Abstract
The extra domain B splice variant of fibronectin (EDB-FN), which is overexpressed in several cancers, is an approved diagnostic and therapeutic target of cancers. The aim of this study was to evaluate the EDB-FN-targeting peptide EDBp as a noninvasive imaging modality for molecular imaging of breast cancer in mice. Western blot, flow cytometry and immunofluorescence were used to assess the expression level of EDB-FN and its binding to EDRp in MCF7, SKBR3, 4T1, EMT6, MDA-MB-231 and MDA-MB-453 cells. Establishment MDA-MB-231-luc cells-based subcutaneous tumor model mice or pulmonary metastasis model mice. The EDRp molecular probes to perform fluorescent probes for near-infrared fluorescence (NIRF)·and PET imaging of model mice. Our results demonstrate that EDBp-Cy5 had a strong binding ability to the MDA-MB-231 cells and exhibited specific tumor accumulation in MDA-MB-231 subcutaneous and pulmonary metastasis model mice. Importantly, the EDBp peptide-based radiotracer [18F]-AlF-NOTA-EDBp provided excellent diagnostic value for positron emission tomography (PET) imaging of breast cancer, especially in subcutaneous model mice. The uptake of [18F]-AlF-NOTA-EDBp in subcutaneous tumors (6.53 ± 0.89%, ID/g) was unexpectedly higher than that in the kidney (4.96 ± 0.20, %ID/g). The high tumor uptake of these probes in mice suggests their potential for application in imaging of EDB-FN-positive breast cancer for disease staging of regional and distant metastases.
Similar content being viewed by others
Introduction
According to the latest data released by the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO), breast cancer has replaced lung cancer as the most common cancer in the world1. Factors such as population aging, delayed childbearing, and fewer births are contributing to the continuing rise in breast cancer cases2. Although the gold standard for the diagnosis of breast cancer is pathological assessment3, which requires pathological tissue obtained by puncture or surgical biopsy, it is an invasive diagnostic method that can easily cause pathogenic microbial infection, damage to the surrounding tissues and bleeding4. Therefore, noninvasive imaging techniques are often carried out before pathological assessment. At present, ultrasound, mammography and magnetic resonance imaging (MRI) are the main clinical diagnostic techniques for breast cancer5. For these techniques, the American College of Radiology reached a consensus and established a Breast Imaging Reporting and Data System (BI-RADS) classification, and as a result, biopsy is considered only if the patient reaches level 4 or above6. Moreover, molecular imaging technology such as 2-deoxy-2-[18F]fluoro-D-glucose[18F]F-FDG) positron emission tomography (PET) can provide real-time monitoring of tumor functional molecular processes6. Therefore, molecular imaging may be the frontier of breast cancer diagnosis.
The extra domain B splice variant of fibronectin (EDB-FN) is an extracellular matrix (ECM) protein deposited by tumor-associated fibroblasts and is associated with tumor growth, angiogenesis, and invasion7,8. EDB-FN, as a tumor-associated ECM protein, is overexpressed in breast cancer9,10, prostate cancer11,12, small cell lung cancer13, and colorectal cancer14. An EDB-FN-targeted peptide(ZD2), Cys-Thr-Val-Arg-Thr-Ser-Ala-Asp-Cys, was initially reported by Lu et al.12. Lu et al. used phage display to screen cyclic heptapeptides that specifically bind to EDB-FN and designed fluorescent probes for near-infrared fluorescence (NIRF) imaging of subcutaneous prostate cancer model mice12. ZD2 was developed for imaging prostate12,15, breast16,17, pancreatic18 and hepatocellular19 cancers. Coincidentally, the amino acid sequence of ZD2 was reported by Feng et al. in an earlier study20. In that study, ZD2 was also identified by phage display screening of the triple-negative breast cancer cell line MDA-MB-23120 Therefore, ZD2, as a EDB-FN-targeted peptide, can be used as an important tool for the molecular imaging of breast cancer.
Recently, Feng et al. optimized ZD2 using alanine scanning technology to increase its binding affinity to EDB-FN from 4.8 µM to 14 nM. The optimized peptide was named EDBp (Ala-Val-Arg-Thr-Ser-Ala-Asp), and its NIRF and PET molecular probes were applied to the molecular imaging of thyroid cancer21. In this study, we first examined the expression levels of EDB-FN in several types of breast cancer cells and their binding to EDBp. Next, we used EDBp molecular probes to perform NIRF and PET imaging of subcutaneous breast cancer model mice and pulmonary metastases model mice.
Results
Design of probe structure
EDBp was connected to NIRF dye Cy5 and radiolabeled chelating agents NOTA by PEG4-Lys as linker to form the EDB-FN-targeted probes EDBp-Cy5 and EDBp-NOTA (Fig. 1). They were synthesized and analyzed through high-performance liquid chromatography (HPLC) and mass spectrometry (MS) (See Supplementary Fig. S1 online).
