Abstract
This study end to develop nanoemulsions of Panax ginseng dry extract and to evaluate the potential impact of these nanoemulsions versus free Panax ginseng dry extract and Vit.E in recovering male infertility induced in rats. Nanoemulsions of Panax ginseng dry extract were prepared by oil in water method. The designed samples were characterized by TEM, zeta sizer, FTIR, and TGA. The in vitro study included DPPH assay to estimate the free radical scavenging activity of the suggested treatments. The in vivo study included 100 adult male Wistar rats which were assigned into 10 equal groups; five groups of young rats weighting (150–200 g) and five groups of aged rats weighting (350–400 g). Group I, negative control. Group II, bisphenol-A (BPA). Group III, BPA+ Panax ginseng dry extract nanoemulsion. Group IV, BPA+ free Panax ginseng dry extract. Group V, BPA +Vit.E. After 40 days, serum total testosterone, free testosterone, MDA, 8-OHdG and AGEs were estimated. Besides, the histological investigation of testicular tissue sections was performed. TEM imaging of Panax ginseng dry extract nanoemulsions indicated spherical shape with diameter range from 2 to 50 nm, and the size distribution was in the range from 62 to 123 d.nm. The zeta potential of the designed nanoemulsions was -32.8 to -38.9 mV. FTIR spectra revealed the common active groups in the prepared nanoemulsions. The thermal stability of the nanoemulsions was up to 207 ºC. The in vitro results of DPPH assay showed % inhibition of DPPH free radical for Panax ginseng nanoemulsions samples was 49.38% (for young-treated group Sample A) and 72.28% (for aged-treated group Sample B), while for free Panax ginseng dry extract samples was 30.27% (for young-treated group Sample C) and 56.76% (for aged-treated group Sample D), for Vit.E samples was 32.36% (for young-treated group Sample E) and 36.39% (for aged-treated group Sample F).Thus the nanoemulsions exhibit free radicals scavenging activity more than free Panax ginseng dry extract and Vit.E. The in vivo findings elucidated that Panax ginseng dry extract nanoemulsions and Vit.E successfully revers the progressive insult of BPA on male fertility by significantly enhance total testosterone (2.87±0.318) and free testosterone (1.63±0.033) serum levels, and significantly decrease MDA (2.77±0.018), 8-OHdG (6.76±0.174) and AGEs (92.60±1.701) serum levels. Interestingly, the most promising outcomes were recorded upon the treatment with Panax ginseng dry extract nanoemulsions. In conclusion the developed Panax ginseng dry extract nanoemulsion could be used as a promising strategy in improving potential male infertility defects by rescuing male sex hormones, neutralizing oxidative stress and retrieving the structural organization of the testes.
Similar content being viewed by others
Introduction
Infertility is a medical condition affecting either male or female reproductive system, marked by the inability to achieve pregnancy following a year of consistent and unprotected sexual intercourse1. It represents a multifaceted crisis that commonly associated with psychological and emotional stress for both partners, as well as the significant economic burdens2. Infertility impacts approximately 15–20% of couples globally, with a male factor contributing to around half of these instances3. According to available data, certain regions like North Africa and the Middle East experience elevated rates of primary infertility prevalence, primary infertility refers to couples who have not had a live birth after at least 1 year having sex without using birth control methods4. In Egypt, a study conducted by the Egyptian Fertility Care Society and supported by the World Health Organization, revealed that infertility impacts 12% of Egyptian couples5.
Male infertility is known as the male inability to successfully impregnate a fertile female, following at least 12 months of unprotected sexual intercourse6. In the last three decades, an expanding body of research has elucidated the adverse effects of environmental, genetic, physiological, and lifestyle factors on fertility of male. Specifically, factors such as smoking, nutritional status, diabetes, psychological stress, overuse of alcohol/drugs, and endocrine disruptors have been highlighted. Research has demonstrated that these factors negatively impact sperm characteristics, DNA integrity and capacitation as well as the production of ATP by mitochondria7,8,9.
Bisphenol A (BPA) is a recognized endocrine disruptor renowned for its estrogenic properties10. It is crystalline chemical compound which extensively employed as a monomer in industrial processes for the manufacture of plastic materials, polyacrylates and polyesters11. Among its myriad applications, this compound is found in numerous everyday items, including linings for food and beverage containers, plastic tableware, kitchen tools, electronic devices (as cell phones and computers), dental sealants and thermal paper12.
Exposure to BPA elicits adverse effects on the male reproductive system, leading to disruptions in spermatogenesis and the quality of sperm13. Exposure to BPA results in a decrease in inhibin B levels, which correlates with a decline in the number of Sertoli cells, thus it directly impacts spermatogenesis10. Additionally, BPA causes a decrease in concentration of sperms, production of ATP and integrity of DNA14. BPA significantly diminishes both the motility and viability of epididymal sperm through the induction of oxidative stress15. In the study conducted by Eshak and Osman16, it was found that the reduced testosterone levels were linked to the elevated malondialdehyde (MDA) levels in rats treated with BPA. Even at the minimal concentrations, the estrogenic effects of BPA disrupt the functioning of the hypothalamic-pituitary-gonadal (HPG) axis17. Furthermore, low concentration of BPA diminishes testosterone levels by directly affecting Leydig cells, hindering their proliferation, and disrupting typical steroidogenesis10.
Korean red ginseng (Panax ginseng) is considered as a perennial herb, which is highly regarded in alternative and complementary medicines worldwide due to its numerous health-enhancing properties18. In traditional practices, ginseng root is utilized to augment male libido and fertility, while also elevating male sex hormone levels in various experimental models. Additionally, it enhances spermatogenesis, sperm count/vitality and restores Leydig/Sertoli cell count19,20. Lin et al.21 demonstrated that Panax ginseng stimulates testosterone synthesis and supports the proper functioning of androgens when administered as a dietary supplement. For an extended period, it has been observed that Panax ginseng exhibits protective characteristics against damage caused by free radicals22. Moreover, Panax ginseng is recognized for its established antioxidant and anti-inflammatory properties within the biological systems23.
Ginseng contains various groups of chemical components, such as ginsenosides, polysaccharides, phenolic acids, alkaloids and glucosides 24. Various reviews have reported that ginsenosides, which are the primary bioactive compounds in ginseng, could influence estrogen and androgen function and exert aphrodisiac effects25. The erectile response of the penis relies on maintaining equilibrium between vasoconstrictor agents, which induce contraction of cavernosal smooth muscle, thereby restricting blood flow, and vasodilator agents, which induce relaxation of cavernosal smooth muscle, thus facilitating increased blood flow and erection26. There is compelling evidence suggesting that ginsenosides can promote penile erection by directly stimulating vasodilation and relaxation of the penile corpus cavernosum27. Although ginseng exhibits promising pharmacological characteristics, its clinical utility is impeded by its inadequate bioavailability and poor solubility28. Nanobiotechnology has received considerable attention for its utilization of nanotechnology to address important medical and biological challenges as well as enhance the effectiveness of related applications29. Different nanodelivery systems, such as nanosuspensions, nanoparticles and nanoemulsions have recently gained attention for their role in improving numerous biophysical functionalities. This includes enhancing poorly soluble compounds dispersibility as well as providing sustained-release capabilities 30. The recent integration of ginseng into nanobiotechnology has led to the investigation of various ginseng-derived nanomaterials and their pharmacological effects, including antioxidant and anti-inflammatory properties31,32. Nanoparticles have exhibited favorable effects on fertility, as their smaller size facilitates their penetration of the testis. Nano-antioxidants have been noted for their ability to decrease reactive oxygen species (ROS), thereby effectively mitigating oxidative damage. Additionally, the nanoformulation of the antioxidant agents has been demonstrated to augment sperm motility and viability, alongside preserving DNA integrity and regulating gene expression levels 33,34. Vitamin E (Vit.E) is the general name of tocopherols, which are a group of phenolic chemical compounds35. It serves as one of the natural antioxidants present in semen, counteracting the actions of free radicals, inhibiting the generation of lipid peroxides, and shielding sperm from damage caused by ROS36. Vit.E opposes the detrimental effects induced by BPA on sperm parameters, testicular tissue, MDA and testosterone levels by enhancing the antioxidant enzymes activity and decreasing lipid peroxidation (LPO) 37. Additionally, it inhibits the initiation of apoptosis in spermatogenic, Leydig, and Sertoli cells38. The focus of our interest was to formulate nanoemulsion of Panax ginseng dry extract standardized to ginsenosides using oil in water method and to appraise the potent role of such nanoformulation against BPA-induced male infertility via hindering the oxidative stress process.
