Abstract
The American Heart Association (AHA) recently redefined cardiovascular health (CVH) with the introduction of Life's Essential 8 (LE8), which encompasses eight areas (diet, physical activity, nicotine exposure, sleep duration body mass index, non-HDL cholesterol, blood glucose, and blood pressure). This study aimed to explore the relationships between both the aggregate and individual CVH metrics, as defined by Life's Essential 8, and cognitive function in older adults in the United States. This cross-sectional, population-based study analyzed data from the National Health and Nutrition Examination Survey conducted between 2011 and 2014, focusing on individuals aged 60 years and older. CVH was categorized as low (0–49), moderate (50–79), or high (80–100). Cognitive function was assessed through the CERAD tests, Animal Fluency test, and Digit Symbol Substitution test. Multivariable logistic models and restricted cubic spline models were employed to investigate these associations. This study included a total of 2279 older adults in the United States. Only 11% of adults achieved a high total CVH score, while 12% had a low score. After further adjustment for potential confounding factors, higher LE8 scores were significantly associated with higher scores on CERAD: delayed recall score (0.02[0.01, 0.03]; P < 0.001), CERAD: total score (3 recall trials) (0.04[0.02, 0.06]; P < 0.001), animal fluency: total score (0.09[0.05, 0.12]; P < 0.001), and digit symbol: score (0.29[0.18, 0.41]; P < 0.001), demonstrating a linear dose–response relationship. Similar patterns were also observed in the associations between health behavior and health factor scores with cognitive function tests. LE8 scores exhibited positive linear associations with cognitive function. Maintaining better levels of CVH may be associated with higher levels of cognitive function in older Americans, but further research is needed to confirm the causal and temporal relationships between LE8 and cognitive function.
Similar content being viewed by others
Introduction
Cognitive function changes in older adults represent a pressing concern in contemporary society, driven by the global aging trend1,2. The increase in life expectancy has resulted in a higher prevalence of cognitive impairments, spanning from mild cognitive decline to severe dementia3,4. From a medical perspective, cognitive decline poses substantial challenges, particularly with conditions like Alzheimer's disease, necessitating specialized care and management that impose a significant financial burden on healthcare systems5. Families often shoulder the responsibility of caring for elderly relatives with cognitive impairments, leading to caregiver burnout and affecting both caregivers' and recipients' quality of life6. Economically, the associated costs of cognitive impairment are staggering, encompassing medical expenses, caregiving, and institutionalization, thereby straining governments and healthcare systems7. Cognitive function changes in older adults present a multifaceted challenge with wide-ranging societal implications.
Despite notable progress in raising awareness of cardiovascular health, the current state of heart health presents a multifaceted landscape8. On one hand, strides have been made in reducing smoking rates, promoting healthy diets, and increasing physical activity levels9,10,11. Advances in medical treatments and interventions have improved outcomes for individuals with heart conditions. Nevertheless, challenges persist. Cardiovascular disease remains the leading global cause of death, exacerbated by sedentary lifestyles, unhealthy dietary habits, and escalating obesity rates. The demands of modern life, compounded by inadequate sleep, further heighten cardiovascular risks12. In 2010, the American Heart Association (AHA) introduced Life's Simple 7 (LS7), a comprehensive cardiovascular health (CVH) assessment comprising elements such as a balanced diet, tobacco abstinence, a healthy body mass index (BMI), regular physical activity, blood pressure control, fasting blood glucose management, and cholesterol levels, all aimed at advancing public health13. More recently, the AHA has enhanced the evaluation of CVH with the introduction of Life's Essential 8 (LE8)14. LE8 introduces updated sleep quality metrics and refined scoring algorithms compared to LS7, offering a more nuanced approach that accounts for individual variations and underscores the importance of social determinants of health and mental well-being in the preservation and enhancement of CVH14. "Life's Essential 8" presents a comprehensive perspective on cardiovascular health, emphasizing the critical role of lifestyle choices and risk factor management. It underscores that a well-balanced diet, regular physical activity, abstaining from tobacco, prioritizing quality sleep, weight maintenance, and effective management of blood lipids, blood glucose, and blood pressure are interconnected elements pivotal for cardiovascular well-being14. The adoption of these principles has the potential to significantly reduce the risk of heart disease and elevate overall quality of life. Notably, research exploring the correlation between LE8 and cognitive function remains limited at present.