Binding of EDBp to breast cancer cells
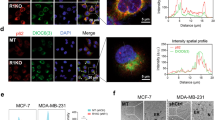
Before investigating the binding ability, we first used western blotting to examine the expression levels of EDB-FN in several breast cancer cells, including MCF7, SK-BR-3, 4T1, EMT6, MDA-MB-231 and MDA-MB-453 cells. High expression of EDB-FN was observed in EMT6 cells, medium expression in MDA-MB-231 cells, and low expression in MCF7, SK-BR-3, 4T1 and MDA-MB-453 cells (Fig. 2a). Then, we used flow cytometry to test the binding ability of EDBp-Cy5 to these breast cancer cells. Similar to the different expression levels of EDB-FN, the cell binding ability of EDBp-Cy5 was high in MDA-MB-231 cells, moderate in MCF7 and EMT6 cells and low in SK-BR-3, 4T1 and MDA-MB-453 cells (Fig. 2b). Furthermore, we used confocal microscopy to scan the fluorescence images, which showed that the EDBp-Cy5 peptide colocalized with EDB-FN in MDA-MB-231 cells (Fig. 2c).
Binding of EDB-FN targeted peptide EDBp. (a) Western blot cropping plot of EDB-FN in breast cancer cell lines. Original blots are presented in Supplementary Fig. S2. (b) Flow cytometry determined the binding between EDBp-Cy5 and breast cancer cell lines. (c) Cellular fluorescence imaging of MDA-MB-231 cells incubated with EDBp-Cy5 at 37 °C for 2 h. Blue, DAPI; red, EDBp-Cy5; green, EDB-FN.
NIRF imaging of EDBp-Cy5
To verify the tumor homing of EDBp-Cy5, MDA-MB-231 cells was injected to form subcutaneous EDB-FN-positive breast cancer model mice and pulmonary metastasis model mice. Then, EDBp-Cy5 was injected into mice through the tail vein. Forty-eight hours later, EDBp-Cy5 was significantly enriched in the subcutaneous tumor, while the uptake in the other organs was low (Fig. 3a and b). At the end of the imaging experiment, the mice were euthanized, and the main organs and tumors were collected and analyzed. EDBp-Cy5 was observed mainly in the kidney and subcutaneous tumor (Fig. 3c-e). For the pulmonary metastases model mice, luminescence images showed that tumor cells had expanded in the lungs 3 weeks after tail vein injection (Fig. 4a). Similar to the subcutaneous tumors, the red fluorescence of EDBp-Cy5 and luminescence of MDA-MB-231-luc cells showed strong colocalization in pulmonary metastases (Fig. 4b). When the organs of mice were collected, the red fluorescence of EDBp-Cy5 in the pulmonary metastases colocalized with the luminescence of MDA-MB-231-luc cells (Fig. 4c-e). Taken together, the results in the MDA-MB-231-luc subcutaneous tumor and pulmonary metastasis model mice further verified the specific tumor homing of EDBp-Cy5 to breast cancer cells.
Tumor-specific homing of EDBp-Cy5 in subcutaneous MDA-MB-231-luc breast cancer model mice. Luminescence imaging (a) and NIRF imaging (b) of the subcutaneous tumor model mice 48 h after injection with EDBp-Cy5 showing tumor-specific homing. Luminescence imaging (c) and NIRF imaging (d) of the subcutaneous tumor model mice 48 h after injection with EDBp-Cy5 showing the tumor and normal organs. (e) Semiquantitative analysis of the fluorescence intensity in the tumor and normal organs, n = 3.
Tumor-specific homing of EDBp-Cy5 in MDA-MB-231-luc breast cancer pulmonary metastasis model mice. Luminescence imaging (a) and NIRF imaging (b) of pulmonary metastasis model mice 48 h after injection with EDBp-Cy5 showing tumor-specific homing. Luminescence imaging (c) and NIRF imaging (d) of the pulmonary metastases model mice 48 h after injection with EDBp-Cy5 showing the pulmonary metastases and normal organs. (e) Semiquantitative analysis of the fluorescence intensity in the pulmonary metastases and normal organs, n = 3.
Radiolabeling and stability of [18F]-AlF-NOTA-EDBp
The radiochemical yield of [18F]-AlF-NOTA-EDBp was approximately 30%, and the radiochemical purity of [18F]-AlF-NOTA-EDBp was > 99% by radio-HPLC (see Supplementary Fig. S3 online). The in vitro stability of [18F]-AlF-NOTA-EDBp was assessed before performing animal studies [18F]-AlF-NOTA-EDBp incubation with FBS at 37 °C for 2 h was still stable, and the purity is > 99% (See Supplementary Fig. S4 online).
