Abstract
The idea of utilizing unused oocytes present in the ovaries has been tested in various ways to produce offspring. However, only a limited number of studies succeeded in offspring generation. They include transplantation of ovaries into autologous or allogeneic animals, and acquisition of pups from oocytes obtained by transplanting mouse ovaries into immunodeficient rats. Here we report successful production of rat oocytes by transplanting rat ovaries under the kidney capsule of immunodeficient mice with addition of hormone administration to the mice. In addition, these oocytes were developed by in vitro fertilization, and transplanted into the oviducts of pseudopregnant rats, resulting in successful delivery of pups. The modified gene of the donor rat was confirmed to be correctly inherited to the pups. These results show that xenotransplantation of ovarian tissue makes it possible to leave offspring, beginning a new phase in developmental engineering.
Similar content being viewed by others
Introduction
Recent progress in gene editing has made it possible to create genetically modified rats of various strains, and to provide them for wide research fields, while there are still critical problems as seen in generation of genetically modified mice. Some genetic modifications result in infertility and other problems in fetal and neonatal development, making it difficult to leave offspring1,2. Compared to mice, the generation of genetically modified rats is more time-consuming and costly. Thus, it is significantly important to develop new technology aiming at effective use of genetically modified rats3.
Various efforts have long been made in reproductive engineering to protect fertility by cryopreservation of ovaries and find methods to mature oocytes and obtain eggs through ovarian transplantation4,5. In practice, however, most attempts have not been successful except for the acquisition of oocytes by autotransplantation6,7. The only exception is the successful acquisition of oocytes by transplanting mouse ovaries into immunodeficient rats and in vitro fertilizing the acquired oocytes8. On the other hand, there is a study comparing gene expression profiles of wild rat ovaries with those of xenotransplanted ovaries, concluding that gene expression of the immune system is elevated in xenotransplanted ovaries, whereas that of the metabolic system is decreased. This paper infers that oocytes derived from xenotransplanted ovaries do not develop to be offspring9.
Recently, we succeeded in developing marmoset oocytes to late-stage blastocysts by transplanting marmoset ovaries to immunodeficient mice, developing the follicles in them, and in vitro inseminating the harvested oocytes10. From this result, we assumed that a combination of appropriate hormone induction and in vitro maturation (IVM) techniques might be able to produce eggs with correct oogenesis occurring in xenotransplanted ovaries.
In this study, we aimed at establishing a new method to produce oocytes by xenotransplantation. We transplanted rat ovaries into mice to obtain pups from the oocytes by in vitro fertilization. For this purpose, we carefully examined the method of follicle maturation in the transplanted ovary, maturation culture of the acquired oocytes, and in vitro fertilization (IVF) methods. As a result, we have confirmed that it is possible to acquire oocytes derived from xenotransplanted ovaries, and to make them become rat pups.
Results
Fertilizing ability of oocytes in rat ovary
Since mature follicles in rat ovaries contain oocytes surrounded by cumulus and granulosa cells, we investigated the fertilizing ability of these pre-ovulatory oocytes. To develop and increase pre-ovulatory follicles, rats were injected with pregnant mare serum gonadotropin (PMSG), and 48 h later human chorionic gonadotropic hormone (hCG) was administrated to induce ovulation. Furthermore, since our aim was to collect oocytes from the ovaries, another set of collection was performed under the condition that PMSG was administered twice, 48 h apart, but no hCG was given to avoid ovulation. They were euthanized 16 h later, and ovaries were removed. There were numerous follicles with a diameter of 0.3 mm or larger remaining in the ovaries after ovulation, containing non-ovulatory oocytes (Fig. 1a). These oocytes were either tightly adherent cumulus oocyte complex (TCOC) (Fig. 1b) or loosely connective cumulus oocyte complex (LCOC) (Fig. 1c), with the morphology of the latter resembling that of the ovulated eggs (Fig. 1d). Contrary to our assumption, however, hCG administration had no predominant effect on the rate of LCOCs that was considered more matured (Fig. 2a) (PMSG plus hCG: 19.50 ± 14.65 vs. PMSG plus PMSG: 22.84 ± 12.58, p = 0.76). To verify that these collected oocytes have the fertility, we performed IVF of oocytes collected from adult female rats treated with PMSG plus hCG and only PMSG twice. The results showed that all oocytes were fertilizable, although there were some differences in the efficiency (Table 1). The percentage of development that made progress to blastocysts (Fig. 2b) was lower in the group treated with PMSG and hCG than in the group with only PMSG twice, and higher in the LCOCs than in the TCOCs (Fig. 2c,d). Compared with the rate of the ovulated eggs, however, their fertilization rates were generally low (Table 1). These results indicate that even non-ovulatory oocytes matured in rat ovaries have the fertilization ability.
