Abstract
To investigate quantitative associations between AI-assessed disease activity and optical coherence tomography angiography (OCTA)-derived parameters in patients with neovascular age-related macular degeneration (nAMD) undergoing anti-VEGF therapy. OCTA and SD-OCT images obtained from multicenter, randomized study data were evaluated. A deep learning algorithm (RetInSight) was used to detect and quantify macular fluid on SD-OCT. Mixed effects models were applied to evaluate correlations between fluid volumes, macular neovascularization (MNV)-type and OCTA-derived MNV parameters; lesion size (LS) and vessel area (NVA). 230 patients were included. A significant positive correlation was observed between SRF and NVA (estimate = 199.8 nl/mm2, p = 0.023), while a non-significant but negative correlation was found between SRF and LS (estimate = − 71.3 nl/mm2, p = 0.126). The presence of Type I and Type II MNV was associated with significantly less intraretinal fluid (IRF) compared to Type III MNV (estimate type I:− 52.1 nl, p = 0.019; estimate type II:− 51.7 nl, p = 0.021). A significant correlation was observed between pigment epithelial detachment (PED) and the interaction between NVA and LS (estimate:28.97 nl/mm2; p = 0.012). Residual IRF at week 12 significantly correlated to baseline NVA (estimate:38.1 nl/mm2; p = 0.015) and LS (estimate:− 22.6 nl/mm2; p = 0.012). Fluid in different compartments demonstrated disparate associations with MNV OCTA features. While IRF at baseline was most pronounced in type III MNV, residual IRF was driven by neovascular MNV characteristics. Greater NVA in proportion to LS was associated with higher amounts of SRF and PED. The correlation between these parameters may represent MNV maturation and can be used as a biomarker for resolution of disease activity. AI-based OCT analysis allows for a deeper understanding of neovascular disease in AMD and the potential to adjust therapeutic strategies to optimize outcomes through precision medicine.
Similar content being viewed by others
Introduction
The recent advancements in imaging technology, specifically retinal imaging, have reached a pinnacle, particularly through the use of high-resolution optical coherence tomography (OCT) and OCT angiography (OCTA). These advancements have resulted in a more precise definition and understanding of age-related macular degeneration (AMD), a condition that is accountable for considerable and progressive loss of vision1. Up to 15% of patients diagnosed with AMD develop macular neovascularization (MNV), which if left untreated, can result in both immediate and long-term vision loss due to exudation and the eventual formation of macular fibrosis and atrophy1,2.
Timely diagnosis and treatment are crucial for limiting photoreceptor degeneration and preventing long-term damage to the neurosensory retina. The advent of new technologies including artificial intelligence (AI), is enabling clinicians to detect, analyze and quantify retinal fluid volume in high-resolution optical coherence tomography (OCT) volume scans. Such clinical decision support systems (CDSS) are capable of monitoring fluid changes and disease activity during treatment3, as well as assisting in the identification of clinically relevant biomarkers for disease activity4,5.
Retinal fluid originates from neovascular structures, which, in the context of advanced image analyses, can be extracted in a defined manner by OCTA technology. Some of these neovascular AMD (nAMD)-specific biomarkers under currently under investigation and can be measured by analyzing OCT-angiography (OCTA) en face scans of MNV membranes6,7,8,9,10. From a pathophysiological perspective, the activity and patterns of retinal fluid exudation as a consequence and MNV-related features as the primary source of leakage should be closely related. A number of studies have been published that associate surrogates such as the presence of fluid or retinal thickness with OCTA findings. However, few of these studies are based on quantified macular fluid volumes11,12,13,14,15. Currently, the selection of suitable retreatment options is primarily guided by the location and activity of retinal fluid as observed in OCT. The majority of treatment guidelines recommend an initial loading dose comprising 3 anti-vascular endothelial growth factor (anti-VEGF) injections, administered at 4 week intervals16,17,18. Subsequent to this, a more individualized treatment plan is initiated, based on the presence of fluid in specific retinal compartments and locations within the central macula. This approach is supported by recent studies which have shown that subretinal (SRF) and intraretinal fluid (IRF) have a different impact on visual acuity19,20.
Given that leakage is derived from the underlying MNV, the association between the MNV condition and disease activity has become an important missing link in the search for optimal treatment outcomes. To this extent, the correlation between OCTA-related MNV biomarkers and fluid volume and dynamics is still unknown. The potential for more personalized treatment to reduce patient visits and associated costs, while simultaneously improving patient management and comfort, is an avenue that warrants further investigation. The objective of this study was to investigate the association between fluid volumes in high-resolution structural OCT and quantitative vascular characteristics of MNV imaged by OCTA utilising consistent data from randomized controlled trials.
