Abstract
Sepsis continues to be a major cause of death and illness globally, posing significant challenges for healthcare professionals. In the pursuit of more accurate and timely monitoring tools, the concept of capnodynamically derived mixed venous oxygen saturation (Capno-SvO2) has emerged as a promising method. Capno-SvO2 provides a non-invasive way to assess and track SvO2 and could serve as an additional tool alongside more invasive methods like the pulmonary artery catheter. This could potentially be of great value in the care of critically ill patients with sepsis, where alternative minimal invasive monitoring methods may vary in reliability. The aim of the current study was to compare capno-SvO2 against values obtained through pulmonary artery blood sample CO-oximetry and continuous fiberoptic SvO2 monitoring, using a well-established porcine experimental sepsis model. Anesthetized pigs were exposed to a standardized endotoxin infusion sepsis protocol, followed by a series of maneuvers typically applied in sepsis care. Simultaneous recordings were done throughout the experiment for all three monitoring methods. Bland–Altman analysis corrected for repeated measurements was used to assess the agreement of absolute values between the paired recording of CO-oximetry and Capno-SvO2 as well as between CO-oximetry and fiberoptic SvO2. The ability of Capno-SvO2 and fiberoptic SvO2 to track changes was assessed by concordance rate. A total of 10 animals and 275 paired datapoints were included in the study. The majority of the animals displayed pronounced hemodynamical instability in response to endotoxin exposure and subsequent treatment interventions. Analysis of all paired data points showed a bias between Capno-SvO2 and CO-oximetry SvO2 of + 1% with 95% limits of agreement of -14% to + 17%. The corresponding numbers for fiberoptic SvO2 and CO-oximetry SvO2 were -4% and -15% to + 8%. The concordance rate as compared to CO-oximetry, were 97% and 93% for Capno-SvO2 and fiberoptic SvO2, respectively. In this experimental sepsis model, continuous, non-invasive Capno-SvO2 generates average absolute values comparable to the gold standard CO-oximetry albeit with relatively wide limits of agreement. Capno-SvO2 displayed a concordance rate of 97% against CO-oximetry and exhibits better trending ability compared to invasive fiberoptic SvO2.
Similar content being viewed by others
Introduction
Sepsis remains a leading cause of mortality and morbidity worldwide, challenging clinicians with its complex pathophysiology and dynamic clinical course. In the pursuit of more accurate and timely monitoring tools, the concept of capnodynamically derived mixed venous oxygen saturation (capno-SvO2) has emerged as a promising method1,2,3. Capno-SvO2 offers a non-invasive mean of monitoring mixed venous oxygen saturation and may be used as a supplement to more invasive SvO2 methods such as Pulmonary Artery Catheter (PAC).
Ever since the introduction of the pulmonary artery catheter in 1970 by Swan and Ganz, mixed venous oxygen saturation (SvO2) has been used as a marker of balance between oxygen delivery and consumption in critically ill patients4. Even though previous literature indicates a co-variability between low SvO2 and poorer outcome, most large, controlled trials have shown variable benefits from the use of PAC regarding clinical outcomes, such as mortality or ICU length of stay5,6,7,8. In addition, concerns regarding catheter-related risks and adverse events still exist9. Recent studies have however again pointed towards a potential beneficial role for PAC in advanced hemodynamic monitoring, provided that the benefits outweigh the risks10. Against this background, it would be valuable to investigate alternative non-invasive techniques intended to monitor mixed venous oxygen saturation.
While previous studies of Capno-SvO2 have been promising in various experimental and clinical settings of both minor and major hemodynamic challenges, the validation against gold standard CO-oximetry in a sepsis model, is lacking. Sepsis is characterized by a state of disturbed mitochondrial function, oxygen extraction and carbon dioxide removal which all together challenges the basis of the capnodynamic method2.
Thus, the primary objective of this study was to compare absolute values of capno-SvO2 against values obtained through pulmonary artery blood sample CO-oximetry and continuous fiberoptic SvO2 monitoring, using a well-established porcine experimental endotoxemic model2. In addition, capno-SvO2 was validated for its ability to detect a change in mixed venous oxygen saturation in response to the development of sepsis and subsequent typical therapeutic interventions.
Methods
This porcine study was carried out at the Hedenstierna Laboratory, Uppsala University, Uppsala, Sweden, in 2023. Authorization was granted by Uppsala Animal Ethics Committee (Uppsala, Sweden case number 5.2.18–8927/16, chairperson Erik Göransson on August 27, 2021). The animals were handled in accordance with the animal experimentation guidelines of the Uppsala Animal Ethics Committee and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
The animals were kept in a light- and temperature-controlled environment, with unlimited access to tap water and food on a standardized schedule. The experiments were done during daytime hours.
