing a more precise assessment of the patient’s health condition than the PCR test90. Perhaps future researchers should consider using more advanced technologies to provide objective assessments of patients.
Conclusion
This study evaluated the effects of training during the recovery period on coagulating factors, inflammation, physical fitness, sleep quality, and quality of life indices in patients recovering from COVID-19. The results suggest that 8 weeks of training can improve the physical condition of COVID-19 patients by enhancing the inflammatory state, coagulation system, and sleep quality, thereby helping patients return to normal conditions more quickly. As the first study to implement exercise intervention for people recovering from COVID-19, this research provides a foundation for developing practical and safe recovery methods for COVID-19 patients.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pal, M., Berhanu, G., Desalegn, C. & Kandi, V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): An update. Cureus 12 (2020).
Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 66, 15–23 (2022).
Ghosh, N., Nandi, S. & Saha, I. A review on evolution of emerging SARS-CoV-2 variants based on spike glycoprotein. Int. Immunopharmacol. 105, 108565 (2022).
Bahat, G. Covid-19 and the renin angiotensin system: Implications for the older adults. J. Nutr. Health Aging 24, 699–704 (2020).
Hamming, I. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637 (2004).
Jia, H., Neptune, E. & Cui, H. Targeting ACE2 for COVID-19 therapy: Opportunities and challenges. Am. J. Respirat. Cell Mol. Biol. 64, 416–425 (2021).
Chaudhry, F. et al. Manipulation of ACE2 expression in COVID-19. Open Heart 7, e001424 (2020).
Bourgonje, A. R. et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 251, 228–248 (2020).
Cascella, M., Rajnik, M., Aleem, A., Dulebohn, S. C. & Di Napoli, R. Features, evaluation, and treatment of coronavirus (COVID-19). Statpearls [internet] (2023).
Paces, J., Strizova, Z., Daniel, S. & Cerny, J. COVID-19 and the immune system. Physiol. Res. 69, 379 (2020).
Hu, B., Huang, S. & Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 93, 250–256 (2021).
Jiang, Y. et al. Cytokine storm in COVID-19: From viral infection to immune responses, diagnosis and therapy. Int. j. Biol. Sci. 18, 459 (2022).
Chen, X. et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. MedRxiv, 2020.2002. 2029.20029520 (2020).
Potere, N. et al. The role of IL-6 and IL-6 blockade in COVID-19. Exp. Rev. Clin. Immunol. 17, 601–618 (2021).
Scheller, J., Chalaris, A., Schmidt-Arras, D. & Rose-John, S. The pro-and anti-inflammatory properties of the cytokine interleukin-6. BBA Mol. Cell Res. 1813, 878–888 (2011).
Wolf, J., Rose-John, S. & Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 70, 11–20 (2014).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
Herold, T. et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 146, 128–136 (2020).
Coomes, E. A. & Haghbayan, H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 30, 1–9 (2020).
Kounis, N. G. et al. “When”,“where”, and “how” of SARS-CoV-2 infection affects the human cardiovascular system: a narrative review. Balkan Med. J. 41, 7 (2024).
Asakura, H. & Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. International journal of hematology 113, 45–57 (2021).
Hadid, T., Kafri, Z. & Al-Katib, A. Coagulation and anticoagulation in COVID-19. Blood Rev. 47, 100761 (2021).
Eljilany, I. & Elzouki, A.-N. D-dimer, fibrinogen, and IL-6 in COVID-19 patients with suspected venous thromboembolism: A narrative review. Vasc. Health Risk Manag. 455–462 (2020).
Rostami, M. & Mansouritorghabeh, H. D-dimer level in COVID-19 infection: A systematic review. Exp. Rev. Hematol. 13, 1265–1275 (2020).
Danese, E., Montagnana, M., Cervellin, G. & Lippi, G. Hypercoagulability, D-dimer and atrial fibrillation: An overview of biological and clinical evidence. Ann. Med. 46, 364–371 (2014).
Crawford, F. et al. D‐dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst. Rev. (2016).
Düz, M. E., Balcı, A. & Menekşe, E. D-dimer levels and COVID-19 severity: systematic review and meta-analysis. (2020).