Materials and methods
Materials
Chemicals and drugs
Bisphenol A; 2, 2, -bis (4-hydroxyphenyl) propane, 4,4’- isopropylidenediphenol with purity ≥ 99% was purchased from Sigma-Aldrich Co-Saint Louis, Mo,63,103 − 2530 USA. Tween 20 (T20) used in the preparation of nanoformulation (stable oil-in-water emulsion) as an emulsifying agent, was procured from Sigma-Aldrich Co-Saint Louis, Mo,63,103 − 2530, USA. The standardized Panax ginseng dry extract (from roots of Panax ginseng C.A Meyer DER3-7:1) was purchased from Mulini, 6934 Bioggio, Switzerland. The content of ginsenosides, the pharmacological active ingredients of Panax ginseng dry extract, was 10% wt/wt. Vitamin E (Vit.E) was supplied from Pharco pharmaceuticals Alexandria, Egypt. The other commercially available reagents and chemicals were of analytical grade.
Animals
Adult male albino rats of Wistar strain were provided from the Animal Care Unit of the National Research Centre (NRC), Egypt. Rats was housed in a light/dark cycles of 12/12 h, a relative humidity of 55% ±5%, and a constant temperature of 22 ± 2˚C. Animals were allowed to have standard chow diet and water ad libitum throughout the experiment. Ethical Committee for Medical Research of the NRC, Egypt has been granted the final approval for the study under registration number of 13104112022.
Methods
Preparation of oil in water (O/W) Panax ginseng dry extract standardized to ginsenosides nanoemulsions
Nanoemulsion of Panax ginseng dry extract standardized to ginsenosides was prepared using the oil-in-water system according to Min et al.39 with some modifications. In brief, 4 mg of ginsenosides (equivalent to 40 mg of Panax ginseng dry extract) was dissolved in 0.75 ml deionized water for preparation of the dose for young adult rats (Sample A), and 7 mg of ginsenosides (equivalent to 70 mg of Panax ginseng dry extract) was dissolved in 0.75 ml deionized water for preparation of the dose for aged adult rats (Sample B). Then Tween 20 was added to the mixture gradually, the mixture was homogenized to form nanoemulsions by a high speed homogenizer (Model: 400ELPC, PRO Scientific Inc., 01-02411ELPC homogenizer, USA) at 20,000 rpm for 8 min. in ice bath to reduce the temperature of the mixture. After that, the emulsions were sonicated by ultrasonicator (Ultrasonic Cleaner MTI Corporation, Model UD150SH3.8LQ, USA) at 750 W for 5 min. for dispersion of particles from each other. Finally, portion of both nanoemulsion samples were subjected to freeze drying using the freeze drier (LABCONCO, FreeZone 12 L freeze-drying system with stopper tray, catalog number: 7759030) to transform nanoemulsion into powder for measuring Fourier transform infrared (FTIR) spectroscopy and Thermogravimetric analysis (TGA).
Characterization of nanoemulsions of Panax ginseng dry extract
All characterization tests were done at the Central Lab of the NRC, Egypt. Panax ginseng dry extract standardized to ginsenosides nanoemulsions were characterized using transmission electron microscope (TEM) Model (JEM-2100, JEOL, Japan) at an accelerating voltage of 200KV. The prepared samples were diluted with distilled water and sonicated for 3 min. in an ultrasonic bath (4HT10146 by CREST ULTRASONICS, Trenton, NJ, USA), then 5 µl from each sample were taken by micropipette and placed on a film-coated 200-mesh copper specimen grids for 10 min. and the excess fluid was eliminated using the filter paper. Finally, the grids were subjected to TEM. The polydispersity index (PDI), zeta sizer and zeta potential of the prepared samples was detected by using Malvern Zetasizer (Nano-ZS, Malvern Instrument, UK). Each sample was dispersed in distilled water under ultra-sonication for 10 min. The size distribution and zeta-potential for each sample were measured using the Malvern zeta sizer Nano-ZS, Malvern Instrument with He/Ne laser at wavelength λ = 633 nm and an angle of 173° using collecting backscatter optics40. Fourier transform infrared (FTIR) spectroscopy was used to determine the functional groups of the prepared samples. A small amount (3.5 mg) of each sample was placed directly on the lens of FTIR (Bruker VERTEX 80, Germany) combined with Platinum Diamond ATR to comprise a diamond disk as an internal reflector in the range 4000–400 cm− 1 with resolution of 4 cm− 1, and refractive index of 2.4 41. The thermogravimetric analysis (TGA) was used to detect the thermal stability of the prepared samples. The samples were heated at 10 °C/min in the atmosphere of nitrogen (SETARAM Instrumentation, model Themys one plus, France) for 22.5 min.
Determination of free radical scavenging capacity in vitro using DPPH assay
The 1,1 - diphenyl − 2 - picrylhydrazyl (DPPH) radical assay was carried out spectrophotometrically (Shimandzu, Tokyo, Japan) to estimate the free radical scavenging activity for samples A (Panax ginseng dry extract nanoemulsion young group), B (Panax ginseng dry extract nanoemulsion aged group), C (free Panax ginseng dry extract young group), D (free Panax ginseng dry extract aged group), E (Vit.E young group), and F (Vit.E aged group). A volume of 50 µl of each sample was combined with 5 ml of a 0.004% ethanol solution of DPPH. Following an incubation period of 30 min. at room temperature, the absorbance was measured against a control (contains all reagents except the tested sample and ascorbic acid) at 517 nm. Ascorbic acid (AA) served as a reference standard and was dissolved in methanol to prepare a stock solution with a concentration of 1 mg/ml.
Where: Abs. of control is the absorbance of the control reaction (contains all reagents except the tested sample and ascorbic acid) and Abs. of sample/standard is the absorbance of the tested sample. I (%) is the inhibition percentage of DPPH radicle. This test was performed in triplicate for validation of the results.
Animal experiment
This study was conducted on one hundred adult male albino rats of Wistar strain which were assigned as follows:
fifty young rats weighting 150–200 g (3 month-old) and fifty aged rat weighting 350–400 g (12 month-old), that were randomly classified as:
negative young control group (young control group) that was orally administered with corn oil (1 ml/rat), negative aged control group (aged control group) that was orally administered with corn oil (1 ml/rat), BPA young group (BPA young-challenged group) that was orally administered with 250 mg/kg/day of BPA dissolved in corn oil according to the study of Malmir et al.42, BPA aged group (BPA aged-challenged group) that was orally administered with 250 mg/kg/day of BPA dissolved in corn oil, BPA + Panax ginseng dry extract nanoemulsion young group (Nano young-treated group) that was orally administered with 250 mg/kg/day of BPA dissolved in corn oil simultaneously with intraperitoneal administration of Panax ginseng dry extract nanoemulsion (20 mg/kg/day Sample A), BPA + Panax ginseng dry extract nanoemulsion aged group (Nano aged-treated group) that was orally administered with 250 mg/kg/day of BPA dissolved in corn oil simultaneously with intraperitoneal administration of Panax ginseng dry extract nanoemulsion (20 mg/kg/day Sample B), BPA + free Panax ginseng dry extract young group (Free young-treated group) that was orally administered with 250 mg/kg/day of BPA dissolved in corn oil simultaneously with intraperitoneal administration of Panax ginseng dry extract dissolved in saline (20 mg/kg/day Sample C) according to method of Kim et al.43, BPA + free Panax ginseng dry extract aged group (Free aged-treated group) that was orally administered with 250 mg/kg/day of BPA dissolved in corn oil simultaneously with intraperitoneal administration of Panax ginseng dry extract dissolved in saline (20 mg/kg/day Sample D), BPA + Vit.E young group (Vit.E young-treated group) that was orally administered with 250 mg/kg/day of BPA dissolved in corn oil simultaneously with oral administration of Vit.E (150 mg/kg/day Sample E) dissolved in corn oil according to Malmir et al.42, BPA + Vit.E aged group (Vit.E aged-treated group) that was orally administered with 250 mg/kg/day of BPA dissolved in corn oil simultaneously with oral administration of Vit.E (150 mg/kg/day Sample F) dissolved in corn oil.
The administration of BPA as well as the different treatments were implemented at the same time and the experiment lasted for 40 days.
Biochemical analyses
After finalizing the experiment (40 days), the rats in different studied groups were fasted overnight and anaesthetized with pentobarbital 45 mg/kg intraperitoneally44 to collect blood samples from the tail vein. Then, the animals were sacrificed and the testes were dissected and washed in saline solution, then fixed in formalin saline (10%) for histological investigation. The serum samples were separated by centrifugation of blood samples at 1800 x g (times gravity) for 10 min. at 4˚C. The serum samples were kept at -20˚C pending biochemical determinations which included total testosterone, free testosterone, 8-hydroxy-2′-deoxyguanosine (8-OHdG) and advanced glycation end products (AGEs) that were measured by enzyme linked immunosorbent (ELIZA) using ELIZA kits (Sunlong, China, Catalogue Number SL1061Ra, SL0293Ra, SL0019Ra, SL0036Ra respectively) according to the operating’s instructions. Serum MDA level was quantified by colorimetric method using a colorimetric kit (Biodiagnostic, Egypt, Catalogue Number MD 25 29) following the manufacturer’s manual45.