PET/CT imaging in subcutaneous tumor model mice
Before the PET/CT scanning, cell uptake was performed to evaluate the affinity of [18F]-AlF-NOTA-EDBp to the MDA-MB-231 cells [18F]-AlF-NOTA-EDBp exhibited significant radioactivity uptake in MDA-MB-231 cells, moderate in EMT6 cells and low in 4T1 cells (See Supplementary Fig. S5 online). This result was similar to the flow cytometry of EDBp-Cy5.
In the subcutaneous MDA-MB-231-luc breast cancer model mice, dynamic PET/CT scanning was acquired after [18F]-AlF-NOTA-EDBp injection. Muscle uptake was consistently low, and tumor uptake was higher than kidney at 5 min continued until the end of the dynamic scanning (Fig. 5a). Static PET/CT imaging showed that [18F]-AlF-NOTA-EDBp was highly accumulated in the tumor, kidney and bladder after 2 h of in vivo circulation (Fig. 5b). In the three-dimensional images [18F]-AlF-NOTA-EDBp exhibited excellent tumor enrichment, and the signal intensity of [18F]-AlF-NOTA-EDBp within the subcutaneous tumor was commensurate with that observed within the kidney. (See Supplementary Fig. S6 online). Subsequently, the mice were euthanized, and the main organs and tumors were collected. The percentage of injected dose per gram (%ID/g) in subcutaneous tumors and normal organs were calculated by a γ counter. Quantification indicated that the tissue uptake of [18F]-AlF-NOTA-EDBp was highest in the tumor (6.53 ± 0.89%, ID/g), which was higher than that in the kidney (4.96 ± 0.20, %ID/g) and liver (1.39 ± 0.12%ID/g) (n = 3) (Fig. 5c). The tumor was subsequently subjected to HE staining and IHC. IHC images showed that EDB-FN was highly expressed in the subcutaneous MDA-MB-231 tumors (Fig. 5d). In the blocking group treated with 500 µg of EDBp-NOTA, no discernible uptake of [18F]-AlF-NOTA-EDBp was observed in the subcutaneous MDA-MB-231 tumors (Fig. 6a). Additionally, the [18F]-AlF-NOTA-EDBp exhibited negligible uptake in the subcutaneous SKBR3 tumors characterized by low-expression of EDB-FN (Fig. 6b).
PET/CT imaging with [18F]-AlF-NOTA-EDBp in MDA-MB-231-luc breast cancer subcutaneous tumor model mice. (a) Representative dynamic PET scanning in the subcutaneous tumor model mice after injection of 3.7 MBq of [18F]-AlF-NOTA-EDBp. Representative dynamic time activity curves were drawn for kidney, muscle and tumor uptake, n = 3. (b) Representative PET/CT imaging of subcutaneous tumor model mice after 2 h injection. (c) The biological distribution of [18F]-AlF-NOTA-EDBp in the tumor and normal organs, n = 3. (d) HE and IHC images of tumors isolated from the subcutaneous tumor model mice. The expression of EDN-FN was analyzed by IHC. Scale bar, 50 μm (left) and 10 μm (right).
PET/CT imaging with [18F]-AlF-NOTA-EDBp in breast cancer subcutaneous tumor model mice. (a) Representative PET/CT images of MDA-MB-231-luc subcutaneous tumor model mice 2 h after injection of 3.7 MBq of [18F]-AlF-NOTA-EDBp, with or without the unlabeled EDBp-NOTA peptide. (b) Representative PET/CT images of SKBR3 (EDB-FN low-expression) subcutaneous tumor model mice 2 h after injection of 3.7 MBq of [18F]-AlF-NOTA-EDBp.
PET/CT imaging in Pulmonary metastases model mice
In the MDA-MB-231-luc breast cancer pulmonary metastasis model mice, PET/CT imaging showed that [18F]-AlF-NOTA-EDBp moderately accumulated in the pulmonary metastases 2 h post intravenous injection (Fig. 7a). The %ID/g in pulmonary metastases was 2.92 ± 0.10%ID/g (Fig. 7b). HE staining confirmed the presence of pulmonary metastases in the lung. IHC images showed relatively high expression of EDB-FN in pulmonary metastases compared to adjacent lung tissues (Fig. 7c).
PET/CT imaging with [18F]-AlF-NOTA-EDBp in MDA-MB-231-luc breast cancer pulmonary metastasis model mice. (a) Representative PET/CT imaging of pulmonary metastases model mice after injection of 3.7 MBq of [18F]-AlF-NOTA-EDBp. (b) The biological distribution of [18F]-AlF-NOTA-EDBp in the pulmonary metastases (lung) and normal organs, n = 3. (c) HE and IHC images of pulmonary metastases isolated from pulmonary metastases model mice. The expression of EDN-FN was analyzed by IHC. Scale bar, 50 μm (left) and 10 μm (right).