Methods
The present post hoc analysis used data from the Vienna Reading Centre imaging database and was conducted at the Department of Ophthalmology and Optometry of the Medical University of Vienna, Vienna, Austria. All data utilized in this analysis were prospectively generated and collected in randomized controlled multicenter studies for anti-VEGF treatment in nAMD. MNV types were graded by reading center proved retina specialists using OCT and in accordance with international guidelines21. More specifically, type 1 MNV appears on OCT as a detachment of the RPE from Bruch's membrane with variable reflectivity. PEDs with type 1 MNV may appear as flat separation (double layer sign) or even dome-shaped PEDs. The sub-RPE reflectivity may be homogeneous with intermediate reflectivity or display a layered structure (onion sign). Exudation associated with type 1 neovascularization includes sub-RPE fluid (serous PEDs or combined fibrovascular/serous PEDs and subretinal fluid). Intraretinal fluid is possible but less common. Type 2 MNV is seen on OCT as subretinal hyperreflective material (SHRM), usually with associated subretinal fluid, subretinal hemorrhage (highly reflective SHRM) and often intraretinal fluid.
Type 3 MNV usually presents on OCT as disorganization of the retinal layers with intraretinal fluid, intraretinal hemorrhages. When a type 3 MNV reaches the level of the RPE, a characteristic notch like PED is seen where it passes through the RPE. Serous and combined serous/fibrovascular PEDs are often seen in association with type 3 MNV.
In the case of a mixed form of type 1&2 MNV, the lesion type that occupies the larger portion of the lesion is defined as the lesion type. In instances where both lesion types occupy 50%, the lesion type closer to the foveal center is designated as the primary lesion type.
The treatment regimen consisted of a 3 × 4-week loading dose followed by a treatment after 8 weeks and a treat-to-control scheme in one study and an 8–12 week treatment interval in another study. The treat-to-control strategy represents a modified treat-and-extend approach, wherein treatment intervals may be adjusted by 4 weeks or maintained at the current level. Total treatment intervals of 8 to 16 weeks were permitted.
Treatment was based on the presence of disease activity, as indicated by the presence of retinal fluid and changes in visual acuity. The treatment medications were aflibercept and brolucizumab.
The multicenter studies, as well as this posthoc analysis were all conducted in accordance with the tenets of the Declaration of Helsinki. Ethical approval was obtained in each country for the multinational core trials, and informed consent was obtained from each subject before inclusion. Approval for this post-hoc analysis was obtained from the Ethics Committee at the Medical University of Vienna (approval number: 1246/2016). The data was provided by the sponsor of each individual trial. The analyses, content and conclusions of this work are solely the responsibility of the authors and have not been modified by the sponsors.
Image acquisition
OCT operator training was standardized as well as comparable device settings were used. Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) was used to acquire all OCT volumes. The Avanti Angiovue (Optovue, Fremont, California, USA), Cirrus 5000 (Carl Zeiss Meditec Inc., Dublin, CA, USA) and Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) were used for OCTA imaging.
For OCT volumes, a fovea-centred high-resolution 97-line raster with an image averaging of 15 images per B-scan was performed in the built-in follow-up mode based on image registration using the HRA + OCT device. The acquisition setting of the Avanti Angiovue OCTA was a 3 × 3 mm scanning area with 304 × 304 A-scans, an A-scan rate of 70 kHz, and split-spectrum amplitude-decorrelation angiography (SSADA) was used with two consecutive B scans at each position.
The acquisition setting of the Cirrus 5000 OCTA was a 3 × 3 mm scanning area with 245 × 245 A-scans, an A-scan rate of 68 kHz, an axial resolution of 5 µm and a transversal resolution of 15 µm.
The acquisition setting of the Spectralis OCTA was a 3 × 3 mm scanning area with 512 B-scans averaging of 5 images per B-scans and an A-scan rate of 85 kHz and a lateral resolution of 5.7 µm.