Anesthesia protocol
The pigs were anaesthetized as previously described11 and mechanically ventilated in a volume-controlled mode (Servo-I; Maquet, Solna, Sweden), with target tidal volume of 10 ml kg-1 and fraction of inspired oxygen (FiO2) 0.3. Positive end-expiratory pressure (PEEP) was kept at 5 cm H2O after an initial 2-min period of lung expansion using PEEP 10 cm H2O and constant driving pressure as previously described. After the lung expansion manoeuvre, an air test using FiO2 0.21 was performed and repeated after an additional lung expansion manoeuvre if necessary, aiming for sustained pulse oximetry saturation > 97% as indicative of open lung conditions12. The animals were given a bolus of Ringers’ acetate solution 20 ml kg-1 after anaesthesia induction and thereafter kept on maintenance infusion of glucose 25 mg ml -1 8 ml kg-1 h-1 and Ringer’s acetate solution 10 ml kg-1 h-1. Exhaled CO2 was measured by a mainstream infrared CO2 sensor (Capnostat-3; Respironics Inc, Wallingford, CT), and ventilation airflow was registered through the regular flow sensor of the servo-I ventilator. Volumetric capnography was utilised for calculating the CO2 elimination rate (VCO2), as described in previous studies13.
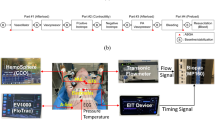
Adequate anesthetic depth and analgesic level were tested regularly during the experiment according to standard procedures of the laboratory as previously described14. The animals were fitted with standard laboratory monitoring devices described in detail in previous studies11. In addition a 7,5F pulmonary artery catheter with fibreoptic SvO2 monitoring (Swan-Ganz pulmonary artery catheter, model 774F75; Edwards Lifesciences, Irvine CA; USA) and a 5F femoral artery cannula for transpulmonary thermodilution cardiac output monitoring (PICCO2™, Pulsion Medical Systems, Munich, Germany), were also included as well as an additional 7,5F pulmonary artery introducer sheath for rapid fluid administration.
The animals were given a bolus dose of intravenous heparin 5000 U (LEO pharma) to minimize the risk of clotting due to the extensive intravascular monitoring setup.
Blood gases were analyzed via blood samples using a CO-oximeter calibrated specifically for porcine hemoglobin (ABL800 Radiometer AS, Copenhagen, Denmark). This also provided the hemoglobin level, necessary for the capnodynamic calculations as well as for calibrations of fiberoptic SvO2.
Assessment of Capno-SvO2
The continuous Capno-SvO2 method, as detailed in previous studies1,2, is based on the differential Fick’s principle. This approach combines the continuous estimation of Effective Pulmonary Blood Flow (EPBF) and oxygen consumption (VO2) within a modified Fick’s equation as illustrated in Eq. 1–3 below2. EPBF is estimated by using a specialized breathing pattern involving variations in respiratory rate. A software (developed for research purposes) in the ventilator produces six breaths with normal rate, followed by three breaths with an approximately 2–3 s expiratory pause. This ventilation pattern induces minor fluctuations in alveolar CO2 concentration and elimination, related to the pulmonary blood flow participating in gas exchange (i.e., EPBF). This enables a breath-by-breath estimation of EPBF15,16,17. (Figures describing the breathing pattern and the setup are available as supplementary material).
The other main determinant of Capno-SvO2, VO2, can be calculated by continuous volumetric capnography measurement of VCO2 combined with the Respiratory Quotient as described in Eq. 1 below.
(Eq. 1, where VO2 is oxygen consumption in ml min-1, VCO2 is CO2 production rate in ml min-1, and RQ is the Respiratory Quotient).
The VCO2 in Eq. 1 is computed as a moving mean value of breath-by-breath CO2 elimination measured over a 20-min period to achieve stable VCO2 values, reflecting an assumed stable metabolism.
Utilizing the classic Fick’s equation18, the oxygen content in mixed venous blood (CvO2) can be derived:
Equation 2, where COEPBF is Effective Pulmonary Blood Flow in L min-1, VO2 is oxygen consumption in ml min-1, CcO2 is pulmonary end capillary oxygen content in ml L-1, and CvO2 is pulmonary mixed venous oxygen content in ml L-1. The pulmonary end capillary blood is assumed to be saturated and in equilibrium with the partial pressure of oxygen in alveolar gas, which was calculated from the alveolar gas equation.
Equation 2 can subsequently be rearranged to obtain CvO2.
CvO2 from Eq. 3 is then used to calculate SvO2, employing the solubility constant for oxygen in blood plasma, Hüfner’s constant, and the hemoglobin value, as described and validated in previous publications, thereby generating breath-by-breath estimations of SvO21,2.
