Abstract
The most cost-effective data collection method is electroencephalography (EEG) to obtain meaningful information about the brain. Therefore, EEG signal processing is very important for neuroscience and machine learning (ML). The primary objective of this research is to detect neonatal seizures and explain these seizures using the new version of Directed Lobish. This research uses a publicly available neonatal EEG signal dataset to get comparative results. In order to classify these EEG signals, an explainable feature engineering (EFE) model has been proposed. In this EFE model, there are four essential phases and these phases: (i) automaton and transformer-based feature extraction, (ii) feature selection deploying cumulative weight-based neighborhood component analysis (CWNCA), (iii) the Directed Lobish (DLob) and Causal Connectome Theory (CCT)-based explainable result generation and (iv) classification deploying t algorithm-based support vector machine (tSVM). In the first phase, we have used a channel transformer to get channel numbers and these values have been divided into three levels and these levels are named (1) high, (2) medium and (3) low. By utilizing these levels, we have created an automaton and this automaton has three nodes (each node defines each level). In the feature extraction phase, transition tables of these nodes has been extracted. Therefore, the proposed feature extraction function is termed Triple Nodes Automaton-based Transition table Pattern (TATPat). The used EEG signal dataset contains 19 channels and there are 9 (= 32) connection in the defined automaton. Thus, the presented TATPat extracts 3249 (= 19 × 19 × 9) features from each EEG segment. To choose the most informative features of these 3249 features, a new feature selector which is CWNCA has been applied. By cooperating findings of this feature selector and the presented DLob, the explainable results have been obtained. The last phase is the classification phase and to get high classification performance from this phase, an ensemble classifier (tSVM) has been presented and the classification results have been obtained using two validation techniques which are 10-fold cross-validation (CV) and leave-one subject-out (LOSO) CV. The proposed EFE model generates a DLob string and by using this string, the explainable results have been obtained. Moreover, the presented EFE model attained 99.15% and 76.37% classification accuracy deploying 10-fold and LOSO CVs respectively. According to the classification performances, the recommended TATPat-based EFE is a good model at EEG signal classification. Also, the presented TATPat-based EFE model is a good model for explainable artificial intelligence (XAI) since TTPat-based EFE is cooperating by the DLob.
Similar content being viewed by others
Introduction
Neonatal seizures are sudden, abnormal bursts of electrical activity in the brain that occur in newborns1,2. It is critical to detect and treat these seizures, which may indicate serious underlying conditions such as brain damage, infections, metabolic disorders, or genetic disorders3. Treating neonatal seizures can lead to long-term neurological damage, developmental delays, and even death4. Traditional methods for detecting neonatal seizures rely on continuous observation by medical professionals, which often involves visual inspection of EEG (electroencephalogram) recordings2. EEG is a technique that measures electrical activity in the brain using electrodes placed on the scalp5. Although effective, this manual process is labor-intensive, time-consuming, and prone to human error6. Additionally, neonatal seizures’ subtle and diverse nature makes their accurate identification difficult, especially in the busy clinical setting7.
With developing technology, the need for automatic and reliable seizure detection systems increases8. Recent advances in artificial intelligence (AI) and machine learning provide new possibilities for the development of these systems9,10,11. These technologies can quickly and accurately analyze large volumes of EEG data, identifying seizure patterns that experts may miss8,12.
Literature review
Some current studies on neonatal seizure detection are given as follows. O’Shea et al.13 suggested investigating varying CNN depths to optimize EEG-based neonatal seizure detection. Their study utilized a large clinical dataset comprising over 800 h of multichannel unedited EEG, containing 1389 seizure events. They attained an AUC90 improvement from 82.6 to 86.8%. Combining the deep CNN with the feature-based shallow SVM further enhanced the AUC90 to 87.6%. O’Shea et al.14 proposed a CNN classifier for detecting neonatal seizures from raw multichannel EEG signals. Their study utilized a large clinical dataset, including over 834 h of continuous EEG recordings with 1389 seizure events. They obtained a 56% relative improvement over a state-of-the-art feature-based baseline, with an AUC of 98.5%. O’Shea et al.15 presented the use of CNNs for the automated detection of neonatal seizures. Their study employed a substantial dataset of 835 h of continuous, multichannel EEG recordings, including 1389 annotated seizure events. The fully CNN demonstrated an AUC of 97.1% and an AUC90 of 82.9%, slightly surpassing the SVM-based system. Hogan et al.16 proposed a novel CNN model for neonatal seizure detection that achieved expert-level performance. Their study used retrospective EEG data from 332 neonates, comprising a development dataset of 202 neonates with over 12,000 seizure events annotated per channel. They achieved an AUC of 0.982. Gramacki and Gramacki17 proposed a framework for detecting epileptic seizures based on neonatal EEG signals. The dataset used in their study consisted of 79 raw EDF files and 3 CSV files annotated by three experts with over ten years of experience in visual EEG interpretation. The CNN architecture employed in the study achieved classification accuracies, with the best test-set accuracy reaching up to 97% using a sliding window design. Pavel et al.18 presented an algorithm for detecting neonatal seizures. Their study utilized EEG data from 264 neonates across multiple centers in Ireland, the Netherlands, Sweden, and the UK, involving 13,827 h of EEG recordings. They attained a sensitivity of 66.0% compared to 45.3% in the non-algorithm group. Temko et al.19 suggested the need for automated neonatal seizure detection systems to aid clinicians. The dataset used comprised EEG recordings from 18 full-term neonates with hypoxic-ischemic encephalopathy collected at Cork University Maternity Hospital, totaling over 834 h and 1389 seizure events. They achieved a mean AUC score of 98.10%. Temko et al.20 developed a personalized real-time diagnosis system for neonatal seizure detection using a combination of patient-adaptive generative and patient-independent discriminative classifiers. Their study utilized a comprehensive dataset of EEG recordings from 18 full-term newborns in the Neonatal Intensive Care Unit (NICU) of Cork University Maternity Hospital, totaling 816.7 h and 1389 seizures. Their results indicated that the adaptive system improved seizure detection rates to 70%. Temko et al.21 suggested an automated solution for neonatal seizure detection. They utilized EEG data recorded from neonatal intensive care units, which included recordings from 18 full-term newborns experiencing seizures due to brain injury associated with hypoxic-ischemic encephalopathy. The dataset used comprised over 834 h of EEG recordings and 1389 seizure events, annotated by two independent experts. Their results demonstrated that the proposed system achieved a sensitivity of 93.00% and a specificity of 86.00%. Yu et al.22 proposed a method using empirical Fourier decomposition (EFD) and improved EFD (IEFD) for EEG signal classification. They worked with datasets of 60 + subjects. They achieved up to 99.82% accuracy in subject-dependent and 82.70% in subject-independent scenarios. Sadiq et al.23 presented an framework for motor imagery EEG classification, focusing on enhancing performance using multidomain features combined with feature selection techniques. They utilized datasets containing over 50 subjects and explored various signal decomposition methods such as variational mode decomposition (VMD) and empirical wavelet transform (EWT). Their framework achieved with classification accuracy reaching up to 99.6% for subject-specific and 98% for subject-independent tasks. Sadiq et al.24 proposed a method utilizing multivariate variational mode decomposition (MVMD) for motor imagery EEG classification. They applied this method to datasets containing over 50 subjects, achieving classification accuracies of 99.8% for subject-dependent tasks and 98.3% for subject-independent tasks. Sadiq et al.25 proposed a framework using multivariate empirical wavelet transform (MEWT) for motor imagery EEG classification. They conducted experiments on datasets with over 50 subjects, achieving classification accuracies of 98% for subject-independent tasks and up to 100% for subject-specific cases. Sadiq et al.26 presented a novel motor imagery BCI classification framework based on empirical wavelet transform (EWT) and two-dimensional (2D) modeling. They worked with a dataset of 5 subjects and achieved a classification accuracy of 95.3%, including 100% accuracy for subjects with very small training samples. Sadiq et al.27 developed a framework for motor imagery EEG classification using multivariate variational mode decomposition (MVMD). They worked with datasets of 50 + subjects and achieved classification accuracies of 99.6% for subject-specific and 98% for subject-independent tasks. Akbari et al.28 proposed a method for depression detection using geometrical features extracted from the second-order differential plot (SODP) of EEG signals. They worked with a dataset consisting of 44 subjects (22 normal and 22 depressed) and applied Binary Particle Swarm Optimization (BPSO) for feature selection. Their method achieved a classification accuracy of 98.79% using the KNN classifier in a ten-fold cross-validation strategy. Akbari et al.29 developed a method for seizure detection using Poincaré plots of EEG signals and novel geometrical features in the discrete wavelet transform (DWT) domain. They applied this method to a dataset of 5 subjects and achieved a classification accuracy of 99.3%. Sadiq et al.30 presented a pre-trained CNN-based framework for EEG signal classification, utilizing datasets with more than 50 subjects. They achieved an impressive 99.52% classification accuracy. Sharma et al.31 proposed an emotion recognition system using EEG signals. They used the DEAP dataset, which includes EEG data from 32 participants watching emotional videos. They attained 82.01% classification accuracy using 10-fold cross-validation. Sharma et al.32 developed a hybrid approach using higher-order statistics and residual wavelet transform to localize epileptic zones. They tested the model on the Bern Barcelona EEG database with 3750 signals. They obtained a classification accuracy of 99.76%. Sharma et al. proposed an emotion recognition system using EEG data from the DEAP dataset. They calculated 78.37% accuracy by applying a hybrid approach with sensitivity analysis. Sharma et al.33 presented a seizure classification system using higher-order statistics and deep neural networks on the Bonn University EEG dataset. Their model achieved 100% accuracy for binary classification and 97.2% for five-class classification. Sharma et al.34 proposed a system for classifying focal and non-focal EEG signals using third-order cumulant features. They attained 99% accuracy on the Bern-Barcelona EEG database.