Histological examination
After fixation of the testicular tissue in 10% formalin saline for 24 h, the tissue samples underwent washing with tap water before being prepared and stained for light microscopy (Leica, Wetzlar, Germany). The process involved dehydrating the fixed tissue through a series of ethyl alcohol dilutions (ranging from 70 to 100%) and subsequently clearing the specimens in xylene. The tissue was then embedded in paraffin wax using a hot air oven set at 56 °C for 6 h. Following this, paraffin wax tissue blocks were sectioned using a microtome (Leica RM2255, Wetzlar, Germany) at a thickness of 5–6 microns. The resulting sections were collected on glass slides, deparaffinized, and stained with hematoxylin and eosin for routine histological examination under a light microscope46.
Statistical analyses
In the present study, all results were expressed as Mean ± S.E (Standard Error) of the mean. The data were analyzed by one-way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (SPSS) program, version 20 followed by least significant difference (LSD) test 47. Difference was considered significant at P < 0.05. The percent of change between groups was calculated according to the equation:
Results
Characterization of nanoemulsions of Panax ginseng dry extract (sample A and sample B)
Transmission electron microscope (TEM)
The obtained data from TEM for samples A and B showed that: sample A of a completely spherical shape of the particles with a diameter ranges from 2 to 8 nm and homogenous distribution (Fig. 1a). Whereas, sample B showed a spherical shape of the particles inclined to hexagonal shape with a diameter ranges from 20 to 50 nm, and heterogeneous distribution (Fig. 1b).
Polydispersity index (PDI), zeta sizer and zeta potential
The calculated values of the polydispersity index (PDI) for the prepared sample A was 0.57 and sample B was 0.6. The particle size distribution and surface charge of sample A and sample B measured by the dynamic light scattering (DLS) technique were represented in Figs. 2 and 3 respectively. As shown in the figures, both sample A (Fig. 2-a) and sample B (Fig. 3-a) exhibit size distribution by number of about 62 and 123 d.nm respectively. Zeta potential of sample A (Fig. 2-b) is -38.9 mV, while Zeta potential of sample B (Fig. 3-b) was − 32.8 mV.
Fourier transform infrared (FTIR)
The FTIR spectrum of sample A (Fig. 4a) and sample B (Fig. 4b) showed almost the same peaks; the absorption band at 3329 cm− 1 of sample A and the absorption band 3347 cm− 1 of sample B describ the stretching mode of hydroxyl (OH) group. The bands at 2917 cm− 1 and 2852 cm− 1 of sample A and the bands at 2918 cm− 1 and 2852 cm− 1 of sample B are characteristic for vibration modes of methylene (CH2) group. The band at 1737 cm− 1 of sample A and the band at 1738 cm− 1 of sample B is unique to the carbonyl (C = O) group. The band at 1612 cm− 1 of sample A and the band at 1614 cm− 1 of sample B corresponds to the alkene (C = C) group.Whereas, the two bands observed at 1458 cm− 1 and 1350 cm− 1 of both samples A and B point to methyl (CH3) and methylene (CH2) groups respectively. Finally, the stretching mode of ether (C-O-C) group is noted band at the absorbtion band 1041 cm− 1 of sample A and 1081 cm− 1 of sample B.
Thermogravimetric analysis (TGA)
The two samples A and B are tested until 225 °C to detect their thermal stability. Sample A shows a stable configuration until 207 °C because of the weight loss of the sample was approximately 8% (Fig. 5). While sample B shows stable configuration until 202 °C because of the weight loss of the sample was approximately 12% (Fig. 5).
Free radical scavenging capacity of the treatment samples in vitro
The free radical scavenging activity of the treatment samples A, B, C, D, E and F was measured by DPPH radical scavenging assay. In comparison with ascorbic acid, the % inhibition of DPPH free radical for ascorbic acid and samples A, B, C, D, E and F was 81.49%, 49.38%, 72.28%, 30.27%, 56.76%, 32.36% and 36.39% respectively (Fig. 6).
Biochemical findings
Total testosterone
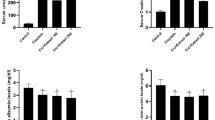
The present data revealed that there is a significant decrease (p < 0.05) in serum total testosterone level in BPA young-challenged group in comparison to young control group by 60.5%. On the other side, a significant increase (p < 0.05) in serum total testosterone level is recorded in nano young-treated group, free young-treated group and Vit.E young-treated group in comparison to BPA young challenged-group by 97.9%, 88.3% and 42.8% respectively. Additionally, there is a significant enhancement (p < 0.05) in serum total testosterone level in nano young-treated group and free young-treated group by contrast with Vit.E young-treated group by 38.6% and 31.9% respectively. Also, the present results indicated a significant reduction (p < 0.05) in serum total testosterone level in BPA aged-challenged group versus aged control group by 60.8%. Meanwhile, there is a significant increase (p < 0.05) in serum total testosterone level in nano aged-treated group, free aged-treated group and Vit.E aged-treated group contrary to BPA aged challenged-group by 109.6%, 92.6% and 39.3%, respectively. The present data also indicated that there is a significant enhancement (p < 0.05) in serum total testosterone level in nano aged-treated group and free aged-treated group in comparison to Vit.E aged-treated group by 50.5% and 38.3% respectively (Table 1).
Free testosterone
The current findings revealed that there is a significant drop (p < 0.05) in serum free testosterone level in BPA young-challenged group in comparison to young control group by 38.9%. On the other side, there is a significant enhancement (p < 0.05) in serum free testosterone level in nano young-treated group and free young-treated group in comparison to BPA young challenged-group by 40.5% and 28.4% respectively. Additionally, there is a significant elevation has been recorded (p < 0.05) in serum free testosterone level in nano young-treated group and free young-treated group versus Vit.E young-treated group by 37% and 25.2% respectively. The present results also indicated a significant reduction (p < 0.05) in serum free testosterone level in BPA aged-challenged group contrary to aged control group by 42.4%. Meanwhile, a significant augmentation (p < 0.05) in serum free testosterone level in nano aged-treated group, free aged-treated group and Vit.E aged-treated group by contrast with BPA aged challenged-group by 38.9%, 25.3% and 21.1% respectively has been observed (Table 2).
Malondialdehyde (MDA)
The present data revealed that there is a significant rise (p < 0.05) in serum MDA level in BPA young-challenged group versus young control group by 78.8%. On the opposite side, a significant depletion (p < 0.05) in serum MDA level in nano young-treated group, free young-treated group and Vit.E young-treated group, as compared to BPA young challenged-group by 36.8%, 21.5% and 15.3% respectively, has been detected. Additionally, the data in present work denoted a significant decrement (p < 0.05) in serum MDA level in nano young-treated group contrary to free young-treated group by 19.5%. Also, the current study found that there is a significant decline (p < 0.05) in serum MDA level in nano young-treated group and free young-treated group in comparison to Vit.E young-treated group by 25.3% and 7.3% respectively. Furthermore, a significant elevation (p < 0.05) in serum MDA level in BPA aged-challenged group in comparison to aged control group by 85.9% has been demonstrated. However, a significant decrease (p < 0.05) in serum MDA level in nano aged-treated group, free aged-treated group and Vit.E aged-treated group in comparison to BPA aged challenged-group by 38.2%, 24.4% and 16.8%, respectively has been determined. Likewise, a significant reduction (p < 0.05) in serum MDA level in nano aged-treated group in comparison to free aged-treated group by 18.3% has been observed. Finally, the findings in present work demonstrated a significant diminution (p < 0.05) in serum MDA level in nano aged-treated group and free aged-treated group by contrast with Vit.E aged-treated group by 25.7% and 9.1% respectively (Table 3).
8-hydroxy-2′-deoxyguanosine (8-OHdG)
The data represented in the present approach revealed that there is a significant elevation (p < 0.05) in serum 8-OHdG level in BPA young-challenged group in comparison to young control group by 84.4%. On the opposite hand, a significant decline (p < 0.05) in serum 8-OHdG level in nano young-treated group, free young-treated group and Vit.E young-treated group versus BPA young challenged-group by 42.8%, 34.5% and 24.3% respectively, has been found. Similarly, a significant decrement (p < 0.05) in serum 8-OHdG level in nano young-treated group in comparison to free young-treated group by 12.7% has been detected. Also, a significant drop (p < 0.05) in serum 8-OHdG level in nano young-treated group and free young-treated group as compared to Vit.E young-treated group by 24.5% and 13.5% respectively has been observed. Moreover, the present results noted a significant enhancement (p < 0.05) in serum 8-OHdG level in BPA aged-challenged group versus aged control group by 89%. Meanwhile, a significant suppression (p < 0.05) in serum 8-OHdG level in nano aged-treated group, free aged-treated group and Vit.E aged-treated group by contrast with BPA aged challenged-group by 42.1%, 36.9% and 22.7% respectively has been recorded. Likewise, the present findings indicated a significant inhibition (p < 0.05) in serum 8-OHdG level in nano aged-treated group and free aged-treated group when compared to Vit.E aged-treated group by 25.2% and 18.3% respectively (Table 4).