Respiratory quotient
For this study, we used the RQ 0.97 calculated in a previous porcine experimental study using a similar setup and cohort of animals1.
Study protocol
Simultaneous recordings of all three modalities for SvO2 i.e. capno-SvO2, CO-oximetry SvO2 and fiberoptic SvO2 were done throughout the protocol.
After the initial preparation, the animals were allowed a 20-min stabilization period before the protocol was started. A blood sample was drawn from the pulmonary artery and the fiberoptic SvO2 monitoring system was calibrated according to the manufacturer’s description. Then a series of five consecutive readings of all three SvO2 methods was done, five minutes apart, to calculate their respective inherent precision. After that, just prior to the initiation of the sepsis protocol, a baseline sample was taken. After the precision measurements, the protocol consisted of two sections, outlined below, where all three SvO2 methods (i.e., CO-oximetry, fiberoptic and capnodynamics) were recorded in parallel.
-
1.
SvO2 reaction under the development of sepsis.
-
2.
The effects on SvO2 of standardized treatment actions in sepsis.
Experimental endotoxemic model
A well-established experimental endotoxemic protocol was used based on an intravenous challenge of endotoxin (lipo-polysaccharide, LPS), given at a rate of 2 µg × kg−1 × h−1 throughout the rest of the experiment. The LPS infusion causes a hemodynamically unstable situation, intended to mimic the accelerating development of sepsis19. A standardized goal-directed intervention protocol was used during this phase of the experiment, to ensure the survival of the subjects for the second part of the protocol (see appendix 1 for goal directed protocol)20.
For the first hour after initiation of the LPS infusion, SvO2 data was recorded every 10 min to capture the development of sepsis and its effect on mixed venous oxygen saturation.
After this initial hour, the animals were allowed approximately one hour of stabilization before the second part of the protocol was carried out. Before commencing the second part of the study protocol, we strived to reach the baseline hemodynamic status recorded prior to the start of LPS challenge since some of the ongoing interventions associated with the goal-directed stabilization (i.e., noradrenaline-infusion and high inspired fraction of oxygen) could interfere with the second part of the study protocol. After a recalibration of the fiberoptic module, the second part of the protocol was started, intended to simulate typical interventions associated with sepsis stabilization in the intensive care setting. Six different challenges were carried out in the order described below.
-
1.
Crystalloid bolus: 15 ml kg-1 of lactated Ringer solution was administered through the PAC introducer over 3–5 min. Simultaneous recordings of all three SvO2 monitoring modalities were done just prior to the infusion and twenty minutes after termination of the fluid bolus.
-
2.
Stepwise increase and decrease in FiO2: The oxygen level was changed every fifth minute from 0.3 – 0.5 – 0.8 – 1.0 –0.3. Recordings of SvO2 were made just before each change in FiO2.
-
3.
Alteration of positive end-expiratory pressure (PEEP): The last recording from the previous step (i.e., 5 cm H2O in step 2 FiO2 0.3) was used as baseline. PEEP was then altered from 10 – 15 – 5 cmH2O and SvO2 recordings were done at the end of each step after a short stabilization period (approximately 1–2 min)
-
4.
Dobutamine: The last reading from the previous step was used for baseline. A central dobutamine infusion running at 15 mcg kg-1 min-1 was started and new recordings were made after 15 min after which the infusion was terminated.
-
5.
Norepinephrine: A new baseline was recorded 15 min after termination of dobutamine (to allow wash out of dobutamine and return to baseline hemodynamics) and a central norepinephrine infusion of 0.1 mcg kg-1 min-1 was started. If norepinephrine was already running due to hemodynamic instability (as per the goal-directed treatment plan described in appendix 1), the infusion rate was increased by 0.1 mcg kg-1 min-1. A recording was done after 10 min, and the infusion was then stopped or returned to the original infusion rate used prior to the increase
-
6.
Hemorrhage: A new baseline was done 10 min after termination of norepinephrine infusion. 15 ml kg-1 blood was then withdrawn from the PA introducer sheath and stored in a plastic bag intended for donor blood, containing 5000 U of Heparin (LEO Pharma). SvO2 values were recorded 10 min after completion of the hemorrhage.
-
7.
Restitution of whole blood: the blood was transfused back over a 5–10 min period and SvO2 data were recorded 10 min after completed transfusion.
After the experiment, the animals were euthanized using intravenous potassium chloride (KCl: 100 mmol) via the central venous catheter as per established standards at the laboratory.