Literature gaps
According to the surveyed literature, the identified gaps are:
-
The well-known feature engineering and deep learning models have generally been used in the literature to get high classification performances. This situation causes stagnation for the new generation models proposing.
-
Most of the models have focused on classification ability. There are the limited number of explainable artificial intelligence (XAI) models in the EEG signal classification.
Motivation and our model
Our essential motivation is to fill the given literature gaps and proposing a new generation EFE model. Moreover, a new version of the Directed Lobish (DLob) has been presented in this research.
The first literature gap is the lack of the new generation EEG signal processing models. In this research, we have inspired by the transformers35 since transformers caused a revolution for the machine learning (ML). Therefore, we have proposed a channel transformer and the output of this transformer is divided into three layers which are (i) high, (ii) medium and (iii) low. By using these layers, we have created an automaton to extract features and demonstrate the relations of these layers. As stated from literature, the histogram-based feature extractors like local binary pattern (LBP)36 are effective feature extractor. Therefore, we have used transition table with the created automaton and we have proposed Triple nodes Automaton Transition table Pattern (TATPat) for feature extraction. Moreover, the second innovation of this model is the Cumulative Weight-based Neighborhood Component Analysis (CWNCA) and this feature selector solved the selection of the optimal number of features by computing cumulative weights. In the classification phase, t algorithm-based support vector machine (tSVM) has been presented. In this aspect, we have proposed an innovative model has been presented for EEG signal classification.
The second literature gap is the limited XAI models. Therefore, we have presented an improved version of the DLob symbolic language and new symbols have been presented in this work. Moreover, the improved version of the DLob is provided by the Causal Connectome Theory (CCT) to give more explainable results. Also, transitions of the used triple nodes automaton have been presented using a graph to present XAI results. Moreover, we have contributed the connectome theory by using the obtained XAI models. The advantages of the DLob and CCT-based XAI model are:
-
Traditional models generally focus on high classification accuracy, and their operations are often defined as black boxes. Understanding the decision processes of these models is very difficult for clinicians and researchers. XAI models, like those using the DLob symbolic language and CCT, offer transparent decision-making processes by explaining which features (e.g., brain regions, signal transitions) contributed to the classification. This makes the models more trustworthy and useful for medical professionals and users.
-
The commonly used XAI models are either feature-based or activation-based. The presented DLob and CCT-based XAI method creates a cortical map for understanding the effects of epilepsy using only EEG signals.
According to above information, our motivations are:
-
To bridge the gap in new generation EEG signal processing models by introducing an innovative channel transformer and automaton-based feature extraction method (proposing TATPat).
-
To advance XAI by improving the DLob symbolic language and using graph-based presentations, contributing to the broader understanding of neural connections and connectome theory.
Innovations
The novelties of this work are:
-
To extract features with high classification capability, a new feature extractor which is TATPat has been proposed.
-
A new generation feature selector which is CWNCA has been used to select the most informative features.
-
tSVM is an ensemble version of the SVM and to obtain classification results, tSVM has been utilized.
-
New symbols have been added to DLob.
-
Automaton-based explainable results have been presented in this research.
-
We have discussed the results using a new theorem called Causal Connectome Theory (CCT).
Contributions
-
By proposing TATPat-based EFE model, we have presented a high accurate EEG signal classification model. In this aspect, the recommended model contributes to EEG signal classification.
-
By cooperating DLob, the explainable results have been given. In this aspect, the proposed model contributes to neuroscience. Moreover, we have explained the string obtained by DLob using CCT.
Material
In this study, we utilized a publicly available dataset37. The dataset comprises neonatal seizures recorded at the Helsinki University Hospital’s Neonatal Intensive Care Unit (NICU). This dataset contains multichannel EEG recordings from 79 newborns. The EEG recordings were made using 19 electrodes (F3, F4, F7, F8, O1, O2, Fp1, C3, C4, Fp2, P3, P4, T3, T4, T5, T6, Fz, Cz, and Pz) placed according to the International 10–20 system. The EEG data were stored in European Data Format (EDF). A NicOne EEG amplifier was used for recording. The sampling frequency was set at 256 Hz. The recordings, with an average duration of 74 min, were reviewed and annotated for seizures by at least three experts using Nicolet reader software. In this research, EEG segments with a length of 30 s (length is 256 × 30 = 7680). The distributions of the used dataset are tabulated in Table 1.
Methods
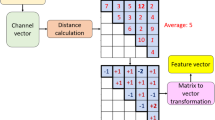
In this model, we have proposed a new generation EFE model and the recommended EFE model is named TATPat-based EFE model. The primary objective of the proposed model is to get high and robust classification performance on the used big dataset since the used dataset has more than 10,000 EEG observations. To better explain the proposed model, the graphical overview of this model is depicted in Fig. 1.
As can be seen from Fig. 1, the proposed model uses a channel transformer and TATPat for feature extraction. In the feature selection phase, the CWNCA feature selection function has been utilized. To classify these selected features, an ensemble classifier which is tSVM has been utilized. These three phases have been defined a feature engineering model. The DLob phase is an explainable phase of the proposed model. Therefore, this model is a EFE model.
By utilizing channel transformer, the channel identities have been obtained from the EEG signal. These transformed signals have been forward to TATPat, an automaton and transition table-based feature extractor. The feature vector generated by TATPat have been fed to CWNCA and this function selected the most informative features. The results of the selected features have been directed to two phases. One of them is the tSVM algorithm and tSVM has been utilized to obtain classification results. The second one of them is XAI phase. By utilizing DLob (it is a symbolic language), the explainable results have been obtained and these results have been analyzed using a new theory which is termed as Causal Connectome Theory (CCT).