Discussion
The use of BI-RADS has several limitations of varying clinical importance. For example, the definition of the “regional distribution” of an abnormal finding varies between mammography and MRI22. Therefore, it is necessary to explore cancer biomarkers that reflect tumor information at the cellular and subcellular levels long before anatomical analysis23 [18F]F-FDG is the most widely used molecular imaging radiotracer for the diagnosis, staging and treatment monitoring of tumors24, including Hodgkin and non-Hodgkin lymphomas25, melanoma26, colorectal cancer27, breast cancer28 and lung cancer29. Based on the available clinical data on breast cancer [18F]F-FDG imaging is substantially beneficial for patients with staging starting from clinical stage IIB and possibly useful value to patients with clinical stage IIA (T1N1 or T2N0) but is not helpful for patients with clinical stage I (T1N0)30. In addition, FDG metabolism reflects the energy metabolism of the body, so the malignant area can be easily confused with inflammation and local infection31. Estrogen receptor (ER) is highly expressed in 70% of breast cancers and plays a central role in prognosis and treatment selection for patients with breast cancer. The ER-targeted radiotracers 16α-[18F]-fluoro-17β-fluoroestradiol [18F]-FES) were approved by the Food and Drug Administration (FDA) in 2020 as diagnostic agents for patients with recurrent or metastatic breast cancer. The limitations of [Deliv Re18F]-FES are that ER-negative tumors are unlikely to be detected and false-positives may also occur32,33.
In addition to the small molecular compounds FDG and FES, peptides are often used as molecular imaging radiotracers in breast cancer, and most of these peptides are identified by phage display34,35,36. Compared to common small molecule compounds, peptides have higher activity and selectivity and have fewer side effects on the human body because their metabolites are amino acids37,38. Compared with antibodies, peptides have the advantages of suitable stability, high purity, low production cost, and low immunogenicity39. Peptides can also improve the affinity, solubility, pharmacokinetic properties, stability and toxicity of drug candidates through chemical modification to support the rapid screening of drug candidates40. The integrin αvβ3-targeted radiotracer Arg-Gly-Asp (RGD) peptide is widely used in PET, single-photon emission computed tomography (SPECT), MRI and ultrasound imaging of breast cancer31. Moreover, integrin α6-targeted radiotracers, such as the CRWYDENAC peptide, have potential value in the diagnosis, staging and prognosis of human breast cancer10. However, in RGD or CRWYDENAC imaging of breast cancer patients, the maximum standardized uptake value (SUVmax) was not as large as that in preclinical mouse trials41. Moreover, the neuropilin-1-targeted peptide CLKADKAKC20 and the sodium pump Na/K ATPase a1 subunit-targeted peptide CSISSLTHC42 showed suitable tumor targeting in the imaging of triple-negative breast cancer-bearing mice, but their effect on patients is unpredictable. Therefore, peptides are an excellent drug form for the diagnosis of breast cancer, but it is necessary to explore more molecular probes that monitor receptors that are overexpressed on breast cancer cells.
EDBp was optimized from the classical EDB-FN-targeting peptide ZD2 by alanine scanning, and its affinity with EDB-FN substantially increased from 4.8 µM to 14 nM21. PET/CT imaging showed that the radiotracer18F]-AlF-NOTA-EDBp had high accumulation in bilateral primary thyroid lesions and bone metastases in two patients with thyroid cancer21. Since the preoptimized ZD2 had a suitable tumor imaging effect on breast cancer16,17, we applied the optimized EDBp to the molecular imaging diagnosis of breast cancer.
Due to its exceptional fluorescence properties, Cy5 is extensively utilized in biolabeling and fluorescence imaging techniques. For instance, it can specifically bind to biological molecules like proteins and peptides, enabling it detecting by advanced devices such as fluorescence microscope or flow cytometer. The longer fluorescent wavelength of Cy7 and ICG make them more suitable for NIRF imaging but limit their application in fluorescence microscopy and flow cytometry, thereby partially restricting their use in early cell studies. In this study, we initially confirmed the tight binding of EDBp-Cy5 to MDA-MB-231 breast cancer cells through both fluorescence microscopy and flow cytometry. (Fig. 2b and c). Subsequently, EDBp-Cy5 was used to detect the breast cancer, including subcutaneous tumor and pulmonary metastasis model mice. EDBp-Cy5 guided the complete position of tumors by NIRF imaging (Figs. 3 and 4). Therefore, EDBp-Cy5 can be used for surgical navigation, which was beneficial for completely removing pulmonary metastasis. Furthermore, the PET tracer18F]-AlF-NOTA-EDBp provided excellent diagnostic value for PET/CT imaging of breast cancer, especially in subcutaneous tumors. The uptake of18F]-AlF-NOTA-EDBp in subcutaneous tumors (6.53 ± 0.89%, ID/g) was unexpectedly higher than that in the kidney (4.96 ± 0.20, %ID/g), which is the main elimination organ of the peptide (Fig. 5c). This phenomenon, in which tumor uptake was higher than in the kidney, was hard-won in most radiotracers, including small molecular compounds, peptides and antibodies. We also acknowledge that the uptake of18F]-AlF-NOTA-EDBp in pulmonary metastases was not as high as that in subcutaneous tumors (Fig. 7a), which may be due to the relatively weaker blood supply to the lungs.