Statistical analysis
Raw data for absolute values of all three SvO2 methods (CO-oximetry, Capno-SvO2 and fibreoptic SvO2), as well as paired differences between Capno-SvO2 and CO-oximetry and between fibreoptic SvO2 and CO-oximetry, were controlled for normal distribution by D’Agostino and Pearson test and visual inspection of the corresponding histograms. Values are presented as mean and 95% confidence interval (CI).
The first five measurements, during hemodynamically stable baseline conditions, were used for the calculation of inherent precision for each SvO2 method respectively. Inherent precision was defined as 2 times the coefficient of variation (CV = SD/mean)21 and was in turn used to quantify the least significant change (LSC) for the reference method CO-oximetry. The LSC reflects the minimum change measured and recognized as a true change21. LSC for the reference method was then used to determine the exclusion zone in the concordance analysis as previously described2.
Bland–Altman analysis corrected for repeated measurements was used to assess the agreement of absolute values between the paired recording of CO-oximetry and Capno-SvO2 as well as between CO-oximetry and fibreoptic SvO2. Bias was defined as the mean difference between the tested methods and the reference method22,23. Limits of agreement were used when presenting the spread of included data points and the corresponding 95% CI calculated. No sample size calculation was done, rather a sample size of 10 animals was used based on previous studies with a similar setup. 12 animals were included for upward margin1. Acceptable limits of agreement against CO-oximetry were set to ± 15% based on previous publications on the performance of fiberoptic SvO2 monitoring which represents an alternative to Capno-SvO2 monitoring24,25,26.
The ability of Capno-SvO2 and fibreoptic SvO2 to track changes in SvO2 compared to the reference method, was assessed by calculating the concordance rate, i.e. the percentage of data points moving in the same direction when comparing two different techniques27. Datapoints representing the anticipated extremes of SvO2 within each intervention were identified, i.e., FiO2 0.3 and 1.0, or the datapoints just before and after whole blood transfusion. Recorded changes between these extremes, before and after each hemodynamic intervention, are displayed in a 4-quadrant plot and used for the calculation of the concordance rate. The concordance rate is presented with a corresponding 95% CI as previously described28. A concordance rate of > 92% was considered clinically useful27.
GraphPad Prism (version 9.0.0 for Windows, GraphPad Software, San Diego, CA, USA) and Medcalc Statistical Software version 16.8.4 (MedCalc Software, Ostend, Belgium) was used for statistical calculations and Microsoft Excel for Mac 2020 version 16.41 for data handling.
Results
Twelve domestic-breed pigs of both sexes (median weight 31.5 kg, range 28.7–33.3 kg, 6–8 weeks of age), from the same breeding colony were used. Two of the animals (on two consecutive days) were highly hemodynamically unstable. One of these two animals died early during the experiment and the second animal suffered a sustained severe pulmonary hypertensive reaction throughout the study. They were both excluded from the analyses since the validity of the data collected was deemed compromised by the ongoing resuscitation of the animals and by the lack of hemodynamic stability before the start of the protocol. Both animals were investigated on a separate week, not related to the remaining subjects. The remaining 10 animals all survived the experiment, and their data were included in the study. Under step 2 in the second part of the protocol, the FiO2 1.0 step was introduced starting from the sixth animal to enable the calculation of any pulmonary shunt (data not shown) and therefore contains data from five animals in total.
Calculated inherent precision and least significant change
The inherent precision was found to be ± 11% for Co-oximetry SvO2 and ± 6% and ± 7% for Capno-SvO2 and fiberoptic SvO2, respectively, which is in line with previous publications1,2. The LSC was calculated to 16%, corresponding to a minimal change in SvO2 of 10 percentage points, i.e., a change in the reference method CO-oximetry SvO2 has to be more than 10 percentage points in magnitude in order to be regarded as a real change and not attributed to imprecision in measurement21.
Response to hemodynamic challenges
The majority of the animals showed a profound hemodynamic response to LPS-infusion and 6 out of 10 animals needed temporary stabilizing actions as per the goal directed protocol (e.g., norepinephrine, increased PEEP, FiO2 and crystalloid fluid resuscitation). The animals typically stabilized hemodynamically after the initial hour after starting LPS, but a few needed interventions later in the protocol during PEEP and hemorrhage interventions. The crystalloid fluid bolus resulted in a slight decrease in SvO2, as measured by the reference method, while the rest of study protocol following the endotoxemic induction affected SvO2, as measured by all methods, in a physiologically anticipated manner. The changes in SvO2 for each hemodynamic intervention is depicted in the event plot (Fig. 1).
Event plot showing SvO2 responses to the various hemodynamic challenges for all three SvO2 monitoring methods. Values are mean (95% CI). N = 10 (refers to number of animals). Vertical black arrows indicate recalibration points for fibreoptic SvO2. BL1-5: Precision Baseline, Induction BL: Baseline before induction sepsis, FiO2: Fraction of inspired oxygen; PEEP: Positive End Expiratory Pressure (cmH2O), Norepi; Norepinephrine, HemBL; Baseline before start hemorrhage.