Quantitative feature analysis
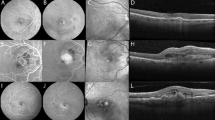
The Fluid Monitor (RetInSight, Vienna, Austria), a clinical decision support system (CDSS) approved by the EU medical device regulation (MDR) (2017/745) was used to detect, localize and quantify distinct fluid volumes in OCT volume scans. The convolutional neural network (CNN) classifies each pixel of the OCT to either normal retina, intraretinal, subretinal or sub-RPE fluid. The number of pixels detected in the OCT are then transformed into volumes and measured in nanoliters3. An example can be seen in Fig. 1, images a, d and g.
illustrates an eye with type II MNV. Images (a–c) were captured at baseline, while images (d–f) were obtained at week 4 and images (g–i) at week 8. Spectral domain optical coherence tomography (SD-OCT) B-scans (images a, d, g) demonstrate fluid segmentation based on automated deep learning. The red colour indicates the presence of intraretinal fluid (IRF), the green colour indicates the presence of subretinal fluid (SRF), and the blue colour indicates the presence of pigment epithelial detachment (PED). In OCT angiography (OCTA) en face images, the magenta colour represents the size of the macular neovascular membrane (MNV) lesion, which corresponds to the area covered by the entire MNV membrane. The yellow colour represents the vessel area, which is the area covered by the pure flow signal, as shown in images (c, f and i). The corresponding areas in square millimetres are shown in the upper right corner. In the OCTA images, the red lines indicate the position of the corresponding SD-OCT B-scan. The fluid volumes at baseline were as follows: The IRF volume was 431 nl, the SRF volume was 461 nl, and the PED volume was 169 nl. The fluid volumes at week 4 were as follows: IRF volume, 10 nl; SRF volume, 43 nl; PED volume, 174 nl. The fluid amounts at week 8 were as follows: IRF volume, 17 nl; SRF volume, 0 nl; PED volume, 163 nl.
A fovea-centered flow volume scan was acquired using three different OCTA devices (Spectralis HRA + OCT, Avanti Angiovue, Cirrus 5000). The acquisition parameters of all devices were predefined to include the central 3 × 3 mm area.
The so called RPE-RPE fit slab (for type I MNV), the outer retina to choriocapillaris slab (for type 2 MNV) and whole retina slab (for type 3MNV) were used to display MNV-membranes in the en-face image22,23. The RPE-RPE fit slab, extends between the actual position of the RPE and the location where the RPE was physiologically positioned on Bruch’s membrane. The outer retina to choriocapillaris slab extends from the outermost parts of the outer plexiform layer to just below Bruch’s membrane. The whole retina slab extends from the inner limiting membrane to Bruch’s membrane. Should it be necessary, manual adjustment of the segmentation was employed to ensure that the orthogonal visualization only contained flow signal from the MNV. The inbuilt projection artifact removal of each device was utilized to remove flow artifacts and improve contrast and quality24. Subsequently the inbuilt software was used to delineate the total MNV area (lesion size, LS) (see Fig. 1, Images b, e, h). If available within the OCTA device software (Angiovue only), the vessel area (NVA), was also calculated, defined as the area covered by the plain MNV flow signal. This is shown in Fig. 1, Images c, f, i.
Statistical testing
For the purposes of statistical testing, SPSS Statistic (Version 25.0; Armonk, NY: IBM Corp.) was employed. The evaluation of normal distribution was conducted via the use of histograms and the Shapiro–Wilk test. The correlation between fluid volumes and MNV parameters (LS, NVA) was calculated using linear mixed models. In one model, fluid volume (SRF, IRF, PED) was designated as the target variable, whereas LS, NVA, the interaction between LS and NVA, MNV type and BSL fluid volume (SRF, IRF, PED) were designated as fixed effects. Study subjects’ identities were designated as a random effect. In this model, OCTA data were only utilised from the Angiovue device, as it is the only device that provides LS and NVA.
In a second model, which was calculated to contain data from all patients, fluid volume (SRF, IRF, PED) was assigned as the target variable. LS, MNV type, OCTA device type and BSL fluid volume (SRF, IRF, PED) were assigned as fixed effects, study subjects’ identities were assigned as a random effect.
In a third model with the same structure as model two, we evaluated only eyes for which a NVA value was also present (Angiovue OCTA only) to evaluate the impact of the NVA parameter.
A further sub-analysis was conducted, comprising only eyes imaged using Angiovue OCTA. This aimed to evaluate the correlation between baseline MNV parameters and week 12 fluid volumes, as well as the correlation between week 8 MNV parameters (end of loading dose) and week 12 fluid volumes. To achieve this, we employed a series of mixed models, wherein the fluid volume (SRF, IRF, PED) at week 12 was designated as the target variable. The baseline fluid volume (SRF, IRF, PED) and MNV type, LS and NVA (BSL or week 12, respectively) were assigned as fixed effects. For all calculations, the level of significance was set to α = 0.05. No correction for multiple testing outside the 3 models was used due to the exploratory character of this work.
Ethics approval and consent to participate
The study was approved by the local ethics committee of the Medical University of Vienna (approval number: 1246/2016).
