Abstract
The purpose of this study is to objectively assess by polysomnography total sleep time and sleep macrostructure in glaucomatous versus non-glaucomatous individuals after adjusting for possible confounding factors affecting the quality of sleep. This is an observational, prospective, single-center, case-controlled study using a sleep research database (MARS e-Cohort) collecting clinical data, comorbidities, medications, and sleep studies of glaucomatous and non-glaucomatous individuals. The diagnosis of glaucoma was confirmed with a full comprehensive ophthalmological examination including a visual field test. Total sleep time and the main sleep parameters (time spent in stages 1, 2, 3 and 4, microarousals, apnea–hypopnea index, and indices of hypoxic burden) were compared in glaucomatous and non-glaucomatous individuals after adjusting for age, anthropometric data, and comorbidities. The study included 33 glaucomatous and 66 non-glaucomatous individuals. The median total sleep time was 325 min [273; 398] for the control group and 311 min [244; 349] for the glaucoma group. After adjusting for the potential confounding factors, there was no significant difference in total sleep time (p = 0.3) and other sleep parameters between the control group and the glaucoma group. The sleep macrostructure was comparable in the glaucomatous and non-glaucomatous individuals after careful adjustment for confounders.
Similar content being viewed by others
Introduction
Glaucoma is one of the most frequent causes of irreversible blindness, and is characterized by the progressive loss of retinal ganglion cells whose axons form the optic nerve1,2,3,4. Most of the retinal ganglion cells transmit image-forming visual information to the brain. A small percentage of retinal ganglion cells, termed “melanopsin-expressing retinal ganglion cells”, do not directly contribute to vision but are photosensitive and constitute the retinohypothalamic tract involved in circadian rhythms5. Thus, glaucoma affects vision but it may also impact the retinohypothalamic pathway informing the suprachiasmatic nuclei that synchronize nychthemeral rhythms6,7,8, with an impact on the secretion of melatonin by the pineal gland9.
It has therefore been suggested that glaucoma could affect sleep structure and quality. Data in the literature are scarce and not consistent regarding sleep quality and architecture in glaucoma patients4. Existing data are either from small case series lacking a control group or from studies based on questionnaires without objective assessment of sleep parameters by polysomnography10,11.
In this study, we used a large prospective research database collecting anthropometric data, comorbidities, medications, and sleep studies of thousands of people, including glaucomatous and non-glaucomatous individuals, to compare the sleep macrostructure of glaucomatous and non-glaucomatous individuals with appropriate adjustment for confounders.
Methods
Study design and participants
The MARS (Multimorbidity Apnea Respiratory failure Sleep) database was designed by the Grenoble Alpes University Hospital Sleep Laboratory and prospectively collects electronic medical records including anthropometric data, Epworth Sleepiness Scale score, Pichot Fatigue Scale score, Pichot Depression Scale score, comorbidities (ICD-10), and medication data (with ATC codes) of thousands of individuals referred for suspicion of sleep disorders. To date, the MARS database has received all the legal authorizations required for its use and scientific exploitation (CCTIRS: Request for advice No. 15.925bis, on 03/23/2016; CNIL: Declaration of conformity to the reference methodology MR003 No. 1996650v0 on 05/10/2016). This database is also managed in compliance with the General Data Protection Regulation (GDPR) legislation of the European Union. All individuals provided written informed consent.
We compared the data of patients with primary open-angle glaucoma (POAG) and healthy controls. The diagnosis of open-angle glaucoma was defined as an intraocular pressure (IOP) of > 21 mmHg during diurnal measurement with or without glaucoma medications, open angle on gonioscopy, consecutive and reliable abnormal standard automated perimetry results with abnormal results on the Glaucoma Hemifield Test and Pattern Standard Deviation outside 95% of normal limits, and optic nerve damage (asymmetric cup/disc ratio > 0.2, rim thinning, notching, excavation, or retinal nerve fiber layer defect). We used the Hodapp–Parrish–Anderson classification12 to classify glaucoma according to severity (early, moderate, and advanced). A reliable visual field test was defined as having a < 20% rate of fixation losses and < 15% false-positive and false-negative results. Exclusion criteria were other forms of glaucoma, including normal-tension glaucoma and pseudoexfoliative glaucoma, as well as non-glaucomatous ocular disease other than cataract.