Agreement of absolute values
A Bland–Altman analysis was performed for all paired data points and showed a bias between Capno-SvO2 and Co-oximetry SvO2 of + 1% with 95% limits of agreement were -14% (95%CI-22 to -10%) to + 17% (95%CI 13 to 25%). The corresponding bias between fiberoptic SvO2 and CO-oximetry SvO2 was -4% with 95% limits of agreement -15% (95%CI -17 to -14) to + 8% (95%CI 7 to 10%). All values refer to absolute percentage points. Bland–Altman plots for both Capno-SvO2 and fiberoptic SvO2 vs Co-oximetry SvO2 are displayed in Fig. 2. The agreement of absolute values for the sepsis induction and each hemodynamic intervention are described in Table 1.
Trending ability
The trending ability for Capno-SvO2 and fiberoptic SvO2 are presented in a 4-quadrant plot, Fig. 3. For Capno-SvO2, 70 datapoints were found outside the exclusion zone. Of these, 68 changed in the same direction as the reference method producing a concordance rate of 97% (95%CI 95 to 99%). Corresponding figures for fiberoptic SvO2 were a total of 70 where 5 were moving in the opposite direction, resulting in a concordance rate of 93% (95%CI 88 to 96%).
Four quadrant plots presenting the concordance between Capno-SvO2 and CO-oximetry SvO2 (A) and between fiberoptic SvO2 and CO-oximetry SvO2 (B). A: 70 paired delta values, B: 70 paired delta values. The central boxed area illustrates the 10% exclusion zone. Dotted line is line of identity. N = 10 (refers to number of animals).
Discussion
The main finding of the current study was that Capno-SvO2 generates average absolute values close to the gold standard CO-oximetry with relatively wide limits of agreements, although still near the predefined limit of 15 percentage points. In addition, Capno-SvO2 has a concordance rate of 97% against CO-oximetry and demonstrates a slightly better ability to detect change than the invasive fiberoptic SvO2with 93%,
Agreement of absolute values between CO-oximetry and the tested methods
The tested methods showed a low variance during stable baseline conditions, with lower calculated inherent precision than the reference method. For the co-oximetry however, the inherent precision was somewhat higher than previously found using a similar model1. The Bland–Altman analysis showed a low mean difference between CO-oximetry and Capno-SvO2 (bias 1%), whereas the fiberoptic module slightly underestimated the SvO2 on average (bias -4%). The 95% limits of agreement for Capno-SvO2 were higher than previously found during major hemodynamic changes during non-sepsis conditions but were still close to the preset acceptable level of agreement of a maximum of 15%. In a similar manner, fiberoptic SvO2 also displayed a wide spread of the limits of agreement but still with values within the preset acceptable limit.
During stable baseline conditions the agreement between Capno-SvO2 and the reference method was within acceptable limits. However, this relation was negatively affected by the introduction of toxin, where bias and limits of agreements increased as outlined in Table 1. Possible reasons are discussed further below. The disturbances were maintained throughout the treatment protocol even though certain maneuvers were associated with better agreement.
Several potential reasons for the wider spread of data points than previously reported with similar protocols using non-sepsis hemodynamic scenarios can be identified.
Firstly, even if the Bland–Altman analysis is considered a standard statistical tool when comparing different monitoring systems, it may not be ideal for the assessment of rapid physiological changes29. Ideally, a study protocol would allow for the biological system to reach equilibrium after hemodynamic interventions and, subsequently, make recordings once stable conditions are reached29. A problem arises when studying unstable subjects with different monitoring techniques characterized by different response times. Theoretically, a new monitoring device producing the exact same values as the reference method, but with a time delay, would therefore in the presence of severe physiological instability, perform poorly in a Bland–Altman plot (explicatory figure available in supplements).
