Abstract
Postmortem metabolomics holds promise for identifying crucial biological markers relevant to death investigations and clinical scenarios. We aimed to assess its applicability in diagnosing hypothermia, a condition lacking definitive biomarkers. Our retrospective analysis involved 1095 postmortem femoral blood samples, including 150 hypothermia cases, 278 matched controls, and 667 randomly selected test cases, analyzed using UHPLC-QTOF mass spectrometry. The model demonstrated robustness with an R2 and Q2 value of 0.73 and 0.68, achieving 94% classification accuracy, 92% sensitivity, and 96% specificity. Discriminative metabolite patterns, including acylcarnitines, stress hormones, and NAD metabolites, along with identified pathways, suggest that metabolomics analysis can be helpful to diagnose fatal hypothermia. Exposure to cold seems to trigger a stress response in the body, increasing cortisol production to maintain core temperature, possibly explaining the observed upregulation of cortisol levels and alterations in metabolic markers related to renal function. In addition, thermogenesis seems to increase metabolism in brown adipose tissue, contributing to changes in nicotinamide metabolism and elevated levels of ketone bodies and acylcarnitines, these findings highlight the effectiveness of UHPLC-QTOF mass spectrometry, multivariate analysis, and pathway identification of postmortem samples in identifying metabolite markers with forensic and clinical significance. The discovered patterns may offer valuable clinical insights and diagnostic markers, emphasizing the broader potential of postmortem metabolomics in understanding critical states or diseases.
Similar content being viewed by others
Introduction
Unraveling the mysteries behind the ultimate trigger for mortality involves navigating through a complex web of physiological, environmental, and contextual intricacies, presenting a multifaceted puzzle demanding comprehensive exploration and understanding. In most countries, unnatural or unexpected deaths shall be reported to the police, which then will request a forensic autopsy. Typically, a forensic autopsy implies a careful dissection of all internal organs and a thorough external examination of the body, and also most often includes toxicological and microscopical analysis of samples collected during the autopsy. Hypothermia, characterized by a critical reduction in core body temperature, caused by extended exposure to low temperature, often outdoors, can present significant challenges in differentiating it from other causes of death, particularly when signs of external trauma or coexisting medical conditions are present1,2,3. In typical cases of fatal hypothermia, sign of undressing at the scene, stress ulcerations in the mucosa of the ventricle (Wieschnieski’s spots), frost erythema in the skin and immuno-positivity for heat shock protein 70 of podocyte cell nuclei in the kidneys can be seen2,3,4. However these findings may be absent, which in part can be dependent on the ambient temperature and the length of the exposure. Moreover, conventional postmortem examinations, relying only on structural macroscopic and microscopic changes may fail to provide conclusive evidence regarding the cause of death in suspected hypothermic cases4,5.
Recent advancements in the field of metabolomics, a branch of systems biology concerned with the comprehensive analysis of endogenous metabolites within biological systems, offer an intriguing approach to unraveling the intricate metabolic alterations associated with hypothermia-related deaths. Postmortem metabolomics stands as a promising frontier in biomarker discovery, presenting an opportunity to unearth novel biological markers that could significantly enhance both clinical practice and investigations into causes of death6,7,8,9,10,11. In cases of complex conditions like hypothermia, where definitive biomarkers are lacking, postmortem metabolomics holds significant promise in providing valuable insights and enhancing diagnostic capabilities12. By analyzing the composition of low-molecular weight molecules present after death, postmortem metabolomics provides a unique opportunity to uncover the pathophysiological changes that occurred leading up to an individual's demise. This method allows us to delve into the metabolic alterations postmortem, potentially unraveling the intricate pathways associated with hypothermia-induced fatalities.
The primary objective of our research is to discern distinct biomarker patterns associated with hypothermia, enhancing the accuracy of its identification during postmortem examinations. Additionally, our study seeks to elucidate the mechanistic underpinnings of these biomarker patterns within physiological pathways, aiming to enhance our comprehension of the biological mechanisms underlying hypothermia.