Non-glaucomatous adults had no ophthalmological history, with a normal ophthalmological examination conducted less than 2 years before the polysomnographic examination, apart from minor refractive disorders (myopia with spherical equivalent less than − 6 diopters or hyperopia with spherical equivalent less than + 3 diopters).
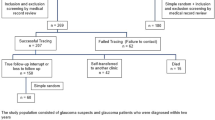
The study flowchart is shown in Fig. 1.
Polysomnography recordings from the MARS database
All individuals included in the MARS database underwent at least one full overnight polysomnographic examination. Continuous recordings were taken with the electrode positions C3/A2-C4/A1-Cz/01 of the International 10–20 System of Electrode Placement measuring eye movements, a chin electromyogram, and an ECG with a modified V2 lead. Sleep was scored manually according to standard criteria13. Air flow was measured on the basis of nasal pressure associated with the sum of buccal and nasal thermistor signals. Respiratory effort was monitored with abdominal and thoracic bands. Oxygen saturation was measured using a pulse oximeter.
Other data recorded in the database included anthropometric data, history, medications, clinical signs that suggest a sleep disorder including reference questionnaires in the field (Epworth Sleepiness Scale (ESS), Pichot Fatigue and Pichot Depression Scale), clinical examination (heart rate, respiratory rate, blood pressure).
Ophthalmic examinations
The patients in the glaucoma arm underwent a full ophthalmic examination including keratometry (Nidek tonoref II, Nidek Co. Ltd., Tokyo, Japan), corneal pachymetry (Cirrus HD-OCT 5000–18541, Carl Zeiss Meditec, Jena, Germany), visual acuity (logMAR) and applanation IOP measurements, slit-lamp examination with gonioscopy, fundus examination with cup/disc ratio, visual field test with a Humphrey Field Analyzer (model 745i, Carl Zeiss Meditec, Jena, Germany), and optical coherence tomography (Cirrus HD-OCT 5000–18541, Carl Zeiss Meditec, Jena, Germany) with measurement of the retinal nerve fiber layer thickness and macular ganglion cell density. Glaucoma evaluation was performed after selection of cases from the MARS e-Cohort. The delay between polysomnography and glaucoma evaluation varied from a few weeks to a few years and was not reported.
The non-glaucomatous individuals were interviewed by telephone to assess whether they had undergone an ophthalmic examination with normal results in the 2 years preceding the polysomnographic examination and to collect the summary of the ophthalmological examination. Those without ophthalmological examinations in the previous 2 years and/or with missing ophthalmological data were not included in the study.
Data were collected after obtaining patient consent except in case of declining participation by the patient or their relatives if unable to decline in person (IMAGEYE cohort, non-interventional study implicating the human person). This study complied with the declaration of Helsinki guidelines for research involving human subjects. All study participants provided written informed consent for conducting all ophthalmologic examinations and using anonymized data for clinical research purpose.
Statistical analysis
A baseline description of patient characteristics was completed by reporting median values with interquartile range (IQR) for quantitative data and frequency and percentage for categorical variables.
We paired two controls for one glaucoma patient. Patients were matched on age, sex, and apnea–hypopnea index (to exclude the impact of associated sleep apnea) measured during diagnostic polysomnography.
A comparison between cases (glaucomatous patients) and controls (non-glaucomatous individuals) was then performed through a chi-square test for the qualitative variables and a non-parametric Wilcoxon test for the quantitative variables.
As few missing values were observed (< 5%), a simple imputation method was used to account for them (including BMI, physical exercise, fatigue score, depression score). Missing values were replaced by the median for quantitative variables and with the most frequent values for qualitative variables. Variables for which the percentage of missing data observed was greater than 20% were not retained for analysis.
Univariable analyses were performed using a generalized linear regression model to identify factors associated with total sleep time (TST) and the other sleep parameters. Variables with a p value ≤ 20% were included in the multivariable analysis. As the multivariable model was explanative, no final variable selection process was performed. Statistical analysis was performed using SAS (V.9.4, SAS Institute). A p-value threshold of 0.05 was considered significant.