Materials and methods
Cell culture
Breast cancer cell lines, including MCF7, SKBR3, 4T1, EMT6, MDA-MB-231 and MDA-MB-453, were obtained from the cell bank of the State Key Laboratory of Oncology in South China. Cells were grown in aseptic conditions at 37 °C and 5% CO2. Cells were cultured in DMEM medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 µg/mL streptomycin. Cells were regularly authenticated and confirmed to be mycoplasma-negative. To facilitate tumor monitoring, MDA-MB-231 cells were stably transfected with luciferase using a lentivirus (OBiO Technology).
Protein extraction and western blotting
Breast cancer cells were washed with phosphate-buffered saline (PBS), and total proteins were isolated using RIPA lysis buffer (Beyotime, #P0013B). The total protein concentrations were determined by the Bradford assay (Thermo Fisher, #23225). The proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes, which were then incubated with anti-EDB-FN monoclonal antibody (1:1000, Abcam, #ab268021) overnight at 4 °C. Finally, goat anti-rabbit IgG HRP antibody (1:10000, Thermo Fisher, #G-21234) was used as the secondary antibody.
Synthesis of the peptides
All peptides involved in this study were synthesized in China Peptide Company which provided complete quality control reports. The NIRF peptide EDBp-Cy5 (chemical structure: AVRTSAD-PEG4-K-Cy5) and CG7C-Cy5 (chemical structure: CGGGGGGGC-Cy5) and the PET precursor peptide EDBp-NOTA (chemical structure: AVRTSAD-PEG4-K-NOTA) were designed by Feng et al.21.
Flow cytometry
To investigate the binding of peptide EDBp-Cy5 to cells, cells were seeded at a density of 1 × 106 cells/ml in a 6-well plate and then stained with 1 µM EDBp-Cy5 or CG7C-Cy5 peptides at 37 °C. After incubating for 60 min, the cells were washed with PBS three times and analyzed on a CytoFLEX S cytometer.
Immunofluorescence
To investigate the binding of peptide EDBp-Cy5 to cells, cells were seeded at a density of 1 × 105 cells/ml in a cell culture dish, which is designed for confocal microscopy (NEST, #801001) and added to 1 µM EDBp-Cy5 peptide. After 2 h incubation at 37 °C, the samples were washed with PBST six times, fixed with 4% paraformaldehyde, incubated with PBS containing 0.25% Triton X-100 (Sigma-Aldrich, #V900502) and blocked with 5% BSA for 30 min. Antibody incubation was performed with anti-EDB-FN monoclonal antibody (1:1000, Abcam, #ab268021) overnight at 4 °C. To detect fluorescence, the samples were incubated with goat anti-rabbit Alexa Fluor 488 secondary antibody (Thermo Fisher, #A-11008) at a concentration of 4 µg/mL in 1% BSA for 1 h at room temperature in the dark. Cell nuclei were stained with DAPI in ProLong Gold Antifade Mountant (Thermo Fisher, #P36930). Finally, fluorescence images were visualized and captured by a confocal microscopy (Olympus FV1000, Japan).
Animal models
The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Guangdong Pharmaceutical University (NO.gdpulac2020215). This study was conducted in adherence to the ARRIVE guidelines. All mice were purchased from Guangdong Medical Laboratory Animal Center (Guangdong, China). To obtain subcutaneous tumor model mice, 6-week-old female BALB/c-nude mice were subcutaneously inoculated with 1 × 106 MDA-MB-231-luc or SKBR3 cells. In order to improve the success rate of subcutaneous tumor model mice, the vehicle of the tumor cell injections was 100 µL PBS supplemented with 30% matrigel (Corning, #354234). Similarly, for pulmonary metastasis model mice, 6-week-old female BALB/c-nude mice were intravenously inoculated with 1 × 106 MDA-MB-231-luc cells. When MDA-MB-231-luc cells could be observed in the lung by luminescence imaging approximately 3 weeks later, the pulmonary metastases mouse model was considered successfully constructed.