Materials and methods
Study population and data selection
All autopsy cases admitted between late June 2017 and November 2020 at the Swedish National Board of Forensic Medicine, aged 18 or older, and that underwent toxicological screening in femoral blood using high-resolution mass spectrometry, were considered for inclusion in this study (n = 17,011). Case information were extracted from the Swedish Forensic Medicine database13. During the study period, we considered cases in which hypothermia was stated as the primary cause of death by the responsible pathologist and without no hospital visits prior to the fatalities or signs of an apparent putrefaction process. Controls were selected from a pool of 3089 femoral blood samples from deceased subjects. The selected causes of death included cardiovascular diseases (e.g., acute myocardial infarction and acute pulmonary heart disease), cerebrovascular diseases (e.g., subarachnoid hemorrhage and intracerebral hemorrhage), aortic rupture, traumatic injuries (e.g., skull fractures, subdural hemorrhage, injury of the thorax), and effects of external causes such as strangulation and drowning. The ICD-9 codes associated with these causes of death were 410K, 415B, 430, 431, 441A, 441B, 441D, 800K, 852M, 861L, 900L, 933, 992X, 994B, 994K, 994N, and 994W (the sufficies are according to the Swedish ICD-9 codes, but some are specific to Swedish forensic pathologist to allow for a better specification of the different medical conditions). The controls were selected based on similarity with the study group, primarily considering sex and age. The distribution of causes of death among the controls is detailed in Supplementary Table S1. The final dataset comprised 150 hypothermia cases and 278 matched controls to be used for metabolite pattern and marker identification.
To evaluate the performance of the markers and to simulate a real-world application a test group was created by pseudo-randomly selecting the first 10 males and 10 females from each month within the inclusion period. This test set consisted of 667 cases after excluding individuals under the age of 18, cases lacking available toxicological screening data, cases admitted to emergency care before their demise and any cases previously included as a hypothermia or control case.
The hypothermia cases and matched controls were randomly divided into a training set (3/4) and a validation set (1/4). The training set was employed for creating and refining the multivariate model, while the validation set was used for evaluation and validation of the model.
Institutional review board statement
This study was approved by the Swedish Ethical Review Authority (Dnr 2019-04530). Due to the retrospective nature of the study, the need of informed consent was waived by Swedish Ethical Review Authority. All methods were carried out in accordance with relevant guidelines and regulations.
Data acquisition and metabolomics analysis
UHPLC-QToF data, from the selected postmortem cases, obtained during drug screening in femoral blood together with multivariate analysis was used to identify postmortem biomarkers. In short, blood samples were prepared and analyzed according to a standardized procedure described elsewhere14. Each sample was prepared by protein precipitation including an addition of three internal standards (amphetamine-D8, diazepam-D5 and mianserin-D3). All samples were injected on a UHPLC-ESI-QToF system. Separation was performed on C18 column using gradient elution (Supplementary Fig. S1). MS-data was collected in positive mode and the total acquisition time for each sample was 12 min. Each analytical run included a blank whole blood sample containing the three internal standards, analyzed in the beginning and at the end of each run. An acceptable run showed absolute areas over 1.2 × 106,1.4 × 106 and 1.6 × 106 for amphetamine-D8, diazepam-D5 and mianserin-D3 respectively, a retention time deviation of maximum ± 0.1 min and a mass accuracy deviation of maximum ± 5 ppm.
The raw LC/MS data from the selected autopsy cases were exported to mzData-files using Masshunter. The postmortem metabolomics analysis was conducted using the 'XCMS' package in R (4.1.2), which integrates the 'CAMERA' package for feature annotation, as previously described6. In XCMS the centWave algorithm were used for feature detection using the following parameters Δm/z of 30 ppm, minimum peak width of 3 s, maximum peak width of 30 s and signal to noise threshold of 3 with noise variable set to 500. Retention time correction was performed using the Obiwarp function and for the grouping an mz width of 0.05, base width of 3 and minimum fraction of 0.6 were used.
