Abstract
The objective of this study was to ascertain the relationship between the weight-adjusted waist index (WWI) and the risk of stroke in an elderly hypertensive population, a relationship that has not been previously elucidated. The Cox regression model was employed to assess the correlation between baseline WWI measurements and the incidence of stroke. To further elucidate the shape of the association between baseline WWI and stroke, restricted cubic splines were employed. Furthermore, subgroup analyses and interaction tests were carried out to investigate potential heterogeneities. Our study cohort comprised 4962 hypertensive individuals aged 60 years or older with no prior history of stroke. Over a median follow-up of 3.2 years, we found 547 new-onset stroke cases. After adjusting for confounding variables, the Cox regression analysis revealed a positive association between baseline WWI and the risk of stroke, with hazard ratios (HRs) escalating progressively as WWI values increased. When compared to the lowest quartile of WWI, the highest quartile demonstrated an HR of 1.87 (95% CI, 1.44–2.42) for stroke. Subgroup analyses confirmed the consistency of this relationship across different demographic and clinical strata. The study findings indicate that an elevated WWI is significantly related with a higher risk of new-onset stroke among elderly patients with hypertension. These results underscore the importance of WWI as a potential risk stratification tool. To confirm these results and explore the causal mechanisms behind the observed correlation, more study is necessary.
Similar content being viewed by others
Introduction
Stroke is a pervasive and devastating condition, constituting the second most frequent cause of mortality and a major source of disability across the globe. In 2019, stroke was responsible for approximately 11.6% of all deaths worldwide1,2. Furthermore, the incidence of stroke is observed to be rising in parallel with the ongoing process of global population aging3. The incidence of stroke in China is disproportionately high, with nearly 4 million new cases reported in 2019. This figure underscores stroke’s status as a leading cause of disability and mortality within the country, highlighting the critical need for proactive public health measures4. According to the Global Burden of Disease 2019 estimate, stroke caused a significant social and economic burden on society in China alone5. The most efficacious strategy for curtailing the incidence of stroke lies in primary prevention measures6. Consequently, to address the escalating burden of stroke, it is imperative to enhance our understanding of the modifiable risk factors linked to this condition. The development of targeted intervention strategies based on these factors is vital. Such an approach will not only contribute to the improvement of clinical practices but also inform public health initiatives aimed at prevention.
Obesity is a prominent public health issue that poses a significant challenge globally, with established correlations to a multitude of non-communicable diseases7,8,9,10,11. Traditional metrics for obesity assessment, such as waist circumference (WC) and body mass index (BMI), are cost-effective, straightforward, and widely accessible12,13. However, both BMI and WC have limitations. Specifically, BMI struggles to distinguish between lean muscle mass and fat, limiting its effectiveness14,15. Additionally, studies have shown that there are sex- and age-specific differences between WC and body fat ratio13,16,17,18. In response to these limitations, Park et al. proposed the weight-adjusted waist index (WWI) in 2018, an innovative and simple anthropometric index for assessing visceral obesity19. The WWI is calculated by dividing WC by the square root of body weight, which allows it to reflect both fat and muscle mass components. Unlike BMI, which only considers weight and height, WWI provides a more comprehensive assessment of an individual’s body type and fat distribution by combining waist circumference with height and weight19. The WWI offers a nuanced measure of body composition by taking into account not only WC but also the square root of body weight. This calculation provides a more holistic view of an individual’s body fat distribution and muscle mass. Unlike the BMI, which can be misleading as it does not differentiate between fat and muscle, WWI offers a clearer distinction by considering both waist circumference and weight. This approach helps to mitigate the obesity paradox, a phenomenon where individuals with higher muscle mass, which is denser than fat, might be incorrectly categorized as having a higher health risk due to a higher BMI. By differentiating between fat and muscle mass, WWI allows for a more accurate assessment of health risks associated with obesity, particularly in the context of cardiovascular diseases19,20. The correlation between the WWI and health outcomes has become a critical area of focus within clinical research21,22,23,24. Specifically, in China, a positive correlation between WWI and the risk of all-cause and cardiovascular mortality has been established, underscoring the importance of this metric in predicting clinical outcomes21. Furthermore, in the United States, a higher WWI has been linked to an increased prevalence of gallstones, highlighting its potential role in assessing risk for a broad spectrum of diseases24. The relationship between WWI and bone health has also been elucidated, with studies demonstrating a strong association with bone mineral density and the risk of osteoporosis, emphasizing the skeletal implications of increased body fat distribution22,23. Given the substantial correlations between WWI and a myriad of diseases, there is a clear imperative for intensified scrutiny and further investigation.
