Abstract
This study evaluated changes in retinal sensitivity (RS) during aflibercept treatment in neovascular age-related macular degeneration (nAMD) patients with good vision. Treatment-naive nAMD patients with visual acuity better than 20/40 were treated with an aflibercept loading regiment, and RS was measured using the MAIA microperimetry device. The study examined RS differences based on visit, region, adjacent local pathology, outer retinal changes and optical coherence tomography angiography (OCTA) data were investigated. Twelve patients completed the protocol. Mean RS improved from 17.9 (3.9) µm at baseline to 20.4 (4.7) µm at final visit, while best-corrected visual acuity (BCVA) did not change. (P = 0.041) RS at baseline is worst in the presence of intraretinal fluid (IRF) and improved after treatment for all retinal pathologies except IRF. RS improvement occurred even with initial outer retinal damage. A Linear mixed model showed subretinal hyperreflective material, baseline IRF height, outer retinal integrity, fibrovascular pigment epithelial detachment height, and local choroidal vascular index as factors associated with RS. In conclusion, RS improved with aflibercept treatment in nAMD eyes with good initial visual acuity, despite no change in BCVA, except when IRF was present.
Similar content being viewed by others
Introduction
Neovascular age-related macular degeneration (nAMD) is a sight-threatening disease with a devastating prognosis if left untreated. Anti-VEGF therapy has become the standard of care for nAMD since its introduction in 2006. Aflibercept, the second approved anti-VEGF agent for nAMD, is currently widely used. The VIEW1 and VIEW2 studies, phase 3 pivotal trials of aflibercept, reported an improvement in visual acuity of 8.3 to 9.3 letters over one year1. In real-world observation, the visual improvement at 1 year with aflibercept showed − 0.19 to 5.1 letters, which is relatively less than that of the VIEW study2,3.
Pivotal studies show more favorable results than real-world clinical practice. This may be due to a relatively smaller number of injections or variable patient severity in real-world data. However, eyes expected to have a worse prognosis, with fibrosis or excessive bleeding, were excluded and only eyes with relatively worse vision, not exceeding 20/40, were included, as the purpose of the study is to demonstrate significant treatment efficacy1,4. Eyes with better visual acuity at baseline are likely to have a favorable outcome while showing less improvement or even deterioration in vision compared to eyes with worse visual acuity5,6. The ceiling effect may limit the visual gain of treatment in the already better-seeing eye in terms of best corrected visual acuity (BCVA). Meanwhile, there are several psychophysical tests that can measure visual function and potentially compensate for visual acuity testing in patients with better vision. Microperimetry, also known as fundus-controlled perimetry, provides a spatially resolved mapping of visual function across the macula and enables the tracking of such function via live fundus image registration. The use of microperimetry as an outcome measure in clinical trials has recently increased because of its ability to detect subtle retinal sensitivity (RS) defects that precede changes in visual acuity and to track disease progression over relatively short periods of time7. Therefore, we designed a prospective study to evaluate the improvement in visual function using microperimetry in treatment-naive nAMD patients with better visual acuity.
Result
Twelve participants completed the study protocol. Patient characteristics are described in Table 1. Mean RS improved significantly from 1 month after the first injection and was maintained until the 3 month visit (last visit) (17.9 (3.9) dB at baseline to 20.4 (4.7) dB at last visit, mean (std), P = 0.041, Wilcoxon signed-rank test). (Fig. 1) However, the mean BCVA did not change. We also compared the RS of the fellow eye between baseline and last visit to investigate whether or not the learning effect of microperimetry is present. Neither BCVA nor RS changed in the fellow eyes. nAMD was not activated in the fellow eye that did not require treatment during the study period.
Boxplot of average best corrected visual acuity (BCVA) and retinal sensitivity change. (A) Average retinal sensitivity change of the study eye (left) and the fellow eye (right) (B) average BCVA change of the study eye (left) and the fellow eye. (right) median and mean value of each visit are presented below x axis. Asterisk (*) indicates difference between baseline and each visit by Wilcoxon signed rank test is statistically significant (P < 0.05) Note that retinal sensitivity improved in study eye from the visit 2 (1 month after the first aflibercept injection) through visit 4 while that of fellow eye showed no change. However, BCVA was not improved.