In order to clarify the proposed model, the pseudocode of this TATPat-based EFE model is given in Algorithm 1.
Algorithm 1: TATPat-based EFE model.
Input: EEG signal: A matrix of size M×19, where each row represents EEG data from 19 channels. Output: The classification and explainable results | |
01 | For each signal block in EEG data do: |
02 | Apply Channel Transformer: |
03 | Sort EEG channels based on signal strength. |
04 | Create a transformed signal by converting EEG data to channel indices. |
05 | Apply TATPat Feature Extraction: |
06 | Divide the transformed signal into three levels: |
07 | High-level: strongest signal channels. |
08 | Medium-level: intermediate signal channels. |
09 | Low-level: weakest signal channels. |
10 | Define an automaton with three nodes: High, Medium, and Low. |
11 | For each combination of levels (High-High, Low-High, etc.) do: |
12 | Compute a transition table between the corresponding levels. |
13 | Fill the transition table with counts of transitions between channels. |
14 | Normalize each transition table by dividing each value by the total transitions in the row. |
15 | Convert each normalized transition table (19 × 19) into a vector of length 361. |
16 | Concatenate all 9 vectors to form a feature vector of length 3249. |
17 | Store the generated feature vector in fv. |
18 | Apply CWNCA Feature Selection: |
29 | Compute qualified feature indices and weights using NCA. |
20 | Calculate cumulative weights to determine the optimal number of features (192 features). |
21 | Select the top 192 most informative features from fv. |
22 | Use tSVM Classification: |
23 | Generate multiple classification outcomes by varying polynomial orders and box constraint values in SVM. |
24 | Use Iterative Majority Voting (IMV) to combine outcomes and select the voted outcome. |
25 | Apply a greedy algorithm to select the outcome with the highest accuracy. |
26 | Generate Explainable Results using DLobV2: |
27 | Map the selected feature indices to DLobV2 symbols (brain regions). |
28 | Compute transition tables for the selected DLobV2 symbols. |
29 | Use Causal Connectome Theory (CCT) to explain brain region transitions and functions. |
30 | Return the final classification result and explainable outcomes. |
The details of the proposed TATPat-based EFE model are provided below, phase by phase.
Feature extraction
In this phase, two functions have been used and these functions are channel transformer and TATPat. The main theoretical background of the presented feature extraction phase is to detect the differences of the channels according to level of the channels. To better clarify this feature extraction phase, we have presented the steps of this model as below.
Step 1 (Channel Transformer)
Apply channel transformer to the EEG signals. The channel transformer is a process that focuses on identifying the importance of different EEG channels rather than analyzing the signal amplitudes directly. The channel transformer is applied to EEG signals. The channel transformer is a simple method that converts EEG signals into channel indices, highlighting the importance of the channels instead of their signal amplitudes. In neuroscience, the significance of channels is often considered more valuable than amplitude. Therefore, we used this transformation in our research. The mathematical definition of this transformation is provided below.
Herein, \(S\): the qualified/sorted values, \(TS\): the transformed signal, \(EEG\): EEG signal, \(argsort(.)\): sorting function and \(\mathcal{L}\): length of each channel of the EEG signal.
Step 2 (TATPat)
Extract features from the transformed signal by deploying the recommended TATPat feature extractor. The theoretical background of the proposed TATPat is to extract features from the differently leveled transformed signals by deploying transition table extraction. This model is also an automaton-based feature extraction function, and this feature extraction function is a simple method. The steps of this feature extractor are.
Step 2.1
Create three levels using the transformed signal. The explanations of these levels are given below.
High level: Defines to the strongest signal channels (e.g., channels 1 to 6).
Medium level: Represents to intermediate signal channels (e.g., channels 7 to 12).
Low level: Corresponds to the weakest signal channels (e.g., channels 13 to 19).
Herein, high (\(high\)), medium (\(medium\)), and low (\(low\))-level bands have been created and utilized as nodes in the automaton shown below to extract features.
The automaton diagram below (see Fig. 2) the levels showcases how the transitions between these levels (High, Medium, and Low) are modeled. The automaton has three nodes, and the transitions between these nodes represent possible connections between different levels of activity in the EEG signal.
The given automaton with three nodes showcases the connections among the nodes, resulting in 9 connections. Consequently, 9 (= 32) transition tables have been computed. The steps for these transition table computations are provided below.
Step 2.2
Compute 9 transition tables by using the three nodes automaton shown in Fig. 2. The mathematical explanations of this step are.
Firstly, 19 × 19 size nine transition tables have been defined.
Herein, \(tt\): transition table with a size of 19 × 19 since the used dataset has 19 channels.
These transition tables defined above have been filled by deploying the below mathematical equations.
By deploying the given process above, the defined transition tables have been filled.
The definitions of these transition tables are: \({tt}_{1}\): High-high transitions, \({tt}_{2}\): Low-high transitions, \({tt}_{3}\): High-low transitions, \({tt}_{4}\): Low-low transitions, \({tt}_{5}\): High-middle transitions, \({tt}_{6}\): Low-middle transitions, \({tt}_{7}\): Middle-high transitions, \({tt}_{8}\): Middle-low transitions, \({tt}_{9}\): Middle-middle transitions.
Step 2.3
Normalize the computed transition tables. Once the transition tables are filled, they are normalized by dividing each value by the total number of transitions in its row, ensuring that the values represent relative transition frequencies between channels.
Herein, each value has been divided to summation of the used row and \(t{t}_{g}^{N}:\) gth normalized transition table.
Step 2.4
Transform the computed transition tables to vectors. Each transition table (19 × 19) is then transformed into a vector of length 361 (19 × 19 = 361), which captures all the transitions between channels for a specific connection (e.g., High-High, Low-Medium).
where \(ve{c}_{g}\): gth vector with a length of 361.
Step 2.5
Concatenate the generated vectors to create feature vector.
where \(fv\): the computed feature vector with a length of 3249 (= 9 × 361) .
As can be seen from steps above, the proposed feature extraction method extract 3249 from each EEG signals.
In conclusion, the transformed signal is divided into three levels (High, Medium, Low) based on signal strength, and an automaton is used to model the transitions between these levels. Transition tables are then computed for all possible transitions between the levels, which are normalized and transformed into feature vectors. The final feature vector, consisting of 3,249 elements, represents the relationships between EEG channels and their transitions across different levels. This method ensures that important patterns in EEG activity, particularly those relevant for detecting seizures, are extracted in a structured and meaningful way.
Feature selection
In order to select the most informative features of the features generated, we have used CWNCA feature selector and which is an improved version of the NCA feature selector38. The objective of the CWNCA is to select the optimal number of features using a simple mathematical formulation (cumulative weight computation). The steps of this feature selector are given below to clarify this function.
Step 3 (CWNCA)
Choose the most informative features from the generated feature vector deploying CWNCA. The details of this feature selector are.
Step 3.1
Compute the qualified indices of the generated feature matrix by deploying NCA feature selector. The first step is to compute the qualified indices of the generated feature matrix by using a Neighborhood Component Analysis (NCA) feature selector. NCA is a method that identifies which features are most important in predicting the outcome by assigning weights to each feature. The mathematical definition of this step is given below.
Herein, \(idx\): the sorted/qualified indices of the features,\(w\): the generated weights of the features by NCA feature selector, \(N(.)\): neighborhood component analysis (NCAS) feature selection function, \(X\): feature matrix and \(y\): real outcome.