The intricate relationship between cognitive function and cardiovascular health in older adults underscores their reciprocal influences on overall well-being15. Cardiovascular health, including key factors such as blood pressure, blood lipid profiles, and vascular function, plays a pivotal role in sustaining optimal cerebral blood flow. Conditions like hypertension and atherosclerosis, prevalent in poor cardiovascular health, can compromise cerebral perfusion, potentially leading to cognitive decline16. Conversely, cognitive function and mental health significantly influence one's capacity to adhere to heart-healthy lifestyle choices. The adoption of healthy habits, including regular exercise, smoking cessation, and a balanced diet, not only reduces cardiovascular risks but also correlates with enhanced cognitive function17. These interconnections emphasize the importance of a comprehensive health approach addressing both cognitive well-being and cardiovascular health in older adults. However, it is worth noting that the association between Life's Essential 8, the latest metric for comprehensively assessing cardiovascular health, and altered cognitive function in older adults requires further research validation. To the best of our knowledge, there is no yet evidence for the association between LE8 and cognitive function. Therefore, this study aims to estimate the correlation between Life's Essential 8 and cognitive function in older adults using data from the National Health and Nutrition Examination Survey (NHANES).
Methods
Study population
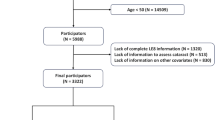
The analysis in this study strictly adhered to the NHANES analytic guidelines, which are overseen by the National Center for Health Statistics at the Centers for Disease Control and Prevention in Maryland. NHANES employs a meticulously designed stratified multi-stage sampling approach, and comprehensive documentation of the sampling and testing procedures can be found in previously published articles18. In summary, systematic health-related interviews and examinations occurred in 2-year cycles, ensuring the inclusion of participants from diverse geographical regions and various racial/ethnic backgrounds, thereby guaranteeing comprehensive representation in the survey. Notably, NHANES protocols have obtained ethical approval from the National Center for Health Statistics research ethics review board, and written informed consent was obtained from all enrolled participants. Cognitive testing was specifically administered to participants aged 60 years and older during the period of 2011–201419. The data utilized in this study were extracted from NHANES surveys conducted between 2011 and 2014. Among the 19,931 subjects who participated in NHANES from 2011 to 2014, individuals were excluded based on the following criteria: (1) those under the age of 60 (n = 16,299), (2) those with incomplete cognitive testing data (n = 698), and (3) those with missing data on LS8 and covariates (n = 221). Consequently, a total of 2279 subjects met the inclusion criteria for this research. The detailed flowchart illustrating this process is presented in Fig. 1.
Cognitive function
Cognitive functioning was evaluated during an interview conducted at a Mobile Examination Center and assessed by skilled interviewers. This assessment comprised three distinct tests: (1) the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word Learning sub-test (CERAD W-L), which measured immediate for 3 times and delayed recall of new verbal information (a memory sub-domain) for 1 times, scores on the CERAD W-L test and the delayed recall test range from 1 to 10; (2) the Animal Fluency test, designed to evaluate categorical verbal fluency (a component of executive function), range from 3 to 39; (3) the Digit Symbol Substitution Test (DSST), which gauged processing speed, sustained attention, and working memory, range from 0 to 105. The CERAD test involved three consecutive learning trials and a delayed recall task. Consequently, the results are reported as three individual trial scores, each ranging from 0 to 10, a total score that combines performance across all three trials, ranging from 0 to 30, and a single score for delayed recall, ranging from 0 to 10. All results were recorded on the cognitive functioning questionnaire (CFQ).
Measurement of Life's Essential 8
The LE8 score comprises four health behaviors (diet, physical activity, nicotine exposure, and sleep duration) and four health factors (BMI, non-HDL (high-density lipoprotein) cholesterol, blood glucose, and blood pressure)14. Detailed scoring criteria for each item can be found in Table S1. Dietary parameters were evaluated using the Healthy Eating Index (HEI) 2015, calculated based on the subject's 24-h dietary recall20. The HEI score was computed as the average of two recall periods, or if only data from the first day was available, that value was used. Information regarding physical activity, nicotine exposure, sleep patterns, diabetes history, and medication history was collected through a self-report questionnaire. Physical examinations included height, weight, and blood pressure measurements, from which BMI was derived by dividing weight (in kilograms) by the square of height (in meters). Non-HDL cholesterol and hemoglobin A1c levels were determined from collected blood samples. Each of the 8 CVH indicators received a score ranging from 0 to 100, and the total LE8 score was calculated as the unweighted average of these 8 indicators. Moreover, participants exhibiting high CVH were classified with LE8 scores between 80 and 100, those with moderate CVH fell within the 50–79 range, and individuals with low CVH were situated between 0 and 4914. In our study, we utilized these same cutoff points to categorize health behavior and health factor scores, enabling further exploration of the relationship between LE8 subscales and cognitive function.
Study covariates
The study's covariates encompassed gender (male and female), age, categorized into age groups (60–69, 70–79, and 80 + years), race/ethnicity (non-hispanic white, non-hispanic black, other hispanic, mexican american, non-hispanic asian, and others), educational attainment (less than 9th grade, college graduate or above, high school graduate/GED or equivalent, less than 9th grade, and some college or AA degree), the family income-to-poverty ratio (the ratio of total household income to the national poverty line, where a larger ratio represents a lower poverty index and a smaller ratio represents a higher poverty index), and alcohol consumption.