Moreover, the geriatric population is often characterized by the confluence of hypertension and metabolic derangements in muscle and fat, which are known to substantially elevate the risk of stroke25,26,27. Despite the recognized impact of obesity on stroke risk, empirical evidence linking WWI specifically to stroke incidence, particularly within the elderly hypertensive cohort, remains scarce. This knowledge gap is of notable clinical importance, as hypertension is a major risk factor for stroke, and the development of effective preventative strategies is contingent upon a comprehensive understanding of the associated risk factors. Accordingly, the primary objective of this study was to explore and elucidate the association between WWI and the incidence of stroke within a well-defined, population-based cohort of elderly hypertensive patients. This investigation aims to fill the existing void in the literature and contribute to the evidence base that informs clinical decision-making and public health policy. By examining the relationship between WWI and stroke risk in this high-risk group, we aim to provide novel insights that could potentially inform targeted interventions and risk reduction strategies. The findings of this study may pave the way for more personalized preventative approaches and enhance the clinical management of stroke risk in elderly hypertensive individuals.
Materials and methods
Study population
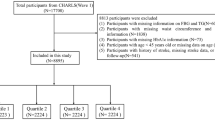
As previously delineated, the participant cohort for this study was derived from the People’s Hospital of Xinjiang Uygur Autonomous Region, as referenced in citation28,29. In this cohort study, all participants were recruited between 1 January 2010 and 31 December 2021 and all had to be at least 60 years of age. The initial cohort comprised 8031 elderly patients with hypertension. We applied the following exclusion criteria: prior stroke (n = 517), loss to follow-up or follow-up time less than six months (n = 874), lack of anthropometric data (n = 330), and presence of severe wasting diseases such as malignancy, autoimmune diseases, severe hepatic disease, and severe renal insufficiency (n = 1348). After these exclusions, 4962 participants were deemed eligible for the study (Supplementary Fig. S1). We conducted a comparison of the baseline characteristics between the excluded participants and those included in the study population. The analysis revealed no significant differences in age, gender, blood pressure levels, or prevalence of comorbidities, indicating that the exclusions were representative of the broader cohort and did not introduce selection bias (SupplementaryTable S1). This process ensured that the study population was reflective of the overall hypertensive cohort from which it was drawn.
The Ethics Board of the People’s Hospital of the Xinjiang Uygur Autonomous Region provided ethical approval for this investigation, under the reference number KY2021031901. All methods are carried out in accordance with current guidelines and all procedures comply with the requirements of the Declaration of Helsinki. All participants provided informed written consent.
Data collection and definitions
Utilizing the electronic medical records, we extracted all pertinent patient data. Alcohol consumption and smoking status were categorized as dichotomous variables, specifically delineated as ‘never/former’ and ‘current’. The anthropometric measurements, including blood pressure, WC, height, and body weight, were conducted following standardized protocols. For the sake of transparency and replicability, the supplementary material provides an exhaustive description of these anthropometric assessments. WWI was computed by dividing WC (in cm) by the weight’s square root (in kg)19. Fasting blood samples were collected from all participants following an overnight fast. An automated biochemical analyzer was employed to measure low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose (FPG), high-density lipoprotein cholesterol (HDL-C), homocysteine (Hcy), uric acid (UA), triglycerides (TG), and hypersensitive C-reactive protein (hs-CRP). The estimated glomerular filtration rate (eGFR) was ascertained using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Medical histories were classified according to the International Classification of Diseases-10 (ICD-10), encompassing coronary heart disease (CHD) (codes I24 and I25), atrial fibrillation (AF) (code I48), diabetes (codes E10–E14), and dyslipidemia (code E78). The overall burden of comorbidities was quantified using the Charlson Comorbidity Index (CCI). Table S2 provides a list of the drugs that were the subject of the investigation.
Outcomes and follow-up
The primary outcome was the occurrence of the first stroke, which could be classified as either ischemic or hemorrhagic. The supplementary material provides a detailed description of the methods used to determine whether an incident stroke occurred. The follow-up period commenced upon participant enrollment and was concluded in December 2021. Participants were recommended to have a minimum of one outpatient visit every three months, with an annual comprehensive health assessment. Follow-ups were conducted through hospital visits and telephonic conversations to accommodate the participants’ needs and to ensure a high follow-up compliance rate. The study outcomes were determined through medical record reviews, participant interviews, liaison with local disease and death registries, and by accessing the database of basic health insurance.
Statistical analysis
Missing data details are outlined in Table S3, with the missForest algorithm in R used for covariate imputation. Divided into four groups according to the WWI quartile. Kaplan-Meier analysis, supplemented by log-rank testing, was performed to assess time-to-event outcomes. Schoenfeld residuals were used to validate the Cox proportional hazards assumption (Fig. S2). Variance inflation factors were used to measure collinearity (Supplementary Table S4). Cox regression was utilized to determine hazard ratios (HRs) and 95% confidence intervals (CIs). The covariates included in the Cox model were age, sex, alcohol consumption, tobacco use, heart rate, BMI, WC, DBP, hypertension duration, SBP, CCI, CHD, AF, diabetes, LDL-C, TG, FPG, Hcy, UA, eGFR, hsCRP, antihypertensive drugs, anti-diabetes drugs, and lipid-lowering drugs. Restricted cubic splines were used to visually evaluate the dose-response correlations between WWI and stroke risk. We conducted tests for interactions and performed subgroup analyses. Sensitivity analysis were carried out to verify how reliable the findings were. The predictive abilities of the WWI and established risk factors for stroke risk were evaluated using the integrated improvement index (IDI), the net reclassification index (NRI), and the C statistic. R version 4.1.1 was used to conduct the analyses, and for two-sided tests, a significance threshold of p = 0.05 was used.