Step 3.2
Compute the optimal number of features by deploying cumulative weight. the cumulative weight for the sorted features is calculated. The goal is to determine the optimal number of features needed to explain 99% of the total weight. This is done by summing the weights of the features incrementally until the cumulative weight reaches or exceeds 0.99 (99% of the total weight). This mathematical details of the cumulative weight computation is demonstrated in below.
where, \(cw\): cumulative weight, \(n\): the length of the features and \(OC\): the optimal number of features. For this work, the optimal number of features has been calculated as 192 for this research. Therefore, the most informative features, with the optimal number being 192, have been selected. Moreover, by deploying these selected 192 features, 384 (= 192 × 2) DLob symbols have been selected since each feature contains two DLob symbols/letters.
Step 3.3
Select the most informative 192 features (as determined by the cumulative weight calculation) of the generated 384 features.
where \(SF\): selected feature vector, \(Dim\): EEG observations count.
Classification
The proposed model is a feature engineering model. Therefore, to demonstrate the high classification performance of this model, shallow classifiers were used for testing. However, using shallow classifiers is not a novel approach. Consequently, we applied the t algorithm to the best-performing shallow classifier, which is the support vector machine (SVM)39,40. The resulting classifier is named tSVM. The tSVM classifier is an ensemble version of the SVM classifier. It generates multiple classification results by using various parameters such as orders of polynomials and box constraint levels. In this context, the classifier can be termed polynomial tSVM. In the second step of tSVM, iterative majority voting (IMV)41 has been applied to the outcomes generated by parameters, producing voted outcomes. In the final step of this phase, the outcome with the highest classification accuracy has been selected using a greedy algorithm. The steps of the tSVM classifier are explained below.
Step 4 (tSVM)
Classify the selected features by deploying the recommended tSVM classifier. The details of the recommended tSVM are explained below.
Step 4.1
Produce the parameter-based outcomes by deploying iterative parameter changing/setting.
where \(po\): the outcomes by generating iterative parameter changing, \(SVM(.)\): SVM classifier, \(pon\): polynomial orders values, \(bcv\): box constraint values. In this version of the tSVM classifier, five polynomial order values and five box constraint values have been used. Thus, 25 (= 5 × 5) paramaters-based SVM outcomes have been created in this work.
Step 4.2
Deploy IMV to the paramaters-based outcomes and create voted outcomes.
where \(acc\): classification accuracy\(\delta (.)\): classification accuracy calculation function, \(id\): the sorted indexes using by descending, \(argsort(.)\): sorting function, \(\omega (.)\): mode function and \(v\): the created voted outcomes. In this step, 23 (= 25 − 3 + 1) voted outcomes have been produces since the range of the used loop for IMV is from 3 to 25.
Step 4.3
Select the final outcome of the tSVM used deploying greedy algorithm.
Herein, \(final\): the generated final outcome of the used tSVM classifier.
The tSVM classifier increases the robustness and accuracy of the classification process by combining the strengths of multiple SVM models with different parameter settings. In this aspect, this classifier is an ensemble classifier. Rather than relying on a single classifier, tSVM generates diverse outcomes from various parameter combinations. The IMV produces voted results by using an iteration and mode function-based majority voting. By using the greedy algorithm, the tSVM classifier selects the outcome with the highest classification accuracy as the final outcome. This configuration demonstrates that the tSVM classifier is an ensemble and self-organized system (selecting the best outcome among the 48 generated outcomes: 25 parameter-based and 23 voted).
Explainable results generation
The recommended model is an EFE model. Therefore, to present explainable results, we have used DLob76,77 symbolic language. This symbolic contains 10 symbols and these symbols and explanations of these symbols have been tabulated in Table 2.
By using the above table (simple letter dictionary) of the DLob, the explainable results have been extracted. However, the EEG signals used have Fz, Cz, and Pz channels. Therefore, we have added three new symbols to the DLob symbol dictionary, and the improved version of this dictionary is tabulated in Table 3.
As can be seen from Tables 2 and 3, in DLob, there are 10 letters and two hemispheres (R: right and L: left). In the presented DLobV2, there are 13 letters and three hemispheres (R: right, L: left, and Z: midline). By using the proposed DLobV2, more information has been generated from the cortical area. The steps of this phase (explainable results generation by deploying DLobV2) are also demonstrated below.
Step 5 (DLobV2 and CCT)
Convert the indices of the selected features and obtain the explainable results. Follow the explainable results computation process outlined below.
Step 5.1
Find the channel and connection of the automaton using the indices of the selected features. In this study, the proposed TATPat uses an automaton that is a fully bipartite graph. Each feature contains information about the graph and two channels (calculated using transitions between the channels). Use the equations below to extract this information.
where \(idx\): indices of the selected features, \(OC\): the number of the selected features, \(dlid\): DLob indexes for the used brain cap. The used brain cap has 19 channels. Therefore, we have used 19 to compute these indices. \(array\): DLobV2 symbols per the used brain cap and \(DLobV2Str\): DLobV2 string generated.
Step 5.2
Compute transition tables of the channels, DLobV2 symbols and connections.
The DLob symbolic language is used to provide explainable results in the EEG signal classification model for this research. It consists of symbols representing different brain regions, with each symbol corresponding to specific cognitive functions. Initially, DLob contained 10 symbols representing different brain lobes and their associated functions, such as logical thinking, motor control, and visual or auditory processing. The model was extended to DLobV2, which includes 13 symbols, adding midline regions like Fz, Cz, and Pz to account for the midline EEG channels. By using this symbolic system, the model translates complex EEG data into interpretable symbols, which are then analyzed to generate a string that explains the neural activity during events like seizures.
Step 5.3
Discuss the effect of the computed findings using CCT. The details of the CCT are.
CCT
The transitions between brain regions and the functions that trigger these transitions are aimed to be understood by CCT. The cause-effect relationships of brain connections are explained by CCT, contributing to the understanding of neurological disorders, learning processes, and overall brain functions.
CCT investigates the transitions between brain lobes and the underlying triggering functions. Below (Table 4) are specific transitions and their associated triggering functions:
The given five steps above have been defined the presented TATPat-based EFE model.
The CCT aims to explain the transitions between different brain regions and the functions that trigger these transitions. It investigates how specific brain lobes interact and what cognitive functions or processes are involved in these transitions, such as decision-making, sensory processing, or memory. The theory provides a framework to understand the cause-effect relationships between brain connections, contributing to the diagnosis and analysis of neurological disorders. In this model, CCT helps explain the transitions captured by the DLobV2 symbols, offering insights into how different brain regions are involved in conditions like seizures and how these interactions lead to specific neurological outcomes.
Experimental results.
The recommended TATPat-based EFE model gave both classification and explainable results. This model was programmed by using a simple configured laptop since this model is a feature engineering model and the time complexity of this model is linear. In order to program this model, MATLAB (version 2024a) was used and central processing unit (CPU) mode was used.
Firstly, we have computed to classification results. We have used classification accuracy, specificity, sensitivity and geometric mean to evaluate the classification performance of this model. Moreover, two validation techniques have been used for the tSVM classifier and the used validation techniques are (i) 10-fold CV and (ii) LOSO CV. These validation techniques have been used to present robust and reliable results. Furthermore, we are the first team to use LOSO CV for this dataset as our knowledge.
The initial parameters of this model is also given in Table 5 below to provide repetition.
By using the above parameters, the presented TATPat-based EFE model was implemented. Moreover, we created an application about Directed Lobish and this application is a custom generative pre-trained transformers (GPT) and the users can reach this custom GPT using https://chatgpt.com/g/g-E3Gvijurs-lobish-eeg-interpreter URL.