Rat oocytes collected from the ovary after ovulation. (a) Rat ovary after superovulation. Follicles are indicated by the arrows (↑). Scale bar = 1 mm. (b) Tightly adherent cumulus oocyte complex (TCOC). (c) Loosely connective cumulus oocyte complex (LCOC). (d) Eggs collected from oviduct ampulla. Scale bar = 200 μm.
Percentage change of LCOCs depending on hormone administrations and oocyte-blastocysts development rates affected by oocyte conditions. (a) Percentage of LCOCs in collected oocytes after PMSG + hCG and two PMSG administrations. PMSG and hCG: 19.50 ± 14.65, n = 3 (total 44); PMSG twice: 22.84 ± 12.58, n = 4 (total 96), p = 0.76. (b) Blastocysts and extended blastocysts after IVF. Scale bar = 200 μm. (c,d) Percentage of oocyte-blastocyst development affected by PMSG + hCG and two PMSG administrations. PMSG and hCG: LCOC: 11.86 ± 4.76, TCOC: 0.00 ± 0.00, p = 0.01. Two PMSG: LCOC: 27.50 ± 19.84, TCOC: 4.53 ± 4.21, p = 0.12. Data are mean ± SD and obtained Student t-test (a,c,d).
Transplantation of rat ovaries into mice
To demonstrate that immature oocytes in rat ovaries can be matured in mice, we selected nude mice as immunocompromised animals. These mice have often been used as recipients for xenografts, because they are relatively inexpensive and maintained without special sterile facilities. Their ovaries were removed before 5 weeks of age to eliminate the effects of sexual maturity. For ovary transplantation to recipient mice, we used three rat strains: EGFP-expressing SD, GCaMP-expressing Lister Hooded, and wild-type SD and Wistar. The ovaries excised from rats were cut into pieces of about 2 mm square, and 3–4 pieces were transplanted under each kidney capsule of mice. Depending on the size of the ovaries, one rat tissues (2 ovaries) were divided and transplanted into 2–6 mice. (Fig. 3b). The mice transplanted with rat ovaries showed vaginal opening on the average day of 8.01 ± 4.34, which was considered to be a good indicator that viable ovaries started to function (Suppl. Fig. 1), then hormones were administered for follicle development and accumulation.
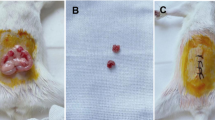
Schematic diagram of hormone administrations for embryo transfer and images of transferred ovarian tissues. (a) Time schedule of three patterns of hormone administrations, IVF and embryo transfer. (b) Rat ovary tissues seen on the mouse kidney immediately after the transfer. (c) Growing follicles (↑) are observed on the transplanted ovary after hormone administrations.
Morphological conditions and fertility of rat oocytes from transplanted ovaries
Three kinds of methods of hormone administration were performed on mice transplanted with ovaries of SD strain wild-type rats as follows (Fig. 3a): (1) 48 h after PMSG (5 IU/mouse) administration, hCG (5 IU/mouse) was given, and 5 h later oocytes were collected from follicles; (2) 48 h after PMSG (5 IU/mouse) administration, the same dose of PMSG was again given, and 21 h later oocytes were collected; (3) Similar to the method (2), 48 h after PMSG (5 IU/mouse), the same dose of PMSG was again given, and 29 h later oocytes were collected. The method (1) was planned to apply general ovulation-inducing procedures for rats to xenotransplantation, but was designed to increase the COC just before ovulation by shortening the time after hCG administration to stimulate ovulation. The methods (2) and (3) were added to suppress ovulation and increase follicles, following the method used to collect oocytes from transplanted ovaries. On examination of the collected oocytes from the follicles (Fig. 3c), however, there were no LCOCs found that were considered more matured in the method (1), and a few LCOCs even in the methods (2) and (3). Therefore, we cultured collected TCOCs for more IVM in medium containing hCG and β-E2, and found the cumulus cells tightly bound to the oocytes were swelling (Fig. 4a,b). These oocytes were given IVF, and the resultant fertilized eggs were transferred into the oviducts of pseudopregnant rats 5–6 h after IVF or 2 days later when development had progressed to the 2-cell stage (Fig. 4c,d). In total, 388 fertilized eggs were implanted into 27 pseudopregnant rats. Two fetuses were observed to form, each from the oocytes treated with the methods (1) and (2), respectively, but failed to be delivered due to malformation (Suppl. Fig. 2a,b). There was only one successful delivery from the oocyte treated with the method (3), but the pup was killed by cannibalism within 24 h. Additionally, there were only 2 implantation scars confirmed (Table 2).