Statistical analysis
Given the intricate NHANES sampling design, we employed appropriate weights for the sample analysis. For initial characterization, we utilized weighted means (IQR) for continuous variables and sample sizes (weighted percentages) for categorical variables. To assess disparities in variable characteristics among the low, moderate, and high CVH groups, we applied ANOVA for differences in weighted means regarding continuous variables and the Rao—Scott χ2 test for distinctions in weighted percentages for categorical variables. First, weighted linear regression was employed to investigate the correlation between LE8 scores and cognitive test results (including CERAD: Trial 1–3 Score, CERAD: Total Score (3 Recall trials), CERAD: Delayed Recall Score, Animal Fluency: Total Score, and Digit Symbol: Score), as well as the association between various CVH levels and cognitive tests. Crude models did not incorporate any potential confounding factors, whereas age-adjusted models were adjusted solely for age. Fully-adjusted models included adjustments for age, sex, race, education level, the family income-to-poverty ratio, and alcohol consumption. Furthermore, we explored the correlations between health behavior, health factor scores, and each of the LE8 scores with cognitive tests using weighted linear regression analyses, while accounting for all confounding variables. Than, the correlation between LE8 metric and cognitive test results, as well as the association between health behavior and health factor scores and cognitive tests. Finally, to further validate the link between LE8 scores and cognitive tests, we employed Restricted Cubic Spline (RCS) and subgroup analyses for sensitivity analyses. Statistical tests were two-sided, with statistical significance set at P < 0.05. All analyses were performed using R software, version 4.2.0 (R Core Team, Vienna, Austria).
Ethics approval and consent to participate
The NHANES was approved by the National Center for Health Statistics Research Ethics Review Board.
Result
Descriptive statistics
The characteristics of the study population according to CVH status were shown in Table 1. A total of 2279 adults aged 60 years or older were included for analysis. Moderate CVH status group contained 76% of all participants. The mean age was 69.01 years, and 47% of the participants were male. Participants with high CVH status were more likely to be Non-Hispanic White, higher educational levels, to have higher poverty impact ratio (PIR), and more alcohol consumption (P < 0.001).
Association between Life’s Essential 8 and cognitive function
Table 2 presented the baseline of Life’s Essential 8 metric scores and cognitive tests scores selected by CVH status. High CVH group had a higher score in Life’s Essential 8 total score, health behaviors score, diet score, physical activity score, sleep health score, tobacco exposure score, health factors score, BMI score, BP score, Blood glucose score and blood lipids (non-HDL cholesterol) score (P < 0.001). High CVH group also showed better performance in cognitive outcomes including CERAD: trial 1–3, CERAD: delayed recall, animal fluency test, and digit symbol test (P < 0.05).
When treating Life’s Essential 8 as a continuous measure, each 1-unit increase in Life’s Essential 8 score was associated with higher CERAD: delayed recall score (Beta: 0.02; 95%CI 0.01, 0.03; P < 0.001), CERAD: total score (3 recall trials) (Beta: 0.04; 95%CI 0.02, 0.06; P < 0.001), animal fluency: total score (Beta: 0.09; 95%CI 0.05, 0.12; P < 0.001), and digit symbol: score (Beta: 0.29; 95%CI 0.18, 0.41; P < 0.001) (Table 3). Similar results were obtained after further adjustment for age (Table 3). After fully adjusting, Life’s Essential 8 score was associated with higher CERAD: total score (3 recall trials) (Beta: 0.02; 95%CI 0.00, 0.04; P = 0.035), animal fluency: total score (Beta: 0.04; 95%CI 0.01, 0.07; P = 0.007), and digit symbol: score (Beta: 0.11; 95%CI 0.02, 0.19; P = 0.015) but not CERAD: delayed recall score (Beta: 0.01; 95%CI 0.00, 0.02; P = 0.052) (Table 3). When grouped according to CVH status, High CVH was significantly associated with higher CERAD: delayed recall score (Beta: 0.82; 95%CI 0.40, 1.2; P < 0.001), CERAD: total score (3 recall trials) (Beta: 1.9; 95%CI 1.1, 2.8; P < 0.001), animal fluency: total score (Beta: 3.5; 95%CI 2.0, 5.1; P < 0.001), and digit symbol: score (Beta: 12; 95%CI 7.0, 17; P < 0.001), compared to Low CVH (Table 3). Similar results were showed after further adjustment for age or fully adjusted (Table 3). Beta coefficients and 95% confidence intervals of Life’s Essential 8 for CERAD: Trial1-3 were provided in Table S2.