Naymagon, L. et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thromb. Res. 196, 99–105 (2020).
Hossain, N. & Paidas, M. J. Seminars in perinatology 257–266 (Elsevier, Berlin, 2007).
Levi, M., Thachil, J., Iba, T. & Levy, J. H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 7, e438–e440 (2020).
Hall, G., Laddu, D. R., Phillips, S. A., Lavie, C. J. & Arena, R. A tale of two pandemics: How will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another?. Prog. Cardiovasc. Dis. 64, 108 (2021).
Stockwell, S. et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: A systematic review. BMJ Open Sport Exercise Med. 7, e000960 (2021).
Sittichai, N. et al. Effects of physical activity on the severity of illness and mortality in COVID-19 patients: A systematic review and meta-analysis. Front. Physiol. 13, 1030568 (2022).
Castoldi, R. C., de Ângelo, J. C., Pereira, T. T., Dias, R. M. & Negrão, F. J. Relationship between physical exercise and COVID-19 (SARS-CoV-2): Systematic review. Sport Sci. Health 19, 55–67 (2023).
Cerasola, D., Argano, C. & Corrao, S. Lessons from COVID-19: Physical exercise can improve and optimize health status. Front. Med. 9, 834844 (2022).
Da Silveira, M. P. et al. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin. Exp. Med. 21, 15–28 (2021).
Tartibian, B., Azadpour, N., Eslami, R. & Khayat, S. M. A. Home-based exercise alters pulmonary function and cellular stress markers in overweight middle-aged men during covid-19 Home quarantine. BMC Sports Sci. Med. Rehabil. 15, 61 (2023).
Nieman, D. C. & Wentz, L. M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 8, 201–217 (2019).
Jesus, I., Vanhee, V., Deramaudt, T. B. & Bonay, M. Promising effects of exercise on the cardiovascular, metabolic and immune system during COVID-19 period. J. Hum. Hypertens. 35, 1–3 (2021).
Zadow, E. K. et al. in Seminars in thrombosis and hemostasis. 807–814 (Thieme Medical Publishers).
Leandro, C. G., Ferreira e Silva, W. T. & Lima-Silva, A. E. Covid-19 and exercise-induced immunomodulation. Neuroimmunomodulation 27, 75–78 (2020).
Ferreira, G. A. et al. The effects of acute and chronic sprint-interval training on cytokine responses are independent of prior caffeine intake. Front. Physiol. 9, 671 (2018).
Goldhaber, S. Z. & Bounameaux, H. Pulmonary embolism and deep vein thrombosis. Lancet 379, 1835–1846 (2012).
Hull, C. M. & Harris, J. A. Cardiology patient page. Venous thromboembolism and marathon athletes. Circulation 128, e469-471. https://doi.org/10.1161/circulationaha.113.004586 (2013).
Gregson, J. et al. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. 4, 163–173. https://doi.org/10.1001/jamacardio.2018.4537 (2019).
Koçyiğit, B. F. & Akyol, A. Physical medicine and rehabilitation approaches in thrombosis associated with covid-19. Central Asian J. Med. Hypoth. Ethics 2, 137–145 (2021).
Elkazzaz, M. R., Alshuwaier, G. & Ahmed, A. K. Crosstalk among strenuous exercise, IL-6 and S-Protein Based Vaccines for COVID-19 may explain the rare adverse effects of myocarditis and thrombosis in recently vaccinated young people. A prospective observational study. (2022).
Chatterjee, S., Sengupta, T., Majumder, S. & Majumder, R. COVID-19: A probable role of the anticoagulant Protein S in managing COVID-19-associated coagulopathy. Aging (Albany NY) 12, 15954 (2020).
Bizuti, M. R. et al. Influence of exercise and vitamin D on the immune system against Covid-19: An integrative review of current literature. Mol. Cell. Biochem. 477, 1725–1737 (2022).
Ali, M. A. & Spinler, S. A. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc. Med. 31, 143–160 (2021).
Sengupta, T., Majumder, R. & Majumder, S. Role of vitamin D in treating COVID-19-associated coagulopathy: Problems and perspectives. Mol. Cell. Biochem. 476, 2421–2427 (2021).