Data preprocessing and multivariate analysis
The training set was normalized in Excel using the probabilistic quotient normalization, and log transformed, scaled with unit variance and subjected to multivariate analysis using SIMCA 17.0.2 (Umetrics, Umeå, Sweden). Features with a retention time < 60 s and > 660 s were excluded. Principal component analysis (PCA) was used to give an overview of the data, enabling identification of outliers and observation of trends. In addition, partial least square (PLS) models for age, sex and BMI were created to investigate systematic differences in the metabolic profiles. Orthogonal partial least square discriminant analysis (OPLS-DA) was used to identify variables contributing to group classification between hypothermia and control cases. Model complexity were reduced by stepwise removing non-contributing features using variable importance for the projection plots (VIP) for visualization and variable selection. The overall goal was to retain a practical and efficient classification model with as few variables a possible.
Experimental reproducibility was assessed by examining the score plots from the principal component analysis (PCA), by cross validation in OPLS-DA model of the training set, and by external validation of the OPLS-DA model using a validation set to assess the predictability of the multivariate model. False positives and false negatives were investigated in depth, together with using a test set with randomly selected control cases, in order to elucidate the usability and predictability of the final model.
Features in the final model were identified and annotated by matching molecular weight (± 5 ppm) and retention time against an in-house database and the online Human Metabolome Database (https://hmdb.ca). All features were also uploaded into MetaboAnalysts (version 6.0) module, functional analysis, usable for untargeted metabolomics data. The basic assumption is that putative annotation at individual compound level can collectively predict changes at functional levels as defined by metabolite sets or pathways15. Statistical variances among the three study groups for both annotated and non-annotated metabolites were validated through univariate analysis via Kruskal–Wallis test, with subsequent Bonferroni correction to compensate for effects of multiple comparisons (SPSS, ver. 29.0, IBM).
Results
Demographic overview and data processing
Table 1 provides a demographic overview of the cases selected for the primary study groups and the test set. Notably, no statistically significant differences were found in sex, BMI, and known PMIs (p > 0.05) between the hypothermia cases and their matched controls. Even though there were no statistical differences between medians, there was a noticeable age distribution difference. In addition it's important to highlight that a considerable portion of the hypothermia cases had unknown PMIs. When assessing the last observed time until the body was found as PMI, differences did indeed emerge. Particularly for the randomly selected controls, significant differences were evident, as they were not matched meticulously with the study groups, resulting in marked demographic disparities. Mass spectra data were processed using XCMS to compile a comprehensive list of chromatographic peaks with specific accurate masses and retention times, termed features. After the exclusion of features with a retention time of < 60 s and > 660 s, this selection resulted in 2526 features being available for multivariate modeling.
Multivariate modeling and model evaluation
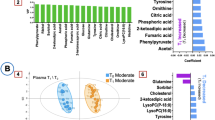
When applying supervised OPLS-DA analyses, we successfully distinguished the study groups based on metabolic features. The OPLS-DA model demonstrated statistical significance, with R2 = 0.83 and Q2 = 0.67, along with a CV-ANOVA p-value of < 0.001. After stepwise removing features from this model. The final OPLS-DA model only contained 44 unique features. This model exhibited a high goodness-of-fit with a comparable predictive performance as the first model, reporting R2 = 0.73 and Q2 = 0.68, along with a CV-ANOVA p-value of < 0.001 (Fig. 1A).