Results
Study population
A total of 99 individuals were finally included, 33 glaucomatous patients and 66 non-glaucomatous controls (Fig. 1). The demographic and medical characteristics of the patients with glaucoma and the controls are summarized in Table 1. After matching, there were no significant differences between the two groups for all the demographic and medical characteristics.
Ophthalmological characteristics of the patients with glaucoma
All the patients had POAG, with 18 cases of early glaucoma, 5 cases of moderate glaucoma, and 10 cases of advanced glaucoma. The worse eye was considered for the staging of glaucoma. Data are summarized in Table 2.
Sleep-related symptoms and objective sleep assessment by polysomnography
The two groups did not differ in terms of sleep-related symptoms (Table 3).
The objective sleep measurements are displayed in Table 4. The median TST was 311 min [244; 349] for the patients with glaucoma and 325 min [273; 398] for the controls. The difference was not statistically significant (p = 0.12).
For all the other parameters studied, the differences between the two groups were not significant, particularly for sleep time in stage 1, sleep time in stage 2, slow-wave sleep, REM sleep, microarousal index, cumulative time spent below 90% of SaO2, and apnea hypopnea index.
These initial analyses provided the trends for univariate and multivariate analysis.
Univariable and multivariable analysis of the predictors of sleep quality
Univariable analysis was performed using a generalized linear regression model to identify factors associated with TST. The results showed that glaucoma was not associated with TST (p = 0.16).
The selected variables at a threshold of 20% presented in Table 5 were included in the multivariable analysis. After adjustment, multivariable analysis showed no significant association between any of the variables and TST (including glaucoma with p = 0.3) except for the ESS score, which was significantly associated with TST (p = 0.02).
Discussion
We used a prospective research database to compare the symptoms and objectively measured sleep parameters of glaucoma patients and non-glaucoma controls. Classic confounders affecting sleep (i.e., gender, age, BMI, sleep habits, and sleep disorders) were accounted for and matched between the two groups. We did not find any significant differences in sleep complaints, TST, and the main sleep architecture parameters objectively measured by polysomnography.
Data in the literature are scarce and not consistent regarding sleep quality and sleep architecture in glaucoma patients4. The existing data are either from small case series lacking a control group or from studies based on questionnaires without objective assessment of sleep parameters by polysomnography10,11.
In 30 patients with POAG and 10 controls, Gracitelli et al. investigated sleepiness using the Epworth Sleepiness Scale (ESS)14 and conducted a polysomnographic evaluation15. The mean ESS score of the glaucoma patients was significantly higher compared with the control group (13.1 ± 5.1 and 9.1 ± 3.7, p = 0.029). The correlations between the ESS score and the main polysomnographic data were moderate to weak. The polysomnographic parameters of the two groups were not displayed in their study, and the matching between the two groups was limited.
In another publication, Gracitelli et al. reported the polysomnographic examination results of 32 patients with POAG and 13 controls16. Patients with glaucoma had significantly lower average values for TST, sleep efficiency, and minimum oxyhemoglobin saturation compared with the healthy controls (p = 0.008, p = 0.002, and p = 0.028, respectively). Patients with glaucoma had significantly higher Wake time After Sleep Onset (WASO) and higher burden of periodic leg movements associated with arousals (p = 0.002 and p = 0.045, respectively). The TST was 370 ± 55 min in healthy controls versus 334 ± 56 min in patients with glaucoma. The hypothesis given by the authors was that the damage to the intrinsically photosensitive retinal ganglion cells caused by glaucoma decreases their input synchronization and could lead to sleep disorders. Confounders that might explain the differences were poorly accounted for in these two studies.
Based on the Pittsburgh Sleep Quality Index (PSQI) questionnaire17, three studies suggested that there was a greater association between glaucoma and sleep disorders than previously reported.
Wang et al. used the PSQI in their study of 99 patients with POAG, 55 with primary angle-closure glaucoma (PACG), and 210 heathy controls10. The proportion of individuals with sleep disturbances (PSQI score > 7) was significantly higher in the POAG and PACG groups than in the controls in three sub-groups classified according to age (33.3% vs. 10.4% in the age group of 18–40, 48.4% vs. 23.4% in the age group of 41–60, 62% vs. 31% in the age group of 61–80). The PSQI significantly decreased with age in healthy controls and POAG patients. In the two groups of glaucoma patients, no significant associations were found between the PSQI and the severity of visual field impairments. These studies, however, lacked an objective measurement of sleep parameters by polysomnography.