The computed results of the presented TATPat-based model have been given in Table 6.
Table 6 depicted that the introduced TATPat-based EFE model attained 76.37% and 99.15% classification accuracies deploying LOSO CV and 10-fold CV respectively. Additionally, the confusion matrices of these results have been depicted in Fig. 3.
In the second step, the explainable results have been computed. In this step, DlobV2 has been used and a string has been computed and the computed string has been demonstrated below.
DLobV2 string
TLPLTRORPZTLFLFLOROLTRORFRPLCLFRFRTLFLFLFRFRCZTLTLORTLTLPLOLFRFLPZFZPRTRPZPZTLFLOLCLFLFLFLTLTLFRCRTRTLCZTLFZFRTRCLPLPRFRFZFZPZTRTRCZPLFLCZPLPLORPZTRFRORFZFLFROLORCZFZTRPZTRTRFROLTRPZCZTLTLTLCLORPRFRFRTRCZCZFLFZFRFRPRPLTLFRPZFZFLOROLTLFRORCRPLOLTLCZTLTLPLFLTLTLCRFRFRTRTLOLOLTLFZTRCRFRTLTLPRFRFLOLORFRPRFRFLFRFZFZCRTLTRFRORFRPLCLFLFRFLTRFZOLCLFRPZCZFRPLCLTRCZTRFLOLPLFRPZORFROLFZTLFRTROLCLCZFLTLFRFLCRTLCLFRTLFLTRFLCRPRTRCZTLTLCLORPRPRPRFLFZCRTLFLTRFRPRTLFLPLFLTLOLPRFRFRPRPLTLORCZFLCRFRPZORCZOLFLTLOLORTRTLCLPRCZTLPZPLFRFLCRTLPZFRORPLFLCZFLFLPLTLTLCRTRPZPRORPLCLOLCRFLFLOLCLFRCRTRPRPRCLCLTLFLTLORFLFRPZFZFLORCZCLPLORPRPRPZFLORPRTLCRFLOLCZPLPRORCRCRFLCLCRFROLCLTLFRFLFZTLOLCZFRTRFRFRPLCZTLFRFRFZTROLFZPRFRPLCLCZPLFRFROLTLFZFLCZORCRCLTRTRFRPLCRFLFLFRCZTLFRPRTROLOLFZTLFL.
Temporal lobe epilepsy is often observed during the neonatal period. These lobes are associated with memory, language, and auditory processing, and epileptic foci usually form in these regions. In your sequence, temporal lobe (TL, TR) activities are noticeably present.
Frontal and Parietal Lobe Transitions (FL, PL): During neonatal epilepsy, frequent transitions between the frontal and parietal lobes can occur. These lobes regulate cognitive and motor functions, and abnormal electrical activity in these regions is observed during epileptic seizures. In your sequence, transitions between the frontal lobe (FL) and parietal lobe (PL) are frequently repeated.
Increased zonal activity in EEG signals (especially in midline regions such as Pz and Cz) is often observed during neonatal epilepsy seizures. These regions may indicate the spread of the seizure and its effects on motor functions.
Repeated symbol groups in the sequence may indicate continuous or frequently recurring seizures. Neonatal epilepsy is characterized by frequent, short seizures, which can create a specific repeating pattern in EEG signals.
The occipital lobe regions, OL and OR, which are responsible for vision, are also active. This may be related to visual disturbances or auras that some newborns may experience during seizures.
The activities of the central regions, especially CL and CR, during seizures indicate that motor functions are significantly affected, which may manifest as convulsions or motor spasms. CZ, representing sensorimotor integration, further emphasizes the involvement of motor functions and sensory feedback during seizure events.
The given symbol sequence is consistent with neonatal epilepsy, as frequent transitions between the temporal, frontal, and parietal lobes, zonal activities, and repeating patterns are observed in the EEG. In neonatal epilepsy patients, such EEG signals typically indicate the presence of epileptic seizures. However, for a definitive diagnosis, this type of analysis should be supported by clinical evaluation and additional tests conducted by neurologists.
Moreover, the transition tables of the channels, symbols, connections/edges of the automaton and hemispheres have been shown in Fig. 4.
Figure 4 demonstrated that the transition tables of the neonatal epileptic seizure detection.
Discussions
The presented TATPat-based model attained 99.15% classification accuracy by deploying 10-fold CV validation technique. Moreover, we have presented LOSO CV results and the introduced TATPat-based model reached 76.37% classification accuracy with LOSO CV. Additionally, the presented EFE model gave explainable results. In this aspect, we have discussed both the classification results and explainable results in this section.
To get classification results, three main phases have been implemented and these phases are (i) feature extraction, (ii) feature selection and (iii) classification. In the feature extraction phase, we have used channel transformer and TATPat together and our presented model selected the most informative 192 out of the generated 3249 features. By using these features, classification results and explainable results have been computed. In order to classify the selected features, the shallow classifiers were tested since we aimed to show the high classification capability of the selected features. In this test, decision tree (DT), linear discriminant (LD), quadratic discriminant (QD), binary generalized linear models logistic regression (BGLMLR), SVM, k-nearest neighbors (kNN), artificial neural network (ANN) and bagged tree (BT) classifiers. The computed classification results have been showcased in Fig. 5.
Figure 5 demonstrated that the SVM classifier is the best among the 8 classifiers used, attaining a 98.62% classification accuracy with 10-fold CV. The worst performer was the DT classifier, which reached 87.27% accuracy. All classifiers achieved over 85% classification accuracy, clearly demonstrating the high classification capabilities of the selected features.
To increase the classification results of the proposed TATPat-based model, the t algorithm was applied to the SVM classifier, resulting in tSVM attaining 99.15% classification accuracy.
To obtain explainable results, we analyzed the selected features. In the feature extraction phase, a triple nodes automaton with 9 (= 32) edges/connections was utilized to extract features. By using these connections, features were extracted, and the transition table of these connections per the selected features is showcased in Fig. 4c. Moreover, the channel, DLobV2 symbol, and hemisphere transition tables are depicted in Fig. 4. Additionally, the histograms of the connections, channels, DLobV2 symbols, and hemispheres according to the selected features are depicted in Fig. 6.
The histogram of channel distributions shows that features are distributed fairly evenly across most channels, with some channels such as T3, F8, and O1 having slightly higher numbers. This means that the selected features are derived from various regions of the brain and capture a wide range of neural activities. T3, F8, and O1 channels are associated with temporal, frontal, and occipital regions, which play important roles in sensory, cognitive, and visual processing, respectively.
Figure 3 demonstrated that the 10-fold CV-based model attained higher classification performances than LOSO CV-based model. The results of this differences have been defined in Table 7.
In 10-fold CV, each fold can contain both training and test data from the same subject. Therefore, the results from 10-fold CV are generally better than those from LOSO CV, but LOSO CV results are more reliable. Therefore, the recommended TATPat-based EFE model achieved a classification accuracy of 99.15% with 10-fold CV and 76.37% with LOSO CV.
The histogram of hemisphere distribution shows a higher number of features from the left hemisphere (169) compared to the right hemisphere (154) and middle (61). This suggests that the left hemisphere, which is typically associated with logical reasoning, language, and analytical tasks, is more affected by the epileptic seizure. The involvement of the right hemisphere means that spatial, creative, and emotional processes are also affected. The middle region, although less represented, still shows that the overall brain activity pattern is affected.
The distribution of DLobV2 symbols shows that FL and FR have the highest numbers, indicating that cognitive activities in the frontal lobes are most affected by epileptic seizures. The intense use of central regions (CL, CR) symbols shows that motor control and coordination are affected more than other functions.