Results
Baseline characteristics
The population of WWI exhibited a normal distribution (Fig. S3). The current study included a total of 4962 participants, as illustrated in Supplementary Fig. S1. Of the total number of participants, 2575 (51.9%) were male. The mean WWI was 9.99 cm/√kg (SD, 1.20). Table 1 presents the characteristics of the study participants according to their WWI quartiles. Individuals exhibiting higher WWI values exhibited elevated DBP, WC, BMI, UA, TG, LDL-C, FPG, and hs-CRP levels. In the upper WWI quartiles, HDL-C levels were observed to be lower. Furthermore, individuals with elevated WWI levels exhibited a higher frequency of insulin utilization.
The relationship between WWI and incident stroke
A total of 547 new cases of stroke were documented during a median follow-up period of 3.20 years. Of these, 114 had hemorrhagic strokes (HS) and 444 had ischemic strokes (IS). The highest quartile of WWI demonstrated a significantly greater cumulative incidence of stroke, as illustrated by the Kaplan-Meier survival curves and confirmed by the log-rank test (P < 0.05; Fig. 1). Overall, there was a positive correlation between WWI and new-onset stroke, after adjusting for all significant variables (per SD cm/√kg increment; HR 1.30; 95% CI 1.18, 1.42) (Fig. 2A; Table 2). Upon stratification of WWI into quartiles, the adjusted HRs for stroke risk were 1.21 (95% CI 0.91, 1.61) for the second quartile, 1.33 (95% CI 1.01, 1.76) for the third quartile, and 1.87 (95% CI 1.44, 2.42) for the fourth quartile, relative to the first quartile (P for trend < 0.001). Similar trends were observed for both new-onset IS (Fig. 2B; Table 2) and new-onset HS (Fig. 2C; Table 2). However, a clear nonlinear dose-response relationship was not established (Fig. 2).
Comparative analysis of WWI with BMI and WC
To compare the superior ability of the WWI with the traditional metrics of BMI and WC, we conducted a receiver operating characteristic curve (ROC) analysis. By calculating the area under the curve (AUC), we determined that the WWI had the largest AUC for stroke prediction, measuring 0.632. This predictive capability was consistent for both haemorrhagic and ischaemic strokes (Fig. 3). These findings further emphasize the excellent predictive performance of the WWI.
Subgroup and sensitivity analysis
Additional stratified analyses were conducted to identify potential moderators of the relationship between WWI (per SD cm/√kg increment) and stroke risk in specific subgroups (Fig. 4A). The relationship between WWI and stroke risk was not substantially altered by age, BMI, hypertension duration, CCI, diabetes, smoking status, or drinking status (all P-values for interactions > 0.05) (Fig. 4A). Given the multiple tests and comparable directionality of effects, even if the P value for interactions by gender was less than 0.05, these findings may not have a significant clinical impact. Similar outcomes were observed for IS and HS (Fig. 4B,C). In the sensitivity analyses, the associations of WWI with stroke risk were not significantly altered after excluding participants with stroke occurring within the first year of the follow-up (Supplementary Table S5), using a competing risk model (Supplementary Table S6), excluding participants with CCI ≥ 2 (Supplementary Table S7), excluding individuals with prevalent atrial fibrillation at baseline (Supplementary Table S8), excluding elderly patients over 85 years old (Supplementary Table S9), or excluding individuals with missing covariates (Supplementary Table S10). Moderate to strong evidence against confounding bias was shown by the E-values (Supplementary Table S11).
Incremental predictive values of WWI
We examined whether WWI could improve the predictive value of established risk factors (Table S12). The C-statistics of the established risk factors for total stroke improved significantly with the inclusion of WWI (from 0.534 to 0.575). In addition, the addition of WWI to the established risk factors increased the discriminative ability of the NRI and IDI (all P < 0.05). For IS and HS, comparable outcomes were seen (Table S12).
Discussion
In the current observational cohort analysis, we demonstrated that, in older patients with hypertension, there was a substantial positive association between WWI and new-onset stroke adjustment for all significant variables. Furthermore, in sensitivity and subgroup analysis, these results and trends remained stable. Meanwhile, WWI exhibited a stronger association with incident stroke compared to other identified indicators of obesity.