We also examined the change in RS in different regions of the retina. RS improved in all eccentricities and quadrants from 1 month after the first injection (Fig. 2). However, RS of the innermost region (S4, RS at the fovea), outermost region (ring 1, RS 5 degrees from the fovea) and supero-nasal quadrant did not improve significantly compared to baseline at the final visit.MNV area decreased from 2.14 (2.72) to 1.67 (2.44) mm2 (mean (standard deviation)) (P value < 0.001, Wilcoxon signed-rank test). (Fig. 3) Patient 8, who had the largest area of MNV, had polypoidal choroidal vasculopathy (PCV). Subretinal fluid (SRF), intraretinal fluid (IRF), pigmented epithelial detachemt [PED, serous PED (SPED) and fibrovascular PED (FPED)] and subretinal hyperreflective material (SHRM) decreased rapidly from the first injection. (Fig. 4, line graph)
Regional differences in retinal sensitivity change. This line plot shows the difference of average retinal sensitivity change depending on the region of same distance from fovea (A) and each quadrant (B). Lineplots are differentiated by color and the legend is at right side. Retinal region is described at right lower part of A. Retinal sensitivity improved from one month after first injection in all the region. The improvement was not statistically significant in S4 (foveal region), ring 1 (outermost ring, 5 degree from the fovea) and supero-nasal region (SN). Asterisk (*) indicates difference between baseline and each visit by Wilcoxon signed rank test is statistically significant (P < 0.05). ST: supero-temporal region; IT: infero-temporal region; IN: infero-nasal region; SN: supero-nasal region.
Boxplot of average retinal sensitivity (RS) depending on the baseline local retinal pathology and line plot showing height of pathologic lesion. Average RS is presented at the bottom of each graph. (mean RS) (A) Average baseline RS. Baseline RS was worst when IRF is present. And RS at SHRM follows. (B) nl: No abnormality is found (C) SRF: Subretinal fluid (D) IRF: Intraretinal fluid (IRF) (E) SPED: Serous pigment epithelial detachment (F) FPED: Fibrovascular pigment epithelial detachment (G) SHRM: Subretinal hyperreflective material (H) NVC: Neovascular complex. Multiple retinal pathology can be present at the same loci simultaneously. Asterisks (*) indicates when P value of Wilcoxon signed rank test is below 0.05, comparing average RS of each visit to that of baseline visit (visit 1). RS over the any kind of retinal pathology improved from the one month after the intravitreal aflibercept injection, except for the IRF and SPED. While RS on the SPED improved at last visit, that of IRF showed improvement only at visit 2. The height of lesion decreased rapidly from one month after the first treatment and almost resolved at last visit in all pathology.
Point-wise analysis showed that baseline RS is worst in IRF. And the average baseline RS of the SHRM lesion follows after IRF. (Fig. 4A) Regarding the change in RS based on retinal pathology, RS showed improvement one month after the first injection (visit 2) compared to baseline (visit 1), and the improvement was maintained until one month after the third injection (visit 4) in all conditions except for IRF. (Fig. 4B-H) When IRF was present, RS improved only at 1 month after treatment. The average RS improvement varied depending on the condition of the measuring point: 0.7 dB for normal areas, 4.3 dB for areas with neovascular complex, 3.9 dB for those with SRF, 1.8 dB for IRF, 3.9 dB for SPED, 2.4 dB for FPED, and 5.2 dB for SHRM.
RS was associated with the integrity of outer retinal structures including external limiting membrane (ELM), inner segment ellipsoid (ISE) and retinal pigmented epithelium (RPE). (Fig. 5A) Mean RS was not different between disrupted or absent ISE, while RPE shows a difference between complete/disrupted/absent structure. RS improved from visit 2 even though retinal structure was disrupted or absent on the optical coherence tomography (OCT) scan at baseline. (Fig. 5B-D) The proportion of outer retinal integrity grade was compared and tracked between visits. (Fig. 6) The overall grade of the ELM and RPE improved, whereas that of the ISE showed mixed results. (Fig. 6, Top row) Integrity grade at the last visit is shown compared to the baseline visit. (Fig. 6, Bottom row) Outer retinal integrity is restored even if it was not visible (absent) at baseline, whereas complete integrity can be damaged.
Boxplot of average local retinal sensitivity (RS) based on the baseline outer retinal structure integrity grading. (A) Average baseline local retinal sensitivity depending on the outer retinal structure integrity grading using optical coherence tomography. ELM: external limiting membrane; ISE: inner segment ellipsoid; RPE: retinal pigment epithelium, ELM is grade as absent/complete while ISE and RPE are graded as absent/disrupted/complete (B-D) Average local retinal sensitivity change depending on the baseline ELM/ISE/RPE integrity grading, respectively. Average local RS was worse as baseline outer retinal structure is damaged. However, disrupted or complete ISE integrity showed no difference.