When comparing Capno-SvO2 to fiberoptic SvO2 and CO-oximetry from a pulmonary artery catheter, it is therefore important to emphasize the different nature of these methods. Whereas the two latter are based on measurements from a catheter positioned in the pulmonary artery, Capno-SvO2 is a mathematically produced number based on continuous measurement of EPBF and FiO2 as described in the methods section. This explains some special features of the Capnodynamic method. In the present capnodynamic algorithm, EPBF is calculated as a moving mean of the nine last breaths and continuously updated for each new breath. Capno-SvO2, in turn, is shown in real time based on the present values of EPBF and FiO2 and is displayed as if the biological system had already reached equilibrium. This means that sudden variations in EPBF and FiO2 will produce a change in Capno-SvO2 immediately, while the in vivo change of actual SvO2 would be reached after a certain time delay. To exemplify this, we made simultaneous SvO2 recordings of all three methods just before and five minutes after euthanasia in one animal; Capno-SvO2 decreased from 29 to 0% (due to the sudden interruption of recorded breath-by-breath VCO2 and therefrom deduced EPBF) within 30 s after cardiac arrest while fiberoptic and CO-oximeter SvO2 increased from 37 to 41% and fiberoptic SvO2 decreased from 44 to 38%. The same rationale applies to some data points recorded in hemodynamically unstable animals during certain maneuvers (i.e. endotoxemic induction, PEEP elevation and hemorrhage) where Capno-SvO2 briefly displayed very low numbers, sometimes even zero. If desired, it would be possible to present Capno-SvO2 as a moving mean of e.g. 50 s rather than nine breaths, to make the method slightly less reactive in situations with rapid hemodynamic change. Also, introducing a delay to compensate for the difference in response time between CO-oximetry and Capno-SvO2 is a possibility, even though this was not further investigated in this study. In Fig. 4, individual continuous data for Capno-SvO2 and fiberoptic SvO2, are presented for animals nr 5 and nr 7. Capno-SvO2 is shown both in the original version and as a filtered (moving mean over 50 s) version. CO-oximetry data are plotted for reference.
Looking at the agreement of absolute values for different parts of the study protocol (Table 1), the wide limits of agreement during the endotoxemic induction are noticeable. Based on the reasoning above, this is not unexpected since the recordings are done strictly every tenth minute during a phase where many animals were highly hemodynamically deranged. This, as opposed to making an intervention, waiting ten minutes for stabilization and then record the data.
Ability to detect change
As in previous studies, Capno-SvO2 showed a reliable ability to detect change (concordance rate 97%) when compared to the reference method. This is reassuring since tracking changes is important in the clinical setting. Fiberoptic SvO2 displayed a concordance rate of 93% which is also above the limit of the preset target of clinical reliability (i.e., > 92%)27. The higher than anticipated inherent precision of the reference method resulted in a somewhat large exclusion zone (10%) used for the concordance analyses. An exclusion zone of 5% would in this material result in a concordance rate of 95% for Capno-SvO2. This could be of interest for the reader since also smaller changes in SvO2 can be of clinical importance.
Limitations
Firstly, the current study uses an experimental model of endotoxemia associated with a relatively fast acceleration of hemodynamic deterioration. In the clinical context, severe sepsis is unlikely to lead to such profound reactions already within 20 min of contact with the infecting agent. In addition, the experimental sepsis model used produces a decrease in SvO2 and not an increase in SvO2 as can be seen with mitochondrial dysfunction30. It is therefore not possible from the current study to fully conclude how the capnodynamic method performs in a situation with high SvO2 resulting from mitochondrial dysfunction.
Secondly, as discussed above the response time of the tested method may interfere with its ability to generate absolute values timed with values obtained from CO-oximetry. This could potentially be compensated for using a time delay even if this was not investigated in detail in the current study. A similar discussion, regarding the difference in response time between a test method and reference method, has previously been debated in the context of cardiac output monitoring29.
Thirdly, the goal-directed therapy protocol used to ensure the survival of the subjects may have led to differences in hemodynamic status between animals. This is likely due to an individual response to LPS, generating variations in inflammatory and hemodynamic response, mirroring the characteristics of sepsis in the clinical setting where the reaction can differ substantially between individuals. However, we believe that the main aim of the study, i.e., comparison of absolute values can still be viewed as valid.
Lastly, in this study RQ was assumed stable throughout the experiment, which might not be true for subjects developing sepsis or under treatment in the intensive care unit. Since reliable RQ measurement is cumbersome to perform and not readily available in most clinical settings we chose to study the performance of Capno-SvO2 using a single, preset RQ value. Ideally, RQ would have been individually measured multiple times throughout the experiment and future monitoring techniques making this feasible could possibly improve the accuracy of Capno-SvO2. Nevertheless, the mostly low variation of bias of Capno-SvO2 between different phases of the experiment (Table 1), indicates a minor influence of shifting RQ under these study settings. A higher or lower RQ value would shift the calculated SvO2, in the same direction but would not affect the spread of datapoints, i.e., the limits of agreement.
Clinical applicability
Despite that Capno-SvO2 in its current form may not fully replace the use of a pulmonary artery catheter for SvO2 monitoring, the method could potentially play a role where invasive assessment of SvO2 is not feasible or warranted. This could be due to patient characteristics (i.e. pediatric patients), availability, staffing, or training issues. Furthermore, initiating Capno-SvO2 monitoring on an already intubated patient under controlled ventilation conditions is fast and normally uncomplicated.