One of the main causes of CVD and its consequences is obesity, particularly visceral obesity30,31,32. Therefore, one of the most important issues in clinical practice is the accurate assessment of body fat. When evaluating visceral adipose tissue in different populations, computed tomography (CT) and magnetic resonance imaging (MRI) are generally more dependable techniques15,33. However, their time-consuming nature, high cost, and ionizing radiation limit their routine clinical use in the largely general population. Previous studies have suggested that traditional indicators of adiposity, such as BMI and WC, may raise the risk of stroke34,35,36,37,38. However, as an index of adiposity, BMI does not truly reflect visceral obesity and does not differentiate between central and peripheral fat14,15. Furthermore, BMI has also demonstrated an obesity paradox for predicting CVD, and the use of BMI is not conducive to identifying high-risk groups39,40. Diabetes and CVD are linked to an increased risk in WC35,40,41,42,43. However, the major drawback is that the cut-points of WC vary with age, sex, and ethnicity in the elderly group16,44. Weight and WC are combined to provide a new, simple anthropometric measure of obesity called WWI. It is associated with a higher probability of having both high fat mass and low muscle mass and raises BMI while maintaining the advantages of WC19,26,45. Kim et al. shown46, WWI probably represents aging-related changes in the abdominal composition in a multiethnic cohort. Park et al. observed that compared to WC, WHR, and WHtR, WWI had a negative connection with muscle mass and was a greater predictor of sarcopenic obesity47.
The relationship between the WWI and a spectrum of adverse health outcomes has garnered considerable research attention. A prospective cohort study, which included a substantial sample of 12,447 Chinese participants, has delineated a significant correlation between elevated WWI values and an increased risk of cardiovascular events and all-cause mortality21. This observation is further supported by the research of Cai et al., who identified a substantial association between WWI and all-cause mortality risk48. In the domain of cognitive health, especially among the geriatric population, WWI has emerged as a relevant factor. Studies have demonstrated that higher WWI values are associated with a more pronounced decline in cognitive function49,50. This association underscores the potential for WWI to serve as a predictive marker for cognitive impairment in older adults. Furthermore, a robust correlation has been established between WWI and bone health, with implications for both bone mineral density and the risk of osteoporosis22,23. This correlation is of particular importance given the prevalence of osteoporosis and its associated morbidity among the aging population. In addition, a study conducted in the United States has identified a link between WWI and the prevalence of gallstone disease24. This finding suggests that WWI may provide insights into the risk of developing gallstone disease, potentially through its association with metabolic dysregulation. Furthermore, the prognostic value of WWI in predicting the 10-year incidence of type 2 diabetes has been underscored by recent research38. This study demonstrated that WWI outperforms other anthropometric indices in predicting diabetes risk. This predictive capability is of significant public health importance, considering the escalating global burden of diabetes and the urgent need for precise risk assessment tools. Despite these associations, the relationship between WWI and stroke remains debated. A research from China with 23,389 individuals showed a link between stroke and WWI. The investigation’s findings suggested that a higher risk of stroke was linked to a bigger WWI51. High WWI was shown to be strongly linked with an elevated risk of CVD in another cross-sectional study of US adults, but further analysis showed no association between WWI and stroke after adjustment for confounders52. The results of the two studies are not only inconsistent but also cross-sectional. Compared with previous studies, ours is a cohort study, in which we demonstrated the association between the level of WWI and incident stroke in senile people with hypertension. After adjusting for blood pressure level, antihypertensive medication, and duration of hypertension, our findings remained stable. Furthermore, these connections imply that, in addition to blood pressure control, WWI may be seen as an intervening factor in the prevention of stroke.
The positive correlation observed between WWI and the incidence of stroke can be attributed to several underlying physiological mechanisms. Firstly, the excessive accumulation of adipose tissue, as indicated by elevated WWI values, is known to precipitate hemodynamic stress, adipokine dysregulation, vascular inflammation, and endothelial dysfunction. Additionally, it has been associated with an imbalance in the gut microbiome, all of which can accelerate the process of atherosclerosis and consequently augment the risk of stroke53,54,55,56. Secondly, obesity is frequently accompanied by insulin resistance, a condition that instigates a proatherogenic state. This metabolic milieu can lead to dysglycemia, dyslipidemia, and arterial hypertension, all of which are well-established risk factors for the development of cerebrovascular disease57,58,59,60. Thirdly, the state of obesity induces neuroendocrine alterations and excites the sympathetic nervous system, potentially resulting in the overactivation of the renin-angiotensin system. These physiological responses may further promote vascular damage and induce vasoconstriction, contributing to the pathogenesis of stroke61,62. Furthermore, obstructive sleep apnea has been found to be a risk factor for CVD and is a common comorbidity among obese people. The relationship between OSA and stroke is well documented, highlighting the interplay between obesity, OSA, and stroke risk63,64,65.