Model trimming overview. (A) Score plot demonstrating group separation between hypothermia cases (blue) and control cases (green) in the training set (R2Y = 0.73 and Q2 = 0.68). (B) Score plot for the validation set in the final OPLS-DA model. (C) Score plot of the randomly selected autopsy cases colored according to density of samples. (D) Score plot of the randomly selected autopsy cases, with acidosis as primary or contributing cause of death colored as red, n = 25. (E) ROC curve for the validation set. (F) Functional analysis with MetaboAnalyst 6.0 using Mummchog and GSEA algorithms.
In the training set, the model correctly classified 93% of the 322 samples, with a sensitivity of 89% and a specificity of 95% for the hypothermia cases. However, 21 samples were misclassified: 15 false negatives and 6 false positives. Notably, one of the false negatives had drowning as primary cause of death and should have been categorized as drowning case from the beginning, except for that no clear trend was observed in the false negatives. Among the false positives, four cases had drowning, and two had subarachnoid hemorrhage listed as the primary cause of death. Two of these six had hypothermia listed as a contributing cause of death.
To further evaluate the model's predictability, the remaining 106 autopsy cases were utilized as an external validation set. Each autopsy case was predicted and classified using the final model with a threshold determined by the true and false positive rates from the training set. The predicted score plot and the ROC curve for the 106 cases in the validation set are shown in Fig. 1. In the validation set, the model accurately classified 94% of the samples, with a sensitivity of 92% and a specificity of 96%. However, six samples were misclassified: three false negatives and three false positives. No discernible pattern was observed for the false negatives, but all three false positives were drowning cases.
To assess the model's applicability in a real setting and to identify any potential differential causes of death exhibiting a similar metabolite pattern to hypothermia, we examined 667 randomly selected control samples. The predicted score plot for these randomly selected controls is displayed in Fig. 1. Since hypothermia, as expected, represent a low proportion of the autopsy cases the vast majority of the samples were correctly predicted as controls, as shown in the density plot in Fig. 1C. Nevertheless, among the samples, 72 (11%) had a predicted score (tPS) above the threshold of 3. This threshold corresponds to achieving a sensitivity of 75% in identifying hypothermia cases in the validation set.
These 72 autopsy cases were categorized into nine classes based on their primary cause of death, including ketoacidosis, brain injury, drowning, drug intoxications, hanging, heart and cardiovascular diseases, pneumonia, other causes of death and an unknown cause of death. The prevalence of cases predicted as hypothermia was roughly similar to or lower than 11% for potential differential diagnoses such as brain injury (4%), drowning (7%), drug intoxication (7%), and heart and cardiovascular diseases (9%) (Table 2). Notably, as many as 17 out of 25 ketoacidosis cases in the random control set were misclassified as hypothermia, suggesting that the model encounters challenges in distinguishing between ketoacidosis and hypothermia (Fig. 1D). It is important to note that, among these 72 cases, five had hypothermia listed as a contributing cause while none of the cases with tPS < 3 had hypothermia as a contributing cause.
Metabolite identification and pathway analysis
In-house and online public database matching led to the identification of the 44 features that discriminate the hypothermia group, resulting in putative metabolite identifications listed in Table 3. These identified metabolites include carnitines, stress hormones, NAD metabolites, purine metabolites, and known biomarkers for renal dysfunction. To provide visual representation of the changes in the hypothermia cases, six specific metabolites—three upregulated and three downregulated across multiple pathways—are depicted as boxplots in Fig. 2, highlighting distinctive differences between the three groups.
For the functional analysis in MetaboAnalyst, all 2256 features were uploaded. MetaboAnalyst identified 230 empirical compounds in the dataset, and the following 7 pathways exhibited a combined p-value, based on the Mummchog and GSEA algorithms, of less than 0.05: C21-steroid hormone biosynthesis and metabolism, vitamin B3 (nicotinate and nicotinamide) metabolism, carnitine shuttle, arginine and proline metabolism, androgen and estrogen biosynthesis and metabolism, and vitamin B12 (cyanocobalamin) metabolism, as shown in Fig. 1B.
