Abstract
To evaluate the effectiveness and biocompatibility of Wistend, a novel Schlemm’s canal (SC) microstent made of Nitinol designed to improve aqueous humor outflow. New Zealand white (NZW) rabbits were divided into blank, sham-operated and Wistend groups. ICare® Tonovet Plus®, swept-source optical coherence tomography (SS-OCT), slit lamp biomicroscopy, retinal camera and scanning electron microscopy (SEM) were used for preoperative and postoperative observations. Hematoxylin and Eosin (H&E) tissue staining was adopted for biocompatibility. A significant difference in intraocular pressure (IOP) between the Wistend group and the control groups was observed during the six-month follow-up. SS-OCT identified arc line internal reflections within the SC in the anterior chamber angle. Conjunctival congestion and edema gradually diminished in the early stages. No corneal vascularization, no anterior chamber inflammatory response and no significant tissue reactions were noted in any groups. SEM showed the Wistend’s windows and orifices remained clear, encircled by minimal incidental ocular tissue and free from blockage. Histopathological examination revealed no discernible differences between the Wistend-implanted and sham-operated eyes. These in vivo studies demonstrate the effectiveness and biocompatibility of the microstent. Our findings suggest a promising potential for Wistend in significantly reducing IOP and effectively facilitating the outflow of aqueous humor.
Similar content being viewed by others
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide1,2, and it is estimated that approximately 120 million people worldwide will be affected by 20403. Intraocular pressure (IOP) is the most important risk factor for optic nerve damage and visual field loss that can be effectively managed4,5. Notably, every 1mmHg increase in IOP elevates the risk of open-angle glaucoma (OAG) increases by 12%6.
Current glaucoma management focuses on lowering IOP and includes various approaches, such as medications, laser procedures, and incisional surgeries7. Topical medications remain the first-line therapy for ocular hypertension and glaucoma8,9. These topical hypotensive agents are generally safe; however, their efficacy can be significantly undermined by low patient adherence, especially when multiple medications are concurrently prescribed over long periods10,11. Additionally, these medications are associated with a spectrum of complications, ranging from mild issues such as conjunctival congestion and ocular surface diseases, to more severe problems such as cardiorespiratory complications in cases of misuse12.
When medication and laser therapy fail to adequately control IOP, surgical options, including trabeculectomy and glaucoma drainage devices, become necessary. While effective, these invasive procedures carry significant postoperative complications such as shallow anterior chamber, hypotony maculopathy, choroidal effusions, postoperative bleb fibrosis, bleb-associated leaks, hypotony, infection, corneal damage, and cataract formation13,14,15,16. For patients with advanced and progressive glaucoma conventional surgery is the only option, these risks may be deemed acceptable; however, for patients with mild-to-moderate glaucoma, the potential benefits from early surgical treatment do not outweigh the substantial risks associated with the more invasive procedures like tube-shunt implantation and trabeculectomy17. The limitations and risks associated with conventional glaucoma treatments have necessitated the development of safer, less invasive alternatives. Minimally invasive glaucoma surgery (MIGS) represents such an advancement, expanding treatment options to include patients with mild-to-moderate glaucoma18. In 2014 the American Glaucoma Society and the US Food and Drug Administration (FDA) defined MIGS as a type of glaucoma surgery that involved the implantation of a surgical instrument to lower IOP through an outflow mechanism, with little or no scleral dissection, using either an internal or ab external approach19. Professors Ahmed and Saheb characterize MIGS by five key attributes: an ab interno microincision to avoid conjunctival loss; minimized tissue trauma; definitive IOP-lowering effect; high safety with minimal serious complications; and rapid recovery with minimal impact on quality of life20.
In terms of the mechanism of action for lowering IOP, MIGS can be divided into two broad categories: increasing aqueous humor drainage or reducing aqueous production21,22. Consistent with the principles of traditional glaucoma surgery, increasing aqueous humor drainage remains a major consideration in the surgical treatment of glaucoma. Depending on their mode of action, MIGS devices facilitating increased aqueous drainage are classified into routes through the trans-Schlemm’s canal (SC), the suprachoroidal chamber, and the subconjunctival space23,24. The trabecular meshwork (TM) and SC were considered to be the main sites of resistance to aqueous humor outflow25,26,27. Intervention in these areas to bypass TM tissue can reduce outflow resistance and potentially to achieving IOP levels close to episcleral venous pressure (EVP)28.
In the present study, the glaucoma microstent Wistend, is designed to reduce outflow resistance of trabecular meshwork tissue by dilating SC, the adjacent TM, and distal collecting ducts. The microstent also penetrates the TM creating an incision into the TM allowing communication between the anterior chamber and SC. This approach aims to lower IOP with minimal tissue destruction. Therefore, investigations into the effectiveness and biocompatibility of the Wistend are necessary to determine its potential as a viable treatment option in glaucoma management.
Methods
Device description
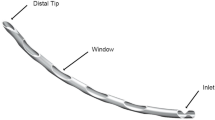
This study utilized a novel glaucoma microstent named Wistend (Model: MIGS90-3, Geneway Biotechnology Co., Ltd, Chengdu, China) and its corresponding injector (illustrated in Supplementary Figs. 1 and 2). Both the Wistend and the injector were irradiated and sterilized for single-use application.