The current investigation is bolstered by several methodological strengths that enhance its credibility and the validity of its findings. Notably, the study benefits from a large cohort, relatively long follow-up, and comprehensive adjustment for a variety of potential confounders. Together, these elements contribute to a robust analysis and increase the reliability of the observed associations. Despite these advantages, several limitations remain. Firstly, our investigation is constrained by its observational nature, which limits the ability to establish causal relationships. While we endeavored to control for potential confounders, the possibility of residual or unmeasured confounding cannot be entirely dismissed. This is an inherent limitation of observational studies, where the complex interplay of factors in the real world may introduce variability that is not accounted for in the analysis. Secondly, the reliance on a single baseline measurement of the WWI is a notable limitation. This approach does not capture the trajectory of changes in WWI over time, which may be pivotal in understanding the dynamic relationship between WWI and the risk of stroke. The lack of longitudinal data on WWI may obscure the long-term impact of fluctuations in this index on stroke risk. Another limitation is the demographic composition of our cohort. Our study exclusively enrolled elderly hypertensive patients from a single geographic region in China, which constrains the generalizability of our findings to younger populations, different ethnicities, and diverse geographical locations. The homogeneity of our study population may not reflect the broader diversity in age, ethnicity, and lifestyle factors that could influence the WWI-stroke association. Moreover, the study did not differentiate between the specific sites of hemorrhagic strokes or subtypes of ischemic strokes. This lack of stratification may mask important nuances in how WWI relates to different stroke subtypes, potentially leading to a generalized understanding that does not fully capture the complexities of stroke etiology in the context of obesity. In addition, our study did not collect comprehensive data on participants’ physical activity levels. The absence of this data limits our ability to assess the independent and interactive effects of physical activity on the relationship between WWI and stroke risk. Furthermore, while we made efforts to account for a wide range of potential confounders, there is always the possibility that certain variables may have been overlooked. For instance, dietary habits, which are known to influence both obesity and stroke risk, were not systematically evaluated in our study. The influence of nutritional factors on our findings cannot be ascertained, representing another limitation. Lastly, the diagnosis of stroke and its classification into ischemic or hemorrhagic subtypes relied on medical record reviews and administrative databases, which may be subject to inaccuracies in documentation or miscoding. The potential for misdiagnosis or incorrect classification, while mitigated through rigorous validation processes, is an inherent limitation of studies reliant on medical records. In conclusion, while our study provides valuable insights into the association between WWI and stroke risk in elderly hypertensive patients, the aforementioned limitations must be taken into account when interpreting the results. Future research should aim to address these limitations through multicenter and longitudinal study designs, inclusion of diverse populations, comprehensive lifestyle assessments, and robust diagnostic methodologies to enhance the validity and generalizability of the findings.
Conclusion
The findings from this study demonstrate a significant positive correlation between an elevated WWI and the incidence of stroke in elderly individuals with hypertension. While these results are compelling, it is imperative to recognize the limits of observational data in establishing causation. The correlation observed does not definitively imply a causal link between WWI and stroke risk. However, the potential of WWI to serve as an independent risk factor for stroke in this demographic is noteworthy. If our findings are substantiated by further research, WWI could be incorporated into clinical assessments to identify elderly hypertensive patients at higher risk of stroke. This could facilitate more personalized prevention strategies and early interventions, ultimately aiding in risk reduction. We acknowledge that our conclusions are based on the current study’s data. Further longitudinal research and interventional studies are required to validate the association between WWI and stroke and to elucidate the mechanisms underlying this relationship. Future studies should also consider the impact of modifiable factors such as physical activity and diet, which could provide additional insights into stroke prevention strategies. In summary, our research underscores the importance of a comprehensive understanding of obesity metrics like WWI in the context of stroke risk. It is our hope that these findings will inspire further investigation and contribute to the development of more effective clinical practices and public health policies aimed at reducing the burden of stroke, particularly among high-risk elderly hypertensive populations.
Data availability
Study data are available on reasonable request to the corresponding author.
References
Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 396, 1223–1249. https://doi.org/10.1016/s0140-6736(20)30752-2 (2020).
Roth, G. A. et al. Global Burden of Cardiovascular diseases and Risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010 (2020).
Global National life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of Disease Study 2015. Lancet (London England). 388, 1459–1544. https://doi.org/10.1016/s0140-6736(16)31012-1 (2016).
Zhou, M. et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet (London England). 394, 1145–1158. https://doi.org/10.1016/s0140-6736(19)30427-1 (2019).
Ma, Q. et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: an analysis for the global burden of Disease Study 2019. Lancet Public. Health. 6, e897–e906. https://doi.org/10.1016/s2468-2667(21)00228-0 (2021).
O’Donnell, M. J. et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet (London England). 376, 112–123. https://doi.org/10.1016/s0140-6736(10)60834-3 (2010).
Riaz, H. et al. Association between obesity and cardiovascular outcomes: a systematic review and Meta-analysis of mendelian randomization studies. JAMA Netw. Open. 1 (e183788). https://doi.org/10.1001/jamanetworkopen.2018.3788 (2018).
Jaakonmäki, N. et al. Obesity and the risk of cryptogenic ischemic stroke in young adults. J. Stroke Cerebrovasc. Dis. 31, 106380. https://doi.org/10.1016/j.jstrokecerebrovasdis.2022.106380 (2022).
Kumral, E., Erdoğan, C. E., Arı, A., Bayam, F. E. & Saruhan, G. Association of obesity with recurrent stroke and cardiovascular events. Rev. Neurol. 177, 414–421. https://doi.org/10.1016/j.neurol.2020.06.019 (2021).
Landsberg, L. et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of the Obesity Society and the American Society of Hypertension. J. Clin. Hypertens. (Greenwich Conn). 15, 14–33. https://doi.org/10.1111/jch.12049 (2013).