Advanced glycation end products (AGEs)
Results of the current investigation revealed that there is a significant increase (p < 0.05) in serum AGEs level in BPA young-challenged group in comparison to young control group by 71.4%. On the other side, the current data showed that significant decrease (p < 0.05) in serum AGEs level in nano young-treated group, free young-treated group and Vit.E young-treated group versus BPA young challenged-group by 39.1%, 36.3% and 19.9% respectively has been noted. Additionally, a significant reduction (p < 0.05) in serum AGEs level in nano young-treated group and free young-treated group by contrast with Vit.E young-treated group by 23.9% and 20.5% respectively has been demonstrated. Furthermore, present results indicated a significant elevation (p < 0.05) in serum AGEs level in BPA aged-challenged group in comparison to aged control group by 74.4%. Meanwhile, a significant decrease (p < 0.05) in serum AGEs level in nano aged-treated group, free aged-treated group and Vit.E aged-treated group in comparison to BPA aged challenged-group by 38.9%, 34.9% and 17.6%, respectively has been recorded. Likewise, a significant drop (p < 0.05) in serum AGEs level in nano aged-treated group and free aged-treated group contrary to Vit.E aged-treated group by 25.8% and 20.9% respectively has been observed (Table 5).
Histological observations
Histological examination of the testicular tissue section of rat in young control group (Fig. 7A) and that in aged control group (Fig. 7B) showed normal testicular architecture of seminiferous tubules that are lined with germinal epithelium which consists of cells that include different developmental stages of germ cells, namely (spermatogonia (Sg), spermatocytes (Sc) and spermatids (Sp)), spermatogonia (Sg) resting on thin basement membrane. The germinal epithelium (spermatogonia, spermatocytes and spermatids) separated by narrow interstitial space with Leydig cells (I).
The testicular tissue section of rat in BPA young-challenged group (Fig. 7) showed disorganized architecture of seminiferous tubules, degenerated germinal epithelium (deteriorated spermatogonia (Sg), spermatocytes (Sc) and spermatids (Sp)), with small dark nucleus and eosinophilic cytoplasm. Other tubules revealed disconnection of spermatogonia (Sg) from the basement membrane. The testicular blood vessels were congested (Bv), and the interstitial tissue appeared degenerated with loss of a large number of Leydig cells (I). Likewise, the testicular tissue section of rat in BPA aged-challenged group (Fig. 7D) showed disturbed architecture of seminiferous tubules, separation of spermatogonia (Sg) from the basement membrane, degenerated, necrotic changes of all germinal epithelium (spermatogonia (SG), spermatocytes (SC) and spermatids (Sp)), with small dark nucleus and eosinophilic cytoplasm. Markedly thickened congested blood vessel and degeneration of interstitial tissue (I) with hemorrhage were also observed (Bv).
Interestingly, the examination of testicular tissue section of rat in nano young-treated group (Fig. 7E) showed nearly normal testicular architecture, the seminiferous tubules with normally germinal epithelium (regular arrangement of spermatogonia (Sg) spermatocytes (Sc) and spermatids (Sp)), the spermatogonia (Sg) normally resting on thin basement membrane. The interstitial tissue was slightly degenerated (I) with mild congested blood vessels (Bv). Likewise, the testicular tissue section of rat in nano aged-treated group (Fig. 7F) showed the seminiferous tubules with apparently nearly normal architecture with nearly normal regular arranged germinal epithelium cells (spermatogonia (Sg), spermatocytes (Sc) and spermatids (Sp)), and the interstitial tissue was slightly degenerated (I) with mild congested blood vessels (Bv).
The examination of the testicular tissue section of rat in free young-treated group (Fig. 7G) showed apparently normal seminiferous tubules lined by multiple layers of spermatogenic cells (spermatogonia (Sg), spermatocytes (Sc) and spermatids (Sp)). The interstitial spaces contain apparently normal Leydig cells (I) with slight vacuolation (V). While the examination of the testicular section of rat in free aged-treated group (Fig. 7H) showed degeneration of spermatogenic cells, and some seminiferous tubules appeared with a well-organized germinal epithelium (spermatogonia (Sg), spermatocytes (Sc) and spermatids (Sp)). The spermatogonia (Sg) resting on thin basement membrane. Also, congested blood vessel (Bv), slight degeneration of interstitial tissue, detached germ layers in some seminiferous tubules, thickness of interstitial tissue (I) and few pyknotic interstitial cells of Leydig have been noted.
Histological examination of the testicular tissue section of rat in Vit.E young-treated group (Fig. 7I) showed degeneration of spermatogenic cells, but the germinal epithelium cells (spermatogonia (Sg), spermatocytes (Sc) and spermatids (Sp)) were more or less organized. However, the interstitial congestion was still observed. Meanwhile, the testicular tissue section of rat in Vit.E aged-treated group (Fig. 7J) showed a degeneration of spermatogenic cells, some seminiferous tubules appeared well organized, and spermatogonia (Sg) of germinal epithelium resting on thin basement membrane. Little intercellular spaces are noticed. Also, the interstitial space still wide with some leydig cells and eosinophilic material.
(A): Photomicrograph of the testicular section of young control group; (B): Photomicrograph of the testicular section of aged control group; (C): Photomicrograph of the testicular section of BPA young-challenged group; (D): Photomicrograph of the testicular section of BPA aged-challenged group; (E): Photomicrograph of the testicular section of Nano young-treated group; (F): Photomicrograph of the testicular section of Nano aged-treated group; (G): Photomicrograph of the testicular section of Free young-treated group; (H): Photomicrograph of the testicular section of Free aged-treated group; (I): Photomicrograph of the testicular section of Vit.E young-treated group; (J): Photomicrograph of the testicular section of Vit.E aged-treated group.
Discussion
The TEM micrograph of the prepared dose of nanoemulsion of Panax ginseng dry extract for young adult rats (sample A) showed efficient distribution and obvious homogeneity. While, TEM micrograph of the prepared dose of nanoemulsion of Panax ginseng dry extract for aged adult rats (sample B) showed agglutination and aggregation clusters among the particles. These findings could be explained by the difference in dilution between the two samples as Petrochenko et al.48 referred that the concentration, referring to the number of particles observed per image, was notably reduced in the 10x diluted nanoemulsion samples in comparison to the undiluted ones. This is in consistent with the results of TEM micrograph of sample A that showed efficient distribution. Also, Wangensteen et al.49 reported that the TEM images from films with lower concentrations distinctly indicate the formation of smaller nanocrystals, characterized by many more boundaries per unit volume. Conversely, in films with higher concentrations, larger particles are observed with fewer boundaries per unit volume. On the other hand, both samples A and B showed almost spherical shape and this is in harmony with Md et al.50 findings which indicated that TEM imaging demonstrates the presence of uniform, spherical-shaped particles in the ginseng-loaded nanohydrogel, with no observable signs of particle aggregation. In addition, Zhang et al.51 observed that the surface morphology of the prepared ginsenoside compound K O-carboxymethyl chitosan nanoparticles (GK–OCMC NPs), as visualized by TEM, exhibits a uniform spherical shape with regular grain diameters and no evidence of aggregation.
The polydispersity index (PDI) is utilized to quantify the extent of non-uniformity of a size distribution of sample particles52. Essentially, it serves as an indicator of the distribution of different size populations within a sample. The PDI value ranges from 0.0, indicating a perfectly uniform sample in terms of particle size, to 1.0, representing a highly polydisperse sample with multiple particle size populations53. Sample A in current investigation had PDI value equal to 0.57 and exhibited a size distribution by number of about 62 d.nm, which indicated that that sample A had a homogenous and wide-size distribution in the prepared nanoemulsion. These findings are also confirmed by TEM micrograph as shown in Fig. 1a). On the other side, sample B had PDI value equal to 0.6 and exhibited a size distribution by number of about 123 d.nm, which denoted that the sample B had agglomerates and a narrow-size distribution in the prepared nanoemulsion. These findings were confirmed by TEM micrograph as shown in Fig. 1b as well.