Association between Life’s Essential 8 components and cognitive function
The LE8 score comprises four health behaviors (diet, physical activity, nicotine exposure, and sleep duration) and four health factors (body mass index, non-HDL cholesterol, blood glucose, and blood pressure). Health behaviors score was associated with higher animal fluency: total score (Beta: 0.04; 95%CI 0.02, 0.06; P < 0.001), and digit symbol: score (Beta: 0.10; 95%CI 0.05, 015; P < 0.001) but not CERAD: delayed recall score (Beta: 0; 95%CI 0.00, 0.01; P = 0.2), CERAD: total score (3 recall trials) (Beta: 0.01; 95%CI 0.00, 0.02; P = 0.081), after fully adjusted (Table 4). Health factors score was associated with higher CERAD: delayed recall score (Beta: 0.01; 95%CI 0.00, 0.01; P = 0.003), CERAD: total score (3 recall trials) (Beta: 0.02; 95%CI 0.01, 0.03; P = 0.002), animal fluency: total score (Beta: 0.03; 95%CI 0.01, 0.05; P = 0.006), and digit symbol: score (Beta: 0.12; 95%CI 0.04, 0.20; P = 0.003) after further adjustment age (Table 4). When fully adjusted, no significant association was found between health factors score and cognitive function scores. Beta coefficients and 95% confidence intervals of health behaviors and health factors for CERAD: Trial1-3 were provided in Table S3. Higher non-HLD cholesterol scores were associated with worse animal fluency: total score (Beta: − 0.01; 95%CI − .02, 0.00; P = 0.047), and digit symbol: score (Beta: − .02; 95%CI − .04, 0.00; P = 0.047), and BMI was not significantly correlated with cognitive function scores (Table S4). Association between all Life’s Essential 8 metric (diet, physical activity, nicotine exposure, sleep duration, body mass index, non-HDL cholesterol, blood glucose, and blood pressure) and cognitive function scores was provided in Table S4.
Sensitivity analyses
The dose–response relationship between Life’s Essential 8 and cognitive function after fully adjusting were shown in Fig. 2. In restricted cubic spline models, Life’s Essential 8 scores were positively associated with CERAD: delayed recall score (P-overall < 0.0001, P-non-linear = 0.3340; Fig. 2A), CERAD: total score (3 recall trials) (P-overall < 0.0001, P-non-linear = 0.7709; Fig. 2B), animal fluency: total score (P-overall < 0.0001, P-non-linear = 0.9974; Fig. 2C), digit symbol: score (P-overall < 0.0001, P-non-linear = 0.3446; Fig. 2D) in a linear manner. The dose–response relationship between health behaviors, health factors and cognitive function after fully adjusting were shown in Figure S1.
Dose–response relationship of Life’s Essential 8 (LE8) and various domains of cognitive function (A: CERAD: Delayed Recall Score, B: CERAD: Total Score (3 Recall trials), C: Animal Fluency: Total Score, D: Digit Symbol: Score). Model is adjusted for age, sex, race, education level, ratio of family income to poverty and alcohol consumption.
The results of subgroup analyses are presented in Table 5. In the male subgroup, LS8 showed a significant positive correlation with animal fluency score (Beta: 0.05; 95%CI 0.01, 0.09; P = 0.014) after fully-adjusted. LS8 was positive correlated with CERAD: total score (3 recall trials) (Beta: 0.02; 95%CI 0.00, 0.04; P = 0.036) and animal fluency score (Beta: 0.04; 95%CI 0.00, 0.07; P = 0.03) in the female subgroup. In the 60–69 years age subgroup, LS8 showed a significant positive correlation with animal fluency score (Beta: 0.04; 95%CI 0.01, 0.08; P = 0.027) after fully-adjusted. In the 70–79 years age subgroup, no significant correlation between LS8 and cognitive tests after fully-adjusted. But in the 80 + years age subgroup, LS8 was positive correlated with animal fluency score (Beta: 0.05; 95%CI 0.00, 0.09; P = 0.036) and digit symbol score (3 recall trials) (Beta: 0.19; 95%CI 0.09, 0.28; P = 0.001). Subgroup analysis of sex and age for CERAD: Trial 1–3 was showed in Table S5.
Discussion
The present study found that High CVH and higher Life’s Essential 8 score was associated with better CERAD: delayed recall score, CERAD: total score (3 recall trials), animal fluency: total score, and digit symbol: score. Health behaviors score was associated with higher animal fluency: total score and digit symbol: score but not CERAD: delayed recall score and CERAD: total score (3 recall trials), but no significant association was found between health factors score and cognitive function scores. In restricted cubic spline models, Life’s Essential 8 scores were positively associated with CERAD: delayed recall score, CERAD: total score (3 recall trials) , animal fluency: total score, digit symbol: score in a linear manner.