Results
Retinal fluid and OCTA parameters over time
A total of 1011 OCT volume scans and 1011 OCTA images from 230 patients with treatment-naïve nAMD collected from baseline up to week 64 were analyzed.
111 patients were imaged using the Cirrus 5000 OCTA, 80 using the Avanti Angiovue OCTA and 39 using the Spectralis HRA + OCTA, respectively.
146 patients (63.5%) were diagnosed with primary type I MNV, 53 (23%) with type II MNV and 31 with type III MNV (13.5%). Type I MNV with the presence of polyps on ICGA were excluded.
Median fluid volumes, lesion size and vessel area at the timepoints studied are shown in Table 1 and Fig. 2.
Correlation between fluid types and volumes with Lesion size and neovascular vessel area
In the linear mixed model that included all visits and both LS and NVA, no significant correlation was found between either MNV parameter (LS: p = 0.825, VA: p = 0.782) and IRF volume. The IRF was found to be significantly correlated with MNV type, with negative estimates for type 1 MNV (− 51.17 nl, p = 0.022) and type 2 MNV (− 49.58 nl, p = 0.026) compared to type 3 MNV (see Table 2). The mean estimated amount of IRF was 68.17 nl in type 3 MNV (95% CI 28.35 to 107.98), followed by type 2 MNV with 18.5 nl. The mean estimated volume of IRF was 9 nl (95% CI − 2.60 to 39.78), while that of type 1 MNV was 16.70 nl (95% CI 2.51 to 31.48).
The volume of SRF was found to be significantly correlated with NVA, with a positive estimate of 199.82 nl/mm2 (p = 0.023). However, no significant correlation was observed between SRF volume and LS, with an estimate of − 71.28 nl/mm2 (p = 0.126). No significant correlation was observed between MNV type and SRF fluid volume (all p > 0.05).
PED was only significantly correlated with the interaction between LS and NVA, with an estimate of 28.97 nl/mm2 (p = 0.012). Further details can be found in Table 2.
Correlation between fluid types and volumes with lesion size
The model for IRF, which included all eyes but only the MNV parameter LS, demonstrated a significant correlation between MNV type and fluid volume. The constant term was 30.21 nl, with estimates of − 27.25 nl for MNV type I (p = 0.004) and − 20.33 nl for MNV type II (p = 0.036) in comparison to the defined reference of MNV type III. The estimated mean values for IRF volume were 16.14 nl (95% CI 8.45 to 23.83) for type I MNV, 23.01 nl (95% CI The estimated mean values for IRF volume were 11.98 to 34.13 nl for type I MNV, 23.01 nl (95% CI 26.40 to 60.37) for type II MNV, and 43.38 nl (95% CI 26.40 to 60.37) for type III MNV.
The models for SRF and PED, which included only LS, demonstrated no statistically significant correlations between any of the parameters and specific fluid volumes.
A comparable, non-significant outcome was observed for the models for SRF and PED, incorporating only eyes for which both LS and NVA were available, if only LS was included alongside MNV type and baseline fluid.
In each calculated mixed-effect model, the employed OCTA device demonstrated no significant impact on IRF (p = 0.935), SRF (p = 0.305) or PED (p = 0.644) volumes, respectively.
Correlation between fluid volumes at week 12 and baseline OCTA parameters—a subcohort analysis
A significant correlation was observed between IRF volume at week 12 and NVA (p = 0.015), with a positive estimate of 38.06 nl/mm2 (95% CI 7.93 to 6.19). Additionally, a negative correlation was observed between IRF volume at week 12 and LS (p = 0.012), with a negative estimate of − 22.56 nl/mm2 (95% CI − 39.85 to − 5.28). No significant correlation was observed between MNV type and IRF volume at week 12. Similarly, no significant correlation was found between SRF volume at week 12 and either MNV parameter (LS: p = 0.830, VA: p = 0.902) or MNV type (p = 0.864).
A significant correlation was observed between PED volume at week 12 and NVA (p = 0.01), with a positive estimate of 847.10 nl/mm2 (95% CI 215.10 to 1479.08). Additionally, a significant negative correlation was noted between PED volume at week 12 and LS (p = 0.011), with an estimate of − 465.97 nl/mm2 (95%CI − 820.60 to − 111.33) and a positive correlation was noted between PED volume and the interaction between LS and NVA (p = 0.019) with an estimate of 45.16 nl/mm2 (95%CI 7.73 to 82.28).
No significant correlation was observed between MNV type and PED volume at week 12 (p = 0.58) (see Table 3).