Proportional stacked bar graph of outer retinal integrity grading and its change from baseline grade. A, B, C show proportion of outer retinal structure (ELM, ISE and RPE) integrity grade at first and last visit side by side. D, E, F show outer retinal structure integrity grade at last visit based on first observation. ELM: external limiting membrane; ISE: inner segment ellipsoid; RPE: retinal pigment epithelium.
In the optical coherence tomography angiography (OCTA) analysis, the mean choroidal thickness (MCT) decreased gradually, while the choroidal vascular index (CVI) did not change in the choroidal quantification. Retinal vessel and perfusion density did not change in either the superficial or deep layers. (Table 2).
The linear mixed effects model showed that RS was associated with age, baseline RS, SHRM and IRF height and outer retinal integrity (ISE and RPE) at each visit in multivariable analysis. (Table 3) RS change was associated with ISE integrity, presence of neovascular complex, SRF height and normal OCT finding at baseline. (Table 4) RS was more improved when ISE was severely disrupted (absent at OCT B scan), neovascular complex is present and SRF was higher at baseline. However, it was declined when OCT was normal looking at baseline.
Discussion
This study showed that RS improved after Aflibercept loading injection in treatment-naive nAMD cases with relatively better vision while BCVA was not changed. To our knowledge, this is the first prospective study showing improvement of visual function.
Visual improvement is typically greater in patients with worse initial vision, and visual prognosis after nAMD treatment is often linked to baseline BCVA. In those with good baseline vision (20/40 or better), no significant improvement in BCVA was seen up to two years after treatment, and in patients with 20/25 vision or better, BCVA actually declined5,6. This has been attributed to a ceiling effect of the BCVA chart. However, the current study showed that RS improved significantly even in the relatively better-seeing eye at baseline. Moreover, MAIA microperimetry, which was used in this study, showed no ceiling effect in healthy subjects in previous report8. Microperimetry measurement showed significant improvement without vision enhancement after anti-VEGF treatment in nAMD in previous reports9,10. In diabetic macular edema patients with visual acuity better than 20/28 showed improvement in RS despite insignificant change in visual acuity after three monthly anti-VEGF injections11. Microperimetry assesses RS across the entire macular region, providing a broader picture of retinal function compared to BCVA, which only reflects central vision. Therefore, microperimetry is increasingly used in clinical trials to detect subtle changes in visual function, even in cases where central vision is not yet impaired like intermediate AMD7,12.
We examined the change in RS in the fellow eye to exclude the possible training effect of repeated microperimetry experience. The mean RS did not change in the fellow eye. (Fig. 1) Although not all fellow eyes were healthy, eyes with nAMD were at least stable during the study period. Fixation stability was analyzed using Bivariate Contour Ellipse Area (BCEA), which measures the area containing 63% and 95% of all fixation points. These areas ranged between 3.4 and 3.9 deg², and 10.2 and 11.7 deg², respectively. These values were smaller than those typically seen in diseased retinas and were also below the reliability threshold suggested in a previous report13. (Supplementary Table 1)
MNV area was significantly reduced, consistent with findings from previous studies14,15. The thickness of SRF, IRF and SPED were decreased to less than 1 μm. Deep learning based volumetric compartment analysis showed that IRF decreased most rapidly after the first anti-VEGF injection and SRF decreased less rapidly. And PED showed a less pronounced decrease with no further reduction with continued treatment16,17. This is consistent with the current study result.
The association between local pathology and RS was investigated. Microperimetry can provide retinal function at specific locations of the retina. Sulzbacher et al. reported that RS was worse at the neovascular complex (NVC) or IRF in a cross-sectional study18. However, the average RS over the NVC was not as bad, but it was worse at the location where IRF is observed in the current study. Furthermore, RS was not improved where IRF is present. Sulzbacher et al. also investigated RS and retinal morphologic characteristics in the treatment of nAMD with aflibercept19. The authors showed a significant functional benefit across all defined retinal morphologic pathologies. However, RS was not improved when IRF was present alone without other abnormalities. Meanwhile, no negative effect of IRF on RS was observed in another microperimetry study20.