The global landscape is witnessing a significant demographic shift, with a burgeoning aging population. By 2050, it is projected that older adults aged 60 years and above will constitute 22% of the global population. This demographic transformation brings forth unique challenges, including the rising prevalence of cognitive impairments and neurodegenerative diseases21. Cognitive function, encompassing memory, attention, executive function, and other mental processes, is a fundamental component of an individual's ability to lead an independent and fulfilling life. Simultaneously, cardiovascular health remains a central concern, given its pervasive impact on mortality and morbidity worldwide8. Cardiovascular disease (CVD) is the leading cause of death globally, underlining the need for comprehensive strategies to mitigate risk factors and promote heart health. LE8 offers a holistic approach to assess cardiovascular health, focusing on lifestyle choices and risk factor management14. In this nationally representative cross-sectional study, we found that LE8 scores and its health behaviors scores and health factors scores showed a significant positive correlation with cognitive test scores of U.S. aged 60 years and older.
Previous study has explored the link between LS7 and cognitive functioning and found that maintaining good LS7 scores showed a significant positive correlation with better cognitive functioning22. Our findings are similar to previous study. The CHV definitions of LS7 were categorized into ideal, intermediate, and poor CVH for each component. This definition is less sensitive to interindividual differences and is unable to be used to assess dose–response effects23. Moreover, we found a significant linear relationship between LS8 and cognitive test scores by RCS analysis (Fig. 2), further suggesting that maintaining good LS8 scores is beneficial to cognitive function.
The use of LE8 as a definition of CVH in this study adds significant evidence of a relationship between CVH and cognitive function. High CVH group (based on LS8 score) was significantly associated with higher CERAD: delayed recall score, CERAD: total score (3 recall trials), animal fluency: total score, and digit symbol: score, compared to Low CVH group. Maintaining good cardiovascular status shows an important positive correlation with good cognitive function performance. One of the primary reasons for the strong connection between cardiovascular health and cognitive function is the presence of shared risk factors. Many risk factors that contribute to cardiovascular diseases, such as hypertension, diabetes, hyperlipidemia, and obesity, have also been implicated in the development of cognitive impairments and neurodegenerative diseases24,25,26,27. The link between cardiovascular health and cognitive function may be explained through the presence of shared risk factors.
The LE8 score comprises four health factors: BMI, non-HDL cholesterol, blood glucose, and blood pressure, to assess individual cardiovascular health. Health factors score was associated with higher CERAD: delayed recall score, CERAD: total score (3 recall trials), animal fluency: total score, and digit symbol: score after further adjustment age. Although our study did not find a significant association between BMI and cognitive function, excessive BMI often represents obesity, obesity can lead to cardiovascular issues, inflammation, insulin resistance, and metabolic disruptions, all of which are associated with cognitive decline. Furthermore, obesity may trigger adverse changes in brain structure and function, increasing the risk of cognitive impairments and dementia28. But there are also studies that link obesity to better cognitive function. Midlife obesity independently increases the risk of cognitive function, whereas late-life obesity, especially metabolically healthy obesity, may be protective against Alzheimer's disease pathology29,30. The results of our study, which was conducted on older adults over the age of 60 years, did not find an association between high body mass index and declining cognitive function scores. And no significant correlation was found after adjusting for the effects of age factors and other relevant covariates. Therefore, with our current results, it is concluded that maintaining a normal and good BMI may promote cardiovascular health and do not adversely affect cognitive function. Elevated blood glucose levels, such as in diabetes, are associated with an increased risk of cognitive decline and dementia. Prolonged high blood glucose can lead to nerve damage, vascular inflammation, and structural brain changes, all of which can impact cognitive function31,32. Optimal blood pressure levels are associated with improved cognitive resilience, while hypertension has been linked to an increased risk of cognitive impairments, including Alzheimer's disease and dementia24,33,34. Managing blood pressure is thus essential for maintaining cognitive function and overall well-being. Health factors not only affect cardiovascular health but also exert a substantial influence on cognitive function. Maintaining favorable health factors can contribute to the preservation of cognitive function.
Interestingly, our findings regarding the relationship between blood lipids and cognitive function revealed a noteworthy result: higher non-HDL cholesterol scores were inversely associated with cognitive function scores, suggesting that elevated non-HDL cholesterol levels in the serum may be indicative of better cognitive performance (Table S4). The brain is the body's highest cholesterol-containing organ, and the total serum cholesterol levels in the blood have a significant impact on brain aging and cognitive abilities35. To date, evidence concerning the relationship between total cholesterol levels and cognitive function in the elderly has yielded ambiguous results without a definitive consensus, which may be influenced by the aging process. Our research results align with some previous studies regarding the association between cholesterol and cognitive function. One study involving 382 individuals examined the link between cholesterol concentration and cognitive abilities, finding that lower cholesterol levels were associated with poorer cognitive function in both non-dementia and dementia patients36. Another study involving 1034 participants revealed that among participants aged 70, higher total cholesterol was associated with higher cognitive ability scores37. However, there are also studies that have reported contrasting trends. A study of 1159 Chinese adults aged 60 and older found that higher blood total cholesterol concentrations were associated with a faster decline in overall cognitive abilities38. A meta-analysis based on eight studies and involving over 21,000 individuals aged 60 and above did not establish any relationship between cholesterol and cognitive decline or dementia in the elderly39. Cholesterol is traditionally regarded as a risk factor for cardiovascular diseases, and lower cholesterol levels are desirable for cardiovascular events40. Nevertheless, recent research has suggested that cholesterol has a protective effect against certain non-cardiovascular diseases and hemorrhagic strokes, particularly among older adults41. Given the complex role of cholesterol, further in-depth research is needed to ascertain the optimal cholesterol levels for individuals to attain maximum benefits.