Discussion
Hypothermia, a potentially life-threatening condition characterized by a dangerously low body temperature, has long been a subject of scientific inquiry1,2,12,16. Its complex pathophysiology has intrigued researchers for years, leading to investigations into the metabolic dysfunctions it induces in search of potential biomarkers12,17,18. Over the years, several biomarkers have been suggested, such as 3-hydroxybutyric acid, cortisol, and arginine. Recognizing the challenges in finding a single marker that might be too unspecific, our approach aims to identify a pattern capable of classifying hypothermia cases with high sensitivity and specificity and explore the potential for incorporating these biomarkers into forensic screening methods. Furthermore, potential biomarkers could also enhance our understanding of the physiological responses during hypothermia and might hold promise for clinical applications. These biomarkers could serve as valuable tools for monitoring and potentially treating hypothermia cases in a clinical setting, thereby advancing our capacity to manage and mitigate the impact of this condition.
Postmortem metabolomics as a screening tool for hypothermia
In the realm of metabolomics, the validation of multivariate models is of paramount importance. Even so, a significant proportion of metabolomics investigations rely exclusively on cross-validation. In this study, we employed a three-set design encompassing a training set, a validation set, and a test set. The final model thereby underwent evaluation not only through cross-validation but also via external validation on unseen samples. Furthermore, testing on randomly selected samples provided insights into the model's real-world performance. This approach provided a robust foundation for the comprehensive validation and evaluation of the applicability of postmortem metabolomics.
The final model exhibited remarkably high predictive power, as demonstrated by both cross-validation and external validation, with sensitivity and specificity exceeding 90%. A noteworthy aspect of our findings is the limited number of metabolites (n = 44) required to achieve this impressive predictive capability. One such environments, hypothermia could have significantly contributed to death, even if it is not explicitly mentioned on death certificates. However, in the test set only 1 out of 14 drowning cases were classified as hypothermic. The interplay between drowning and hypothermia presents a diagnostic challenge, as both conditions might share overlapping metabolic profiles.
Moreover, we employed a test set to investigate whether other causes of death shared a similar metabolomic profile with hypothermia. This test set validated the model's high sensitivity by classifying all cases where hypothermia was a contributing cause of death. Notably, in the test set, 17 out of 25 ketoacidosis cases were classified as hypothermia cases, while the remaining 8 cases teetered on the borderline of being classified as a hypothermia case. The correlation between hypothermia and ketoacidosis is intriguing, given that conditions known to induce ketoacidosis often serve as triggers for secondary hypothermia19. Secondary hypothermia often occurs in the context of underlying clinical conditions or concurrent medications that affect the body’s ability to maintain its internal core temperature (e.g. malnutrition, underlying diseases such as diabetes or alcohol that impair central thermoregulation), which are also known to cause ketoacidosis20,21,22. It could therefore be argued that the test set showed no potential differential diagnosis as the ketoacidosis cases might be hypothermic as well. However, differentiating different types of ketoacidosis represents a crucial area for future research in postmortem metabolomics. As different types of ketoacidosis (e.g., due to diabetes, alcohol, starvation) can exhibit distinct metabolic profiles, comparing these with the metabolic signatures of hypothermia could uncover specific biomarkers unique to each condition. For example, metabolites related to alcohol metabolism, such as ethyl glucuronide, may help differentiate alcoholic ketoacidosis from other forms. Similarly, markers of nutritional status and stress response could be informative in cases of starvation and hypothermia, respectively. Even so, the model’s predictive power for the validation set and the limited set of differential diagnosis demonstrates in the test set, proves the potential of postmortem metabolomics as a screening tool for hypothermia.