Post-hoc studies on the association between fluid compartment and visual acuity showed consistent results with the current study. OCT volume measurement study showed that baseline VA is negatively affected by volume of IRF, SHRM, SRF and FPED in order with univariable analysis21. Absence of fluid is associated with favorable visual outcome after loading treatment with brolucizumab or aflibercept16,22. Fluid analysis from the HARBOR and ARIES studies showed that IRF consistently correlates with decreased function and recovery throughout treatment, but SRF is associated with more pronounced functional improvement23,24. IRF is thought to indicate Müller cell dysfunction and damage to the external limiting membrane (ELM). IRF seems more closely related to photoreceptor function and neural transmission while SRF may be associated with intact, functioning photoreceptors and ELM25. The current study is consistent with previous studies on pathology and visual function in the treatment of nAMD. Interesting finding in this study is that RS improved even in the location where it looks normal, no fluid and intact outer retina. It might be caused by improvement of abnormality present at baseline but not detected in OCT B-scan or improvement of connecting neural circuits on the pathway to the optic nerve head.
Outer retinal layer alteration is an important imaging biomarker related to visual function and can predict visual outcome in variable retinal diseases. Outer retinal integrity of ELM, ISE and RPE is associated with baseline RS. And the difference in average RS is maintained until the last visit depending on the baseline, although it improves after treatment. This is consistent with previous studies in nAMD18,19,26. Outer retinal integrity is also altered in our investigation. Indistinguishable outer retinal structure (absent ELM, ISE and RPE) was observed at the last visit and vice versa is also detected. Because the OCT image is a reflection of light, the absence of normal structure may be obscured by attenuated light from fluid or exudation. Although the recovery of outer retinal structure should be clarified by further studies, clinicians shouldn’t be disappointed by OCT structural abnormalities at baseline.
OCTA analysis showed that MCT decreased by 12%, but CVI did not. Previous reports showed that both MCT and CVI decreased after short-term anti-VEGF treatment27,28. One possible explanation is that the higher proportion of PCV (5 of 12) in the current study affected different change in CVI with previous data. CVI has been reported to be increased in PCV after treatment29.
We observed the association between RS and local retinal structures including retinal pathology and outer retinal alterations. These abnormalities can exist simultaneously, which requires multivariable analysis. In addition, retinal structural abnormality changes after treatment rapidly. Therefore, we generated a linear mixed model including demographic data, retinal pathology and outer retinal alteration at each visit. Retinal vascular parameters (perfusion density and vessel density) was not included because layer segmentation was consistent as to treatment status. FPED, SHRM height and local CVI showed reciprocal interactions with visit. Negative effect of baseline IRF and SHRM are consistent with previous reports21,25. The negative effect of age was also observed previously21.
The weaknesses of the current study are relatively small sample size and short-term follow-up limited to anti-VEGF loading phase. Further prospective study of large pariticipants and longer extended duration required to corroborate this study result. Meanwhile, strengths of this study are prospective study to explore retinal function in patients with better baseline vision using microperimetry. Fundus controlled perimetry (microperimetry) allows direct analysis of RS with local pathology observed on OCT B-scan and OCTA at 37 locations in one examination and serial follow-up examinations in addition.
This study demonstrated a significant improvement in RS with aflibercept loading injection in treatment-naive nAMD with better than 20/40 at baseline. Mean RS improved although BCVA was not changed. This result suggests the efficacy of aflibercept in nAMD in the better-seeing eye. Furthermore, it should be emphasized that BCVA has limitations as a representative marker of visual function.
Methods
Participants
Patients with treatment-naive nAMD were recruited at a single center. Patients were included if they had better than 20/40 vision in the study eye and were older than 50 years. Exclusion criteria were as follows Choroidal neovascularization due to other ocular diseases, concomitant macular abnormality other than AMD in the study eye, concomitant ocular disease affecting visual function, history of intraocular surgery other than cataract surgery. Written informed consent was obtained from all patients prior to examination or initiation of any study-related procedures. This study adhered to the tenets of the Declaration of Helsinki, and the study protocol was approved by the Independent Ethics Committee/Institutional Review Board of Seoul National University Hospital (approval number: 2010-015-1161). This clinical trial was registered in clinicaltrial.gov (03/18/2024, NCT06330220).