Comparison of lesion size between MNV types
A comparison of the differences in LS between the three different MNV types revealed that type II MNV exhibited the greatest LS, with a value of 1.07 mm2 (95% CI 0.77 to 1.37). This was followed by type I, with a value of 0.83 mm2 (95% CI 0.64 to 1.02) and type III with a value of 0.24 mm2 (95% CI − 0.18 to 0.65), (p = 0.007).
Discussion
In this post-hoc analysis of 230 eyes from 230 patients with treatment-naïve nAMD followed over one year, we comprehensively evaluated the correlation between quantitative OCTA-derived MNV parameters and distinct retinal fluid volumes. Retinal fluid volumes by compartment type were detected, localized and quantified using an approved deep learning algorithm to provide highly reliable parameters. Compared to previous studies published by our group11,15,23,25, in which third-party software was used to analyze OCTA en face images of MNV membranes for collecting several complex MNV parameters, this study was based on the two basic OCTA parameters: Lesion size (LS) and neovascular vessel area (NVA). All OCTA scans were acquired in the 3 × 3 mm area, as this field was shown to contain more details due to higher resolution, which might be even more important in spectral domain OCTA devices26.
In our study cohort, fluid volumes, distribution and progression over time and under treatment were comparable to the findings of the HARBOR study, where fluid was measured in OCT volume scans of 1095 patients using a fully automated deep learning-based algorithm5. The consistency with the HARBOR nAMD population indicates good generalizability for nAMD-related disease activity.
We found that the amount of IRF at each time point was not correlated with either of the two most popular MNV parameters, but only with the MNV type, with type III MNV demonstrating the highest IRF volumes (68.17 nl; 95% CI 28.35 to 107.98). The fact that type III MNV is dominantly associated with IRF has been suggested previously5, yet the correlation between MNV parameters and fluid in type III MNV is rather unknown, as this type is often excluded from OCTA analyses due to the vertical rather than horizontal27,28 orientation of the vessels, leading to a significantly smaller LS, with less detail, neither are objective quantitative data available.
When investigating SRF, however, we found that the amount of SRF at each timepoint was significantly correlated with NVA (estimate: 199.82, p = 0.023), but not significantly correlated with LS (estimate: − 71.28 p = 0.126). Different models investigating only LS, did not produce a significant result, confirming the fact that for SRF NVA is of highest importance. These results are in line with one of our recent publications, in which we evaluated the correlation between microstructural MNV parameters derived from swept source OCTA and fluid in a smaller monocentric cohort. We found that the number of vessel endpoints, total vessel length and vessel density (the quotient of NVA and LS) were significantly, but weakly associated with the amount of SRF15. A recent paper by Told found correlations between retinal thickness in OCT and total vessel length, total number of junctions and the NVA11. Similar results were found by Faatz, who also evaluated the effect of fractal dimension on central retinal thickness12. All these complimentary parameters except LS and NVA, are obtained by further processing of the OCTA enface scans and therefore require high OCTA image quality. However, they could be summarized by the two simple and more quality-independent parameters LS and NVA. The relationship between LS and NVA indirectly includes parameters such as number of junctions, number of endpoints, total vessel length and fractal dimension. Higher sprouting activity within a MNV membrane leads to more vessel per area, which leads to a higher total vessel length, more endpoints, a higher fractal dimension and all together to a denser packed MNV membrane with more flow signal and consequently to a higher NVA within the LS. However, this simplification may also lead to a loss of information, referring to a paper by Spaide in which he showed that the formation of vessel endpoint loops, anastomoses and loss of sprouts reflect maturation of neovascular networks29. Subsequently, a more differentiated evaluation of vessel endpoints, junctions, and the relationship between these two parameters may provide additional insight into the pathophysiology of MNV activity.
Faatz et al.30 stated that vessel density or flow density, a value which is often calculated automatically by OCTA devices, must not be used to describe MNV architecture. Our results reflect the same notion, as we found that the absolute values of LS and NVA play an important role when considering the amount of fluid. The vessel density only represents a relative value, for example: in an eye with a LS of 1 mm2 and a NVA of 1 mm2 the vessel density is 1 and in an eye with LS of 3 mm2 and NVA of 3 mm2 it is also 1, but according to our model the total SRF volume is approximately 3 × higher in the eye with LS and NVA of 3 mm2.
Besides the quantitative correlations between OCTA-derived MNV parameters and fluid amounts, the influence of baseline MNV parameters on the therapeutic response in terms of fluid reduction and residual fluid at a given time point is of great interest for understanding the biological base of disease activity and therapeutic response patterns in nAMD. This is especially relevant for IRF as it is responsible for vision loss over time compared to non-active persistent SRF, which might be tolerated19,20.