Zeta potential quantifies the effective electric charge present on the surface of nanoparticles. The magnitude of the zeta potential offers insights into particle stability. A higher zeta magnitude indicates an electrostatic repulsion between particles in given sample, thus indicating increased stability54. Kumar and Dixit55 stated that if the zeta potential of the sample falls within the range of 0 to ± 5 thus, it indicates flocculation or coagulation in sample, within the range of ± 10 to ± 30, the sample shows incipient instability. For values ranging from ± 30 to ± 40, the sample exhibits moderate stability. If zeta potential falling between ± 40 to ± 60, the sample demonstrates good stability. Lastly, if the zeta potential range for the surface exceeds ± 60, the sample is deemed to possess excellent stability. In the present study, zeta potential recorded − 38.9 mV for sample A and − 32.8 mV for sample B. This can be explained as sample A has a high stability as it has larger zeta potential so the particles have a tendency to repel from each other and don’t aggregate as shown in TEM micrograph Fig. 1a. Whereas, sample B has smaller zeta potential, thus particles have a tendency to attract to each other again and form agglomerate as shown in TEM micrograph Fig. 1b.
The characteristic bands appeared in the FTIR of samples A and B in current approach showed the presence of hydroxyl (OH) group at 3329 cm− 1 and 3347 cm− 1 respectively, and this is in consistent with Hou56 findings that the characteristic band of ginsenosides in IR appeared approximately at wavelength 3380 cm− 1 representing OH stretch. Moreover, sample A showed the presence of bands at wavelengths 2917 cm− 1 and 2852 cm− 1, sample B showed the presence of bands at wavelengths 2918 cm− 1 and 2852 cm− 1 denoting for vibration modes of methylene (-CH2) group. These findings were proved by Owaid et al.57 and Choi et al.58 who reported the presence of two bands in FTIR of Panax ginseng at approximately 2923 cm− 1 and 2889 cm− 1 due to the asymmetric and symmetric vibration of methylene group respectively. The presence of carbonyl (C = O) group at 1737 cm− 1 of sample A, and at 1738 cm− 1 of sample B. In addition to the existence of alkene (C = C) group at 1612 cm− 1 of sample A and 1614 cm− 1 of sample B in FTIR are in agreement with Hou56 findings that IR of ginseng indicated the presence of band at approximately 1740 cm− 1 assigns to the stretching vibration of COOR group and band at 1620 cm− 1 clarifies C = C group. Moreover, the existence of bands at 1458 cm− 1 and 1350 cm− 1 of both samples A and B assigned to methyl (CH3) and methylene (-CH2) groups, as Liu et al.59 demonstrated that FTIR of ginseng shows two band approximately at 1458 cm− 1 and 1370 cm− 1 specific for the bending vibration of methyl and methylene groups. Furthermore, the bands appeared at 1041 cm− 1 and 1081 cm− 1 in FTIR of sample A and sample B respectively, assigned to ether (C-O-C) group comes in line with Choi et al.58 results that recorded the FTIR analysis of ginseng samples reveals the presence of numerous C-O-C groups, which are identifiable by characteristic bands within the spectral range of 1150 to 911 cm− 1.
Thermogravimetric analysis (TGA) is an analytical method utilized to evaluate the thermal stability of the material and assess its proportion of volatile components. This is achieved by monitoring the changes in weight as the sample undergoes controlled heating at a constant rate 60. The results of the present study denoted that sample A showed thermal stability until 207 °C and sample B showed thermal stability until 202 °C. Both samples showed a TGA curve profile of two weight loss phases; the first phase may be due to evaporation. Jiang et al.61 demonstrated that, the ginseng insoluble dietary fiber experiences evaporation at around 120 °C, leading to a slight weight loss of less than 2%. This observation suggests that this weight loss is likely attributable to the evaporation of moisture absorbed on the outer surface of the sample. While the second phase may be due to thermal dehydration as Hwang et al.62 reported that, upon heating ginseng at 150 °C, a significant reduction in weight occurs rapidly. This phenomenon is attributed to the additional dehydration of the glycosyl moiety at the C-3 and C-20 positions.
The DPPH assay is widely utilized as in vitro method for evaluating the plant extracts free radical scavenging activity 63. The higher value of inhibition percent (% inhibition), the greater antioxidant activity exhibited by the extract64. In our results, nanoemulsion of Panax ginseng dry extract samples A and B showed higher inhibition percent of 49.38% and 72.27% respectively. However, Panax ginseng dry extract free samples C and D showed lower inhibition percent of 30.26% and 56.75% respectively. These data fit with those of Rice-Evans et al.65 and Chang et al.66 findings that nano-grinding substantially enhanced the radical scavenging activity of ginseng in the DPPH assay as the nano-powdered ginseng demonstrating a higher inhibitory percentage compared to conventionally powdered ginseng. This can be elucidated by alterations in the molecular structure and positioning of polyphenol hydroxyl groups, which significantly impact the antioxidant activity of ginseng powder. Ultimately, this leads to modifications in radical scavenging activity by facilitating the donation of phenolic hydrogen and stabilizing the phenolic radical. Moreover, the inhibition percent (% inhibition) of sample B was higher than inhibition percent of sample A and the % inhibition of sample D was higher than % inhibition of sample C this may be due to different of concentration as El-Atawy et al.67 demonstrated that for all compounds, the % inhibition of DPPH increasing by increasing the concentration.
In in vivo experiment, oral administration of BPA caused significant reduction in both serum total and free testosterone levels in BPA young-challenged group and BPA aged-challenged group. This finding is in agreement with Nakamura et al.68 who reported that the administration of BPA at doses exceeding 100 mg/kg results in a reduction of plasma testosterone levels to approximately one-third in comparison to rats in control group. Biosynthesis of testosterone commences with the entry of cholesterol into the mitochondria this step is facilitated by the help of steroidogenic acute regulatory protein (StAR). Then inside the mitochondria, cholesterol is enzymatically converted into pregnenolone by the cholesterol side chain cleavage enzyme (P450scc)68. García et al.69 reported that elevated levels of ROS diminish the activity of P450scc, consequently hindering testosterone production in vitro. Also, Tsai et al.70 stated that hydrogen peroxide (H2O2) inhibits both basal and human chorionic gonadotropin-induced testosterone production from primary Leydig cells. This inhibition occurs through two mechanisms: firstly, by suppressing the activity of P450scc, and secondly, by reducing the expression of StAR in vivo. Additionally, studies have indicated that exposure to BPA induces oxidative stress in the testes of rodents71. As Olukole et al.72 noted that in the testes of rats treated with BPA, superoxide dismutases (SOD) were involved in the conversion of superoxide anion radicals into H2O2. Consequently, H2O2 accumulated in the testes due to its diminished elimination. Moreover, testosterone is synthesized through the canonical androgen production pathway, and it is playing a vital role in normal masculinization and the proper functioning of the testes. Within this canonical pathway, 17β-hydroxysteroid dehydrogenase type 3 (HSD17B3) is recognized as a pivotal enzyme involved in the biosynthesis of testosterone. It expressed solely within the testes and triggers the conversion of dehydroepiandrosterone (DHEA) into either androstenediol or androstenedione. Subsequently, both androstenediol and androstenedione undergo conversion into the biologically active androgen (testosterone) 73. At a low dosage, BPA exhibited a direct inhibitory effect on the activity of human and rat HSD17B3 enzymes by approximately 50% and 30%, respectively, leading to a decrease in testosterone synthesis7475.
Currently, the elevated levels of serum total and free testosterone in nano young-treated group as well as nano aged-treated group are higher than their levels in Free young-treated group and free aged-treated group. These findings are in agreement with Kamel et al.76 and Linjawi77 results where the nanoparticles of Panax ginseng (PGNPs) in the small dose was better than free Panax ginseng (PG), as PGNPs markedly elevate serum levels of both total and free testosterone compared to free PG. Researchers observed that PGNPs have greater efficacy than free PG in enhancing symptoms of fertility in male rats by augmenting levels of male sex hormones. Linjawi77 proposed that the Panax ginseng nanoparticles enhance the efficacy of this botanical extract in reaching the target cells of the hypothalamic-pituitary-testicular axis, thereby enhancing male fertility. Furthermore, experimental results proved that the augmentative effect of nano-ginseng on the secretion of testicular hormones and the enhancement of sperm production78. It is known that ginseng or its derivatives have antioxidative properties through scavenging of free radicals, thereby inhibiting LPO79 and reducing production of H2O2 in rat testes80.
In the current work, groups treated with Vit.E displayed significant elevation in both serum total and free testosterone levels. Vit.E is a potent lipid-soluble antioxidant naturally occurring within cells, tends to accumulate in the membranes of mitochondria and endoplasmic reticulum. Its role involves safeguarding testicular cells against oxidative damage and LPO Gavazza and Catalá81 and Aydilek et al.82. Rats administered with 25 mg/kg of Vit.E exhibited an elevation in activity of antioxidant enzymes and concentrations of glutathione (GSH), alongside a reduction in LPO. In this context, Vit.E scavenges free radicals to maintain crucial cell membrane functions such as ion transport and membrane fluidity 83. Moreover, it safeguards molecules of testosterone from damage by impeding the polyunsaturated fatty acids (PUFA) oxidation84.