Examinations
The study was carried out in accordance with the approved guidelines and regulations. Participants were treated with 2 mg intravitreal aflibercept injection. The study schedule consisted of three monthly visits for loading injections and one month after the third injection. BCVA, microperimetry, fundus photography, OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) and OCTA (PLEX Elite 9000, Carl Zeiss Meditec Inc., Dublin, CA, USA) were performed before each injection and at a final visit (one month after the third injection). BCVA was measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart with standardized refraction. Fluorescein and indocyanine green angiography were performed at baseline and at the final visit. MAIA microperimetry (CentreVue, Padova, Italy) was used to assess RS. The target size was Goldmann III and the background luminance was 4 apostib. The measurement scale was 0 to 36 dB. Threshold algorithm was standard 4 − 2 staircase. Grid points covered 10º of the central macular region, corresponding to the inner circle of the ETDRS grid, and consisted of 37 measurement points, corresponding to one point centrally and 12 points on each 1, 3 and 5 degree radius circle. (Figures 2 and 7-A inlet)
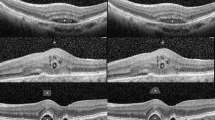
Pointwise analysis of retinal sensitivity and corresponding retinal pathology. Representative example of image analysis process in the same patient. (A) Retinal sensitivity map was superimposed on OCT IR image semi-automatically based on retinal landmarks. (Bifurcation of major vessel in most of cases) (B) Corresponding OCT B scan along the green arrow of (A). Both OCT IR image and superimposed retinal sensitivity map of (A) were cut and superimposed on corresponding OCT B scan. Arrow heads indicate each retinal sensitivity measurement loci. (C) Macular neovascularization (MNV) area was segmented in OCT angiography (D) MNV is also superimposed on retinal sensitivity map.
Analysis
OCT examination was performed using 97 frames of B-scans for 30 degrees of the macula to precisely match the loci of the corresponding RS measurement grid. The RS map was semi-automatically superimposed on the OCT IR images based on retinal landmarks (bifurcation of major retinal vessels). More than three landmarks were manually selected on each corresponding fundus image containing RS map (MAIA microperimetry) and IR image (OCT IR image). Then, the optimization to minimize the total distance between the landmarks of the corresponding image was calculated using the Python library ‘scipy optimize minimize’30. Two images were superimposed to this optimization using affine transformation. (Fig. 7A) Then we can recognize the location of RS grid points on the OCT scan. (Fig. 7B) Retinal pathology was graded according to fluid status (SRF and IRF), PED type (SPED and FPED), SHRM using OCT and fundus photo. The height of each pathological lesion on the microperimetry grid point was measured on the OCT scan. Outer retinal integrity (integrity of ISE, ELM and RPE) was graded as complete/absent for ELM and complete/disrupted/absent for ISE and RPE using OCT B-scans. Macular neovascular area was manually segmented using ICG angiography and OCT angiography. Each segmented MNV was superimposed and combined. This segmented area was also overlaid on the RS map and designated as NVC in the retinal pathology analysis. (Fig. 7-C, D) Regarding BCVA, RA, MNV and retinal lesion size, value at specific time point was compared to baseline value using nonparametric pairwise test (Wilcoxon signed rank test).
To quantify the OCTA parameters, we first uploaded the raw data to the Advanced Retina Imaging Network at the Advanced Retina Imaging Network Hub (https://arinetworkhub.com). The data were then analyzed using the choroid quantification (v20220224-B) and macular density (v20210824-B) algorithms, which automatically quantified the choroid and retinal slabs31.
A multilevel linear mixed effects model was generated to analyze the longitudinal effect of clinical parameters and retinal pathology on the RS measurements of each grid. A multilevel analysis was performed with 1776 microperimetry measurements (37 measuring points at each examination, 4 visits, 12 patients) at level 1 (the lowest level), which are nested within 4 visits at level 2, which are nested within 12 patients at level 3 (the highest level). Clinical parameters (age and sex), outer retinal integrity grade, OCTA parameters and retinal lesion height were fixed effects. Two models were fitted with RS measurement and change in RS between baseline and final visit as outcomes. Coefficients were nested in patients as a random effect. The model for RS change used both baseline value and change in retinal pathology lesion height between baseline (visit 1) and second visit (visit 2) to reflect response to treatment.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author, Chang Ki Yoon, upon reasonable request.
References
Heier, J. S. et al. Intravitreal Aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 119 (12), 2537–2548. https://doi.org/10.1016/j.ophtha.2012.09.006 (2012).