The LE8 score also includes four health behaviors: diet, physical activity, nicotine exposure, and sleep duration. Health behaviors score was associated with higher animal fluency: total score, and digit symbol: score but not CERAD: delayed recall score, CERAD: total score (3 recall trials), after fully adjusted. Research has shown that diet has a significant impact on brain health. Adopting a balanced diet rich in antioxidants, including fruits, vegetables, whole grains, and healthy fats, can reduce inflammation, oxidative stress, and neuronal damage, thus contributing to the protection of cognitive function42,43. Conversely, high sugar, saturated fat, and processed food consumption have been linked to an increased risk of Alzheimer's disease and dementia44. Furthermore, diet can also influence cardiovascular health, which in turn affects cognitive function10. Poor cardiovascular health can lead to inadequate blood supply to the brain, impacting memory, thinking, and attention, among other blood supply abilities45. Therefore, adopting healthy dietary habits is not only beneficial for the heart but also helps maintain clear thinking and memory. Physical activity promotes increased blood flow to the brain, stimulates the release of neurotrophic factors, and enhances neuronal connectivity and communication, all of which contribute to the protection and promotion of cognitive function46. Moreover, physical activity holds significant potential for preventing cognitive impairments and dementia47. Studies have consistently shown that an active lifestyle can reduce the risk of cognitive impairments in older adults, enhancing cognitive reserve capacity and making the brain more resilient48,49. Nicotine, the addictive component of tobacco, acts on the brain's neurotransmitter systems, disrupting their normal functioning and potentially leading to cognitive deficits50. Long-term smoking has also been linked to an increased risk of neurodegenerative conditions such as Alzheimer's disease and dementia51,52. It accelerates brain aging processes and may contribute to the accumulation of harmful proteins in the brain associated with these conditions. Quality sleep plays a crucial role in maintaining and enhancing cognitive abilities, including memory, attention, problem-solving, and decision-making53. During sleep, the brain undergoes essential processes that consolidate and organize information acquired during wakefulness. It helps strengthen neural connections, optimize memory storage, and clear waste products from brain cells54. Insufficient or poor-quality sleep disrupts these processes, leading to cognitive impairments. Chronic sleep deprivation has been linked to decreased cognitive performance, mood disturbances, and increased risk of neurodegenerative conditions such as Alzheimer's disease55. Based on our findings, it is evident that maintaining healthy behaviors has a more significant impact on improving cognitive function. However, this should not overshadow the crucial role that healthy elements play in sustaining cognitive function at its current level.
To the best of our knowledge, this is the first large-scale, nationally representative cohort study that delves into the relationship between Cardiovascular Health (CVH) and cognitive function using Life's Essential 8. Additionally, we examined the dose–response correlation between Life's Essential 8 and cognitive function scores. Nevertheless, it's crucial to acknowledge several potential limitations. Firstly, the four health behaviors, encompassing dietary intake, physical activity, nicotine exposure, and sleep health, rely on self-reported data, which could introduce recall bias. Secondly, despite making multivariate adjustments, unmeasured or residual confounding factors might impact the observed associations. Thirdly, our findings among U.S. adults may not be directly generalizable to other populations. Lastly, the cross-sectional study design prevents us from establishing causal and temporal relationships between CVH and cognitive function. Given the promising findings and the acknowledged limitations of this study, further validation through extensive prospective cohort studies is warranted.
Conclusion
To summarize, a higher level of adherence to the Life's Essential 8 (LE8) criteria is associated with improved cognitive function in older adults in the United States. Future studies to investigate this topic across different countries and populations, and develop evidence using data from low-middle-income countries to understand cross-cultural differences in the relationship between health behavior, health factors, and cognition are needed.
Data availability
Data from the National Health and Nutrition Examination Survey (NHANES) 2011–2014 are publicly available online (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
Abbreviations
- AHA:
-
American Heart Association
- LS7:
-
Life's Simple 7
- CVH:
-
Cardiovascular health
- LE8:
-
Life's Essential 8
- NHANES:
-
National Health and Nutrition Examination Survey
- CERAD:
-
Consortium to Establish a Registry for Alzheimer’s Disease.