Maturation culture of xenotransplanted oocytes and in vitro fertilized embryos. TCOCs (a) before IVM, and (b) after IVM. (c) Embryos in a pronuclear stage, 5–6 h after IVF; (d) Embryos in a 2-cell stage, 2 days after IVF. (e) Percentage of LCOCs after IVM culture of oocytes collected under three different hormone administration conditions (1–3). (1) PMSG and hCG administered 48 h apart, and oocytes collected 5 h after hCG. 26.70 [17.69, 35.86], n = 4; (2) PMSG administered twice 48 h apart, and oocytes collected 21 h after PMSG. 0.00 [0.00, 9.09], n = 3; (3) PMSG administered twice 48 h apart, and oocytes collected 29 h after PMSG. 0.00 [0.00, 12.77], n = 5, p = 0.07. Data are median and interquartile range and obtained Mann–Whitney U test.
Maturation of oocytes in the transplanted ovary
These results suggested further maturation of the oocytes would be necessary by improving the condition of hormone administration. While examining the various conditions, we noticed that the action of hCG is important in vivo. Since a greater number of LCOCs was collected after IVM in the previous experiment when the PMSG and hCG were administered (Fig. 4e), we extended the time after hCG administration to make maturation further progress in the ovaries. We also stripped cumulus and granulosa cells from the oocytes after IVM in order to accurately evaluate the oocyte maturation. Briefly, 48 h after PMSG administration hCG was dosed to mice implanted with rat ovaries from three strains, Wistar, SD, and Lister Hooded, and 16 h later COCs were collected (Fig. 5a). The average number of the collected COCs was not much different among each strain (Wistar: 10.14 ± 5.16; SD: 10.00 ± 3.32; Lister Hooded: 12.20 ± 5.32; p = 0.32), white the percentage of each LCOC was highest in Wistar, but no significant difference was observed (Fig. 5b). Furthermore, the remaining TCOCs were treated with IVM for 25 h, and more than 60% of the eggs from every strain made progress to the later stages MI–MII (Fig. 5c,d and Table 3).
Improved schedule for hormone administrations, IVM, IVF and transplantation. (a) Schematic schedule of hormone administrations, oocyte harvest, IVM, IVF and oviduct transplantation. (b) Percentage of collected COCs varies according to rat strains. Gray, TCOC; white, LCOC. Wistar: 10.14 ± 5.16; SD: 10.00 ± 3.32; Lister Hooded: 12.20 ± 5.32; p = 0.32. Data are mean ± SD and obtained ANOVA. (c) Percentage of COCs after IVM of TCOCs. White, M1–M2 eggs; gray, GV oocytes; black, degenerated eggs. (d) Eggs in various developmental stages are seen after IVM of TCOC. White arrow, M1; black arrow, GV; white arrowhead, M1–M2; black arrowhead, M2; *, degenerated egg.