Lotery, A., Griner, R., Ferreira, A., Milnes, F. & Dugel, P. Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set. Eye. 44 (March), 1–10. https://doi.org/10.1038/eye.2017.143 (2017).
Talks, J. S. et al. First-year visual acuity outcomes of providing Aflibercept according to the VIEW Study Protocol for Age-Related Macular Degeneration. Ophthalmology. 123 (2), 337–343. https://doi.org/10.1016/j.ophtha.2015.09.039 (2016).
Rosenfeld, P. J. et al. Ranibizumab for neovascular age-related macular degeneration. N Engl. J. Med. 355 (14), 1419–1431. https://doi.org/10.1056/NEJMoa054481 (2006).
Ho, A. C. et al. The Potential Importance of Detection of Neovascular Age-Related Macular Degeneration when Visual Acuity is relatively good. JAMA Ophthalmol. 135 (3), 268. https://doi.org/10.1001/jamaophthalmol.2016.5314 (2017).
Ho, A. C. et al. Baseline visual acuity at Wet AMD diagnosis predicts long-term vision outcomes: an analysis of the IRIS Registry. Ophthalmic Surg. Lasers Imaging Retina. 51 (11), 633–639. https://doi.org/10.3928/23258160-20201104-05 (2020).
Yang, Y. & Dunbar, H. Clinical perspectives and trends: Microperimetry as a Trial Endpoint in Retinal Disease. Ophthalmologica. 244 (5), 418–450. https://doi.org/10.1159/000515148 (2021).
Pfau, M. et al. Test-retest reliability of scotopic and mesopic fundus-controlled Perimetry using a modified MAIA (Macular Integrity Assessment) in normal eyes. Ophthalmol. J. Int. Ophtalmol Int. J. Ophthalmol. Z. Augenheilkd. 237 (1), 42–54. https://doi.org/10.1159/000453079 (2017).
Michalska-Małecka, K. et al. Evaluation of retinal function improvement in neovascular age-related macular degeneration after intravitreal aflibercept injections with the use of the assessment of retinal sensitivity: the use of the assessment of retinal sensitivity in anti-VEGF treatment – a STROBE-compliant observational study. Med. (Baltim). 98 (44), e17599. https://doi.org/10.1097/MD.0000000000017599 (2019).
Squirrell, D. M., Mawer, N. P., Mody, C. H., Brand, C. S. & Visual outcome after intravitreal ranibizumab for wet age-related macular degeneration: a comparison between best-corrected visual acuity and microperimetry. Retina. 30 (3), 436–442. https://doi.org/10.1097/IAE.0b013e3181bd2f29 (2010).
Sugimoto, M. et al. Effectiveness of microperimetry in evaluating anti-vascular endothelial growth factor therapy for diabetic macular edema patients with relatively good vision: a retrospective observational study. Med. (Baltim). 100 (51), e28404. https://doi.org/10.1097/MD.0000000000028404 (2021).
Pondorfer, S. G. et al. Detecting vision loss in intermediate agerelated macular degeneration: a comparison of visual function tests. PLoS ONE. 15 (4), 1–12. https://doi.org/10.1371/journal.pone.0231748 (2020).
Josan, A. S. et al. Microperimetry Reliability assessed from fixation performance. Transl Vis. Sci. Technol. 12 (5), 21. https://doi.org/10.1167/tvst.12.5.21 (2023).
Sarwar, S. et al. Aflibercept for neovascular age-related macular degeneration. Cochrane Eyes and Vision Group. ed. Cochrane Database Syst. Rev. 2016 (2). https://doi.org/10.1002/14651858.CD011346.pub2 (2016).
Woo, S. J. et al. Efficacy and safety of the Aflibercept Biosimilar SB15 in Neovascular Age-Related Macular Degeneration. JAMA Ophthalmol. 141 (7), 668–676. https://doi.org/10.1001/jamaophthalmol.2023.2260 (2023).
Schmidt-Erfurth, U. et al. Therapeutic response in the HAWK and HARRIER trials using deep learning in retinal fluid volume and compartment analysis. Eye. 37 (6), 1160–1169. https://doi.org/10.1038/s41433-022-02077-4 (2023).
Schmidt-Erfurth, U., Vogl, W. D., Jampol, L. M. & Bogunović, H. Application of automated quantification of Fluid volumes to Anti–VEGF therapy of Neovascular Age-Related Macular Degeneration. Ophthalmology. 127 (9), 1211–1219. https://doi.org/10.1016/j.ophtha.2020.03.010 (2020).