Wistend is made from a medical-grade nickel-titanium shape memory alloy, conforming to the ASTM F2063-2018 standards, and comes preloaded in a hand-held injector29. Nitinol, as a shape memory alloy, has superelastic properties and is suitable for use in SC support structures30,31. The Wistend features a crescent shape with drainage windows on both sides of its tubular body, creating an outflow channel for the aqueous humor. The ends of the Wistend are designed with a distal tip for insertion and an inlet for attaching the injector. Wistend is engineered to occupy approximately 90˚of the SC (equivalent to 3 clock hours), with a robust tube body to provide sufficient structural support of the SC. This design ensures both the support strength of Wistend within the SC and an optimal diameter for aqueous humor drainage, which allows the aqueous humor to enter the SC along the drainage cavity and smoothly enter the distal collector channel. During the implantation process, the Wistend is advanced into position using an injector which applies force to its distal tip. Once implanted, the apical end of Wistend passes through the TM and is securely positioned in the SC. Meanwhile, the proximal tip of Wistend remains positioned in the anterior chamber, providing an unhindered outflow of aqueous humor fluid from the anterior chamber into the SC.
Effectiveness study
Rabbit model
The study was approved by the Institutional Animal Care and Use Committee of the Henan Eye Hospital (Zhengzhou, China) with ethical approval (ID: HNEECA-2022-30), and all the animals used in this study were treated in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and ARRIVE guidelines and the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Twelve 5-month-old male New Zealand white (NZW) rabbits, weighing between 2.2 and 2.8 kg, were purchased from Zhengzhou University Animal Experiment Centre (Zhengzhou, China). Selection criteria included overall health and the absence of any signs of eye irritation. The rabbits were acclimatized for 7 days prior to the experiments. They were then divided into three groups: A (blank group), B (sham-operated group), and C (Wistend group). The right eyes of the rabbits received either Wistend implantation (n = 6) or sham surgery (n = 6), and the contralateral eyes served as the blank control. The rabbits were housed individually under controlled environmental conditions (20–23 °C) and maintained on a 12-hour light/dark cycle. They had unrestricted access to water and a standard diet with no fasting required on the experimental day.
Implantation procedure
The surgery was performed by an experienced ophthalmologist under general anesthesia. The anesthesia was induced by subcutaneous preadministration of ketamine (10 mg/kg, Beikang Pharma, Jiangsu, China) and xylazine (2 mg/kg, Huamu Animal Company, Jilin, China) mixture. Then isoflurane (RWD Company, Shenzhen, China) was inhaled and the concentration was maintained at 3%32,33.
In the Wistend group, the Wistend was implanted via an ab-interno approach. The surgeon’s preference and the eye’s condition determined the primary incision location (supratemporal or temporal) for nasal side Wistend implantation. A clear corneal incision was created with a 3.0 mm knife Slit SB (Beaver Visitec International, Bidford-on Avon, UK), and the inferior nasal quadrant anterior chamber and the TM angle were visualized with a gonioscope (Volk Surgical ACS Gonio Lens, Mentor, OH, USA). Ophthalmic viscoelastic device (OVD, 15 mg/ml sodium hyaluronate, Shanghai Qi Sheng Biologics Co., Ltd) was used to maintain anterior chamber depth. The Wistend, preloaded in a push-injector, was inserted through this incision and advanced into the SC via the TM under direct gonioscope guidance. The push-injector was then actuated to deploy the stent. The Wistend was positioned to span 90° within the SC, ensuring its proximal end facilitated the flow of aqueous humor from the anterior chamber into the SC. Following implantation, the anterior chamber was irrigated with a balanced salt solution and the incision was sealed watertight.
In the sham-operated group, a similar clear corneal incision was also created with the 3.0 mm knife Slit SB, and the inferior nasal quadrant anterior chamber and the TM angle were also visualized with a gonioscope. The anterior chamber depth was maintained by ophthalmic viscoelastic device. The push-injector, without the Wistend, was inserted through the incision and the tip of the push-injector was passed through the TM under direct gonioscope guidance. Thereafter, the push-injector was withdrawn without the placing of the Wistend. After the procedure, the anterior chamber was irrigated with a balanced salt solution and the incision was sealed watertight.
The left eyes served as blank controls without any operation. Postoperative Wistend eyes and sham-operated eyes received topical antibiotic eye drops (ofloxacin, Renhe Pharmacy Co, Nanchang, China) three times daily for one week.
Examination procedures
IOP measurement procedure
IOP was measured using the ICare® Tonovet Plus® (Icare Finland Oy, Helsinki, Finland)34,35. After initial eye condition assessments, IOP readings were taken 7 days preoperatively and on days 1, 7, 14, 30, 60, 90, 120, 150, and 180 postoperatively. The average value of six measurements was recorded as the final IOP value.
Noninvasive imaging by swept-source optical coherence tomography
SS-OCT (VG200D, SVision Imaging Co. Ltd., China) was performed on all groups at days 7, 30, 60, 90, 120 and 180 days postoperatively36,37,38. Both anterior segment OCT and posterior segment OCT of rabbit eyes were acquired, and only images with a signal intensity > 8 (0 = poor, 10 = excellent) were selected. The same experienced operator completed all the scans. Each anterior segment scan centered the apex of the rabbit’s cornea, utilizing 18 radial scanning lines, and 16 B-scan images per line to enhance signal quality39. The surgical site (i.e., the nasal side) was examined for SC dilatation and Wistend hyper-reflectivity. Posterior segment OCT was centered on the optic disc and assessed the retinal nerve fiber layer and fundus abnormalities40.
Color fundus photography
A retinal camera (ClearView, Optibrand, Fort Collins, Colorado) was used for fundus photography41. Fundus photography was performed to detect any abnormal changes in the retinal morphology and structure on both eyes at preoperative, and postoperative days 7, 30, 60, 90, 120, and 180. The presence of edema, detachment, hemorrhage, and pigmentation abnormalities in the retinal pigment epithelium (RPE) layer, as well as the presence of optic nerve atrophy and enlargement of the cup-to-disc ratio, were visualized and documented.