Ayaki et al. also used the PSQI in their study of 69 patients with various types of glaucoma and 71 heathy controls18. Only patients with advanced glaucoma (MD ≤ − 12 dB in the worse eye) had significantly worse PSQI scores than the controls (p < 0.05). In multivariate analysis, PSQI was significantly correlated with the mean deviation in the worse eye, the number and frequency of medications, and anxiety and depression scores after adjusting for age and sex. Depression, which was included in the matching in our study, is a major confounder for sleep and probably explained their results.
Ma et al. studied 80 PACG patients, 120 POAG patients, and 120 healthy controls19. They also found a significantly worse sleep score in the two glaucoma groups compared with the controls, and a worse score in the PACG group compared with the POAG group. Similarly to the aforementioned study, self-rated anxiety and depression were significantly higher in the two groups of glaucoma patients compared with the controls, and could explain the differences in the subjective evaluations of sleep quality. Furthermore, the glaucoma patients were older than the controls (mean age of 62 and 50 years in the PACG and POAG groups, respectively, vs. 48 years in the healthy controls).
Several factors could explain the differences between our study and the studies performed to date. Compared to the only study that reports the results of full objective polysomnographic examinations for POAG and healthy controls16, the individuals in our study were significantly older (mean age of 69.5 and 72 years in the control group and glaucoma group, respectively, vs. 56.8 and 61.5 in the study by Gracitelli et al.). Many studies have demonstrated a strong negative correlation between age, melatonin secretion, TST, and sleep quality19. This could have led to a lower TST in the control groups, and may have reduced the relative difference between the glaucoma and control groups. Also, our patients had significantly less advanced glaucoma than those included in Gracitelli and colleagues’ study. The median, first and third quartiles of MD with standard automated perimetry were − 2.5, − 0.2, and − 10.1 dB, respectively, in our study whereas the mean and range MD with standard automated perimetry were − 9.6 and + 2.5–32.3 dB in the Gracitelli et al. study. The studies using subjective sleep scores suggested a relationship between the severity of glaucoma visual field impairments and sleep quality. The fact that we had mostly patients with early glaucoma and only a few patients with moderate and advanced glaucoma could be a limitation of our study and could have contributed to the lack of differences in sleep macrostructure compared with healthy controls. Regarding the other studies performed with sleep quality questionnaires, the comparison of our results with those of these other studies that were performed using subjective sleep quality scores is most likely less relevant because these studies did not report on objective sleep parameters. They reported altered sleep quality and more prevalent sleep disorders in glaucoma patients compared to controls; however, the reported higher depression and anxiety in the glaucoma groups could have impacted the subjective evaluation of sleep, and therefore represent a potential major bias. Studies have shown that clinical depression and depression disorders could have a greater impact on patients’ self-reported sleep quality than on objective polysomnographic evaluations of sleep quality20.
Another limitation of our study could be that we did not perform a full ophthalmic examination of the controls in the hospital ophthalmology department. However, we carefully checked that all the individuals included in the analysis had undergone an ophthalmological examination in the 2 years preceding the polysomnographic examination that excluded the presence of glaucoma. Only data from individuals with minor refractive errors were included in the analysis. The possibility of having controls with undiagnosed glaucoma therefore seems very unlikely.
In conclusion, we compared the sleep structure of glaucomatous and non-glaucomatous individuals using a full objective polysomnographic examination and by controlling for any possible confounding factors that can affect the quality of sleep. We found no significant differences in TST and in all the other sleep parameters studied. The two groups comprised mostly older adults, and most of the glaucoma patients had early glaucoma. A similar study could be performed in a group of patients with moderate and advanced glaucoma.
Data availability
Data supporting the results of this study are available from CHU Grenoble-Alpes upon request (contact Prof. C. CHIQUET, cchiquet@chu-grenoble.fr) and with CHU Grenoble-Alpes permission.
References
Weinreb, R. N., Aung, T. & Medeiros, F. A. The pathophysiology and Treatment of Glaucoma. JAMA. 311, 1901–1911 (2014).