Therefore, we investigated the correlation between MNV parameters and residual fluid at week 12 (4 weeks after the last intravitreal injection of the loading dose). In our models, which included the target fluid volume at week 12 and the MNV parameters as well as lesion type and baseline fluid volume, we found significant correlations for IRF at week 12 and PED at week 12. MNV membranes presenting with a larger NVA at baseline (estimate: 38.06 nl/mm2, p = 0.015) in relationship to a smaller LS (estimate: − 22.56 nl/mm2, p = 0.012) are related to more residual IRF at week 12. Again, the absolute values of LS and NVA provide more information in this matter compared to vessel density as already discussed above. However, it seems that the proportion between LS and NVA is linked to MNV exudation activity and that larger LS with relatively smaller NVA represent less active MNV membranes. Thus, eyes with larger and denser packed MNV membranes, might need another 4-week treatment interval after loading dose, instead of switching to a 6 or 8 week interval immediately. Similar results were found considering PED volume at week 12 (LS: estimate: − 465.97 nl/mm2, p = 0.011; VA: estimate: 847.10 nl/mm2, p = 0.010). This result is empirically plausible as a larger and more complex MNV membranes leads to a larger PED. It was previously shown that PEDs stay relatively unchanged under treatment compared to SRF and IRF. Furthermore, it is assumed that MNV membranes keep growing even under treatment in their sub-RPE space23. Despite a reduction of serous fluid in a PED, there might be an increase of the fibrovascular component. In a related analysis of the VIEW study data, PED growth was mostly followed by the development of IRF31. Considering SRF at week 12 no significant correlation to any baseline parameter was found. Our results concerning SRF and PED differ from what was recently published by Faatz et al.30. They found that patients with residual or persisting SRF and PED after loading dose had significantly higher lesion size, vessel length and fractal dimension compared to eyes without residual fluid. They found no differences considering IRF. However, a different approach was chosen compared to our analysis. They compared baseline MNV parameters based on a purely qualitative dichotomic criterium fluid (present or absent) after the loading dose, whereas we correlated baseline MNV parameters with distinct fluid volume at week 12 and corrected for the amount of baseline fluid volume. To appropriately elucidate correlations between retinal parameters, quantification is much superior to mere present/absent statements.
A significant limitation of this study was the use of spectral domain OCTA devices, which have a wavelength-dependent lower penetration depth and slower imaging speed compared to swept-source OCTA. This constraint impairs the visibility of MNV flow signal, particularly in eyes with large serous PEDs, large amounts of SRF, and type I MNV. At baseline, MNV LS and NVA may be underestimated due to fluid obscuring the flow signal, leading to reduced visibility. As fluid, especially substantial subretinal fluid and serous PED, decreases, the flow signal becomes clearer, improving the visualization of the MNV membrane. Consequently, this enhanced visibility can result in an increase in LS and NVA following the initial anti-VEGF injection in these eyes.
The use of 3 different OCTA devices each device having its specific strengths and weaknesses, such as different segmentation algorithms and possibilities for manual segmentation, is another limitation, however, in our analysis considering LS we found that the device type did not play a significant role in our models32. Another limitation was the rather small number of patients who were imaged every 4 weeks up to week 12 with the Angiovue OCTA, providing NVA and LS compared to the total number of patients. Additionally, the use of two different study medications (aflibercept and brolucizumab) as well as different treatment regimen (treat to control, every 8 or every 12 weeks) bear limitations. However, the main strengths of this study were: the use of AI to localize fluid by compartment and by volumes in a most accurate manner, data collection by Reading Centre-certified retinal specialists, and the multicentre nature of the study data.
In conclusion, we found that IRF volume correlated with lesion type, SRF volume correlated with vessel area, and PED correlated with the interaction between lesion size and vessel area. Furthermore, we found that within the study cohort, larger and denser MNV lesions at baseline are correlated with more residual IRF following the loading dose, and the same was found when controlling for PED. The current controversy in the debate about the impact of different fluid compartments such as IRF and SRF, the question about how dry the retina should be kept during long-term treatment and whether SRF prevents the development of macular atrophy can only lead to robust conclusions if all relevant parameters are defined by objective measurements20,33,34. In routine practice, treatment decisions are largely based on OCT imaging with the presence of active retinal fluid triggering retreatment. OCTA will not replace the standard SD-OCT for decision-making, it is, however relevant to understand the origin and intensity of exudation, i.e. the pathophysiology of neovascular disease. Quantification of fluid types by compartment using AI-based fluid analysis allows precision medicine in an area where anatomical alteration leads to irreversible functional loss.