Clinical ophthalmic examinations and slit lamp biomicroscopy
Clinical exams and slit lamp biomicroscopy (MediWorks portable slit lamp S150, Shanghai, China) were performed on Oculus Uterque (OU) at preoperative (before micro-stent implantation), and postoperative days 7, 30, 60, 90, 120 and 18042,43. Conjunctival congestion and edema, corneal vascularization, anterior chamber flare, and anterior chamber inflammatory reaction were observed and photographed. Ocular changes were evaluated according to the Eaton scoring system and Draize scoring criteria44,45.
Termination and scanning electron microscopy (SEM) analysis
Six months postoperatively, the rabbits were euthanized in compliance with the American Veterinary Medical Association Euthanasia Guidelines, using an intravenous overdose of pentobarbital46. Following euthanasia, both eyes (whole globes) were promptly excised and the sites of the Wistend and sham surgical incisions were marked with sutures. The eyes were then immersed and fixed in 4% paraformaldehyde (Biosharp, Anhui, China) for further histological processes47.
For SEM examination, the Wistend was carefully removed from the eye, leaving a split at the base of the canal. The cannula’s opening was enlarged to expose the microstent’s drainage collection channels. Tissues were prepared with dehydration and gold sputtering48. The insertion point, most distal extension, passage area, and drainage window of the Wistend were examined with SEM, comparing the implanted area with the adjacent non-implanted area. Special attention was given to detecting any irregular granular fragments indicating tissue damage. Additionally, the Wistend itself was examined for fibrin and debris formation.
Statistical analyses
Laboratory data of IOP were presented as mean ± standard deviation (SD) and subjected to Kolmogorov-Smirnov (K-S) normality test. One-way analysis of variance (ANOVA) was performed on parameters that conformed to a normal distribution to determine if there were statistical differences between groups. The SPSS software (SPSS version 26.0, International Business Machines Corporation, New York, USA) was used for all statistical analyses. The statistical significance was defined as P < 0.05.
Biocompatibility study
Rabbit model
This study adhered to ethical guidelines (GB/T 35892 − 2018, Guidelines for Ethical Review of Experimental Animal Welfare; GB/T 35823 − 2018, Laboratory Animals General Requirements for Animal Experiments, and Regulations of Guangdong Province on the Administration of Experimental Animals) with ethical approval (ID: 2022E031). All animals were treated in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and ARRIVE guidelines and the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Seven healthy, 8-month-old female New Zealand white rabbits (2.2 to 2.8 kg) were acclimatized for 7 days. Each rabbit underwent a Wistend implantation in the right eye and a sham operation in the left. Rabbits were housed individually under controlled conditions (20–23 °C, 12-hour light/dark cycle), with free access to food and water, and no fasting on the experimental day.
Implantation procedure
The Wistend microstent was implanted in contact with tissues including the sclera, orbital tissues, and the conjunctiva, to simulate the most challenging conditions for eliciting an inflammatory response. The implantation procedure was conducted under general anesthesia. The anesthesia methods used were the same as those described in Sect. 1 of the Effectiveness Study. The anesthesia was induced by subcutaneous preadministration of ketamine (10 mg/kg) and xylazine (2 mg/kg) mixture. Then isoflurane was inhaled and the concentration was maintained at 3%32,33.
Surgery was performed on the right eye for the Wistend microstent implantation and on the left eye for the sham surgery. The surgeon used a 0.7*30 TWLB needle (Jiangsu Changcheng Medical Equipment Co., Ltd., China) as a cannula, which was slightly bent for the procedure. The Wistend microstent was delivered via the cannula to the interscleral layer and subconjunctiva at the 10 o’clock position, 1.5 mm from the corneal limbus. The cannula with Wistend was then advanced partially into the anterior chamber before being slowly withdrawn. The anterior chamber was reformed to full depth with balanced salt solution. In the sham-operated group, the same procedure was followed, but no Wistend was implanted, and the cannula was empty. Postoperatively, the rabbits recovered smoothly and were safely returned.
Termination and histological processes of hematoxylin and eosin (H&E) stain
Six months postoperatively, the rabbits were euthanized in compliance with the American Veterinary Medical Association Euthanasia Guidelines, using an intravenous overdose of pentobarbital. Following euthanasia, both eyes (whole globes) were promptly excised and the sites of the Wistend and sham surgical incisions were marked with sutures. The eyes were then immersed and fixed in 4% paraformaldehyde for further histological processes.
For H&E staining, eyeballs were sectioned sagittally parallel to the surgical sites of the Wistend implant or sham site. Following tissue preparation, sections were stained for standard histological examination31,49. Tissue reactions, such as neovascularization, fibrosis, fatty infiltration, as well as responses from various cell types (polymorphonuclear leukocytes, lymphocytes, plasma cells, macrophages, giant cells, and cellular necrosis) in the cornea, conjunctiva, and sclera near the implantation site were noted and evaluated semi-quantitatively. The fibroblast layer thickness and tissue inflammatory response to the Wistend, including the presence of giant cells were also assessed and photographed.
Result
Effectiveness study
All surgeries in Groups B and C were successful, with less than 0.1 ml of hemorrhage. There were no cases of corneal edema, significant corneal damage, iris root or ciliary body dissections, anterior chamber collapse, or significant anterior chamber hemorrhage. Postoperatively, all corneal incisions achieved watertight closure. The Wistend maintained its integrity without deformation, fracture, or implantation difficulties. There was no misalignment of the Wistend, with the distal end in the inferior nasal quadrant SC, and the proximal end visible in the anterior chamber.