Offspring production from transplanted ovary-derived oocytes
Out of the oocytes collected from transplanted ovaries of the three strains, LCOCs were given IVF immediately after the collection and fertilized eggs were implanted to the oviducts of pseudopregnant rats next day, while TCOCs were stripped naked 25 h after IVM to check their condition, followed by IVF and transplantation the next day (Table 3). Their fertilization rates were determined as follows: in Wistar rats, TCOC: 18.87%; LCOC: 21.43%; p = 0.98; in EGFP-SD rats, TCOC: 4.76%; LCOC: 18.18%; p = 0.16; and in GCaMP-Hooded rats, TCOC: 5.88%; LCOC: 36.84%; p = 0.16 with no significant differences between TCOC and LCOC. Other comparisons were also made between three rat strains (LCOC: p = 0.25; LCOC: p = 0.50), with no significant differences (Table 3). About 21 days of gestation period after implantation, those pregnant rats were examined for fetuses from natural delivery, and those with no sign of delivery were given Caesarean section. Rats with fertilized eggs of a Wistar strain transplanted had one implantation scar, but no pups from TCOC; those with EGFP-SD strain eggs had three implantation scars and one pup only from LCOC; and those with GCaMP-Hooded strain eggs had two implantation scars from TCOC, and six implantation scars and three pups, out of which one died within 1 day, from LCOC (Table 3). Out of the three pups obtained by this technique, two derived from a GCaMP-Hooded strain were males retaining a GCaMP gene, developed to adulthood showing healthy reproductive performance, and the retained transgene was transmitted to their offspring (Fig. 6). The rats produced in this study have been living healthily for 10 months and their pups are now 5 months old showing no defects.
Pups from oocytes derived from xenotransplanted ovaries. (a) An SD rat at 1 day of age, (b) LH rats at 1 day of age and (c) LH rats at 23 days of age. (d) Second generation of the LH rat pups. (e) A targeting vector of GCaMP. (f) PCR products from GCaMP-LH parents and their pups. The gene for ovarian donor GCaMP-LH is correctly inherited. M, F0 father rat; F, LH mother rat; TV, targeting vector.
These results show that a critical key to obtaining offspring from transplanted ovary-derived oocytes is to mature the oocytes in the ovary and make as many as oocytes progress to the form of LCOC. In this condition, the eggs can develop to the mid-MI or later stages, and proceed to fertilization. It was also found that implanting these fertilized eggs into the oviducts of pseudopregnant rats leads to the production of offspring. This study has proved that oocytes obtained from transplanted ovaries have the ability to produce gametes.
Discussion
The purpose of this study is to prove that rat oocytes in ovaries transplanted to different species have the ability to produce pups, and to contribute to technological development for the production and maintenance of genetically modified rats. To this end, we obtained the oocytes by transplanting rat ovaries under the renal capsule of mice, and successfully produced rat pups (Fig. 7). Our results demonstrated that ovaries from different species of animals can be used for the production of offspring, when the oocytes are matured under appropriate conditions. This is an important progress in reproductive technology in laboratory animals, livestock production, breeding of rare species, as well as development of reproductive medicine.
Various attempts have been made to utilize ovarian oocytes for the purpose of producing offspring. These techniques have been tried in such cases where a female mother has problems in maturing oocytes, mating, maintaining the pregnancy, and raising the offspring. Although the technique of obtaining oocytes from mouse ovaries implanted subcutaneously or into ovaries of the same species is established6,7, there is only one report of pup production from ovaries implanted in a different species of animal. They obtained pups from the oocytes collected from mouse ovaries that had been transplanted into immunocompromised rats8. It has been considered that the reason for this limited success of just one case is that the gene expression pattern of oocytes matured in xenotransplanted ovaries is different from that of the original oocytes, thus preventing normal development9. In addition, there is a report that even if immature oocytes are fertilized and proceed to egg division, they do not implant and become fetuses11. The oocytes recovered after xenotransplantation had a higher rate of polyspermy due to delayed or dysfunctional zona pellucida reactions. The embryos that became triploid were either malformed or died during development in vivo12.