Nguyen, N. T., Magno, C. P., Lane, K. T., Hinojosa, M. W. & Lane, J. S. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J. Am. Coll. Surg. 207, 928–934. https://doi.org/10.1016/j.jamcollsurg.2008.08.022 (2008).
Nuttall, F. Q. & Body mass index, obesity, BMI, and Health: a critical review. Nutr. Today. 50, 117–128. https://doi.org/10.1097/nt.0000000000000092 (2015).
Ross, R. et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on visceral obesity. Nat. Rev. Endocrinol. 16, 177–189. https://doi.org/10.1038/s41574-019-0310-7 (2020).
Rådholm, K. et al. The impact of using sagittal abdominal diameter to predict major cardiovascular events in European patients with type 2 diabetes. Nutr. Metabolism Cardiovasc. Dis. NMCD. 27, 418–422. https://doi.org/10.1016/j.numecd.2017.02.001 (2017).
Snijder, M. B., van Dam, R. M., Visser, M. & Seidell, J. C. What aspects of body fat are particularly hazardous and how do we measure them? Int. J. Epidemiol. 35, 83–92. https://doi.org/10.1093/ije/dyi253 (2006).
Stevens, J., Katz, E. G. & Huxley, R. R. Associations between gender, age and waist circumference. Eur. J. Clin. Nutr. 64, 6–15. https://doi.org/10.1038/ejcn.2009.101 (2010).
Kuerban, A., Beyond Asian-Specific & Cutoffs Gender effects on the predictability of body Mass Index, Waist circumference, and Waist circumference to height ratio on Hemoglobin A1c. J. Racial Ethnic Health Disparities. 8, 415–421. https://doi.org/10.1007/s40615-020-00796-6 (2021).
Xu, F. et al. The sex and Race/Ethnicity-Specific Relationships of Abdominal Fat Distribution and anthropometric indices in US adults. Int. J. Environ. Res. Public Health. 19https://doi.org/10.3390/ijerph192315521 (2022).
Park, Y., Kim, N. H., Kwon, T. Y. & Kim, S. G. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci. Rep. 8, 16753. https://doi.org/10.1038/s41598-018-35073-4 (2018).
Zhao, J. et al. J-Shaped relationship between weight-adjusted-waist index and cardiovascular disease risk in hypertensive Patients with obstructive sleep apnea: a Cohort Study. Diabetes, metabolic syndrome and obesity : targets and therapy. 17, 2671-2681, doi:10.2147/dmso.S469376 (2024)
Ding, C. et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in China: a prospective cohort study. Nutr. Metab. Cardiovasc. Dis. NMCD. 32, 1210–1217. https://doi.org/10.1016/j.numecd.2022.01.033 (2022).
Wang, X., Yang, S., He, G. & Xie, L. The association between weight-adjusted-waist index and total bone mineral density in adolescents: NHANES 2011–2018. Front. Endocrinol. 14, 1191501. https://doi.org/10.3389/fendo.2023.1191501 (2023).
Guo, M. et al. The relationship between weight-adjusted-waist index and total bone mineral density in adults aged 20–59. Front. Endocrinol. 14https://doi.org/10.3389/fendo.2023.1281396 (2023).
Ke, B., Sun, Y., Dai, X., Gui, Y. & Chen, S. Relationship between weight-adjusted waist circumference index and prevalence of gallstones in U.S. adults: a study based on the NHANES 2017–2020. Front. Endocrinol. 14, 1276465. https://doi.org/10.3389/fendo.2023.1276465 (2023).
Harris, T. B. et al. Waist circumference and sagittal diameter reflect total body fat better than visceral fat in older men and women. The Health, Aging and Body Composition Study. Ann. N. Y. Acad. Sci. 904, 462–473. https://doi.org/10.1111/j.1749-6632.2000.tb06501.x (2000).
Kim, N. H., Park, Y., Kim, N. H. & Kim, S. G. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 50, 780–786. https://doi.org/10.1093/ageing/afaa208 (2021).
Santulli, G. et al. Prediabetes increases the risk of Frailty in Prefrail older adults with hypertension: Beneficial effects of Metformin. Hypertens. (Dallas Tex. : 1979). 81, 1637–1643. https://doi.org/10.1161/hypertensionaha.124.23087 (2024).
Cai, X. et al. Association between the Sarcopenia index and the risk of stroke in elderly patients with hypertension: a cohort study. Aging. 15, 2005–2032. https://doi.org/10.18632/aging.204587 (2023).
Cai, X. et al. Systemic inflammation response index as a predictor of Stroke Risk in Elderly patients with hypertension: a Cohort Study. J. Inflamm. Res. 16, 4821–4832. https://doi.org/10.2147/jir.S433190 (2023).
Cai, X. et al. Body roundness index improves the predictive value of cardiovascular disease risk in hypertensive patients with obstructive sleep apnea: a cohort study. Clin. Experimental Hypertens. (New York N Y : 1993). 45 (2259132). https://doi.org/10.1080/10641963.2023.2259132 (2023).