All rabbits experienced a weight loss of 6.7 ± 3.4% in the first 10 postoperative days, but regained weight after 15 days, reaching or surpassing their initial weights by day 30 (19 ± 4.6% increase). No signs of generalized discomfort were observed and all rabbits survived the study duration, indicating overall comfort and health during the in vivo phase of the study.
IOP measurements
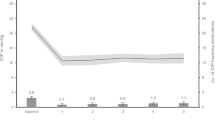
Preoperative IOP measurements were comparable across all groups (Group A: mean = 17.50, SD = 0.55; Group B: mean = 17.83, SD = 0.75; Group C: mean = 18.33, SD = 0.52; p > 0.05). Notably, by the first postoperative day, Group C exhibited a significant decrease in IOP (mean = 9.33, SD = 1.03), which was considerably compared to Group A (mean = 17.17, SD = 0.41; p < 0.001) and B (mean = 17.67, SD = 0.52; p < 0.001). Subsequent multiple comparisons showed that IOP in Group C was significantly lower than that in Groups A (t (15) =-19.19, p < 0.05, Cohen’s d=-11.07) and B (t (15) =-20.41, p < 0.05, Cohen’s d=-11.78), whereas no significant difference was observed between Groups A and B (p > 0.05). Longitudinal assessments at 7, 14, 30, 60, 90, 120, 150, and 180 days post-surgery demonstrated persistent, significant differences in IOP among the groups (p < 0.001). Specifically, Group C consistently exhibited lower IOP compared to Groups A and B, with no significant variance between Group B and Group C (p > 0.05). Notably, the decrease of IOP in group C one day after Wistend implantation was 8.67 ± 1.03 mmHg, equating to 39-52% of their preoperative levels. Despite a gradual rebound over time, by day 180, the IOP in Group C still remained about 4.83 ± 0.41 mmHg below the baseline, indicating a substantial and sustained reduction of approximately 30% from the preoperative levels. In contrast, Groups A and B maintained IOP within the normal range for rabbits (16.69 ± 2.85 mmHg, i.e., 13.84–19.54 mmHg)50, with no statistically significant differences between preoperative and postoperative periods (Fig. 1).
OCT images
OCT imaging was conducted on three groups of rabbit eyes at intervals of 7, 30, 60, 90, 120, and 180 days postoperatively to verify the correct placement of the Wistend and to detect any early-stage dislocation. Both anterior and posterior segments of each rabbit eye underwent thorough scanning.
During the 180-day follow-up, OCT imaging revealed no evidence of microstent detachment or displacement in Group C. The Wistend implant was stably positioned within the rabbit’s eyes, and was consistently observed within the SC, positioned in the inferior nasal quadrant of the anterior chamber angle (Fig. 2). This placement of the Wistend was characterized by a unique metallic hyperreflective shadow, masking the underlying structure. Wistend probably has the potential to enlarge and dilate the SC as subjectively observed in this study. Importantly, no instances of ciliary body detachment, choroidal detachment, or narrow anterior chamber angle were observed. The anterior chamber depth remained normal with no signs of inflammatory exudates or hemorrhages. Posterior segment OCT analysis further confirmed the absence of retinopathy or optic nerve abnormalities throughout the follow-up duration as noted subjectively by the observer, no objective measurements were obtained.
Slit-lamp biomicroscopy examination
Slit lamp biomicroscopy was used to evaluate postoperative eye responses in the animals. As shown in Tables 1, 2, 3, 4 and 5, conjunctival congestion (0 to 3 points), and conjunctival edema (0 to 2 points), were observed in the eyes of postoperative Groups B and C. These symptoms gradually subsided over time, diminishing to a score of one or less within one month postoperatively, with a validity of 100%. Notably, no corneal vascularization, anterior chamber flare and inflammatory response were observed in either group throughout the study (Fig. 3).
In the Wistend group (n = 6), postoperative week 1 revealed signs of an inflammatory reaction: mild conjunctival congestion in three eyes (1 point), severe conjunctival congestion in one eye (3 points), mild conjunctival edema in two eyes (1 point), and moderate conjunctival edema in 1 eye (2 points). By week 2, these symptoms showed a marked decrease: mild conjunctival congestion was observed in one eye (1 point), moderate conjunctival congestion in another (2 points), mild conjunctival edema in four eyes (1 point), and moderate conjunctival edema in one eye (2 points). By the end of the first month, the symptoms further subsided, with mild conjunctival congestion in three eyes (1 point), and mild conjunctival edema in four (1 point).
In the sham group (n = 6), similar patterns were observed. One week after surgery, there was mild conjunctival congestion in three eyes (1 point) and moderate congestion in one eye (2 points); light conjunctival edema in three eyes (1 point) and moderate conjunctival edema in one eye (2 points). After 2 weeks, the symptoms slightly reduced: one eye had mild congestion (1 point) and one had moderate congestion (2 points); three exhibited mild conjunctival edema (1 point) and one moderate conjunctival edema (2 points). By the one-month mark, only mild congestion in one eye (1 point) and mild conjunctival edema in six eyes (1 point) were observed.
Throughout this study, the blank group (control) did not exhibit any abnormalities. Overall, slit-lamp biomicroscopy revealed no significant differences between the Wistend and sham-operated groups, suggesting that the observed changes were more likely attributable to the surgical procedure rather than the Wistend implant itself.