Wu, K., Van Name, J. & Xi, L. Cardiovascular abnormalities of long-COVID syndrome: Pathogenic basis and potential strategy for treatment and rehabilitation. Sports Med. Health Sci. (2024).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. https://doi.org/10.3758/bf03193146 (2007).
Webb, C., Vehrs, P. R., George, J. D. & Hager, R. Estimating VO2max using a personalized step test. Meas. Phys. Educ. Exercise Sci. 18, 184–197 (2014).
Buysse, D. J., Reynolds, C. F. III., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
Organization, W. H. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: Field trial version, December 1996. (World Health Organization, 1996).
Gholami, A., Jahromi, L. M., Zarei, E. & Dehghan, A. Application of WHOQOL-BREF in measuring quality of life in health-care staff. Int. J. Prevent. Med. 4, 809 (2013).
Qu, L. et al. Ace2 and innate immunity in the regulation of SARS-CoV-2-induced acute lung injury: A review. Int. J. Mol. Sci. 22, 11483 (2021).
Roshanravan, N., Seif, F., Ostadrahimi, A., Pouraghaei, M. & Ghaffari, S. Targeting cytokine storm to manage patients with COVID-19: A mini-review. Arch. Med. Res. 51, 608–612 (2020).
Vatansever, H. S. & Becer, E. Relationship between IL-6 and COVID-19: To be considered during treatment. Future Virol. 15, 817–822 (2020).
Romano, G. et al. Physical training effects in renal transplant recipients. Clin. Transplant. 24, 510–514 (2010).
Kragstrup, T. W. et al. Plasma ACE2 predicts outcome of COVID-19 in hospitalized patients. PloS one 16, e0252799 (2021).
Gue, Y. X. & Gorog, D. A. Reduction in ACE2 may mediate the prothrombotic phenotype in COVID-19. Eur. Heart J. 41, 3198–3199 (2020).
Pai, W.-Y., Lo, W.-Y., Hsu, T., Peng, C.-T. & Wang, H.-J. Angiotensin-(1–7) inhibits thrombin-induced endothelial phenotypic changes and reactive oxygen species production via NADPH oxidase 5 downregulation. Front. Physiol. 8, 994 (2017).
Mehta, P. et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 (2020).
Klöting, N., Ristow, M. & Blüher, M. Effects of exercise on ACE2. Obesity 28, 2266–2267 (2020).
Dwyer, M. J., Pasini, M., De Dominicis, S. & Righi, E. Physical activity: Benefits and challenges during the COVID-19 pandemic. Scand. J. Med. Sci. Sports 30, 1291 (2020).
Jurak, G. et al. Physical activity recommendations during the coronavirus disease-2019 virus outbreak. J. Sport Health Sci. 9, 325 (2020).
Chen, A.-T., Wang, C.-Y., Zhu, W.-L. & Chen, W. Coagulation disorders and thrombosis in COVID-19 patients and a possible mechanism involving endothelial cells: A review. Aging Dis. 13, 144 (2022).
Teimury, A., Khameneh, M. T. & Khaledi, E. M. Major coagulation disorders and parameters in COVID-19 patients. Eur. J. Med. Res. 27, 25 (2022).
Sixt, S. et al. Exercise training but not rosiglitazone improves endothelial function in prediabetic patients with coronary disease. Eur. J. Prevent. Cardiol. 15, 473–478 (2008).
Furukawa, F., Kazuma, K., Kojima, M. & Kusukawa, R. Effects of an off-site walking program on fibrinogen and exercise energy expenditure in women. Asian Nurs. Res. 2, 35–45 (2008).
Bizheh, N. & Jaafari, M. Effects of regular aerobic exercise on cardiorespiratory fitness and levels of fibrinogen, fibrin D-dimer and uric acid in healthy and inactive middle aged men (2012).
Myint, P. K. et al. Physical activity and fibrinogen concentrations in 23,201 men and women in the EPIC-Norfolk population-based study. Atherosclerosis 198, 419–425 (2008).