In current study, administration of BPA caused significant increase in serum MDA levels, and this observation aligns with the findings of Zhang et al.85, wherein exposure to BPA led to a notable increase in serum levels of MDA compared to the control group in vivo. Lipid peroxidation (LPO) refers to the oxidation of unsaturated fatty acids, with MDA being the resultant end product. MDA serves as a well-known parameter to substantiate the occurrence of oxidative stress 42. El Henafy et al.86 observed that BPA decreases the activity of testicular antioxidant enzymes [including glutathione reductase, superoxide dismutase, glutathione peroxidase (GPx) and catalase (CAT)], hence it significantly elevates LPO as well as MDA levels. Moreover, investigators asserted that BPA stimulates myriad oxidative damage within the testes of rats, leading to markedly elevated concentrations of MDA in both serum and sperm of male rats87. Thus, the assessment of MDA concentration could be valuable for evaluating male infertility 88.
Our findings showed a significant decrease in serum MDA levels in nano young-treated group and Nano aged-treated group more than Free young-treated group and Free aged-treated group. Studies have substantiated that Panax ginseng, known for its abundance of antioxidants, plays a vital role in regulating numerous pathways related to oxidative stress89. Ginsenosides, the primary active components found in Panax ginseng, exhibit potent antioxidant properties and mitigate oxidative stress by effectively scavenging ROS. Several in vitro and in vivo studies have proved that both Panax ginseng and its constituent ginsenosides augment the activity of antioxidant enzymes, consequently resulting in decreased levels of MDA90. Interestingly, ginseng berry silver nanoparticles (AgNPs) exhibited superior free radical scavenging activity in comparison to free ginseng berry. The heightened antioxidant activity observed in ginseng berry AgNPs might be attributed to the adsorption of bioactive compounds from ginseng onto the great surface area of spherical nanoparticles32. So this may be the reason for the better effect of ginseng nanoformulation than free ginseng.
In the present work, treatment with Vit.E brought about significant reduction in serum MDA levels both young and aged groups. In the study conducted by Malmir et al.42, the level of MDA exhibited a significant reduction in the BPA + Vit.E group compared to the BPA group. Through its vital role in augmentation of antioxidant enzyme activity, Vit.E can mitigate LPO, thereby ameliorating the detrimental alterations induced by BPA on testosterone levels, MDA concentrations and the testicular tissue. As noted by Babaei et al.37, the primary roles of Vit.E include inhibiting phospholipid membrane peroxidation and safeguarding cell membranes via its antioxidative properties.
In the current research, BPA induced a significant increase in serum 8-OHdG levels in BPA young challenged-group and BPA aged-challenged group. These findings are in harmony with those of Tiwari et al.91 which demonstrated that exposure to BPA raises plasma concentrations of 8-OHdG, augments LPO and reduces GSH activity. Omari et al.92 noted that 8-OHdG is among the primary forms of ROS, thus it is frequently employed as a biomarker to assess DNA oxidative damage. It represents one of the adducts resulting from the hydroxyl radical (HO•) assault on the deoxyguanosine residue93. The outcomes of in vivo investigation suggested that BPA augments the generation of HO•, which subsequently bind to DNA, resulting in the formation of 8-OHdG94.
Currently, the reduction of serum 8-OHdG levels in nano young-treated group as well as nano aged-treated group is more than the reduction of 8-OHdG in free young-treated group and free aged-treated group. Panax ginseng demonstrates robust antioxidant properties in both in vitro and in vivo studies, this is primarily attributable to its ginsenosides content95. Moreover, El-Banna et al.96 noted that ginsenoside Rg3 nanoparticles (Rg3-NPs) proved to be more efficacious than free ginsenoside Rg3 in mitigating DNA damage and dramatically reducing SOD as well as glutathione peroxidase (GPx) levels in mice. Scientists observed that utilizing liposomal nanovesicles as a nanocarrier for ginseng extract resulted in heightened intracellular antioxidant activity compared to administering free ginseng extract 97. Furthermore, the findings from both in vitro and in vivo studies conducted by Ullah et al.98 revealed a significant reduction in the levels of 8-OHdG in groups treated with red ginseng extract (RGE) and red ginseng oil (RGO). This suggests that both RGE and RGO possess the capability to protect DNA against oxidation and facilitate the recovery from DNA damage.
In our results, groups of rats (young and aged) treated with Vit.E showed significant decrement in serum 8-OHdG levels. Vitamin E plays a crucial role in scavenging free oxygen radicals such as perhydroxyl radical (HO2•) and hydroxyl radical (HO•) within the testes of adult rats 99. In Hassanien et al.100 study, Vit.E led to a notable reduction in serum levels of 8-OHdG, attributed to its known antioxidant properties. Furthermore, Oblette et al.101 demonstrated that the addition of Vit.E to cultures of fresh testicular tissues did not lead to an increase in the production of spermatozoa containing 8-OHdG adducts.
In the present work, groups administered BPA showed significant increase in serum AGEs level. Advanced glycation end products (AGEs) are the outcomes of non-enzymatic glycosylation of either endogenous or exogenous proteins102. They represent a dietary risk factor contributing to impaired spermatogenesis and reduced sperm quality in rodents. This is attributed to their pro-inflammatory and pro-oxidant properties, which significantly affect the sperm function 103. Advanced glycation end products (AGEs) bind to the AGE receptor (RAGE), thereby trigger the production of ROS and proinflammatory cytokines such as NF-κB104. Activation of NF-κB leads to the transcription of various target genes, including additional cytokines and the RAGE itself. One of the distinctive characteristic of RAGE-induced NF-κB activation is the establishment of a positive feedback loop, whereby the RAGE signal is sustained and reinforced105. The activation of NF-κB leads to the upregulation of RAGE expression, resulting in sustained stimulation and the promotion of signaling mechanisms that contribute to cellular damage106. Recently, Tekin and Çelebi107 reported a significant rise in NF-κB levels in testicular tissue following the administration of BPA compared to control groups in male rats. Hence, BPA indirectly elevates AGEs levels through NF-κB activation, subsequently leading to the upregulation of RAGE expression.
In the current approach, groups of rats (young and aged) treated with Panax ginseng either in nano form or free form exhibited significant decrease in serum AGEs levels. Ali et al.108 showed that Panax ginseng extract, particularly ginsenoside Rh2, inhibits AGEs formation and decreases fructosamine level. Additionally, it prevents oxidation of protein by reducing formation of protein carbonyl and modification of protein thiol groups. Thus, ginsenosides exhibit an effective role as anti-glycation agent for mitigating diabetic complications by inhibiting the formation of AGEs and oxidation-induced protein damage. The advantageous influence of Panax ginseng on oxidative damage induced by AGEs suggests that ginseng downregulates RAGE expression, consequently inhibits NF-κB activation109. Additionally, Kim110 discovered that ginsenosides Re and Rp1 directly inhibit the NF-κB signaling pathway.
Our results reported that the groups of young and aged rats treated with Vit.E experienced significant reduction in serum AGEs levels. Reckelhoff et al.111 reported that rats administered a high-Vit.E diet showed a downregulation in the expression of RAGE. This observation could potentially be attributed to the influence of Vit.E on the activation of NF-κB which subsequently impacts the expression of RAGE. Glauert112 noted that Vit.E inhibits NF-κB activation both in vitro and in vivo. The mechanisms through which Vit.E suppresses NF-κB activation likely pertain to its antioxidative properties. If NF-κB activation is triggered by oxidative stress, the antioxidant function of Vit.E is likely to be implicated. Additionally, Vit.E might impede the formation of lipoxygenase metabolites generated during LPO, which could otherwise activate NF-κB. Moreover, Vit.E blocks low-density lipoprotein (LDL) oxidation as LDL in their oxidized form has been observed to be stimulators of NF‐κB.
In the present investigation, photomicrograph of testicular tissue section of rat in BPA young-challenged group showed disorganized architecture of seminiferous tubules, degenerated germinal epithelium (spermatogonia, spermatocytes and spermatids), with small dark nucleus and eosinophilic cytoplasm, others tubules revealed disconnection of spermatogonia from the basement membrane. The testicular blood vessels were congested, and the interstitial tissue appeared degenerated with loss of a large number of Leydig cells. In addition, photomicrograph of testicular tissue section of rat in BPA aged-challenged group showed disturbed architecture of seminiferous tubules, present separation of spermatogonia from the basement membrane and degenerated germinal epithelium (spermatogonia, spermatocytes and spermatids), with small dark nucleus and eosinophilic cytoplasm, others tubules reveal reduction in the number of germinal epithelium cells, degeneration of interstitial tissue and thickened congested blood vessel. These data constitute Eid and Ahmed113 results which observed through histological examination of testicular tissue from the BPA-challenged group that there was a decrease in the typical organization of the germinal epithelium, characterized by mal-arrangement and abnormal cellular attachment. Furthermore, there was degeneration and necrosis in the spermatogenic cells. Also, Malmir et al.42 reported that the group exposed to a daily dose of 250 mg/kg of BPA showed a deterioration of the seminiferous tubule, disruption of spermatogenesis, and shedding of immature cells into the lumen. According to Nakamura et al.68 results, the examination of testicular tissue section from rat exposed to a 200 mg/kg dose of BPA revealed a decrease in the number of Leydig cells.