Color fundus photography
Fundus photographs of three groups were captured at 7, 30, 60, 90, 120, and 180 days after surgery. Across all time points, the images consistently revealed a clear, well-defined optic disc with normal branching of retinal vessels in all eyes. No signs of hemorrhages, exudates, or retinal pigment epithelial abnormalities were observed at any of these intervals (Fig. 4).
SEM images
At 180 days after implantation, SEM showed that the Wistend maintained its primary position with no visible irregular granular debris in the surrounding tissue. The Wistend’s windows and orifices remained clear, encircled by minimal incidental ocular tissue and free from blockage. Additionally, no changes were observed in its surface and interior compared to its pre-implantation state (Supplementary Fig. 3).
Biocompatibility study
After 180 days, H&E staining showed one Wistend-implanted eye exhibited slight capillary hyperplasia and localized fibrosis at the periphery (1 point), without any signs of fat infiltration or inflammation. The other six Wistend implanted eyes showed no significant tissue reaction such as fibrosis, neovascularization, fatty infiltration, or inflammation (0 point). No corneal or scleral abnormalities were evident. Importantly, no polymorphonuclear leukocytes, plasma cells, macrophages, giant cells, lymphocytes, and necrosis were observed in any of these seven Wistend-implanted eyes (Fig. 5).
In the contralateral eyes underwent sham operations, there were no significant signs of fibrosis, neovascularization, fatty infiltration, or inflammatory reaction in the tissues surrounding the sham-operated site, nor any notable corneal or scleral abnormalities (0 point). Furthermore, no polymorphonuclear leukocytes, plasma cells, macrophages, macrophages, giant cells, lymphocytes, or necrosis was seen in any of the seven contralateral eyes. The control group’s eyes also showed no tissue reactions (Fig. 6).
Histopathological examination of sections around both the Wistend and sham surgical sites yielded scores ranging from 0 to 1 for all histological evaluation indices. The difference between Wistend eyes and sham-operated eyes was not statistically significant (p > 0.05).
Discussion
Our study demonstrated the Wistend microstent’s efficacy in significantly reducing IOP in a rabbit model over six months, achieving a sustained 30% reduction from baseline levels. SS-OCT showed SC enlargement due to the Wistend implantation. Postoperative symptoms, including conjunctival congestion, conjunctival edema, and corneal vascularization diminished rapidly to minimal levels within the first month.
In this study, we implanted the Wistend by ab-interno approach, which increased the difficulty of the surgery but remarkably eliminated the events associated with conjunctival and scleral incisions, shortening the surgical time. Moreover, it has a more meaningful reference value for future applications in humans. Fixation of the microstent in vivo has been the focus of research51, and the Wistend did not require additional suture fixation, and OCT results suggested stability of its position during the follow-up period. Wistend works through the trans-TM/SC mechanism, which is the most physiological method for the natural circulation of fluid in the eye52.
Wistend, made from a biocompatible nickel-titanium alloy (Nitinol), exhibited excellent compatibility with ocular tissues. Nitinol has previously been shown to be well tolerated and biocompatible in anterior segment surgeries, as noted in animal studies by Jeffrey L. Olson, and Ian Grierson, among others31,53. Previous studies show that nitinol has been widely used not only in ophthalmology, but also in orthopedics, cardiovascular surgery, neurosurgery, gastrointestinal surgery, and orthodontics, attesting to its suitability and safety for human use54,55,56,57. Nitinol’s unique properties, such as shape memory, superelasticity, and high damping properties, combined with the continuous advancement of surface modification and coating technology, show great promise for clinical use58,59. Our histological analyses further corroborated minimal tissue reactions post-implantation, such as fibrosis and neovascularization.
The Hydrus microstent (Ivantis Inc., Irvine, CA, USA), which also acts on the SC/TM, is characterized by an interchange of windows and spines with a curvature consistent with SC. The Hydrus had a scaffold design to dilate SC, without obstructing outflow access to collector channel ostia60,61. Unlike the Hydrus microstent, the Wistend is designed with a crescent-shaped, curved tubular body, with large enough windows without obstructing the outflow pathway. We speculate that the tubular design in the Wistend theoretically has a potentially more effective ability to stretch the trabecular mesh and support the schlemm tubes, and more drainage windows allow for better collection and drainage of aqueous humor.
At 180 days postoperatively, the IOP in Wistend implanted animal eyes was 13.5 ± 0.55 mmHg, a nearly 30% reduction from baseline IOP, which is lower than the IOP reductions achieved in two prospective randomized clinical trials of the iStent (Glaukos Corporation, Laguna Hills, CA, USA) and Hydrus microstent62. Hypotony postoperative low IOP syndrome, which in turn will cause optic papillae edema and visual acuity loss. The Incidence of hypotony after CyPass (Transcend Medical, Menlo Park, CA, USA) implantation was about 13.8% ~ 15.4%63,64. The hypotensive events in iStent combined cataract surgery was about 1%65. In the present study, the lowest postoperative IOP in Wistend eyes was not less than 8 mmHg. Hypotony and choroidal detachment were not found in this study suggesting Wistend might be likely to be safer.
However, it is worth noting that the subjects selected for these two RCTs were primary open-angle glaucoma patients with an IOP of 23–39 mmHg after preoperative elution of all antihypertensive medications, and the study animals selected for this experiment were normotensive rabbits with a preoperative IOP that lay in the normal range of 13.84–19.54 mmHg, this comparison does not suggest that the Wistend’s ability to lower IOP is weaker than other MIGS devices with similar mechanisms of action.