There has been a vast accumulation of studies about the development process of oocytes and the acquisition of eggs fertilization potential13,14. PMSG contains both FSH and LH activities, and is involved in follicle maturation and accumulation of pre-ovulatory follicles. LH (hCG), on the other hand, is involved in maturation of eggs in follicles and their ovulation. Therefore, the activities of PMSG and LH are combined and used in this study. We confirmed that ovulated ovaries of rats treated under superovulation-inducing conditions still contained oocytes that could be fertilized and embryogenic. Thus, we harvested oocytes from rat ovaries implanted in mice using a combination of three different hormone administration methods and in vitro culture in order to mature the oocytes. The results showed that both the two-time PMSG and the PMSG plus hCG treatments almost failed to collect matured LCOCs, and most of the collected oocytes were in the state of TCOCs. These TCOCs were changed to become fertile by IVM in medium containing hCG and β-E2, but few healthy offspring were obtained (Table 2, Suppl. Fig. 2). In this process, as development from secondary follicles to early follicles proceeds, FSH receptors are expressed in the granulosa cells surrounding the oocyte, triggering ligand-stimulated cAMP-dependent signaling that leads to differentiation into the cell layer lining the follicle membrane (granulosa cells) and the cumulus cells lining the oocyte15. Furthermore, it has been shown that the cumulus cells not only supply energy to the oocyte via gap junctions, but also regulate its meiosis16. When the follicles become large enough, LH receptors are expressed on the granulosa cells, and EGF-like factors synthesized by LH stimulation release meiotic inhibition of the oocyte via the EGF receptor-ERK1/2 system in the cumulus cells. This hormonal stimulation makes the oocyte reach the mid-second meiosis, which is required for the oocyte to obtain the fertilization ability and proceed to subsequent embryo development. In addition, LH stimulation causes the cumulus cells to accumulate extracellular matrices composed mainly of hyaluronan in preparation for ovulation17.
Based on these findings in the literature, we tried to grow the follicles as much as possible in the implanted ovaries, taking longer time after hCG administration to collect LCOCs with swollen cumulus cells. As a result, we succeeded in obtaining pups from embryos artificially inseminated with LCOCs.
Here we have made it clear that in order for transplanted ovary-derived oocytes to be inseminated and develop to offspring, follicles must be developed in the transplanted ovary and the peri-ovarian cumulus cells must be grown to a swollen state (LCOC) just prior to ovulation. We also must wait longer after hCG stimulation to obtain viable oocytes, because oocyte development in xenotransplanted ovaries needs more time compared to normal development in vivo. This may be due to delayed increase in hormone concentration caused by inadequate blood flow to the transplanted tissue. Another reason may be a lack of timely supply of brain-derived hormones, etc. that affect oocyte development. All of these factors contribute to better conditions for oocyte maturation, where fertilization potential can be acquired and subsequent embryonic development occurs via cross-talk with the cumulus cells. Under the IVM conditions tested, we failed to mimic the phenomena that occur within the implanted oocyte (Table 3). If we could make adequate conditions similar to those in vivo such as reduced partial pressure of oxygen18, it may be possible to allow even TCOCs develop without defects.
There have been some attempts to utilize oocytes effectively in the ovaries for decades. Cross and Brister collected fully grown oocytes from mature follicle in mouse ovaries in 1970 and resumed meiosis in vitro to obtain mature oocytes19. Eppig et al. developed a follicle culture system in which developing mouse follicles were cultured for about 2 weeks in a medium containing follicle-stimulating hormone and fetal bovine serum to develop oocytes20. In 1996, they further developed a 2-step method, in which newborn mouse ovaries were cultured in organ culture to grow primordial follicles, followed by follicle culture by isolating fully grown follicles from the cultured ovaries21. Hikabe et al. differentiated mouse iPS cells into primordial germ cell-like cells and succeeded in obtaining offspring using the 2-step method22. These attempts were made with mice, but their culture processes were extremely complicated and time-consuming. In contrast, the ovarian transplant method presented here needs a simple process for obtaining eggs, and is inexpensive. Our study using a mouse as an “incubator” has certain advantage, and suggests possibilities of producing mature oocytes in various mammals in the future.
In this experiment, we were able to produce rat oocytes and pups by transplanting rat ovaries to immunodeficient mice. Thus this technique would make it possible to obtain genetically modified rat oocytes and produce pups from the ovaries of valuable genetically modified rats that have died, if the ovarian tissue can be collected. In other words, it is expected to shorten the time to produce genetically modified animals at a low cost. In addition, since we have successfully harvested oocytes from refrigerated and frozen ovarian tissue (Supplementary Table 1), even ovaries from animals that had died at a remote location could be used, if the ovarian tissue was appropriately transported. These results may provide a new production and maintenance method for genetically modified rats, which can be widely used in medical and pharmaceutical research.