Kim, S. H. et al. Association of obesity, visceral adiposity, and Sarcopenia with an increased risk of metabolic syndrome: a retrospective study. PloS One. 16, e0256083. https://doi.org/10.1371/journal.pone.0256083 (2021).
Chartrand, D. J. et al. Overweight, obesity, and CVD risk: a focus on Visceral/Ectopic Fat. Curr. Atheroscler. Rep. 24, 185–195. https://doi.org/10.1007/s11883-022-00996-x (2022).
van der Kooy, K. & Seidell, J. C. Techniques for the measurement of visceral fat: a practical guide. Int. J. Obes. Relat. Metab. Disor. J. Int. Association Study Obes. 17, 187–196 (1993).
Bardugo, A. et al. Body Mass Index in 1.9 million adolescents and stroke in Young Adulthood. Stroke. 52, 2043–2052. https://doi.org/10.1161/strokeaha.120.033595 (2021).
Mitchell, A. B. et al. Obesity increases risk of ischemic stroke in young adults. Stroke. 46, 1690–1692. https://doi.org/10.1161/strokeaha.115.008940 (2015).
Liu, L. et al. Family history, waist circumference and risk of ischemic stroke: a prospective cohort study among Chinese adults. Nutr. Metab. Cardiovasc. Dis. NMCD. 33, 758–769. https://doi.org/10.1016/j.numecd.2023.01.009 (2023).
Strazzullo, P. et al. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 41, e418–426. https://doi.org/10.1161/strokeaha.109.576967 (2010).
Hafezi, S. G. et al. Prediction of the 10-year incidence of type 2 diabetes mellitus based on advanced anthropometric indices using machine learning methods in the Iranian population. Diabetes Res. Clin. Pract. 214, 111755. https://doi.org/10.1016/j.diabres.2024.111755 (2024).
Nevill, A. M., Stewart, A. D., Olds, T. & Holder, R. Relationship between adiposity and body size reveals limitations of BMI. Am. J. Phys. Anthropol. 129, 151–156. https://doi.org/10.1002/ajpa.20262 (2006).
Xia, X. et al. Roles of general and central adiposity in cardiometabolic multimorbidity: revisiting the obesity paradox using a multistate model. Obes. (Silver Spring Md). 32, 810–821. https://doi.org/10.1002/oby.23980 (2024).
Wang, L. et al. A prospective study of waist circumference trajectories and incident cardiovascular disease in China: the Kailuan Cohort Study. Am. J. Clin. Nutr. 113, 338–347. https://doi.org/10.1093/ajcn/nqaa331 (2021).
Zhang, F. L. et al. Strong Association of Waist Circumference (WC), Body Mass Index (BMI), Waist-to-Height Ratio (WHtR), and Waist-to-Hip Ratio (WHR) with Diabetes: A Population-Based Cross-Sectional Study in Jilin Province, China. J. Diabetes Res. 8812431. https://doi.org/10.1155/2021/8812431 (2021).
Hu, J. et al. Association between triglyceride glucose Index-Waist circumference and risk of first myocardial infarction in Chinese hypertensive patients with obstructive sleep apnoea: an Observational Cohort Study. Nat. Sci. Sleep. 14, 969–980. https://doi.org/10.2147/nss.S362101 (2022).
Seo, D. C., Choe, S. & Torabi, M. R. Is waist circumference ≥ 102/88 cm better than body mass index ≥ 30 to predict hypertension and diabetes development regardless of gender, age group, and race/ethnicity? Meta-analysis. Prev. Med. 97, 100–108. https://doi.org/10.1016/j.ypmed.2017.01.012 (2017).
Kim, K. J., Son, S., Kim, K. J., Kim, S. G. & Kim, N. H. Weight-adjusted waist as an integrated index for fat, muscle and bone health in adults. J. Cachexia Sarcopenia Muscle. 14, 2196–2203. https://doi.org/10.1002/jcsm.13302 (2023).
Kim, J. Y. et al. Associations between weight-adjusted waist index and abdominal fat and muscle Mass: multi-ethnic study of atherosclerosis. Diabetes Metab. J. 46, 747–755. https://doi.org/10.4093/dmj.2021.0294 (2022).
Park, M. J. et al. A Novel Anthropometric parameter, weight-adjusted waist index represents sarcopenic obesity in newly diagnosed type 2 diabetes Mellitus. J. Obes. Metab. Syndr. 32, 130–140. https://doi.org/10.7570/jomes23005 (2023).
Cai, S. et al. Association of the weight-adjusted-Waist Index with risk of all-cause mortality: a 10-Year Follow-Up study. Front. Nutr. 9, 894686. https://doi.org/10.3389/fnut.2022.894686 (2022).
Mone, P. et al. Empagliflozin improves cognitive impairment in Frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes care. 45, 1247–1251. https://doi.org/10.2337/dc21-2434 (2022).
Huang, X. T., Lv, X. & Jiang, H. The weight-adjusted-waist index and cognitive impairment among U.S. older adults: a population-based study. Front. Endocrinol. 14, 1276212. https://doi.org/10.3389/fendo.2023.1276212 (2023).