Ahmadizad, S., El-Sayed, M. S. & MacLaren, D. P. Effects of water intake on the responses of haemorheological variables to resistance exercise. Clin. Hemorheol. Microcircul. 35, 317–327 (2006).
Kordi, M. R., Gaini, A. A., Ahmadi, A. & Veysi, K. Effect of resistance exercise on coagulation and fibrinolytic factors in inactive aged men. Internal Med. Today 18, 103–108 (2012).
Rashidlamir, A., Javaheri, A. H. & Jaafari, M. The effect of regular aerobic training with weight loss on concentrations of fibrinogen and resistin in healthy and overweight men. Tehran Univ. Med. J. 68 (2011).
Rezaeimanesh, D. The effects of high intensity interval training on fibrinolytic factors, D-dimer, and fibrinogen in men with Type 2 diabetes. Arch. Pharma. Pract. 11 (2020).
Sobhani, V., Mohammadi, M., Shirvani, H. & Amini, A. Long-term effect of high-intensity interval and concurrent exercise on blood coagulation and fibrinolysis parameters in non-athlete healthy young men. Internal Med. Today 22, 329–336 (2016).
Khademi, A., Tofighi, A., Tolouei Azar, J., SaifyNabiabad, H. & Nouri Habashi, A. The effect of aerobic and resistance training on some coagulation (PAI-1, fibrinogen, PT, PTT) and fibrinolysis (t-PA, plasminogen and D-dimer) factors in sedentary obese women. J. Sport Biosci. 13, 39–57 (2021).
Irwin, M. R., Olmstead, R. & Carroll, J. E. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80, 40–52 (2016).
Lau, H.M.-C. et al. A randomised controlled trial of the effectiveness of an exercise training program in patients recovering from severe acute respiratory syndrome. Aust. J. Physiother. 51, 213–219 (2005).
Sprod, L. K. et al. Exercise, sleep quality, and mediators of sleep in breast and prostate cancer patients receiving radiation therapy. Commun. Oncol. 7, 463 (2010).
Elavsky, S. & McAuley, E. Lack of perceived sleep improvement after 4-month structured exercise programs. Menopause 14, 535–540 (2007).
Courneya, K. S. et al. A randomized trial of aerobic exercise and sleep quality in lymphoma patients receiving chemotherapy or no treatments. Cancer Epidemiol. Biomark. Prevent. 21, 887–894 (2012).
Khazen, A. & Mousavinezhad, M. The effect of physical activity on the quality of life in individuals with hypertension. Med. J. Mashhad Univ. Med. Sci. 63, 2130–2137 (2020).
Esazadeh, L., Hosseini Kakhk, A., Khajeie, R. & Hejazi, S. M. The effect of concurrent training order (resistance-aerobic) on some factors of physical fitness, functional capacity and serum levels of myostatin and follistatin hormones in postmenopausal women (clinical trial). J. Sport Biosci. 12, 189–206 (2020).
Khademosharie, M., Tadibi, V., Behpor, N. & Hamedinia, M. Effect of 12-week endurance-resistance training on motor and muscular function, degree of disability, fatigue, and quality of life in Multiple Sclerosis patients. (2018).
Ai, T. et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 296, E32–E40 (2020).
Godazandeh, G. A., Majidi, H., Bani-Mostafavi, E.-S. & Godazandeh, F. CT scan findings in patients with COVID-19. J. Mazandaran Univ. Med. Sci. 31, 20–26 (2021).
Acknowledgements
The authors are very grateful to the participants for their time and effort.
Funding
No sources of funding were sought or awarded for this study.
Author information
Authors and Affiliations
Contributions
S.B. and M.R. Prepared the original draft, performed the experiments, supervised the training sessions, collected and analyzed the data. H.R., D.G., M.K., R.R., and A.A. Designed Methodology, controlled and supervised the stages of the study and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Binabaji, S., Rahimi, M., Rajabi, H. et al. Effects of physical training on coagulation parameters, interleukin-6, and angiotensin-converting enzyme-2 in COVID-19 survivors. Sci Rep 14, 18968 (2024). https://doi.org/10.1038/s41598-024-67522-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-67522-8