NIRF imaging
NIRF imaging was performed using an in vivo imaging system (IVIS) Spectrum (PerkinElmer, USA) at 48 h after 1 µmol EDBp-Cy5 was intravenously injected into subcutaneous tumor model mice or pulmonary metastasis model mice. NIRF images were acquired with excitation at 640 nm and emission at 680 nm, and luminescence images were obtained starting 10 min after intraperitoneal injection of 150 mg/kg luciferin (Promega, #P1041). After NIRF imaging, the mice were euthanized by cervical dislocation and immediately dissected. Luminescence and NIRF signals from the vital organs and tumors were recorded.
Synthesis of18F]-AlF-NOTA-EDBp
The PET precursor peptides EDBp-NOTA (50 µg) and AlCl3·6H2O (3 µg) were previously freeze-dried in the reaction vial as a lyophilized kit18. F] ion (radioactivity concentration: 1400 MBq/mL) come from China Isotope and Radiation Corporation. Ethanol (330 µL), acetic acid (5 µL) and18F] ion (85 MBq, 65 µL) were added to the lyophilized kit. The mixture was heated at 100 °C for 10 min and cooled to room temperature. Subsequently, the mixture was diluted to 10 mL, passed through a C18 light column (Waters Sep-Pak, #WAT023501) and eluted with ethanol (400 µL) after washing with sterilized water (10 mL). The eluted solution was evaporated with nitrogen, and final product was dissolved with saline before injection.
Cell uptake study of18F]-AlF-NOTA-EDBp
A total of 1 × 106 4T1, EMT6 and MDA-MB-231 cells were added into 200 µL of PBS separately (n = 4). The cells were incubated with 370 kBq18F]-AlF-NOTA-EDBp at 37 °C for 1 h before washed with 1 mL of cold PBS three times. The radioactivity of each sample was measured with a γ-counter. The affinity of the18F]-AlF-NOTA-EDBp to cells was calculated as the percentage of total added dose per 106 cells (%AD/106 cell).
PET/CT imaging
Once mice were intravenously injected with approximately 3.7 MBq of18F]-AlF-NOTA-EDBp, dynamic PET/CT scanning (small animal PET/CT, Albira II, Bruker) was performed. Static PET/CT scanning was performed after 1–2 h of in vivo circulation. For the blocking experiment, the blocking group received an injection of 500 µg of EDBp-NOTA 10 min prior to the administration of18F]-AlF-NOTA-EDBp. Medical imaging data PMOD analysis software was utilized to analyze the enrichment intensity of18F]-AlF-NOTA-EDBp in the tumor.
Biodistribution
To investigate the biodistribution of18F]-AlF-NOTA-EDBp in vivo, the mice were euthanized and immediately dissected after PET/CT imaging. The normal organs and tumors were weighed and measured by a γ counter. Radioactivity was expressed as a percentage of the injected dose per gram (%ID/g).
Hematoxylin and Eosin (HE) staining and immunohistochemistry (IHC)
Tumors and pulmonary metastases from model mice were carefully dissected, fixed with formalin, and embedded in paraffin. Paraffin blocks were cut into 3 mm sections. One of the sections was stained with HE according to routine histological procedures. One of the sections was used for IHC with an anti-EDB-FN monoclonal antibody (1:1000, Abcam, #ab268021). IHC was performed following the conventional procedure as previously reported21. Finally, images were visualized and captured by a confocal microscopy confocal scanning system (Olympus FV1000, Japan).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 6.0 software. Statistical evaluations were performed using one-way ANOVA, and the results are shown as the mean ± standard deviation (SD). Statistical analyses of the survival experiments were performed using log-rank tests. p values are denoted as follows: ns, p > 0.05; *p < 0.05; **p < 0.01; and ***p < 0.001.
Conclusion
In this study, we provided an EDB-FN-targeted peptide for the noninvasive molecular imaging of breast cancer. The high tumor uptake of these tracers in mice bearing EDB-FN-positive breast cancer suggests its potential for application in NIRF and PET imaging of EDB-FN-positive breast cancer, which may improve the disease staging of regional and distant metastases and relapse monitoring.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- BI-RADS:
-
Breast Imaging Reporting and Data System
- [18F]F-FDG:
-
2-deoxy-2-[18F]fluoro-D-glucose
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- EDB-FN:
-
Extra domain B splice variant of fibronectin
- IARC:
-
International Agency for Research on Cancer
- WHO:
-
World Health Organization
- MRI:
-
Magnetic resonance imaging
- ECM:
-
Extracellular matrix
- NIRF:
-
Near-infrared fluorescence
- PBS:
-
Phosphate-buffered saline
- HPLC:
-
High-performance liquid chromatography
- MS:
-
Mass spectrometry
- IACUC:
-
Institutional Animal Care and Use Committee
- Cy5:
-
Cyanine5
- NOTA:
-
1,4,7-triazacyclononane-1,4,7-triacetic acid
- IVIS:
-
In vivo imaging system
- HE:
-
Hematoxylin and eosin
- IHC:
-
Immunohistochemistry
- SD:
-
Standard deviation
- [18F]-FES:
-
16α-[18F]-fluoro-17β-fluoroestradiol
- FDA:
-
Food and Drug Administration
- RGD:
-
Arg-Gly-Asp
- SPECT:
-
Single-photon emission computed tomography
- SUVmax:
-
Maximum standardized uptake value
- ABD:
-
Albumin binding domain
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021).