It is also important to emphasize that the preset acceptable limits of agreement of + /- 15 percentage points do not necessarily apply in clinical context where such divergence might be unacceptable. The wider limits of agreements for Capno-SvO2 in this sepsis model, as compared to previous published data, is probably at least partly due to differences in response time in the investigated monitoring techniques, as lined out in the previous text. However, this was not á priori addressed in the experimental setup and individual, absolute values of Capno-SvO2 should be interpreted cautiously and in conjunction with other available hemodynamic parameters for the subject. On the other hand, Capno-SvO2 reliably detects changes and has the potential to early identify significant hemodynamic change, also in subjects with sepsis.
In its current form, Capno-SvO2 requires an intubated patient and controlled ventilation in order to generate controlled fluctuations in exhaled CO2, which may be a limitation for widespread use. However, these prerequisites would often already be met when a subject is deemed in need of SvO2 monitoring.
Conclusions
In the current experimental endotoxemic model, continuous, non-invasive Capno-SvO2 generates average absolute values close to the gold standard CO-oximetry, but with relatively wide limits of agreement. Furthermore, Capno-SvO2 displayed a concordance rate of 97% compared to CO-oximetry and exhibits slightly better performance in trending ability compared to invasive fiberoptic SvO2.
Data availability
Data will be available from the corresponding author upon reasonable request.
Abbreviations
- SvO2 :
-
Mixed venous oxygen saturation
- PAC:
-
Pulmonary artery catheter
- VO2 :
-
Oxygen consumption
- FiO2 :
-
Fraction of inspired oxygen
- VCO2 :
-
CO2 elimination rate
- LPS:
-
Lipo-polysaccharide
- PEEP:
-
Positive end expiratory pressure
- EPBF:
-
Effective pulmonary blood flow
- RQ:
-
Respiratory quotient
- CcO2 :
-
Pulmonary end capillary oxygen content
- CvO2 :
-
Oxygen content in mixed venous blood
References
Svedmyr A, Konrad M, Wallin M, Hallbäck M, Lönnqvist PA, Karlsson J. Non-invasive capnodynamic mixed venous oxygen saturation during major changes in oxygen delivery. J Clin Monit Comput. 2021.
Karlsson J, Lönnqvist PA, Wallin M, Hallbäck M. A Continuous Noninvasive Method to Assess Mixed Venous Oxygen Saturation: A Proof-of-Concept Study in Pigs. Anesth Analg. 2020.
Svedmyr A, Steiner K, Andersson A, Sjöberg G, Hallbäck M, Wallin M, et al. Validation of a Novel Method for Noninvasive Mixed Venous Oxygen Saturation Monitoring in Anesthetized Children. Anesth Analg. 2024; Oct 1;139(4):781–788..
Swan, H. J. et al. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 283(9), 447–451 (1970).
Holm, J., Håkanson, E., Vánky, F. & Svedjeholm, R. Mixed venous oxygen saturation predicts short- and long-term outcome after coronary artery bypass grafting surgery: a retrospective cohort analysis. Br J Anaesth. 107(3), 344–350 (2011).
Goonasekera, C. D. A., Carcillo, J. A. & Deep, A. Oxygen Delivery and Oxygen Consumption in Pediatric Fluid Refractory Septic Shock During the First 42 h of Therapy and Their Relationship to 28-Day Outcome. Front Pediatr. 6, 314 (2018).
Harvey, S. et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. The lancet. 366(9484), 472–477 (2005).
Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane library. 2013;2013(2):CD003408.
Kalra, A., Heitner, S. & Topalian, S. Iatrogenic pulmonary artery rupture during Swan-Ganz catheter placement–a novel therapeutic approach. Catheter Cardiovasc Interv. 81(1), 57–59 (2013).
Szabo, C., Betances-Fernandez, M., Navas-Blanco, J. R. & Modak, R. K. PRO: The pulmonary artery catheter has a paramount role in current clinical practice. Ann Card Anaesth. 24(1), 4–7 (2021).
Karlsson, J. et al. Validation of capnodynamic determination of cardiac output by measuring effective pulmonary blood flow: a study in anaesthetised children and piglets. Br J Anaesth. 121(3), 550–558 (2018).
Ferrando, C. et al. The accuracy of postoperative, non-invasive Air-Test to diagnose atelectasis in healthy patients after surgery: a prospective, diagnostic pilot study. BMJ Open. 7(5), e015560 (2017).
Suarez-Sipmann, F., Bohm, S. H. & Tusman, G. Volumetric capnography: the time has come. Curr Opin Crit Care. 20(3), 333–339 (2014).