Currently, photomicrograph of the testicular tissue section of rat in Nano young-treated group showed apparently nearly normal testicular architecture, the seminiferous tubules with normally germinal epithelium (regular arrangement of spermatogonia, spermatocytes and spermatids), Interstitial tissue was slightly degenerated with mild congested blood vessels. Additionally, photomicrograph of the testicular tissue section of rat in Nano aged-treated group showed seminiferous tubules apparently nearly normal architecture with nearly normal regular arranged germinal epithelium (spermatogonia, spermatocytes and spermatids), the interstitial tissue was slightly degenerated with mild congested blood vessels. These results are in line with those of Kamel et al.76, where the administration of ginseng nanoparticles to rats notably mitigated the harmful effects of methotrexate (MTX) on testicular tissue and restored its normal histological structure. The ginseng nanoparticles effectively reversed impaired spermatogenesis, decreased disruption of seminiferous tubules, reduced detachment of germ cells, alleviated congestion, mitigated damage to Sertoli cells, and counteracted the effects of MTX on Leydig cells.
In present work, photomicrograph of the testicular tissue section of rat in Free young-treated group showed apparently normal seminiferous tubules lined by multiple layers of spermatogenic cells; spermatogonia, spermatocytes and spermatids. The interstitial spaces contain apparently normal Leydig cells with slight vacuolation. While, photomicrograph of the testicular tissue section of rat in Free aged-treated group showed degeneration of spermatogenic cells, some seminiferous tubules appeared with a well-organized germinal epithelium (spermatogonia, spermatocytes and spermatids). The spermatogonia resting on thin basement membrane. Also, congested blood vessel, slight degeneration of interstitial tissue, vacuolation, detached germ layers in some seminiferous tubules, thickness of interstitial tissue and few pyknotic interstitial cells of Leydig. These data come in line with those of with Kim et al.114 and Ali et al.115 that histological examination of the testicular tissue from rats treated with ginseng revealed the restoration of Leydig cells and spermatogenic layers. Additionally, there was a decrease in interstitial space, less basal membrane thickness, and a return to a typical arrangement of germinative cells within the seminiferous tubules.
Finally, photomicrograph of the testicular tissue section of rat in Vit.E young-treated group showed degeneration of spermatogenic cells. The germinal epithelium cells (spermatogonia, spermatocytes and spermatids) were more or less organized. However, interstitial congestion was still observed. Photomicrograph of the testicular tissue section of rat in Vit.E aged-treated group showed degeneration of spermatogenic cells, some seminiferous tubules appeared well organized and spermatogonia of germinal epithelium resting on thin basement membrane. Little intercellular spaces are noticed. Also, interstitial space still wide with some Leydig cells and eosinophilic material. These histological changes are in agreement with those of Malmir et al.42 which denoted that the group treated with BPA + Vit.E showed almost normal arrangement of germinal epithelium cells and regular spermatogenesis. In El Gharabawy et al.116 study, the examination of testicular tissue sections from rats treated with Vitamin E revealed the presence of typical seminiferous tubules, interspersed with a thin interstitial tissue containing Leydig cells and blood vessels. These tubules were encased by a consistent, delicate basement membrane and were lined with layers of Sertoli cells and spermatogenic cells (including spermatogonia, primary spermatocytes, spermatids, and spermatozoa). Therefore, the nanoemulsion of Panax ginseng dry extract proved to be the superior treatment. In particular, young rats treated with Panax ginseng dry extract nanoemulsion had the most promising results.
Conclusion
According to our knowledge this is the first study for development of Panax ginseng dry extract nanoemulsion by oil in water (O/W) method. The evolved nanoemulsion formulization of Panax ginseng dry extract in the present study exhibited potential effect as male anti-infertility agent via normalizing the male sex hormones and regulating various pathways associated with oxidative stress. Importantly, the beneficial effect of nanoemulsion of Panax ginseng dry extract surpasses that of free Panax ginseng dry extract, resulting in a superior mitigation of BPA-induced male infertility. This is could be attributed to the small particles size and large surface area of the nanoemulsion.
Data availability
the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Change history
27 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-94138-3
References
Borght, M. V. & Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 62, 2–10 (2018).
Sharma, A. & Shrivastava, D. Psychological problems related to infertility. Cureus. 14, e30320 (2022).
Mazzilli, R. et al. Male factor infertility and assisted reproductive technologies: Indications, minimum access criteria and outcomes. J. Endocrinol. Investig. 46, 1079–1085 (2023).
Borumandnia, N., Majd, H. A., Khadembashi, N. & Alaii, H. Worldwide trend analysis of primary and secondary infertility rates over past decades: A cross-sectional study. Int. J. Reprod. Biomed. 20, 37 (2022).
Ramadan, S. A. & Said, A. R. Effect of an educational intervention for infertile women regarding natural fertility methods and sexual skills for improving sexual function. Am. J. Nurs. 6, 1–11 (2018).
Huang, B., Wang, Z., Kong, Y., Jin, M. & Ma, L. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: An analysis of global burden of disease study. BMC Public Health. 23, 2195 (2023).
Shi, X. et al. Lifestyle and demographic factors associated with human semen quality and sperm function. Syst. Biol. Reprod. Med. 64, 358–367 (2018).
Badar, A., Khilwani, B., Lohiya, N. K. & Ansari, A. S. Etiology of male infertility: A review. Int. J. Reprod. Contracept. Obstet. Gynecol. 11, 2320–2328 (2022).
Hanna, D. H., Beshay, S. N., El. Shafee, E., & El‐Rahman, H. A. A. The protective effect of aqueous extract of Stevia rebaudiana against tartrazine toxicity in male Wistar rat. Cell Biochem. Funct. 41, 1462–1476 (2023).
Santiago, J., Silva, J. V., Santos, M. A. & Fardilha, M. Fighting bisphenol A-induced male infertility: The power of antioxidants. Antioxidants. 10, 289 (2021).
El-Shimi, B. I. et al. Mechanistic insights into bisphenol a-mediated male infertility: Potential role of panax ginseng extract. Chem. Biodivers. e202400480 (2024).
Fenichel, P., Chevalier, N. & Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. Endocrinol. 74, 211–220 (2013).
Murata, M. & Kang, J. H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 36, 311–327 (2018).
Li, N. et al. Physiologically detectable bisphenol A impairs human sperm functions by reducing protein-tyrosine phosphorylation. Ecotoxicol. Environ. Saf. 221, 112418 (2021).
Barbonetti, A. et al. In vitro exposure of human spermatozoa to bisphenol A induces pro-oxidative/apoptotic mitochondrial dysfunction. Reprod. Toxicol. 66, 61–67 (2016).
Eshak, M. G. & Osman, H. F. Biological effects of chitosan against bisphenol-A induced endocrine toxicity and androgen receptor gene expression changes in male rats. Int. J. Pharm. Clin. Res. 6, 300–311 (2014).
Grami, D. et al. Protective action of eruca sativa leaves aqueous extracts against bisphenol a-caused in vivo testicular damages. J. Med. Food. 23, 600–610 (2020).
Mei, T. I. A. N., Lin-Nan, L. I., Zheng, R. R., Li, Y. A. N. G. & Zheng-Tao, W. A. N. G. Advances on hormone-like activity of Panax ginseng and ginsenosides. Chin. J. Nat. Med. 18, 526–535 (2020).
Kopalli, S. R. et al. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp. Gerontol. 69, 94–102 (2015).
Park, H. J., Choe, S. & Park, N. C. Effects of Korean red ginseng on semen parameters in male infertility patients: A randomized, placebo-controlled, double-blind clinical study. Chin. J. Integr. Med. 22, 490–495 (2016).
Lin, H., Zhao, J., Liu, Z., Liu, Z. & Lin, Z. Efficacy of Panax ginseng supplementation on androgen deficiency rats via metabolomics and gut microbiota. J. Funct. Foods. 87, 104810 (2021).
Zhu, D. et al. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J. Cell Biochem. 108, 117–124 (2009).
Oyewopo, A. O. et al. Panax ginseng supplementation protects against testicular damage induced by radiofrequency electromagnetic radiation from cell phone. Nutrire 48, 47 (2023).