Previous MIGS devices have also used rabbits as preclinical models proving their clinical translatability31,66. Compared to humans, rabbits are highly sensitive to ocular injuries and show aggressive wound-healing responses67,68,69,70,71. Ian Grierson et al. found histologic analysis demonstrated there was no evidence of an acute or chronic inflammatory response in the Hydrus nickel-titanium alloy microstent31. Mild conjunctival congestion and conjunctival edema, which were expected as early postoperative reactions and largely disappeared within one month of surgery. The incidence and severity of postoperative adverse events did not differ significantly between the Wistend group and the sham-operated group, suggesting that the adverse reactions were mainly related to the implantation procedure rather than to the implant itself. Furthermore, Wistend implantation was positioned in closer proximity to corneoscleral tissues such as the sclera, and conjunctiva which were considered more likely to get inflammatory reactions. Even so, Wistend has excellent biocompatibility with intraocular tissues, did not cause infiltration of inflammatory cells, and effectively avoided and reduced postoperative tissue reactions.
There were several limitations in the present study. Firstly, primate studies could be considered. Secondly, the model chosen for this study was rabbits with normal IOP, and in order to further evaluate the effectiveness of the Wistend when applied to patients with high IOP, a specialized study is currently underway to assess the device’s performance in lowering IOP in rabbits with high IOP models. Thirdly, endothelial cell count is an important analysis to assess the safety of Wistend and should be included in future studies.
Conclusion
In this study, long-term implantation of Wistend implantation in NZW rabbit eyes demonstrated the effectiveness and biocompatibility of implants fabricated from Nitinol for intraocular implantation. Our study suggests Wistend, is intended to obtain more basic and clinical research evidence for the prevention and treatment of blinding eye diseases caused by glaucoma and to develop new minimally invasive anti-glaucoma treatments that will benefit more patients.
IOP measurements showing blank eyes, sham-operated eyes and Wistend eyes during the trial period of 6 months, presented as mean ± SD. The − 7 on the time axis referred to 7 days preoperatively, and 1, 7, 14, 30, 60, 90, 120, 150, and 180 each refer to 1, 7, 14, 30, 60, 90, 120, 150 and 180 days postoperatively. * represented a statistical difference between the Wistend group and the remaining two groups.
Fundus photography. (A1.A2.A3) Fundus photography at 1 week, 1 month, and 6 months postoperatively in the blank group; (B1.B2.B3) Fundus photography at 1 week, 1 month, and 6 months postoperatively in the sham-operated group; (C1.C2.C3) Fundus photography at 1 week, 1 month and 6 months postoperatively in the Wistend group.
Histological evaluation by H&E staining near implant microstent. Figure B was a partial enlargement of Figure A. There was no significant fibrosis, neovascularization, fat infiltration, or inflammatory reaction around the implant. Very minimal fatty infiltration was observed at the corneoscleral rim (±). No polymorphonuclear leukocytes, lymphocytes, plasma cells, macrophages, or giant cells were seen.
Histological evaluation by H&E staining near the sham-operated site. Figure B was a partial enlargement of Figure A. It was similar to the Wistend-implanted eyes. There was only limited fat cell infiltration at the corneoscleral rim (±). Neither abnormal tissue reaction nor abnormal cells were found.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Weinreb, R. N. & Khaw, P. T. Primary open-angle glaucoma. Lancet. 363 (9422), 1711–1720 (2004).
Flaxman, S. R. et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 5 (12), e1221–e34 (2017).
Tham, Y. C. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 121 (11), 2081–2090 (2014).
Leske, M. C. et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 114 (11), 1965–1972 (2007).
Musch, D. C. et al. Visual field progression in the collaborative initial Glaucoma treatment study the impact of treatment and other baseline factors. Ophthalmology. 116 (2), 200–207 (2009).
Nemesure, B. et al. Incident open-angle glaucoma and intraocular pressure. Ophthalmology. 114 (10), 1810–1815 (2007).
Lusthaus, J. & Goldberg, I. Current management of glaucoma. Med. J. Aust. 210 (4), 180–187 (2019).
Wang, T. et al. Topical Medication Therapy for Glaucoma and ocular hypertension. Front. Pharmacol. 12, 749858 (2021).
El Hoffy, N. M. et al. Glaucoma: management and future perspectives for nanotechnology-based treatment modalities. Eur. J. Pharm. Sci. 158, 105648 (2021).
Kompella, U. B., Hartman, R. R. & Patil, M. A. Extraocular, periocular, and intraocular routes for sustained drug delivery for glaucoma. Prog Retin Eye Res. 82, 100901 (2021).
Occhiutto, M. L. et al. Nanotechnology for Medical and Surgical Glaucoma Therapy-A review. Adv. Ther. 37 (1), 155–199 (2020).
Weinreb, R. N., Aung, T. & Medeiros, F. A. The pathophysiology and treatment of glaucoma: a review. Jama. 311 (18), 1901–1911 (2014).
Razeghinejad, M. R., Havens, S. J. & Katz, L. J. Trabeculectomy Bleb-associated infections. Surv. Ophthalmol. 62 (5), 591–610 (2017).
Park, J. et al. Device-modified trabeculectomy for glaucoma. Cochrane Database Syst. Rev. 3 (3), Cd010472 (2023).
Schwartz, G. F. et al. Resuturing the scleral flap leads to resolution of hypotony maculopathy. J. Glaucoma. 5 (4), 246–251 (1996).
Schrieber, C. & Liu, Y. Choroidal effusions after glaucoma surgery. Curr. Opin. Ophthalmol. 26 (2), 134–142 (2015).