The used automaton connections indicate that high-level signals are used more for diagnosis and low-level signals carry hidden patterns.
Also, the entropy values of the channels and DLobV2 symbols were computed as 4.2054 and 2.9110, respectively. There are 19 channels and 13 DLobV2 symbols. Therefore, the maximum entropy values for these metrics are 4.2479 (log2(19)) and 3.7004 (log2(13)), respectively. This indicates that the distributions of the channels are complex for neonatal epilepsy seizure detection.
Comparative results
To show the high classification ability of the presented model, we have compared the computed results to the cutting-edge models, and these comparative results are tabulated in Table 8.
Per Table 8, the proposed model reached the best classification performance among to the state-of-the-art models. Moreover, we have used LOSO CV to get classification results.
Analysis by CCT
A new explainable neuroscience theory called CCT is being developed. Using DLobV2, CCT aims to predict the causes of brain activities, and detailed results of this theory are given in the Appendix section.
The recommended TATPat-based EFE model is used to understand how neonatal epilepsy activates in different brain regions and which triggers may be associated with these processes. CCT is employed to provide a better understanding of the cause-effect relationships underlying these findings and the generated DLob string has been utilized to obtain the CCT-based findings and these are given as below.
-
Abnormal activities in the frontal lobe can lead to impairment of higher cognitive functions (attention, decision-making).
-
Abnormal activities in the temporal lobe can lead to auditory hallucinations and language processing disorders.
-
Abnormal activities in the parietal lobe can affect sensory information processing and spatial awareness functions.
-
Abnormal activities in the occipital lobe can lead to visual perception disorders and auras.
-
Abnormal activities in central areas can lead to motor control disorders and convulsions.
Moreover, based on the DLobV2 string and the regions it highlights, brain development disorders (such as cortical dysplasia) and genetic factors are likely considered significant contributors to the observed patterns of neonatal epilepsy. The high activity in various lobes is suggested to indicate widespread neural disruption, which aligns with these causes, as they have the potential to affect multiple brain functions during critical periods of development.
Highlights
Findings:
-
The recommended TATPat-based EFE model achieved a classification accuracy of 99.15% with 10-fold CV and 76.37% with LOSO CV.
-
By utilizing an automaton and transition table-based model, the proposed TATPat extracts 3,249 features from each EEG signal, and each feature represents transitions of two channels.
-
The introduced model provided detailed and interpretable explanations of EEG signal patterns and their relation to neonatal epilepsy by utilizing DLobV2.
-
Our analyses highlighted that the frontal lobe is the most affected lobe.
-
It was among the first findings of the Causal Connectome Theory, which is being developed and presents insights into the cause-effect relationships underlying neonatal epilepsy.
Advantages:
-
According to the results, the proposed TATPat captures complex EEG signal patterns.
-
A new XAI method was presented in this research, and this XAI method is DLobV2.
-
The recommended TATPat-based model achieved high classification performance.
-
A lightweight model has been presented, and this lightweight model can simply integrate with the hardware.
-
The model’s ability to analyze and explain activity in various brain regions provides descriptive information about the neural mechanisms of neonatal seizures using only EEG signals.
-
By using both 10-fold and LOSO CVs, robust and reliable results have been presented.
-
This research contributes to the development of CCT, revealing new and meaningful findings regarding neural connections and the causes of neonatal epilepsy.
-
This research contributes both feature engineering and neuroscience.
Limitations:
-
More and larger datasets can be used to show the generalizable capability of the proposed model.
-
The computed classification results with LOSO CV are relatively low.
Future works:
-
One of the primary future directions is to test the TATPat-based EFE model on larger and more diverse EEG datasets.
-
We are planning to apply the proposed model to other EEG signal datasets to obtain classification and explainable results of other brain-related disorders.
-
The details of the CCT will be presented since we are still developing the presented CCT model.
-
New symbols can be added to DLob, and new generation versions can be proposed by utilizing a more comprehensive brain cap.
-
We are planning to create a DLob dictionary for other brain conditions.
-
New generation TATPat-based explainable deep learning models will be created.
Real-world applications:
-
The model can assist in detecting and diagnosing neurological conditions like neonatal epilepsy and Alzheimer’s disease.
-
EEG2DLob2CCT applications will be implemented to help medical professionals in the near future.
-
A new neuroscience education platform will be developed for students to enhance their information levels.
-
Its lightweight design makes it suitable for real-time monitoring and diagnosis in clinical and home-care settings.
-
The DLobV2-based XAI framework helps healthcare professionals understand EEG signal patterns for better decision-making.
-
The model’s high accuracy can support early detection and personalized treatment plans for patients.
-
The CCT can advance research into brain functions and connections, aiding in the understanding of complex neurological interactions.
-
The presented TATPat-based EFE model could be used in neuroscience education platforms to help students and professionals learn more about brain signals and disorders.
Conclusions
The proposed TATPat-based EFE model achieved high accuracy rates of 99.15% with 10-fold CV and 76.37% with LOSO CV in classifying neonatal EEG signals using a dataset containing more than 10,000 EEG signals. The results clearly show that the proposed feature engineering model outperforms even deep learning algorithms. The model effectively captures transitions between channels in the complex EEG signal using a next-generation approach that combines automata and transition table-based feature extraction. The detailed and interpretable descriptions using the developed DLobV2 demonstrate the model’s ability to elucidate the underlying neural mechanisms associated with neonatal epilepsy, particularly highlighting the critical role of the frontal lobe. These findings show that the proposed TATPat-based model adds valuable insights to neuroscience and machine learning in EEG signal processing and neonatal seizure detection.
Moreover, this research shows that the integration of the lightweight design of the proposed model with hardware systems can be achieved, making our model a promising tool for real-time medical applications. This study also provides information that will enable a more in-depth understanding of neural connections and the causes of neonatal epilepsy, contributing to the development of CCT and paving the way for future research and applications.
Appendix
A1. CCT and neonatal epilepsy
Based on the TATPat model, CCT helps understand how neonatal epilepsy occurs in different brain regions and what triggers these processes. The TATPat model supports CCT’s principles and assists in identifying the transitions and triggering factors responsible for epileptic seizures. This model provides explainable results, helping to understand the impact of epileptic seizures on various brain functions.
A1.1. Causes of neonatal epilepsy according to CCT and TATPat model
-
Frequent FL and FR: Indicates impairment of higher cognitive functions (attention, decision-making).
-
Potential Cause: Brain development disorders (e.g., cortical dysplasia).
-
Frequent TL and TR: Indicates auditory hallucinations and language processing disorders.
-
Potential Cause: Genetic factors and prenatal/postnatal factors.
-
Frequent PL and PR: Indicates sensory information processing and spatial awareness functions are affected.
-
Potential Cause: Metabolic disorders.
-
Frequent OL and OR: Indicates visual perception disorders and auras.
-
Potential Cause: Prenatal and postnatal factors.
-
Frequent CL and CR: Indicates motor control disorders and convulsions.
-
Potential Cause: Traumatic brain injuries during birth.
The proposed CCT aids in better understanding the mechanisms behind neonatal epilepsy and its triggers, contributing significantly to the diagnosis and treatment of neurological disorders.
Data availability
We utilized a publicly available dataset37. The dataset used in this study can be downloaded using this link https://zenodo.org/records/2547147.
References
Abend, N. S., Wusthoff, C. J., Jensen, F. E., Inder, T. E. & Volpe, J. J. Neonatal seizures. Volpe’s Neurology of the Newborn, 381–448. e317 (2025).
El-Dib, M. et al. Neuromonitoring in neonatal critical care part I: neonatal encephalopathy and neonates with possible seizures. Pediatr. Res.94, 64–73 (2023).