We have recently transplanted primate marmoset ovaries into the kidneys of immunocompromised mice and administered repeated FSH doses to the transplanted mice to grow follicles in the implanted ovaries. Consequently, we successfully made these oocytes develop to blastocysts by maturation culture and in vitro fertilization10. These results suggested that even xenotransplanted tissues can produce oocytes that can develop, if appropriate measures such as proper administration of hormones are taken. Furthermore, we have shown that fertilizable oocytes can be obtained from deceased marmoset ovaries in a previous study. This is more beneficial to the animal welfare than obtaining oocytes from living organisms by over-ovulation. The effective use of ovarian tissue is in the interest of animal welfare.
Materials and methods
Animals
All animal experiments were reviewed and approved by the Animal Welfare Committee and the Ethics Committee of Niigata University (approval number: SA01190), and carried out in accordance with the institutional guiding principles for the care and use of laboratory animals. The study was carried out in compliance with the ARRIVE guidelines (https://arriveguidelines.org).
Rats and mice were housed in plastic cages (polysulfone) in clean racks with 54 ventilation cycles/h in a room temperature of 21–24 °C, humidity of 40–75%, ventilation frequency of about 15 cycles/h, and a 12-h light/dark cycle (light period 7–19 h), and were fed solid feed (Oriental MF for rats and NMF for mice, Oriental Yeast Industry Co., Ltd., Tokyo, Japan) and water ad libitum. Rats were euthanized by cervical cord dislocation after anesthesia with carbon dioxide inhalation, and mice were euthanized by cervical cord dislocation.
Donor rats
Wild-type adult SD and Wistar rats were used. EGFP-expressing transgenic rats of SD strain23 and GCaMP-expressing knock-in Lister Hooded strain rats (unpublished) were also used as genetically modified donor rats. Adult ovaries were harvested from euthanized rats, and their fat and blood were removed at room temperature. The ovaries were placed in an ice-cold tissue preservation solution Lifor (Lifeblood Medical Inc., NJ, USA) dish, and then shredded into approximately 2 mm squares in Lifor for transplantation. A total of 68 rats were used for this study.
Recipient mice
A total of 131 KSN/slc female nude mice, aged 4 weeks, were purchased from Japan SLC Co., Ltd., and used as immunodeficient mice. Before these mice reached 5 weeks of age, bilateral ovariectomies were performed as described10. To explain briefly, the ovary of an anesthetized mouse lying on its side is pulled out of the body and removed using a heat cutter and scissors.
Interspecies ovarian transplantation
Three to four weeks after the ovary removal, the mice, aged 8 weeks or over, were given a mixture of three anesthetics10. They were placed on their side, and the flank skin was incised about 1.0 cm checking the bulge of the kidney, followed by opening of the fascia, to one central side of which a 3.0 thread (Polysorb, Covidien, MS, USA) was applied. The thread was pulled to widen the opening and expose the kidney carefully so that no damage was made on the kidney. A small hole was made using tweezers in the center of the kidney capsule, and a glass capillary with a rounded tip was inserted through the hole to peel off the capsule and create a pocket. A total of three to four shredded rat ovary fragments were inserted into the pocket, then the kidney was placed back into the body with special care so that no pressure was applied on the kidney, and the fascia was sutured. While the skin was autoclipped, the other kidney was given ovary transplantation in the same way, then antagonist drugs were administered, and the animal was awakened on a heating plate.
Follicle development and oocyte retrieval from ovaries
For retrieval of rat oocytes directly from rat ovaries, rats were administered 300 IU/kg each of PMSG (Serotropin, ASKA Pharmaceutical, Tokyo, Japan) and hCG (gonatropin 3000, ASKA Pharmaceutical, Tokyo, Japan) or PMSG and PMSG with a 48 h interval. The ovaries were removed 16 h later, and placed in a POE-CM solution (Functional Peptide Institute, Yamagata, Japan) containing 10 IU/ml sodium heparin (Mochida Pharmaceutical, Tokyo, Japan). Follicles that had developed to over 0.3 mm in diameter were broken with tweezers and a 26 G needle, and a mixture of oocytes and cumulus cells was collected. The mixture was washed 3 times with mHTF (Transgenic, Fukuoka, Japan) for later use.