Radcliffe, N. The case for standalone micro-invasive glaucoma surgery: rethinking the role of surgery in the glaucoma treatment paradigm. Curr. Opin. Ophthalmol. 34 (2), 138–145 (2023).
Nichani, P. et al. Microinvasive glaucoma surgery: a review of 3476 eyes. Surv. Ophthalmol. 66 (5), 714–742 (2021).
Caprioli, J. et al. Special Commentary: Supporting Innovation for Safe and Effective Minimally Invasive Glaucoma Surgery: Summary of a Joint Meeting of the American Glaucoma Society and the Food andWashington, DC, 26, Ophthalmology, 2015, 122 9: 1795 – 801. (2014).
Saheb, H. & Ahmed, I. I. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr. Opin. Ophthalmol. 23 (2), 96–104 (2012).
Rowson, A.C., Hogarty, D.T., Maher, D. et al. Minimally invasive glaucoma surgery: Safety of individual devices [J]. j. Clin. Med. 11(22), 6833 (2022).
Soohoo, J. R. et al. Minimally invasive glaucoma surgery: current implants and future innovations. Can. J. Ophthalmol. 49 (6), 528–533 (2014).
Richter, G. M. & Coleman, A. L. Minimally invasive glaucoma surgery: current status and future prospects. Clin. Ophthalmol. 10, 189–206 (2016).
Shah, M. Micro-invasive glaucoma surgery - an interventional glaucoma revolution. Eye Vis. (Lond). 6, 29 (2019).
Qin, M., Yu-Wai-Man, C. & Glaucoma Novel antifibrotic therapeutics for the trabecular meshwork. Eur. J. Pharmacol. 954, 175882 (2023).
Tamm, E. R. The trabecular meshwork outflow pathways: structural and functional aspects. Exp. Eye Res. 88 (4), 648–655 (2009).
Braunger, B. M., Fuchshofer, R. & Tamm, E. R. The aqueous humor outflow pathways in glaucoma: a unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 95 (Pt B), 173–181 (2015).
Kasahara, M. & Shoji, N. Effectiveness and limitations of minimally invasive glaucoma surgery targeting Schlemm’s canal. Jpn J. Ophthalmol. 65 (1), 6–22 (2021).
Pu, Z. et al. Study on the role of Carbon in Modifying Second Phase and Improving Tensile Properties of NiTi Shape Memory Alloys Fabricated by electron beam Directed Energy Deposition (Additive Manufacturing, 2023).
Alipour, S. et al. Nitinol: From historical milestones to functional properties and biomedical applications. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine, 236: 1595 – 612. (2022).
Grierson, I. et al. A Novel Schlemm’s Canal Scaffold: histologic observations. J. Glaucoma. 24 (6), 460–468 (2015).
Chae, J. J., Prausnitz, M. R. & Ethier, C. R. Effects of General Anesthesia on intraocular pressure in rabbits. J. Am. Assoc. Lab. Anim. Sci. 60 (1), 91–95 (2021).
Parikh, K. S. et al. Nano-structured glaucoma drainage implant safely and significantly reduces intraocular pressure in rabbits via post-operative outflow modulation. Sci. Rep. 10 (1), 12911 (2020).
Gloe, S. et al. Validation of the Icare(®) TONOVET plus rebound tonometer in normal rabbit eyes. Exp. Eye Res. 185, 107698 (2019).
Lorenzo-Soler, L. et al. Angiotensin receptor blockers in cyclodextrin nanoparticle eye drops: ocular pharmacokinetics and pharmacologic effect on intraocular pressure. Acta Ophthalmol. 99 (4), 376–382 (2021).
Cho, B. J. et al. Monocular retinal degeneration induced by intravitreal injection of sodium iodate in rabbit eyes. Jpn J. Ophthalmol. 60 (3), 226–237 (2016).
Park, J. et al. Biocompatibility evaluation of bioprinted decellularized collagen sheet implanted in vivo cornea using swept-source optical coherence tomography. J. Biophotonics. 12 (11), e201900098 (2019).
Li, H. et al. Investigation of Macular Structural and Microcirculatory Characteristics of Posterior Staphyloma in high myopic eyes by swept source Optical Coherence Tomography Angiography. Front. Physiol. 13, 856507 (2022).
Xin, X. et al. High-resolution image analysis reveals a decrease in Lens Thickness and Cone Density in a cohort of Young myopic patients. Front. Med. (Lausanne). 8, 796778 (2021).
Cheng, D. et al. Characteristics of the Optic nerve head in myopic eyes using swept-source Optical Coherence Tomography. Invest. Ophthalmol. Vis. Sci. 63 (6), 20 (2022).
de Paiva, M. R. B. et al. Assessment of the safety of intravitreal injection of metoprolol tartrate in rabbits. Doc. Ophthalmol. 142 (1), 75–85 (2021).
Li, R. et al. LensAge index as a deep learning-based biological age for self-monitoring the risks of age-related diseases and mortality. Nat. Commun. 14 (1), 7126 (2023).
Dutt, S. et al. Design and performance characterization of a Novel, Smartphone-Based, Portable Digital Slit lamp for Anterior Segment Screening using Telemedicine. Transl Vis. Sci. Technol. 10 (8), 29 (2021).
Eaton, J. S. et al. The SPOTS System: an ocular scoring system optimized for use in modern preclinical drug development and toxicology. J. Ocul Pharmacol. Ther. 33 (10), 718–734 (2017).
Wilhelmus, K. R. The Draize eye test. Surv. Ophthalmol. 45 (6), 493–515 (2001).
Jia, H. Z., Pang, X. & Peng, X. J. Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits. Int. J. Ophthalmol. 14 (1), 26–31 (2021).