Soerjomataram, I. & Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol.18, 663–672 (2021).
Yerushalmi, R., Woods, R., Ravdin, P. M., Hayes, M. M. & Gelmon, K. A. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol.11, 174–183 (2010).
Asdourian, M. S. et al. Precautions for breast cancer-related lymphoedema: Risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol.17, e392–405 (2016).
McDonald, E. S., Clark, A. S., Tchou, J., Zhang, P. & Freedman, G. M. Clinical diagnosis and management of breast Cancer. J. Nucl. Med.57 (Suppl 1), 9S–16S (2016).
Sedgwick, E. L. et al. BI-RADS update for breast cancer caregivers. Breast Cancer Res. Treat.150, 243–254 (2015).
Menrad, A. & D Menssen, H. ED-B fibronectin as a target for antibody-based cancer treatments. Expert Opin. Ther. Targets. 9, 491–500 (2005).
Ronca, R., Sozzani, S., Presta, M. & Alessi, P. Delivering cytokines at tumor site: The immunocytokine-conjugated anti-EDB-fibronectin antibody case. Immunobiology. 214, 800–810 (2009).
D’Ovidio, M. C. et al. Intratumoral microvessel density and expression of ED-A/ED-B sequences of fibronectin in breast carcinoma. Eur. J. Cancer. 34, 1081–1085 (1998).
Matsumoto, E., Yoshida, T., Kawarada, Y. & Sakakura, T. Expression of fibronectin isoforms in human breast tissue: Production of extra domain A+/extra domain B + by cancer cells and extra domain A + by stromal cells. Jpn J. Cancer Res.90, 320–325 (1999).
Petrini, I. et al. ED-B fibronectin expression is a marker of epithelial-mesenchymal transition in translational oncology. Oncotarget. 8, 4914–4921 (2017).
Han, Z. et al. EDB Fibronectin specific peptide for prostate cancer targeting. Bioconjug. Chem.26, 830–838 (2015).
Salge-Bartels, U., Heiden, M., Seitz, R. & Gieseler, F. PO-18 - Fibronectin EDA/EDB is expressed in adherent SCLC NCI-H69 cells and in pleural effusions of lung cancer patients: Possible implication for drug resistance. Thromb. Res.140 (Suppl), S182–S183 (2016).
Pujuguet, P. et al. Expression of fibronectin ED-A + and ED-B + isoforms by human and experimental colorectal cancer. Contribution of cancer cells and tumor-associated myofibroblasts. Am. J. Pathol.148, 579–592 (1996).
Ayat, N. R. et al. Optimization of ZD2 peptide targeted Gd(HP-DO3A) for detection and risk-stratification of prostate cancer with MRI. ACS Med. Chem. Lett.9, 730–735 (2018).
Ye, X. X. et al. EDB Fibronectin-Specific SPECT Probe (99m)Tc-HYNIC-ZD2 for breast cancer detection. ACS Omega. 2, 2459–2468 (2017).
Zhang, L. et al. ZD2-Engineered Gold Nanostar@Metal-Organic Framework Nanoprobes for T(1) -Weighted magnetic resonance imaging and photothermal therapy specifically toward triple-negative breast Cancer. Adv. Healthc. Mater.7, e1801144 (2018).
Gao, S. et al. Synthesis and assessment of ZD2-((68)Ga-NOTA) specific to extradomain B fibronectin in tumor microenvironment for PET imaging of pancreatic cancer. Am. J. Nucl. Med. Mol. Imaging. 9, 216–229 (2019).
Sergeeva, O. et al. PET imaging of hepatocellular carcinoma using ZD2-((68)Ga-NOTA). J. Hepatocell Carcinoma. 10, 291–301 (2023).
Feng, G. K. et al. SPECT and near-infrared fluorescence imaging of breast cancer with a neuropilin-1-targeting peptide. J. Control Release. 192, 236–242 (2014).
Li, R. et al. EDB-FN targeted probes for the surgical navigation, radionuclide imaging, and therapy of thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging. https://doi.org/10.1007/s00259-023-06147-x (2023).
Eghtedari, M., Chong, A., Rakow-Penner, R. & Ojeda-Fournier, H. Current status and future of BI-RADS in multimodality imaging, from the AJR special series on radiology reporting and data systems. AJR Am. J. Roentgenol.216, 860–873 (2021).