Ye, J. et al. Association between the weight-adjusted waist index and stroke: a cross-sectional study. BMC Public. Health. 23https://doi.org/10.1186/s12889-023-16621-8 (2023).
Fang, H., Xie, F., Li, K., Li, M. & Wu, Y. Association between weight-adjusted-waist index and risk of cardiovascular diseases in United States adults: a cross-sectional study. BMC Cardiovasc. Disord. 23, 435. https://doi.org/10.1186/s12872-023-03452-z (2023).
Unamuno, X. et al. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 48, e12997. https://doi.org/10.1111/eci.12997 (2018).
Koliaki, C., Liatis, S. & Kokkinos, A. Obesity and cardiovascular disease: revisiting an old relationship. Metab. Clin. Exp. 92, 98–107. https://doi.org/10.1016/j.metabol.2018.10.011 (2019).
Bleau, C., Karelis, A. D., St-Pierre, D. H. & Lamontagne, L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diab./Metab. Res. Rev. 31, 545–561. https://doi.org/10.1002/dmrr.2617 (2015).
Koenen, M., Hill, M. A., Cohen, P., Sowers, J. R. & Obesity Adipose tissue and vascular dysfunction. Circ. Res. 128, 951–968. https://doi.org/10.1161/circresaha.121.318093 (2021).
Tounian, P. et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet (London England). 358, 1400–1404. https://doi.org/10.1016/s0140-6736(01)06525-4 (2001).
Fink, J., Seifert, G., Blüher, M., Fichtner-Feigl, S. & Marjanovic, G. Obesity surgery. Deutsches Arzteblatt Int. 119, 70–80. https://doi.org/10.3238/arztebl.m2021.0359 (2022).
Meex, R. C. R., Blaak, E. E. & van Loon, L. J. C. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 20, 1205–1217. https://doi.org/10.1111/obr.12862 (2019).
Kotsis, V. et al. Obesity and cardiovascular risk: a call for action from the European Society of Hypertension Working Group of Obesity, diabetes and the high-risk patient and European Association for the Study of Obesity: part A: mechanisms of obesity induced hypertension, diabetes and dyslipidemia and practice guidelines for treatment. J. Hypertens. 36, 1427–1440. https://doi.org/10.1097/hjh.0000000000001730 (2018).
Balasubramanian, P., Hall, D. & Subramanian, M. Sympathetic nervous system as a target for aging and obesity-related cardiovascular diseases. GeroScience. 41, 13–24. https://doi.org/10.1007/s11357-018-0048-5 (2019).
Kalil, G. Z. & Haynes, W. G. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens. Res. 35, 4–16. https://doi.org/10.1038/hr.2011.173 (2012).
Bonsignore, M. R. Obesity and obstructive sleep apnea. Handb. Exp. Pharmacol. 274, 181–201. https://doi.org/10.1007/164_2021_558 (2022).
Javaheri, S., Peker, Y., Yaggi, H. K. & Bassetti, C. L. A. Obstructive sleep apnea and stroke: the mechanisms, the randomized trials, and the road ahead. Sleep Med. Rev. 61, 101568. https://doi.org/10.1016/j.smrv.2021.101568 (2022).
Brill, A. K. et al. CPAP as treatment of sleep apnea after stroke: a meta-analysis of randomized trials. Neurology. 90, e1222–e1230. https://doi.org/10.1212/wnl.0000000000005262 (2018).
Funding
The Xinjiang Uygur Autonomous Region’s Key Research and Development Project (2022B03009, 2022B03009-1) provided funding for this study.
Author information
Authors and Affiliations
Contributions
J.H. and X.C. both contributed equally to this project. The research was done by X.C., J.H., and N.L. Data analysis and interpretation were done by Q.Z., W.Y., and J.H.. The paper was written by J.H. and X.C., and it was critically corrected by Q.L., S.S., D.S., and N.L. N.L. directed the entire research process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, J., Cai, X., Song, S. et al. Association between weight-adjusted waist index with incident stroke in the elderly with hypertension: a cohort study. Sci Rep 14, 25614 (2024). https://doi.org/10.1038/s41598-024-76709-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76709-y
Keywords
This article is cited by
-
Association between normal-weight central obesity and asymptomatic hyperuricemia in Korean adults: a cross-sectional study
BMC Public Health (2025)
-
Association between conicity index (C-index), relative fat mass (RFM), and osteoarthritis (OA): evidence from NHANES 2003–2018
Lipids in Health and Disease (2025)
-
Association between weight-adjusted-waist index and the prevalence of gallstone disease in Minhang District, Shanghai: a cross-sectional study
Journal of Health, Population and Nutrition (2025)
-
Inositol supplementation efficacy in improving key cardiometabolic and anthropometric indices: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials
Diabetology & Metabolic Syndrome (2025)
-
Association between triglyceride glucose (TyG)-atherogenic index of plasma (AIP) index and new-onset stroke risk in middle-aged and older Chinese individuals: a nationwide prospective cohort study
BMC Neurology (2025)