Meanwhile, for retrieval of rat oocytes from the mice implanted with rat ovarian fragments, we checked vaginal opening in the mice as an indicator of successful implantation. This phenomenon indicates that the implanted rat ovaries start to function (Supplemental Fig. 1). With the appearance of vaginal opening, hormone administration was performed; 48 h after the administration of 5 IU PMSG, 5 IU hCG or 5 IU PMSG was intraperitoneally administered. Five, 16, 21 and 29 h after the hormone administration, the mice were euthanized, and their kidneys were quickly removed. Follicles that developed to larger than 0.3 mm on the transplanted ovaries were collected and incised in a solution of 2 ml POE-CM containing 10 IU/ml sodium heparin, and the contents were washed three times with mHTF for later use.
In vitro maturation
Some of the collected oocytes were cultured for maturation on base medium of MEMα (12571063, Thermo Fisher Scientific, MA, USA) supplemented with 3 mg/ml BSA (Albumin, bovine, F-V, Nacalai Tesque, Inc., Kyoto, Japan), 0.1 μg/ml of β-E2 (β-Estradiol, FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) or/and 10 IU/ml hCG, under 5% CO2 and 95% air at 37 °C, for 0, 19, 23 and 47 h. After IVM, the eggs were stripped by using 0.1% hyaluronidase to remove cumulus and granulosa cells surrounding the eggs, and used for IVF.
Sperm collection and IVF
SD or Wistar male rats were euthanized and promptly incised at the center of the right and left testes to remove cauda epididymis. After blood and fat were wiped out, incisions were made with ophthalmic scissors, and while pressure was being applied with tweezers, emerging spermatozoa were collected. The collected sperm were placed in mHTF and incubated in 5% CO2 at 37 °C for 5 min. After the sperm spread was visually checked, pre-culture was started and continued for 1 h or 4–5 h. The mixture of oocytes and cumulus cells from rat ovaries or the matured eggs were fertilized with pre-cultured sperm 1–5 × 105/ml for 4–5 h in mHTF medium. For in vitro embryogenesis, the medium was changed to rKSOM (Transgenic, Fukuoka, Japan) every 48 h after IVF. The total number of male rats used was 38.
Transfer of fertilized eggs to recipient rats
SD or Wistar female rats aged over 8 weeks were used as recipients. Their estrus cycles were checked for about 2 weeks prior to transplantation. They were mated with vasectomized male rats, and female with plug were used for transplantation. Pronuclear-stage embryos with male and female nuclei confirmed 5–6 h after insemination were transferred to the recipient rats, while two-cell stage embryos were transferred on the morning of the second day after IVF. The recipient rats were intraperitoneally given a mixture of three anesthetics, after CO2 absorption. They were laid on their side, with flank hair clipped, skin and fascia cut open, ovarian fat body drawn out and fixed with a cramp. Then the ovarian side of the ampulla of uterine tube was pinched with tweezers and punctured with a 30G needle to make a small hole, into which aspirated embryos with a glass capillary tube were transferred. After successful embryo transfer was confirmed by air bubbles appearing in the end of the tube in the fallopian tube, the fascia was sutured, the skin autoclipped, antagonist drugs administered, and the animals were awaked on a heating plate.
Statistical processing
EZR (Easy R, Jichi Medical University), version 1.6124 was used. For comparison between the two groups, the student t-test and the Mann–Whitney U test were used for statistical processing. For comparison between the three groups, the one-way ANOVA was employed, and multiple comparisons were performed using the Bonferroni method. A p value < 0.05 was considered a significant difference.
Data availability
All data are available from the corresponding authors upon reasonable request.
References
Petrich, B. G. et al. c-Jun N-terminal kinase activation mediates downregulation of connexin43 in cardiomyocytes. Circ. Res. 91, 640–647 (2002).
Raja, M. A., Maldonado, M., Chen, J., Zhong, Y. & Gu, J. Development and evaluation of curcumin encapsulated self-assembled nanoparticles as potential remedial treatment for PCOS in a female rat model. Int. J. Nanomed. 16, 6231–6247 (2021).