Lee, S. Y., Jeon, S. I., Jung, S., Chung, I. J. & Ahn, C. H. Targeted multimodal imaging modalities. Adv. Drug Deliv. Rev.76, 60–78 (2014).
Kahle, X. U. et al. Review molecular imaging in lymphoma beyond 18F-FDG-PET: Understanding the biology and its implications for diagnostics and therapy. Lancet Haematol., 7, e479–e489 (2020).
Barrington, S. F. & Kluge, R. FDG PET for therapy monitoring in Hodgkin and non-hodgkin lymphomas. Eur. J. Nucl. Med. Mol. Imaging. 44, 97–110 (2017).
Ayati, N. et al. The value of (18)F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging. 48, 428–448 (2021).
Mirshahvalad, S. A. et al. Diagnostic performance of [(18)F]-FDG PET/MR in evaluating colorectal cancer: a systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging. 49, 4205–4217 (2022).
Avril, S. et al. 18F-FDG PET/C for monitoring of treatment a response in breast B cancer. J. Nucl. Med.57 (Suppl 1), 34S–9S (2016).
Vaz, S. C. et al. Joint EANM/SNMMI/ESTRO practice recommendations for the use of 2-[(18)F]FDG PET/CT external beam radiation treatment planning in lung cancer V1.0. Eur. J. Nucl. Med. Mol. Imaging. 49, 1386–1406 (2022).
Groheux, D. & Hindie, E. Breast cancer: Initial workup and staging with FDG PET / CT. Clin. Transl Imaging. 9, 221–231 (2021).
Chakravarty, R., Chakraborty, S. & Dash, A. Molecular imaging of breast cancer: Role of RGD peptides. Mini Rev. Med. Chem.15, 1073–1094 (2015).
Katzenellenbogen, J. A. The quest for improving the management of breast cancer by functional imaging: the discovery and development of 16α-[(18)F]fluoroestradiol (FES), a PET radiotracer for the estrogen receptor, a historical review. Nucl. Med. Biol.92, 24–37 (2021).
Ulaner, G. A. 16α-18F-fluoro-17β-Fluoroestradiol (FES): Clinical applications for patients with breast Cancer. Semin Nucl. Med.52, 574–583 (2022).
Melo, C. M. et al. The Heparan sulfate binding peptide in tumor progression of triple-negative breast cancer. Front. Oncol.11, 697626 (2021).
Leung, K. (99m)Tc-Diamine dioxime-lys-cys-arg-gly-asp-cyc-phe-cys-polyethylene glycol (2004).
Larimer, B. M. & Deutscher, S. L. Development of a peptide by phage display for SPECT imaging of resistance-susceptible breast cancer. Am. J. Nucl. Med. Mol. Imaging. 4, 435–447 (2014).
Shi, M. & McHugh, K. J. Strategies for overcoming protein and peptide instability in biodegradable drug delivery systems. Adv. Drug Deliv. Rev.199, 114904 (2023).
Varanko, A., Saha, S. & Chilkoti, A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv. Drug Deliv. Rev.156, 133–187 (2020).
Sun, X. et al. Peptide-based imaging agents for cancer detection. Adv. Drug Deliv. Rev.110–111, 38–51 (2017).
Wang, W. & Hu, Z. Targeting peptide-based probes for molecular imaging and diagnosis. Adv. Mater.31, e1804827 (2019).
Yoon, H. J. et al. Correlation of breast cancer subtypes, based on estrogen receptor, progesterone receptor, and HER2, with functional imaging parameters from 68Ga-RGD PET/CT and 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 41, 1534–1543 (2014).
Wang, Q. et al. Identification of a sodium pump na(+)/K(+) ATPase α1-targeted peptide for PET imaging of breast cancer. J. Control Release off J. Control Release Soc.281, 178–188 (2018).
Acknowledgements
We thank G.F., X. Y. and Q. W. for their excellent technical assistance.
Funding
This work has been supported by grants from Guangzhou Basic and Applied Basic Research Project (202201010663).
Author information
Authors and Affiliations
Contributions
Y.Z.: Writing-original drafts, Conceptualization, Funding acquisition. X.Z.: Writing – review & editing, Validation.Yanfang Huang: Writing – review & editing, Methodology. S.L.: Software, Data curation. X.L.: Writing – review & editing, Methodology, Formal analysis. L.Z.: Writing – review & editing , Resources, Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Zheng, X., Huang, Y. et al. EDB-FN-targeted probes for near infrared fluorescent imaging and positron emission tomography imaging of breast cancer in mice. Sci Rep 14, 22056 (2024). https://doi.org/10.1038/s41598-024-73362-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73362-3