- DSST:
-
Digit Symbol Substitution Test
- HEI:
-
Healthy Eating Index
- BMI:
-
Body mass index
- HDL:
-
High-density lipoprotein
- RCE:
-
Restricted Cubic Spline
- CVD:
-
Cardiovascular disease
References
Lenze, E. J. et al. Effects of mindfulness training and exercise on cognitive function in older adults: A randomized clinical trial. JAMA 328, 2218–2229 (2022).
Zhang, R. et al. Association between life’s essential 8 and biological ageing among US adults. J. Transl. Med. 21, 622 (2023).
Anderson, N. D. & Craik, F. I. 50 years of cognitive aging theory. J. Gerontol. B Psychol. Sci. Soc. Sci. 72, 1–6 (2017).
Spreng, R. N. & Turner, G. R. The shifting architecture of cognition and brain function in older adulthood. Perspect. Psychol. Sci. 14, 523–542 (2019).
Scheltens, P. et al. Alzheimer’s disease. Lancet 397, 1577–1590 (2021).
Luiu, A. L., Favez, N., Betrancourt, M., Szilas, N. & Ehrler, F. Family relationships and alzheimer’s disease: A systematic review. J. Alzheimers. Dis. 76, 1595–1608 (2020).
2023 Alzheimer's disease facts and figures. Alzheimers Dement. 19, 1598–1695 (2023).
Kivimaki, M. & Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 15, 215–229 (2018).
Hadley, M. B., Henderson, S. B., Brauer, M. & Vedanthan, R. Protecting cardiovascular health from wildfire smoke. Circulation 146, 788–801 (2022).
Ros, E. et al. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study. Adv. Nutr. 5, 330S-336S (2014).
DeFina, L. F. et al. Physical activity versus cardiorespiratory fitness: Two (partly) distinct components of cardiovascular health?. Prog. Cardiovasc. Dis. 57, 324–329 (2015).
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021 (2020).
Lloyd-Jones, D. M. et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121, 586–613 (2010).
Lloyd-Jones, D. M. et al. Life’s Essential 8: Updating and enhancing the American heart association’s construct of cardiovascular health: A presidential advisory from the American heart association. Circulation 146, e18–e43 (2022).
Vogel, B. et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 397, 2385–2438 (2021).
Poti, F., Santi, D., Spaggiari, G., Zimetti, F. & Zanotti, I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: A review and meta-analysis. Int. J. Mol. Sci. https://doi.org/10.1530/endoabs.63.P637 (2019).
Muscaritoli, M. The impact of nutrients on mental health and well-being: Insights from the literature. Front. Nutr. 8, 656290 (2021).
Borrud, L. et al. National health and nutrition examination survey: National youth fitness survey plan, operations, and analysis, 2012. Vital. Health Stat. 2, 1–24 (2014).
Christensen, K., Gleason, C. E. & Mares, J. A. Dietary carotenoids and cognitive function among US adults, NHANES 2011–2014. Nutr. Neurosci. 23, 554–562 (2020).
Krebs-Smith, S. M. et al. Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet. 118, 1591–1602 (2018).
Prince, M. J. et al. The burden of disease in older people and implications for health policy and practice. Lancet 385, 549–562 (2015).
Wei, J., Wang, L., Kulshreshtha, A. & Xu, H. Adherence to Life’s Simple 7 and cognitive function among older adults: The national health and nutrition examination survey 2011 to 2014. J. Am. Heart Assoc. 11, e22959 (2022).
Wang, L., Yi, J., Guo, X. & Ren, X. Associations between life’s essential 8 and non-alcoholic fatty liver disease among US adults. J. Transl. Med. 20, 616 (2022).
Ungvari, Z. et al. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 17, 639–654 (2021).
Dove, A. et al. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimers Dement 17, 1769–1778 (2021).
Buie, J. J., Watson, L. S., Smith, C. J. & Sims-Robinson, C. Obesity-related cognitive impairment: The role of endothelial dysfunction. Neurobiol. Dis. 132, 104580 (2019).
Jia, L. et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 5, e661–e671 (2020).
Gunstad, J., Sanborn, V. & Hawkins, M. Cognitive dysfunction is a risk factor for overeating and obesity. Am. Psychol. 75, 219–234 (2020).
Xu, W. et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 86, 1299–1306 (2015).
Ly, M. et al. Neuroinflammation: A modifiable pathway linking obesity, Alzheimer’s disease, and depression. Am. J. Geriatr. Psychiatry 31, 853–866 (2023).
Ji, Y. et al. Lactobacillus paracasei ameliorates cognitive impairment in high-fat induced obese mice via insulin signaling and neuroinflammation pathways. Food Funct. 12, 8728–8737 (2021).
Chen, Y. et al. Identifying type 2 diabetic brains by investigating disease-related structural changes in magnetic resonance imaging. Front Neurosci. 15, 728874 (2021).
Daugherty, A. M. Hypertension-related risk for dementia: A summary review with future directions. Semin. Cell Dev. Biol. 116, 82–89 (2021).