Kaneko, T. Reproductive technologies for the generation and maintenance of valuable animal strains. J. Reprod. Dev. 64, 209–215 (2018).
Lee, D. M. et al. Live birth after ovarian tissue transplant. Nature 428, 137–138 (2004).
Youm, H. W. et al. Transplantation of mouse ovarian tissue: Comparison of the transplantation sites. Theriogenology 83, 854–861 (2015).
Gunasena, K. T., Villines, P. M., Critser, E. S. & Critser, J. K. Live births after autologous transplant of cryopreserved mouse ovaries. Hum. Reprod. Oxf. Engl. 12, 101–106 (1997).
Meirow, D. et al. Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: Endocrine studies, in vitro fertilization cycles, and live birth. Fertil. Steril. 87(418), e7-418.e15 (2007).
Snow, M., Cox, S.-L., Jenkin, G., Trounson, A. & Shaw, J. Generation of live young from xenografted mouse ovaries. Science 297, 2227–2227 (2002).
Agca, C., Lucy, M. C. & Agca, Y. Gene expression profile of rat ovarian tissue following xenotransplantation into immune-deficient mice. Reprod. Camb. Engl. 137, 957–967 (2009).
Hirayama, R. et al. Production of marmoset eggs and embryos from xenotransplanted ovary tissues. Sci. Rep. 13, 18196 (2023).
Noyes, R. W. Fertilization of follicular ova. Fertil. Steril. 3, 1–12 (1952).
Yamazaki, W., Takahashi, M. & Kawahara, M. Restricted development of mouse triploid fetuses with disorganized expression of imprinted genes. Zygote 23, 874–884 (2015).
Matzuk, M. M., Burns, K. H., Viveiros, M. M. & Eppig, J. J. Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science 296, 2178–2180 (2002).
Hamazaki, N. et al. Reconstitution of the oocyte transcriptional network with transcription factors. Nature 589, 264–269 (2021).
Hunzicker-Dunn, M. & Maizels, E. T. FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cell. Signal. 18, 1351–1359 (2006).
Sela-Abramovich, S., Chorev, E., Galiani, D. & Dekel, N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146, 1236–1244 (2005).
Sen, A. & Caiazza, F. Oocyte maturation: A story of arrest and release. Front. Biosci. Sch. Ed. 5, 451–477 (2013).
Shimamoto, S. et al. Hypoxia induces the dormant state in oocytes through expression of Foxo3. Proc. Natl. Acad. Sci. U.S.A. 116, 12321–12326 (2019).
Cross, P. C. & Brinster, R. L. In vitro development of mouse oocytes. Biol. Reprod. 3, 298–307 (1970).
Eppig, J. J. & Schroeder, A. C. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol. Reprod. 41, 268–276 (1989).
Eppig, J. J. & O’Brien, M. J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 54, 197–207 (1996).
Hikabe, O. et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 539, 299–303 (2016).
Ito, T., Suzuki, A., Imai, E., Okabe, M. & Hori, M. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J. Am. Soc. Nephrol. JASN 12, 2625–2635 (2001).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Acknowledgements
We express our deepest condolences to one of the authors, Dr. E. Nakatsukasa, who passed away during the progress of this study. We thank Mss. M. Obata, N. Ishimoto, K. Hayasaka and Yabe, I. for technical assistance. We are grateful to Prof. T. Sasaoka for the employment of H. Taketsuru with his AMED JP23dm0207091h0005 research fund. This work was financially supported by AMED JP23dm0207091h0005 (to E.N., M.A. and K.S.), JSPS KAKENHI Grant Number JP 16H06276 (AdAMS) (to K.S. and M.A.), 18KK0458 (to M.A.), and JST SPRING, Grant Number JPMJSP2145 (to R.H. and K.T.).
Author information
Authors and Affiliations
Contributions
H.T., R.H. and E.N. designed and performed the experiments. R.N. provided technical assistance. R.H., E.N. M.A. and K.S. conceived the study and analyzed the results. H.T., R.H., K.T. and K.S. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Taketsuru, H., Hirayama, R., Nakatsukasa, E. et al. Generation of rat offspring from ovarian oocytes by xenotransplantation. Sci Rep 14, 20109 (2024). https://doi.org/10.1038/s41598-024-71030-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71030-0