Metabolic changes and affected pathways during hypothermia
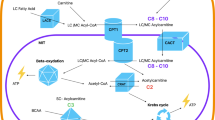
When exposed to cold conditions, the body often initiates a stress response, leading to an increase in cortisol production as it attempts to maintain core body temperature and adapt to the cold environment, which might be why we see upregulated level of cortisol and the observed pattern and C21 hormone response in the functional analysis. The rise in cortisol levels might reflect the biological stress response to cold, and cortisol has been suggested as a marker for cold exposure23. Another significant mechanism during hypothermia induced stress is vasoconstriction, where peripheral blood vessels constrict to minimize heat loss. This change in blood flow can lead to reduced renal perfusion and glomerular filtration rates, possibly explaining the observed alterations in metabolic markers related to renal function, such as N-methyl-2-pyridone-5-carboxamide (2PY), N-methyl-2-pyridone-5-carboxamide (4PY), phenacetylglutamine, and hippuric acid24,25.
As the body fights the cold, its metabolic rate significantly increases. This increased metabolism is an energy-intensive process aimed at generating heat and preserving core body temperature. Thermogenesis consumes NADH, which may explain the observed patterns in nicotinamide metabolism. Metabolites, including 1-(beta-d-ribofuranosyl)-1,4-dihydronicotinamide (a precursor of nicotinamide), s-adenosylmethionine (SAM, a vital co-substrate in the nicotinamide pathway), and the end products 2PY and 4PY, were the precursor and co-factor are downregulated while the waste products are up-regulated. This pattern aligns with observations in living subjects24,25. Furthermore, the thermogenesis in brown adipose tissue could account for the accumulation of end products from the Krebs cycle and β-oxidation, such as hippuric acid, phenylacetylglutamine, and hydroxybutyric acid. Additionally, there's a consensus in the literature regarding increased levels of blood ketone bodies, including β-hydroxybutyrate, acetone, isopropyl alcohol, and increased cortisol levels2,12,17,26 Moreover, the increase in β-oxidation in brown adipose tissue may also contribute to the elevated levels of circulating acylcarnitines, and might be why the carnitine shuttle seems affected. Interestingly a model restricted to cases aged 70 or younger (Supplementary Fig. 1), outperformed the model in Fig. 1. This might be explained by the amount and activity of brown adipose tissue (BAT) which is expected to decline with age27. As BAT is important in energy homeostasis and thermogenesis, the metabolome differences in younger individuals are expected to be greater between the groups in comparison to older individuals. Furthermore, acylcarnitines have been proposed as a trigger and a fuel source for brown fat thermogenesis28. To conclude, these results aligns with findings from a previous targeted metabolomics study on forensic hypothermia cases by Rousseau and colleagues in 201912.
Our investigation into the metabolomic profile differences between hypothermia cases and controls cases revealed distinct variations in several metabolites, indicating potential biomarkers for accurate identification. A Summary of affected metabolites and their relation to thermogenesis and renal dysfunction is found in Fig. 3. Notably, the study identified key metabolic pathways associated with hypothermia pathophysiology, shedding light on underlying mechanisms. Additionally, the observed differences, especially in metabolites linked to specific pathways, present promising avenues for developing targeted treatments or interventions. These findings not only hold diagnostic implications for hypothermia but also offer insights into potential therapeutic approaches. Understanding the altered metabolic pathways could pave the way for treatment strategies aimed at mitigating the effects of hypothermia and improving patient outcomes.
Potential insights and limitation
Postmortem metabolomics presents a novel avenue for exploring potential biomarkers that offer insights into the mechanisms of states or diseases. This approach provides an opportunity to investigate aspects that might be unfeasible to explore in clinical settings due to practical or ethical constraints. It is essential to highlight the clinical implications of these findings. Beyond revealing the potential to probe disease mechanisms using postmortem samples, an approach potentially more ethical than clinical investigations and closer to actual human conditions than animal models, the results underscore the possibility of identifying crucial markers for various diseases or conditions.