Valverde, A. & Mitrofanis, J. Photobiomodulation for hypertension and Alzheimer’s disease. J. Alzheimers. Dis. 90, 1045–1055 (2022).
Pang, K. et al. Higher total cholesterol concentration may be associated with better cognitive performance among elderly females. Nutrients 14, 4198 (2022).
Thorvaldsson, V., Skoog, I. & Johansson, B. Cholesterol and cognitive aging: Between-person and within-person associations in a population-based representative sample not on lipid-lowering medication. Psychol. Aging 35, 508–516 (2020).
Corley, J., Starr, J. M. & Deary, I. J. Serum cholesterol and cognitive functions: The Lothian Birth Cohort 1936. Int. Psychogeriatr. 27, 439–453 (2015).
Ma, C. et al. Blood cholesterol in late-life and cognitive decline: A longitudinal study of the Chinese elderly. Mol. Neurodegener. 12, 24 (2017).
Peters, R. et al. Evaluation of high cholesterol and risk of dementia and cognitive decline in older adults using individual patient meta-analysis. Dement. Geriatr. Cogn. Disord. 50, 318–325 (2021).
Jeong, S. M. et al. Effect of change in total cholesterol levels on cardiovascular disease among young adults. J. Am. Heart Assoc. 7, e008819 (2018).
Wang, X., Dong, Y., Qi, X., Huang, C. & Hou, L. Cholesterol levels and risk of hemorrhagic stroke: A systematic review and meta-analysis. Stroke 44, 1833–1839 (2013).
Martinez-Lapiscina, E. H. et al. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 84, 1318–1325 (2013).
Scarmeas, N., Anastasiou, C. A. & Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 17, 1006–1015 (2018).
Hoscheidt, S. et al. Mediterranean and Western diet effects on Alzheimer’s disease biomarkers, cerebral perfusion, and cognition in mid-life: A randomized trial. Alzheimers Dement 18, 457–468 (2022).
Santisteban, M. M. et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension 76, 795–807 (2020).
White, R. L. et al. Domain-specific physical activity and mental health: A meta-analysis. Am. J. Prev. Med. 52, 653–666 (2017).
Yang, W., Liang, X. & Sit, C. H. Physical activity and mental health in children and adolescents with intellectual disabilities: A meta-analysis using the RE-AIM framework. Int. J. Behav. Nutr. Phys. Act. 19, 80 (2022).
Krivanek, T. J., Gale, S. A., McFeeley, B. M., Nicastri, C. M. & Daffner, K. R. Promoting successful cognitive aging: A ten-year update. J. Alzheimers. Dis. 81, 871–920 (2021).
Bliss, E. S., Wong, R. H., Howe, P. R. & Mills, D. E. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow Metab. 41, 447–470 (2021).
Waterhouse, U., Brennan, K. A. & Ellenbroek, B. A. Nicotine self-administration reverses cognitive deficits in a rat model for schizophrenia. Addict. Biol. 23, 620–630 (2018).
Durazzo, T. C., Mattsson, N. & Weiner, M. W. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimers Dement 10, S122–S145 (2014).
Grande, G., Qiu, C. & Fratiglioni, L. Prevention of dementia in an ageing world: Evidence and biological rationale. Ageing Res. Rev. 64, 101045 (2020).
Taillard, J., Sagaspe, P., Philip, P. & Bioulac, S. Sleep timing, chronotype and social jetlag: Impact on cognitive abilities and psychiatric disorders. Biochem. Pharmacol. 191, 114438 (2021).
Boyce, R., Williams, S. & Adamantidis, A. REM sleep and memory. Curr. Opin. Neurobiol. 44, 167–177 (2017).
Irwin, M. R. & Vitiello, M. V. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 18, 296–306 (2019).
Acknowledgements
We thank the National Center for Health Statistics of the Centers for Disease Control and Prevention for sharing the NHANES data.
Funding
This research was funded by the the National Natural Science Foundation of China (NSFC) (No. 82260248) and the Doubel Thousand Talents Plan of Jiangxi (No. Jxsq2023101024).
Author information
Authors and Affiliations
Contributions
Z.L. and B.H. designed the study and is the principal investigator. W.Z., B.H. and J.C.T. drafted the manuscript. H.X.Z. conducted the data analysis. B.H., W.Z., Y.Y.Z., J.C.T., and M.H.L. critically revised the manuscript for important intellectual content and interpreted the data. Z.L. and H.X.Z. accessed and verified the data. All authors approved the final version of the manuscript. M.H.L. is the guarantor. The corresponding author (Z.L.) has access to and responsibility for the raw data associated with the study. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, H., Hei, B., Zhou, W. et al. Association between Life's Essential 8 and cognitive function among older adults in the United States. Sci Rep 14, 19773 (2024). https://doi.org/10.1038/s41598-024-70112-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70112-3