Resnikoff, S. et al. Global data on visual impairment in the year 2002. Bull. World Health Organ. 9 (2004).
Quigley, H. A. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 80, 389–393 (1996).
Gracitelli, C. P. B. & Paranhos, A. Jr. Can glaucoma affect sleep quality? Arq. Bras. Oftalmol 78 (2015).
Provencio, I. et al. A novel human opsin in the inner retina. J. Neurosci. Off J. Soc. Neurosci. 20, 600–605 (2000).
Li, R. S. et al. Melanopsin-expressing retinal ganglion cells are more Injury-Resistant in a chronic ocular hypertension model. Invest. Ophthalmol. Vis. Sci. 47, 2951–2958 (2006).
Feigl, B., Mattes, D., Thomas, R. & Zele, A. J. Intrinsically photosensitive (Melanopsin) retinal ganglion cell function in Glaucoma. Invest. Ophthalmol. Vis. Sci. 52, 4362–4367 (2011).
Obara, E. A., Hannibal, J., Heegaard, S. & Fahrenkrug, J. Loss of Melanopsin-expressing retinal ganglion cells in severely staged Glaucoma patients. Invest. Ophthalmol. Vis. Sci. 57, 4661–4667 (2016).
Møller, M. & Baeres, F. M. The anatomy and innervation of the mammalian pineal gland. Cell. Tissue Res. 309, 139–150 (2002).
Wang, H., Zhang, Y., Ding, J. & Wang, N. Changes in the circadian rhythm in patients with primary Glaucoma. PLOS ONE. 8, e62841 (2013).
Ra, S., Ayaki, M., Yuki, K., Tsubota, K. & Negishi, K. Dry eye, sleep quality, and mood status in glaucoma patients receiving prostaglandin monotherapy were comparable with those in non-glaucoma subjects. PLoS ONE. 12, e0188534 (2017).
Hodapp, E., Parrish, R. K. & Anderson, D. R. Clinical Decisions in Glaucoma (Mosby, 1993).
Berry, R. B. et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated events. Deliberations of the Sleep Apnea definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep. Med. JCSM off Publ Am. Acad. Sleep. Med. 8, 597–619 (2012).
Johns, M. W. A New Method for Measuring Daytime Sleepiness: the Epworth Sleepiness Scale. Sleep. 14, 540–545 (1991).
Gracitelli, C. P. B. et al. Relationship between Daytime Sleepiness and Intrinsically Photosensitive Retinal Ganglion Cells in Glaucomatous Disease. J. Ophthalmol. 5317371 (2016). (2016).
Gracitelli, C. P. B. et al. Intrinsically photosensitive retinal ganglion cell activity is Associated with decreased sleep quality in patients with Glaucoma. Ophthalmology. 122, 1139–1148 (2015).
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
Ayaki, M., Shiba, D., Negishi, K. & Tsubota, K. Depressed visual field and mood are associated with sleep disorder in glaucoma patients. Sci. Rep. 6, 25699 (2016).
Ma, X. P., Shen, M. Y., Shen, G. L., Qi, Q. R. & Sun, X. H. Melatonin concentrations in serum of primary glaucoma patients. Int. J. Ophthalmol. 11, 1337–1341 (2018).
Alvaro, P. K., Roberts, R. M. & Harris, J. K. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and Depression. Sleep. 36, 1059–1068 (2013).
Acknowledgements
The MARS database received all the legal authorizations required for its use and scientific exploitation (CCTIRS: Request for advice No. 15.925bis, on 03/23/2016; CNIL: Declaration of conformity to the reference methodology MR003 No. 1996650v0 on 05/10/2016). This database is also managed in compliance with the General Data Protection Regulation (GDPR) legislation of the European Union. Written informed consent was obtained from all participants.
Author information
Authors and Affiliations
Contributions
LB, RH, CC, FA, JLP, SB wrote the main manuscript. JLP, RT, SB, and THVN use the database and have done statistical analysis. All authors reviewed the manuscript. JLP and CC have designed the study. LB and RH contributed equally to this work. Christophe Chiquet is the corresponding author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bec, L., Herber, R., Bailly, S. et al. Sleep quality in glaucoma patients. Sci Rep 14, 25593 (2024). https://doi.org/10.1038/s41598-024-76368-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76368-z