Carapancea, E. & Cilio, M. R. A novel approach to seizures in neonates. Eur. J. Pediatr. Neurol. (2023).
Yan, K. et al. Incidence of neonatal seizures in China based on electroencephalogram monitoring in neonatal neurocritical care units. JAMA Netw. Open6, e2326301 (2023).
Da Silva, F. L. EEG-fMRI: Physiological Basis, Technique, and Applications 23–48 (Springer, 2023).
Xue, Z. et al. Instrumentation, measurement, and signal processing in electroencephalography-based brain–computer interfaces: situations and prospects. IEEE Trans. Instrum. Meas. (2024).
Cheng, S., Wang, J., Tian, J., Zhu, A. & Fan, J. Using humanoid robots to obtain high-quality motor imagery electroencephalogram data for better brain–computer interaction. IEEE Trans. Cogn. Dev. Syst.16, 706–719 (2023).
Ein Shoka, A. A., Dessouky, M. M., El-Sayed, A. & Hemdan, E. E.-D. EEG seizure detection: concepts, techniques, challenges, and future trends. Multimed. Tools Appl.82, 42021–42051 (2023).
Rubinger, L., Gazendam, A., Ekhtiari, S. & Bhandari, M. Machine learning and artificial intelligence in research and healthcare. Injury. 54, S69–S73 (2023).
Kumar, K., Kumar, P., Deb, D., Unguresan, M. L. & Muresan, V. In Healthcare 207 (MDPI).
Higgins, O., Short, B. L., Chalup, S. K. & Wilson, R. L. Artificial intelligence (AI) and machine learning (ML) based decision support systems in mental health: an integrative review. Int. J. Ment. Health Nurs.32, 966–978 (2023).
Islam, M. R., Zhao, X., Miao, Y., Sugano, H. & Tanaka, T. Epileptic seizure focus detection from interictal electroencephalogram: a survey. Cogn. Neurodyn.17, 1–23 (2023).
O’Shea, A., Lightbody, G., Boylan, G. & Temko, A. In 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 5862–5865 (IEEE, 2018).
O’Shea, A., Lightbody, G., Boylan, G. & Temko, A. Neonatal seizure detection from raw multi-channel EEG using a fully convolutional architecture. Neural Netw.123, 12–25 (2020).
O’Shea, A., Lightbody, G., Boylan, G. & Temko, A. in 2017 IEEE 27th International Workshop on Machine Learning for Signal Processing (MLSP). 1–6 (IEEE).
Hogan, R. et al. Scaling convolutional neural networks achieves expert-level seizure detection in neonatal EEG. arXiv preprint arXiv:2405.09911 (2024).
Gramacki, A. & Gramacki, J. A deep learning framework for epileptic seizure detection based on neonatal EEG signals. Sci. Rep.12, 13010 (2022).
Pavel, A. M. et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child. Adolesc. Health. 4, 740–749 (2020).
Temko, A., Thomas, E., Marnane, W., Lightbody, G. & Boylan, G. EEG-based neonatal seizure detection with support vector machines. Clin. Neurophysiol.122, 464–473 (2011).
Temko, A. et al. Toward a personalized real-time diagnosis in neonatal seizure detection. IEEE J. Translational Eng. Health Med.5, 1–14 (2017).
Temko, A., Thomas, E., Marnane, W., Lightbody, G. & Boylan, G. B. Performance assessment for EEG-based neonatal seizure detectors. Clin. Neurophysiol.122, 474–482 (2011).
Yu, X., Aziz, M. Z., Sadiq, M. T., Fan, Z. & Xiao, G. A new framework for automatic detection of motor and mental imagery EEG signals for robust BCI systems. IEEE Trans. Instrum. Meas.70, 1–12 (2021).
Sadiq, M. T. et al. Toward the development of versatile brain–computer interfaces. IEEE Trans. Artif. Intell.2, 314–328 (2021).
Sadiq, M. T. et al. Motor imagery BCI classification based on multivariate variational mode decomposition. IEEE Trans. Emerg. Top. Comput. Intell.6, 1177–1189 (2022).
Sadiq, M. T. et al. Motor imagery EEG signals decoding by multivariate empirical wavelet transform-based framework for robust brain–computer interfaces. IEEE Access.7, 171431–171451 (2019).
Sadiq, M. T., Yu, X., Yuan, Z. & Aziz, M. Z. Motor imagery BCI classification based on novel two-dimensional modelling in empirical wavelet transform. Electron. Lett.56, 1367–1369 (2020).
Sadiq, M. T., Akbari, H., Siuly, S., Li, Y. & Wen, P. Alcoholic EEG signals recognition based on phase space dynamic and geometrical features. Chaos Solitons Fractals. 158, 112036 (2022).
Akbari, H. et al. Depression Detection based on geometrical features extracted from SODP shape of EEG signals and binary PSO. Traitement Du Signal.38 (2021).
Akbari, H. et al. Recognizing seizure using Poincaré plot of EEG signals and graphical features in DWT domain. Bratislava Med. J. (2023).
Sadiq, M. T. et al. Exploiting pretrained CNN models for the development of an EEG-based robust BCI framework. Comput. Biol. Med.143, 105242 (2022).
Sharma, R., Pachori, R. B. & Sircar, P. Automated emotion recognition based on higher order statistics and deep learning algorithm. Biomed. Signal Process. Control. 58, 101867 (2020).
Sharma, R. Localization of epileptic surgical area using automated hybrid approach based on higher-order statistics with sensitivity analysis and residual wavelet transform. Biomed. Signal Process. Control. 86, 105192 (2023).
Sharma, R., Pachori, R. B. & Sircar, P. Seizures classification based on higher order statistics and deep neural network. Biomed. Signal Process. Control. 59, 101921 (2020).
Sharma, R., Sircar, P. & Pachori, R. B. Automated focal EEG signal detection based on third order cumulant function. Biomed. Signal Process. Control. 58, 101856 (2020).
Vaswani, A. et al. Attention is all you need. Adv. Neural. Inf. Process. Syst. 30 (2017).
Ojala, T., Pietikainen, M. & Maenpaa, T. Multiresolution gray-scale and rotation invariant texture classification with local binary patterns. IEEE Trans. Pattern Anal. Mach. Intell.24, 971–987 (2002).
Stevenson, N. J., Tapani, K., Lauronen, L. & Vanhatalo, S. A dataset of neonatal EEG recordings with seizure annotations. Sci. data. 6, 1–8 (2019).
Goldberger, J., Hinton, G. E., Roweis, S. & Salakhutdinov, R. R. Neighbourhood components analysis. Adv. Neural. Inf. Process. Syst. 17 (2004).
Vapnik, V. In Nonlinear Modeling55–85 (Springer, 1998).
Vapnik, V. The Nature of Statistical Learning Theory (Springer science & business media, 2013).
Dogan, A. et al. PrimePatNet87: Prime pattern and tunable q-factor wavelet transform techniques for automated accurate EEG emotion recognition. Comput. Biol. Med.138, 104867 (2021).
Yadav, V. P. & Sharma, K. K. Automatic classification framework for neonatal seizure using Wavelet scattering transform and nearest component analysis. IRBM, 100842 (2024).
Raeisi, K. et al. A graph convolutional neural network for the automated detection of seizures in the neonatal EEG. Comput. Methods Programs Biomed.222, 106950 (2022).
Raab, D., Theissler, A. & Spiliopoulou, M. XAI4EEG: spectral and spatio-temporal explanation of deep learning-based seizure detection in EEG time series. Neural Comput. Appl.35, 10051–10068 (2023).
Raeisi, K. et al. A class-imbalance aware and explainable spatio-temporal graph attention network for neonatal seizure detection. Int. J. Neural Syst.33, 2350046 (2023).