Data availability
The datasets generated during and/or analyzed in this study are not publicly available due to privacy regulations set by the Medical University of Vienna but are available from the corresponding author on reasonable request.
References
Wong, W. L. et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2(2), e106–e116. https://doi.org/10.1016/S2214-109X(13)70145-1 (2014).
Schmidt-Erfurth, U. et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 98(9), 1144–1167. https://doi.org/10.1136/bjophthalmol-2014-305702 (2014).
Schlegl, T. et al. Fully automated detection and quantification of macular fluid in OCT using deep learning. Ophthalmology 125(4), 549–558. https://doi.org/10.1016/J.OPHTHA.2017.10.031 (2018).
Bogunovic, H. et al. Prediction of anti-VEGF treatment requirements in neovascular AMD using a machine learning approach. Investig. Opthalmol. Vis. Sci. 58(7), 3240. https://doi.org/10.1167/iovs.16-21053 (2017).
Schmidt-Erfurth, U., Vogl, W. D., Jampol, L. M., Bogunovi, H. & Bogunović, H. Application of automated quantification of fluid volumes to anti-VEGF therapy of neovascular age-related macular degeneration. Ophthalmology 127, 1211–1219. https://doi.org/10.1016/j.ophtha.2020.03.010 (2020).
Bae, K., Kim, H. J., Shin, Y. K. & Kang, S. W. Predictors of neovascular activity during neovascular age-related macular degeneration treatment based on optical coherence tomography angiography. Sci. Rep. 9(1), 19240. https://doi.org/10.1038/s41598-019-55871-8 (2019).
Liang, M. C. et al. Correlation of spectral domain optical coherence tomography angiography and clinical activity in neovascular age-related macular degeneration. Retina 36(12), 2265–2273. https://doi.org/10.1097/IAE.0000000000001102 (2016).
Told, R. et al. Swept source optical coherence tomography angiography, fluorescein angiography, and indocyanine green angiography comparisons revisited: Using a novel deep-learning-assisted approach for image registration. Retina https://doi.org/10.1097/IAE.0000000000002695 (2020).
Novais, E. A. et al. Choroidal neovascularization analyzed on ultrahigh-speed swept-source optical coherence tomography angiography compared to spectral-domain optical coherence tomography angiography. Am. J. Ophthalmol. 164, 80–88. https://doi.org/10.1016/j.ajo.2016.01.011 (2016).
Cicinelli, M. V. et al. Swept-source and spectral domain optical coherence tomography angiography versus dye angiography in the measurement of type 1 neovascularization. Retina https://doi.org/10.1097/iae.0000000000002452 (2020).
Told, R. et al. Correlation of retinal thickness and swept-source optical coherence tomography angiography derived vascular changes in patients with neovascular age-related macular degeneration. Curr. Eye Res. https://doi.org/10.1080/02713683.2020.1849734 (2020).
Faatz, H. et al. Changes in the OCT angiographic appearance of type 1 and type 2 CNV in exudative AMD during anti-VEGF treatment. BMJ Open Ophthalmol. 4, 369. https://doi.org/10.1136/bmjophth-2019-000369 (2019).
Coscas, F. et al. Quantitative optical coherence tomography angiography biomarkers for neovascular age-related macular degeneration in remission. PLoS One 13(10), e0205513. https://doi.org/10.1371/journal.pone.0205513 (2018).
Roberts, P. K., Nesper, P. L., Gill, M. K. & Fawzi, A. A. Semiautomated quantitative approach to characterize treatment response in neovascular age-related macular degeneration: A real-world study. Retina 37(8), 1492–1498. https://doi.org/10.1097/IAE.0000000000001400 (2017).
Schranz, M. et al. Correlation of vascular and fluid-related parameters in neovascular age-related macular degeneration using deep learning. Acta Ophthalmol. 101(1), e95–e105. https://doi.org/10.1111/aos.15219 (2023).
Hatz, K. & Prünte, C. Treat and extend versus Pro Re Nata regimens of ranibizumab in neovascular age-related macular degeneration: A comparative 12 month study. Acta Ophthalmol. 95(1), e67–e72. https://doi.org/10.1111/aos.13031 (2017).
Berg, K., Roald, A. B., Navaratnam, J. & Bragadóttir, R. An 8-year follow-up of anti-vascular endothelial growth factor treatment with a treat-and-extend modality for neovascular age-related macular degeneration. Acta Ophthalmol. 95(8), 796–802. https://doi.org/10.1111/aos.13522 (2017).
Haga, A., Kawaji, T., Ideta, R., Inomata, Y. & Tanihara, H. Treat-and-extend versus every-other-month regimens with aflibercept in age-related macular degeneration. Acta Ophthalmol. 96(3), e393–e398. https://doi.org/10.1111/aos.13607 (2018).