Karlsson, J., Svedmyr, A., Wallin, M., Hallbäck, M. & Lönnqvist, P. A. Validation of an alternative technique for RQ estimation in anesthetized pigs. Intensive Care Med Exp. 12(1), 11 (2024).
Peyton, P. J., Wallin, M. & Hallbäck, M. New generation continuous cardiac output monitoring from carbon dioxide elimination. BMC Anesthesiol. 19(1), 28 (2019).
Gedeon, A., Krill, P. & Osterlund, B. Pulmonary blood flow (cardiac output) and the effective lung volume determined from a short breath hold using the differential Fick method. J Clin Monit Comput. 17(5), 313–321 (2002).
Hällsjö Sander, C. et al. Novel continuous capnodynamic method for cardiac output assessment during mechanical ventilation. Br J Anaesth. 112(5), 824–831 (2014).
Fick A. Uber die messung des Blutquantums in den Hertzvent rikeln. Sitzber Physik Med Ges Wurzburg 1870. p. 290–91.
Lipcsey, M., Larsson, A., Eriksson, M. B. & Sjölin, J. Inflammatory, coagulatory and circulatory responses to logarithmic increases in the endotoxin dose in the anaesthetised pig. J Endotoxin Res. 12(2), 99–112 (2006).
Otterbeck, A. et al. Intravenous anti-. Innate Immun. 28(7–8), 224–234 (2022).
Cecconi, M., Rhodes, A., Poloniecki, J., Della Rocca, G. & Grounds, R. M. Bench-to-bedside review: the importance of the precision of the reference technique in method comparison studies–with specific reference to the measurement of cardiac output. Crit Care. 13(1), 201 (2009).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1(8476), 307–310 (1986).
Bland, J. M. & Altman, D. G. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 17(4), 571–582 (2007).
Herner, A. et al. Accuracy and precision of ScvO2 measured with the CeVOX-device: A prospective study in patients with a wide variation of ScvO2-values. PLoS One. 13(4), e0192073 (2018).
Kissoon, N., Spenceley, N., Krahn, G. & Milner, R. Continuous central venous oxygen saturation monitoring under varying physiological conditions in an animal model. Anaesth Intensive Care. 38(5), 883–889 (2010).
Armaganidis, A. et al. Accuracy assessment for three fiberoptic pulmonary artery catheters for SvO2 monitoring. Intensive Care Med. 20(7), 484–488 (1994).
Critchley, L. A., Lee, A. & Ho, A. M. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 111(5), 1180–1192 (2010).
Axiak Flammer, S. M. et al. Reliability of lithium dilution cardiac output in anaesthetized sheep. Br J Anaesth. 111(5), 833–839 (2013).
Magnúsdóttir, S. O., Simonsen, C., Rasmussen, B. S., Lund, P. E. & Kjaergaard, B. Continuous cardiac output measured with a Swan-Ganz catheter reacts too slowly in animal experiments with sudden circulatory failure. Animal Model Exp Med. 5(3), 258–265 (2022).
Wittayachamnankul, B. et al. High central venous oxygen saturation is associated with mitochondrial dysfunction in septic shock: A prospective observational study. J Cell Mol Med. 24(11), 6485–6494 (2020).
Acknowledgements
To the faculty at the Hedenstierna Laboratory in Uppsala, Sweden.
Funding
Open access funding provided by Karolinska Institute. This work was supported by the Karolinska Institute and partially by unrestricted grants from Maquet Critical Care AB and the regional agreement on medical training and research (ALF) between Stockholm County Council and the Karolinska Institute.
Author information
Authors and Affiliations
Contributions
A.S. and J.K.–Study design, data collection, statistical analysis, figures and manuscript writing. M.H.–Study design, data collection, statistical analysis and critical manuscript review. M.W., PA.L., J.H. and M.L.–Study design and critical manuscript review. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.H. is employed by Maquet Critical Care AB, M.W. is a previous employee of Maquet Critical Care AB, PA.L. received an unrestricted research grant from Maquet Critical Care AB. The other authors declare no conflict of interest.
Ethics approval and consent to participate
Authorization was granted by Uppsala Animal Ethics Committee (Uppsala, Sweden case number 5.2.18–8927/16, chairperson Erik Göransson on August 27, 2021). The animals were handled in accordance with the animal experimentation guidelines of the Uppsala Animal Ethics Committee and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Consent for publication
N/A.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Svedmyr, A., Hedov, J., Lipcsey, M. et al. Capnodynamic assessment of mixed venous oxygen saturation in a porcine experimental endotoxemic model. Sci Rep 14, 26807 (2024). https://doi.org/10.1038/s41598-024-77483-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77483-7