Dong, C. et al. Ex vivo cultivated retinal pigment epithelial cell transplantation for the treatment of rabbit corneal endothelial dysfunction. Eye Vis. 10 (1), 34 (2023).
Sakuma, T. et al. Safety of in vivo pharmacologic vitreolysis with recombinant microplasmin in rabbit eyes. Investig. Ophthalmol. Vis. Sci. 46 (9), 3295–3299 (2005).
Grierson, I. et al. A novel suprachoroidal microinvasive glaucoma implant: in vivo biocompatibility and biointegration. BMC Biomed. Eng. 2, 10 (2020).
Zhang, H. et al. Age-Related Variations of Rabbit Corneal Geometrical and Clinical Biomechanical Parameters. Biomed Res Int, 2017: 3684971. (2017).
Siewert, S. et al. Development of a novel valve-controlled drug-elutable microstent for microinvasive glaucoma surgery: in vitro and preclinical in vivo studies. Transl Vis. Sci. Technol. 12 (3), 4 (2023).
Duke-Elder, W. S. The ocular circulation: its normal pressure relationships and their physiological significance. Br. J. Ophthalmol. 10 (10), 513–572 (1926).
Olson, J. L., Velez-Montoya, R. & Erlanger, M. Ocular biocompatibility of Nitinol intraocular clips. Invest. Ophthalmol. Vis. Sci. 53 (1), 354–360 (2012).
Nagaraja, S. & Pelton, A. R. Corrosion resistance of a Nitinol ocular microstent: implications on biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 108 (6), 2681–2690 (2020).
Mani, G. et al. Surface finishing of Nitinol for implantable medical devices: a review. J. Biomed. Mater. Res. B Appl. Biomater. 110 (12), 2763–2778 (2022).
Karsy, M. et al. Emerging technologies in Flow diverters and stents for Cerebrovascular diseases. Curr. Neurol. Neurosci. Rep. 17 (12), 96 (2017).
Kaidar-Person, O. et al. Compression anastomosis: history and clinical considerations. Am. J. Surg. 195 (6), 818–826 (2008).
Marashi-Najafi, F., Khalil-Allafi, J. & Etminanfar, M. R. Biocompatibility of hydroxyapatite coatings deposited by pulse electrodeposition technique on the Nitinol superelastic alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 76, 278–286 (2017).
Mohammadi, F. et al. Chitosan-heparin nanoparticle coating on anodized NiTi for improvement of blood compatibility and biocompatibility. Int. J. Biol. Macromol. 127, 159–168 (2019).
Samet, S., Ong, J. A. & Ahmed, I. I. K. Hydrus microstent implantation for surgical management of glaucoma: a review of design, efficacy and safety. Eye Vis. (Lond). 6, 32 (2019).
Johnstone, M. A. et al. Effects of a Schlemm canal scaffold on collector channel ostia in human anterior segments. Exp. Eye Res. 119, 70–76 (2014).
Ahmed, I. I. K. et al. A prospective randomized trial comparing hydrus and iSTENT microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma: the COMPARE study. Ophthalmology. 127 (1), 52–61 (2020).
Pillunat, L. E. et al. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin. Ophthalmol. 11, 1583–1600 (2017).
Höh, H. et al. Two-year clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary micro-stent. Klin. Monbl Augenheilkd. 231 (4), 377–381 (2014).
Samuelson, T. W. et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 118 (3), 459–467 (2011).
Kao, B. W. et al. Biocompatibility and feasibility of VisiPlate, a novel ultrathin, multichannel glaucoma drainage device. J. Mater. Sci. Mater. Med. 32 (12), 141 (2021).
Bouhenni, R. A. et al. Animal models of glaucoma. J Biomed Biotechnol, 2012: 692609. (2012).
de Feo, F. et al. Histological biocompatibility of a stainless steel miniature glaucoma drainage device in humans: a case report. Toxicol. Pathol. 37 (4), 512–516 (2009).
Khaw, P. T. et al. Effects of intraoperative 5-fluorouracil or mitomycin C on glaucoma filtration surgery in the rabbit. Ophthalmology. 100 (3), 367–372 (1993).
Schultz, G. et al. Growth factors and ocular wound healing. Eye (Lond). 8 (Pt 2), 184–187 (1994).
Norton, J. N. et al. Ocular biocompatibility testing of intraocular lenses: a 1 year study in pseudophakic rabbit eyes. J. Cataract Refract. Surg. 25 (11), 1467–1479 (1999).
Acknowledgements
The authors would like to thank the medical writers, proof-readers and editors for their assistance.
Funding
QL received support from the Henan Medical Science Foundation of China (SBGJ2018083), Basic Research Project of Henan Provincial Eye Hospital (No. 21JCQN005), Natural Science Foundation of Henan Province (222300420196), and 23456 Talent Project of Henan Provincial People’s Hospital.
Author information
Authors and Affiliations
Contributions
MMH and YZ and QL and CGL and JJW and XMF and WJC and QYW and YFW performed the initial research. MMH and YZ completed the statistical analysis. MMH, YZ and ZC prepared the first draft of the manuscript and tables. All authors contributed to the study revision and edited the manuscript. ZC and QL completed critical revision of the manuscript. MMH and YZ were co-first authors. All authors reviewed the manuscript. QL supervised the study and contributed to the final version sent for approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, M., Zhang, Y., Chen, Z. et al. Effectiveness and biocompatibility of a novel Schlemm’s canal microstent for glaucoma management. Sci Rep 14, 24919 (2024). https://doi.org/10.1038/s41598-024-76789-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76789-w