Reiter, G. S. et al. Analysis of fluid volume and its impact on visual acuity in the FLUID study as quantified with deep learning. Retina 41(6), 1318–1328. https://doi.org/10.1097/IAE.0000000000003023 (2021).
Guymer, R. H. et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology 126(5), 723–734. https://doi.org/10.1016/j.ophtha.2018.11.025 (2019).
Spaide, R. F. et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology https://doi.org/10.1016/j.ophtha.2019.11.004 (2020).
Parravano, M. et al. A comparison among different automatically segmented slabs to assess neovascular AMD using swept source OCT angiography. Transl. Vis. Sci. Technol. 8(2), 8. https://doi.org/10.1167/TVST.8.2.8 (2019).
Told, R. et al. Profiling neovascular age-related macular degeneration choroidal neovascularization lesion response to anti-vascular endothelial growth factor therapy using SSOCTA. Acta Ophthalmol. 99, 1–7. https://doi.org/10.1111/aos.14554 (2020).
Zhang, Q. et al. Projection artifact removal improves visualization and quantitation of macular neovascularization imaged by optical coherence tomography angiography. Ophthalmol. Retina 1(2), 124–136. https://doi.org/10.1016/j.oret.2016.08.005 (2017).
Told, R. et al. OCTA biomarker search in patients with nAMD: Influence of retinal fluid on time-dependent biomarker response. Curr. Eye Res. 48(6), 600–604. https://doi.org/10.1080/02713683.2023.2184318 (2023).
Faatz, Id. H. et al. Morphologic analysis of macular neovascularizations by OCT angiography-technical limitations in the comparison of 3×3 mm and 6×6 mm images. Plos one https://doi.org/10.1371/journal.pone.0237785 (2020).
Berlin, A. et al. Correlation of optical coherence tomography angiography of type 3 macular neovascularization with corresponding histology. JAMA Ophthalmol. 140(6), 628. https://doi.org/10.1001/jamaophthalmol.2022.0890 (2022).
Cho, H. J. et al. Assessing the long-term evolution of type 3 neovascularization in age-related macular degeneration using optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 259(9), 2605–2613. https://doi.org/10.1007/s00417-021-05163-7 (2021).
Spaide, R. F. Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. Am. J. Ophthalmol. 160(1), 6–16. https://doi.org/10.1016/j.ajo.2015.04.012 (2015).
Faatz, H. et al. The architecture of macular neovascularizations predicts treatment responses to anti-VEGF therapy in neovascular AMD. Diagnostics 12(11), 2807. https://doi.org/10.3390/diagnostics12112807 (2022).
Waldstein, S. M. et al. Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age-related macular degeneration in the VIEW trials. Ophthalmology https://doi.org/10.1016/j.ophtha.2016.03.037 (2016).
Munk, M. R. et al. OCT-angiography: A qualitative and quantitative comparison of 4 Oct-A devices. PLoS One 12(5), e0177059. https://doi.org/10.1371/journal.pone.0177059 (2017).
Pawloff, M. et al. Systematic correlation of central subfield thickness with retinal fluid volumes quantified by deep learning in the major exudative macular diseases. Retina 42(5), 831–841. https://doi.org/10.1097/IAE.0000000000003385 (2022).
Grechenig, C. et al. Impact of residual subretinal fluid volumes on treatment outcomes in a SRF-tolerant treat & extend regimen. Retina https://doi.org/10.1097/IAE.0000000000003180 (2021).
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this work. All authors read and approved the final manuscript. M.S. wrote the main manuscript, prepared figures H.B. statistics, analysis and interpretation of the data G.D. reviewed the manuscript, interpretation of the data A.S. OCT fluid quantification G.R. statistics, analysis and interpretation of the data U.S. supervising, reviewing, interpretation of the data and results, final correction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Schranz, M., Bogunovic, H., Deak, G. et al. Linking disease activity with optical coherence tomography angiography in neovascular age related macular degeneration using artificial intelligence. Sci Rep 14, 19278 (2024). https://doi.org/10.1038/s41598-024-70234-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70234-8
Keywords
This article is cited by
-
Exploratory investigation of OCTA-VISTA for longitudinal monitoring of neovascular age-related macular degeneration treatment
Graefe's Archive for Clinical and Experimental Ophthalmology (2026)
-
Aflibercept, ranibizumab, and bevacizumab for macular neovascularization secondary to age-related macular degeneration: a retrospective OCT-angiography study
International Journal of Retina and Vitreous (2025)