Mumenin, K. M., Biswas, P., Khan, M. A. M., Alammary, A. S. & Nahid A.-A. A modified aquila-based optimized XGBoost framework for detecting probable seizure status in neonates. Sensors. 23, 7037 (2023).
Tanveer, M. A., Khan, M. J., Sajid, H. & Naseer, N. Convolutional neural networks ensemble model for neonatal seizure detection. J. Neurosci. Methods. 358, 109197 (2021).
Diykh, M. et al. Texture analysis based graph approach for automatic detection of neonatal seizure from multi-channel EEG signals. Measurement. 190, 110731 (2022).
Gomez-Quintana, S., O’Shea, A., Factor, A., Popovici, E. & Temko A. A method for AI assisted human interpretation of neonatal EEG. Sci. Rep.12, 10932 (2022).
Caliskan, A. & Rencuzogullari, S. Transfer learning to detect neonatal seizure from electroencephalography signals. Neural Comput. Appl.33, 12087–12101 (2021).
Tapani, K. T., Nevalainen, P., Vanhatalo, S. & Stevenson, N. J. Validating an SVM-based neonatal seizure detection algorithm for generalizability, non-inferiority and clinical efficacy. Comput. Biol. Med.145, 105399 (2022).
Webb, L., Kauppila, M., Roberts, J. A., Vanhatalo, S. & Stevenson, N. J. Automated detection of artefacts in neonatal EEG with residual neural networks. Comput. Methods Programs Biomed.208, 106194 (2021).
He, H., Liu, X. & Hao, Y. A progressive deep wavelet cascade classification model for epilepsy detection. Artif. Intell. Med.118, 102117 (2021).
Isaev, D. Y. et al. Attention-based network for weak labels in neonatal seizure detection. Proceedings of machine learning research 126, 479 (2020).
Debelo, B. S., Thamineni, B. L., Dasari, H. K. & Dawud, A. A. Detection and severity identification of neonatal seizure using deep convolutional neural networks from multichannel EEG signal. Pediatr. Health Med. Ther., 405–417 (2023).
Frassineti, L., Ermini, D., Fabbri, R. & Manfredi, C. in 2020 IEEE 20th Mediterranean Electrotechnical Conference (MELECON). 344–349 (IEEE).
Wang, Y. et al. Combining EEG features and Convolutional Autoencoder for neonatal seizure detection. Int. J. Neural Syst., 2450040–2450040 (2024).
Li, Z. et al. in. IEEE International Conference on Systems, Man, and Cybernetics (SMC). 196–201 (IEEE). (2023).
Visalini, K., Alagarsamy, S. & Nagarajan, D. Neonatal seizure detection using deep belief networks from multichannel EEG data. Neural Comput. Appl.35, 10637–10647 (2023).
Jiang, X., Meng, L., Wang, Z. & Wu, D. Deep source semi-supervised transfer learning (DS3TL) for cross-subject EEG classification. IEEE Trans. Biomed. Eng. (2023).
Hermans, T. et al. A multi-task and multi-channel convolutional neural network for semi-supervised neonatal artefact detection. J. Neural Eng.20, 026013 (2023).
Nagarajan, V., Muralidharan, A., Sriraman, D. & Kumar, S. P. in 2nd International Conference on Artificial Intelligence and Signal Processing (AISP). 1–6 (IEEE). (2022).
Amrani, G., Adadi, A. & Berrada, M. An explainable hybrid DNN model for seizure vs. non-seizure classification and seizure localization using multi-dimensional EEG signals. Biomed. Signal Process. Control. 95, 106322 (2024).
Daly, A., Lightbody, G. & Temko, A. in 2023 31st European Signal Processing Conference (EUSIPCO). 1100–1104 (IEEE).
Zeedan, A., Al-Fakhroo, K. & Barakeh, A. EEG-based seizure detection using feed-forward and LSTM neural networks based on a neonates dataset. Authorea Preprints (2023).
He, Z. et al. Unified Convolutional Sparse Transformer for Disease diagnosis, monitoring, Drug Development, and Therapeutic Effect Prediction from EEG Raw Data. Biology. 13, 203 (2024).
Urbina Fredes, S. et al. Enhanced epileptic seizure detection through Wavelet-based analysis of EEG Signal Processing. Appl. Sci.14, 5783 (2024).
Frassineti, L., Manfredi, C., Olmi, B. & Lanatà, A. in 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). 471–474 (IEEE). (2021).
Purnima, P., Suresh, M. & Kattepura, S. in 2023 International conference on inventive computation technologies (ICICT). 135–141 (IEEE).
Udayantha, D. S. et al. Using Explainable AI for EEG-based Reduced Montage Neonatal Seizure Detection. arXiv preprint arXiv:2406.16908 (2024).
Nsugbe, E. Artificial Intelligence-assisted care for human newborns with neurological impairments. Digit. Technol. Res. Appl.1, 76–88 (2022).
Larbi, A., Abed, M., Nahi, A. & Ouahabi, A. in 8th International Conference on Image and Signal Processing and their Applications (ISPA). 1–6 (IEEE). (2024).
Fredes, S. U. et al. in. 9th International Conference on Signal Processing and Communication (ICSC). 538–543 (IEEE). (2023).
Smith, G. A., Henry, J. & van Drongelen, W. Detecting heterogeneous seizures in newborn infants using triple correlation. medRxiv, 2006. 2009.23291216 (2023). (2023).
Borovac, A., Runarsson, T. P., Thorvardsson, G. & Gudmundsson, S. in Curr. Dir. Biomedical Eng. 604–607 (De Gruyter).
Tuncer, T., Dogan, S., Tasci, I., Baygin, M., Barua, P. D., & Acharya, U. R. Lobish: Symbolic Language for Interpreting Electroencephalogram Signals in Language Detection Using Channel-Based Transformation and Pattern. Diagnostics 14(17), 1987 (2024).
Tuncer, T., Dogan, S., Baygin, M., Tasci, I., Mungen, B., Tasci, B., Barua, P.D., & Acharya, U.R. TTPat and CWINCA-based explainable feature engineering model using Directed Lobish: A new EEG artifact classification model. Knowledge-Based Systems, 112555 (2024).
Funding
This study was supported by the Scientific Research Projects Coordination Unit of Firat University. Project number TEKF.24.48.
Author information
Authors and Affiliations
Contributions
Author Contributions: Conceptualization, TT, SD, IT, BT, RH; formal analysis, TT, SD, IT, BT, RH; investigation, TT, SD, IT, BT, RH; methodology, TT, SD, IT, BT, RH; software: TT, SD; project administration, TT; resources, TT, SD, IT, BT, RH; supervision, TT; validation, TT, SD, IT, BT, RH; visualization, TT, SD, IT, BT, RH; writing—original draft, TT, SD, IT, BT, RH; writing—review and editing, TT, SD, IT, BT, RH. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tuncer, T., Dogan, S., Tasci, I. et al. TATPat based explainable EEG model for neonatal seizure detection. Sci Rep 14, 26688 (2024). https://doi.org/10.1038/s41598-024-77609-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77609-x
Keywords
This article is cited by
-
DCPat-XFE: an explainable EEG model for psychogenic nonepileptic seizure detection
Cognitive Neurodynamics (2026)
-
Novel accurate classification system developed using order transition pattern feature engineering technique with physiological signals
Scientific Reports (2025)
-
Neonatal seizure detection from EEG using inception ResNetV2 feature extraction and XGBoost optimized with particle swarm optimization
Scientific Reports (2025)
-
A multi-interactive learning model for sleep staging based on polysomnography signals
Discover Computing (2025)