However, it is important to mention that our analytical method was primarily optimized for forensic toxicological screening, which has implications for the width of metabolome coverage. Expanding the screening to include various chromatographic conditions and both positive and negative ionization methods could potentially unveil more markers related to hypothermia. It is therefore important to not overinterpret the metabolite changes and relate them to the mechanism of hypothermia. However, the decision to utilize the current forensic toxicological screening analytic method was guided by the aim of creating a practical and efficient classification model. A simple and straightforward model, employing as few metabolites as necessary for prediction, was considered more important than to unravel the mechanism behind hypothermia. It is likely that further refinements of the data may provide additional insights into the mechanisms underlying hypothermia.
In the context of postmortem metabolomics, little is known about factors such as postmortem interval, postmortem degradation, and postmortem redistribution and their influence on the metabolome29. However, to mitigate these issues, we only included autopsy cases showing no putrefaction, aiming to minimize the potential impact of these factors on the metabolome and no apparent differences in postmortem interval were observed between the study groups. Having said that, examination of samples from decomposed samples are important to find out if the results obtained can be applied on such cases. We recognize the analytical method's limitations and the need for further research to elucidate the mechanisms underlying hypothermia and the impact of postmortem factors on the metabolome.
Conclusions
In conclusion, our study's utilization of a three-set design, strong predictive capabilities, and intriguing metabolite correlations in the mechanism of hypothermia, highlights the potential of postmortem metabolomics. This study serves as evidence that postmortem metabolomics could offer means to delve into the mechanisms underlying critical states or diseases which might hold relevance beyond forensic applications.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available, due to legal and ethical considerations.
References
Turk, E. E. Hypothermia. Forensic Sci. Med. Pathol. 6, 106–115 (2010).
Palmiere, C., Teresiński, G. & Hejna, P. Postmortem diagnosis of hypothermia. Int. J. Legal Med. 128, 607–614 (2014).
Byard, R. W. & Bright, F. M. Lethal hypothermia: A sometimes elusive diagnosis. Forensic Sci. Med. Pathol. 14, 421–423 (2018).
Sakurada, M. et al. Estimates of exposure to cold before death from immunohistochemical expression patterns of HSP70 in glomerular podocytes. Int. J. Legal Med. 127(4), 783–790 (2013).
Doberentz, E. & Madea, B. Microscopic examination of pituitary glands in cases of fatal accidental hypothermia. Forensic Sci. Res. 2, 132–138 (2017).
Elmsjö, A., Vikingsson, S., Söderberg, C., Kugelberg, F. C. & Green, H. Post-mortem metabolomics: A novel approach in clinical biomarker discovery and a potential tool in death investigations. Chem. Res. Toxicol. 34, 1496–1502 (2021).
Elmsjö, A., Söderberg, C., Jakobsson, G., Green, H. & Kronstrand, R. Postmortem metabolomics reveal acylcarnitines as potential biomarkers for fatal oxycodone-related intoxication. Metabolites 12, 109 (2022).
Ward, L. J. et al. Postmortem metabolomics of insulin intoxications and the potential application to find hypoglycemia-related deaths. Metabolites 13, 5 (2023).
Bohnert, S. et al. Metabolomics in postmortem cerebrospinal fluid diagnostics: A state-of-the-art method to interpret central nervous system-related pathological processes. Int. J. Legal Med. 135(1), 183–191 (2021).
Chighine, A. et al. Infant urinary metabolomic profile in a fatal acute methadone intoxication. Int. J. Legal Med. 136(2), 569–575 (2022).
Nariai, M., Abe, H., Hoshioka, Y., Makino, Y. & Iwase, H. Biomarker profiling of postmortem blood for diabetes mellitus and discussion of possible applications of metabolomics for forensic casework. Int. J. Legal Med. 136(4), 1075–1090 (2022).
Rousseau, G. et al. A serum metabolomics signature of hypothermia fatalities involving arginase activity, tryptophan content, and phosphatidylcholine saturation. Int. J. Legal. Med. 133, 889–898 (2019).