Sulzbacher, F. et al. Correlation of SD-OCT features and retinal sensitivity in Neovascular Age-Related Macular Degeneration. Investig Opthalmology Vis. Sci. 53 (10), 6448. https://doi.org/10.1167/iovs.11-9162 (2012).
Sulzbacher, F. et al. Relationship of retinal morphology and retinal sensitivity in the treatment of neovascular age-related macular degeneration using aflibercept. Invest. Ophthalmol. Vis. Sci. 56 (2), 1158–1167. https://doi.org/10.1167/iovs.14-14298 (2015).
Roh, M. et al. Microperimetry in age-related macular degeneration: Association with macular morphology assessed by optical coherence tomography. Br. J. Ophthalmol. 103 (12), 1769–1776. https://doi.org/10.1136/bjophthalmol-2018-313316 (2019).
Moraes, G. et al. Quantitative Analysis of OCT for Neovascular Age-Related Macular Degeneration using deep learning. Ophthalmology. 128 (5), 693–705. https://doi.org/10.1016/j.ophtha.2020.09.025 (2021).
Eichenbaum, D. et al. A treatment agnostic analysis of the HAWK and HARRIER studies. Retina. 43 (4), 632–640. https://doi.org/10.1097/IAE.0000000000003699 (2023).
Chaudhary, V. et al. Association between Visual Acuity and Fluid compartments with treat-and-extend Intravitreal Aflibercept in Neovascular Age-Related Macular Degeneration: an ARIES Post Hoc Analysis. Ophthalmol. Ther. 11 (3), 1119–1130. https://doi.org/10.1007/s40123-022-00491-1 (2022).
Riedl, S., Vogl, W. D., Waldstein, S. M., Schmidt-Erfurth, U. & Bogunović, H. Impact of intra- and Subretinal Fluid on Vision based on volume quantification in the HARBOR Trial. Ophthalmol. Retina. 6 (4), 291–297. https://doi.org/10.1016/j.oret.2021.12.007 (2022).
Chaudhary, V. et al. Impact of fluid compartments on functional outcomes for patients with neovascular age-related macular degeneration. Retina Phila. Pa. 42(4), 589–606. https://doi.org/10.1097/IAE.0000000000003283 (2022).
Landa, G., Su, E., Garcia, P. M. T., Seiple, W. H. & Rosen, R. B. Inner segment-outer segment junctional layer integrity and corresponding retinal sensitivity in dry and wet forms of age-related macular degeneration. Retina Phila. Pa. 31 (2), 364–370. https://doi.org/10.1097/IAE.0b013e3181e91132 (2011).
Pellegrini, M. et al. Short-term choroidal vascular changes after aflibercept therapy for neovascular age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 259 (4), 911–918. https://doi.org/10.1007/s00417-020-04957-5 (2021).
Shen, M. et al. Choroidal changes after Anti-VEGF therapy in AMD eyes with different types of Macular Neovascularization using swept-source OCT angiography. Investig Opthalmology Vis. Sci. 64 (13), 16. https://doi.org/10.1167/iovs.64.13.16 (2023).
Shen, M. et al. Choroidal changes in eyes with Polypoidal Choroidal Vasculopathy after Anti-VEGF therapy imaged with swept-source OCT angiography. Invest. Ophthalmol. Vis. Sci. 62 (15), 5. https://doi.org/10.1167/iovs.62.15.5 (2021).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods. 17 (3), 261–272. https://doi.org/10.1038/s41592-019-0686-2 (2020).
Zhou, H. et al. Age-related changes in Choroidal Thickness and the volume of vessels and stroma using swept-source OCT and fully automated algorithms. Ophthalmol. Retina. 4 (2), 204–215. https://doi.org/10.1016/j.oret.2019.09.012 (2020).
Acknowledgements
None.
Funding
This study was supported by the research grant with an investigator-initiated trial supported by Bayer. The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
Hyeong Gon Yu and Chang Ki Yoon designed and performed the experiments. Chang Ki Yoon and Jae Hyup Lee analyzed the data. Hyeong Gon Yu supervised the work. Chang Ki Yoon and Jae Hyup Lee wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, J.H., Yu, H.G. & Yoon, C.K. A pilot study : retinal sensitivity change in neovascular age-related macular degeneration patients with better baseline visual acuity. Sci Rep 14, 27035 (2024). https://doi.org/10.1038/s41598-024-77485-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77485-5