Druid, H., Holmgren, P. & Löwenhielm, P. Computer-assisted systems for forensic pathology and forensic toxicology. J. Forensic Sci. 41, 830–836 (1996).
Roman, M., Ström, L., Tell, H. & Josefsson, M. Liquid chromatography/time-of-flight mass spectrometry analysis of postmortem blood samples for targeted toxicological screening. Anal. Bioanal. Chem. 405, 4107–4125 (2013).
Li, S. et al. Predicting network activity from high throughput metabolomics. PLoS Comput. 9, e1003123 (2013).
Hirvonen, J. Necropsy findings in fatal hypothermia cases. Forensic Sci. 8, 155–164 (1976).
Palmiere, C. & Mangin, P. Hyperthermia and postmortem biochemical investigations. Int. J. Legal Med. 127, 93–102 (2013).
Rousseau, G. et al. Preliminary metabolomic profiling of the vitreous humor from hypothermia fatalities. J. Proteome Res. 20, 2390–2396 (2021).
Paal, P. et al. Accidental Hypothermia: 2021 Update. Int. J. Environ. Res. Public Health 19, 501 (2022).
Brooke, O. G. Influence of malnutrition on the body temperature of children. Br. Med. J. 5, 331–333 (1972).
Kalant, H. & Lê, A. D. Effects of ethanol on thermoregulation. Pharmacol. Ther. 23, 313–364 (1983).
Tran, C. et al. Hypothermia is a frequent sign of severe hypoglycaemia in patients with diabetes. Diabetes Metab. 38, 370–372 (2012).
Shida, A. et al. Cortisol levels after cold exposure are independent of adrenocorticotropic hormone stimulation. PLoS ONE 15, e0218910 (2020).
Okamoto, H. et al. Effects of stress on the urinary excretory pattern of niacin catabolites, the most reliable index of niacin status, in humans. J. Nutr. Sci. Vitaminol. 48, 417–419 (2002).
Okamoto, H. et al. Diurnal variations in human urinary excretion of nicotinamide catabolites: Effects of stress on the metabolism of nicotinamide. Am. J. Clin. Nutr. 77, 406–410 (2003).
Tani, N., Michiue, T., Chen, J. H., Oritani, S. & Ishikawa, T. Usefulness of postmortem biochemistry in identification of ketosis: Diagnosis of ketoacidosis at the onset of autoimmune type 1 diabetes in an autopsy case with cold exposure and malnutrition. Leg Med. 22, 23–29 (2016).
Tarantini, S. et al. Revisiting adipose thermogenesis for delaying aging and age-related diseases: Opportunities and challenges. Ageing Res. Rev. 87, 101912 (2023).
Simcox, J. et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 26, 509–522 (2017).
Chighine, A., Locci, E., Nioi, M. & d’Aloja, E. Looking for post-mortem metabolomic standardization: Waiting for godot—the importance of post-mortem interval in forensic metabolomics. Chem. Res. Toxicol. 34, 1946–1947 (2021).
Author information
Authors and Affiliations
Contributions
A.E., K.H., S.W., and H.D. conceived the study. A.E., L.J.W., F.C.K., H.D., and H.G. designed the study. A.E. and L.J.W. developed the metabolomics workflow. H.D. selected and characterized cases. F.C.K. and H.G. acquired funding and provided infrastructure. A.E. wrote the manuscript. A.E. and L.J.W. designed the figures in the manuscript. All authors edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Elmsjö, A., Ward, L.J., Horioka, K. et al. Biomarker patterns and mechanistic insights into hypothermia from a postmortem metabolomics investigation. Sci Rep 14, 18972 (2024). https://doi.org/10.1038/s41598-024-68973-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-68973-9
Keywords
This article is cited by
-
Hypothermia drives fatty acid transporter 1 upregulation and lipid accumulation in renal tubules: evidence from forensic cases
International Journal of Legal Medicine (2025)