Ru, W. et al. Chemical constituents and bioactivities of Panax ginseng (CA Mey). Drug Discov. Ther. 9, 23–32 (2015).
Park, J. et al. Effects of ginseng on two main sex steroid hormone receptors: Estrogen and androgen receptors. J. Ginseng Res. 41, 215–221 (2017).
Mills, T. M., Chitaley, K. & Lewis, R. W. Vasoconstrictors in erectile physiology. Int. J. Impot. Res. 13, 29–34 (2001).
Murphy, L. L. & Lee, T. J. F. Ginseng, sex behavior, and nitric oxide. Ann. NY. Acad. Sci. 962, 372–377 (2002).
Ganesan, P., Ko, H. M., Kim, I. S. & Choi, D. K. Recent trends of nano bioactive compounds from ginseng for its possible preventive role in chronic disease models. RSC Adv. 5, 98634–98642 (2015).
Shah, M., Fawcett, D., Sharma, S., Tripathy, S. K. & Poinern, G. E. J. Green synthesis of metallic nanoparticles via biological entities. Materials. 8, 7278–7308 (2015).
Chuacharoen, T. & Sabliov, C. M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf. A Physicochem. Eng. Asp. 503, 11–18 (2016).
Ahn, S. et al. Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory-mediators production via blockade of NF-κB activation in macrophages. Artif. Cells Nanomed. Biotechnol. 45, 270–276 (2017).
Jiménez Pérez, Z. E. et al. Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of their in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines. Int. J. Nanomedicine.12, 709–723 (2017).
Narlıçay, S., & Kıvrak, M. B. Nanotechnology in the treatment of infertility. In Nanotechnology in Reproduction (e.d Öztürk, A. E.) 139 (Özgür Publications, 2023).
Klein, J. P. et al. Impact of nanoparticles on male fertility: What do we really know? A systematic review. Int. J. Mol. Sci. 24, 576 (2022).
Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 94, 651–715 (2020).
Ahmed, A. E. et al. Vitamin E and selenium administration synergistically mitigates ivermectin and doramectin-induced testicular dysfunction in male Wistar albino rats. Biomed. Pharmacother. 124, 109841 (2020).
Babaei, A., Kheradmand, N., Baazm, M., Nejati, N. & Khalatbari, M. Protective effect of vitamin E on sperm parameters in rats infected with Candida albicans. Andrologia. 52, e13593 (2020).
Mumtaz, S. et al. Therapeutic role of garlic and vitamins C and E against toxicity induced by lead on various organs. Environ. Sci. Pollut. Res. Int. 27, 8953–8964 (2020).
Min, J. Y., Ahn, S. I., Lee, Y. K., Kwak, H. S. & Chang, Y. H. Optimized conditions to produce water-in-oil-in-water nanoemulsion and spray-dried nanocapsule of red ginseng extract. Food Sci. Technol. 38, 485–492 (2018).
El-Kattan, N. et al. Curcumin assisted green synthesis of silver and zinc oxide nanostructures and their antibacterial activity against some clinical pathogenic multi-drug resistant bacteria. RSC Adv. 12, 18022–18038 (2022).
Embaby, M. A., Haggag, E. S. A., El-Sheikh, A. S. & Marrez, D. A. Biosorption of Uranium from aqueous solution by green microalga Chlorella sorokiniana. Environ. Sci. Pollut. Res. Int. 29, 1–17 (2022).
Malmir, M., Mehranjani, M. S., Faraji, T. & Noreini, S. N. Antioxidant effect of Vitamin E on the male rat reproductive system by a high oral dose of Bisphenol-A. Toxicol. Res. Appl. 5, 23978473211005560 (2021).
Kim, Y. O. et al. Panax ginseng improves functional recovery after contusive spinal cord injury by regulating the inflammatory response in rats: An in vivo study. Evid. Based Complement. Alternat. Med. 2015, 817096 (2015).
Debuf, Y. The veterinary formulary handbook of medicines used in veterinary practice. J. S. Afr. Vet. Assoc. 62, 181 (1991).
Sayed, A. A. et al. Oral drug delivery vehicle for insulin and curcumin based on egg albumin-dextran conjugate: In vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 100, 106052 (2024).
Bancroft, J. D., & Gamble, M. Theory and Practice of Histological Techniques. (Elsevier health sciences, 2008).
Armitage, P., Berry, G. & Matthews, J. N. S. Comparison of several groups. Stat. Methods Med. Res. 2, 202–207 (1987).
Petrochenko, P. E. et al. Analytical considerations for measuring the globule size distribution of cyclosporine ophthalmic emulsions. Int. J. Pharm. 550, 229–239 (2018).
Wangensteen, T. et al. Growth of nanoparticulate films of Ca3Co4O9 by a microwave plasma –assisted spray process. J. Mater. Res. 26, 1940–1946 (2011).
Md, S. et al. Sustained-release ginseng/sodium alginate nano hydrogel formulation, characterization, and in vivo assessment to facilitate wound healing. J. Drug Deliv. Sci. Technol. 74, 103565 (2022).
Zhang, J. et al. Characterization of ginsenoside compound K loaded ionically cross-linked carboxymethyl chitosan–calcium nanoparticles and its cytotoxic potential against prostate cancer cells. J. Ginseng Res. 45, 228–235 (2021).
Bera, B. Nanoporous silicon prepared by vapour phase strain etch and sacrificial technique. Int. J. Comput. Appl. 975, 8887 (2015).
Danaei, M. et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 10, 57 (2018).
Selvamani, V. Stability studies on nanomaterials used in drugs. In Characterization and Biology of Nanomaterials for Drug Delivery (eds. Mohapatra, S., Ranjan, S., Dasgupta, N., Thomas, S., & Mishra, R. K.) 425–444. (Elsevier, 2019).
Kumar, A., & Dixit, C. K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids (eds. Nimesh, S., Chandra, R., & Gupta, N) 43–58 (Woodhead Publishing, 2017).
Hou, J. P. The chemical constituents of ginseng plants. Am. J. Chin. Med. 5, 123–145 (1977).
Owaid, M. N., Zaidan, T. A., Muslim, R. F. & Hammood, M. A. Biosynthesis, characterization and cytotoxicity of zinc nanoparticles using Panax ginseng roots. Araliaceae. Acta Pharmaceutica Sciencia 57, 19–32 (2019).
Choi, J. Y. et al. Rapid discrimination of Panax ginseng powder adulterated with various root plants by FT-IR spectroscopy coupled with multivariate analysis. Food Sci. Biotechnol. 33, 1–11 (2023).
Liu, D., Li, Y. G., Xu, H., Sun, S. Q. & Wang, Z. T. Differentiation of the root of cultivated ginseng, mountain cultivated ginseng and mountain wild ginseng using FT-IR and two-dimensional correlation IR spectroscopy. J. Mol. Struct. 883, 228–235 (2008).
Rajisha, K. R., Deepa, B., Pothan, L. A., & Thomas, S. Thermomechanical and spectroscopic characterization of natural fibre composites. In Interface Engineering of Natural Fibre Composites for Maximum Performance (ed. Zafeiropoulos, N. E.), 241–274 (Elsevier, 2011).
Jiang, G. et al. Modification of ginseng insoluble dietary fiber by enzymatic method: structural, rheological, thermal and functional properties. Foods 12, 2809 (2023).
Hwang, I. G. et al. Changes in ginsenosides and antioxidant activity of Korean ginseng (Panax ginseng CA Meyer) with heating temperature and pressure. Food Sci. Biotechnol. 19, 941–949 (2010).
Nagarajan, J., Ramanan, R. N., Raghunandan, M. E., Galanakis, C. M., & Krishnamurthy, N. P. Carotenoids. In Nutraceutical and functional food components: Effects of innovative processing techniques (ed. Galanakis, C. M.) 313–353 (Academic Press, 2021).
Olszowy-Tomczyk, M. How to express the antioxidant properties of substances properly?. Chemical Papers. 75, 6157–6167 (2021).
Rice-Evans, C. A., Miller, N. J. & Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 20, 933–956 (1996).
Chang, Y. H. & Ng, P. K. W. Extraction of ginsenosides from a blend of wheat flour and ginseng powder. Food Chem. 115, 1512–1518 (2009).
El-Atawy, M. A. et al. Synthesis, characterization, antioxidant, and anticancer activity against colon cancer cells of some cinnamaldehyde-based chalcone derivatives. Biomolecules. 14, 216 (2024).
Nakamura, D. et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 194, 16–25 (2010).
García, M. M. S. et al. Cisplatin inhibits testosterone synthesis by a mechanism that includes the action of reactive oxygen species (ROS) at the level of P450scc. Chem. Biol. Interact. 199, 185–191 (2012).
Tsai, S. C., Lu, C. C., Lin, C. S. & Wang, P. S. Antisteroidogenic actions of hydrogen peroxide on rat Leydig cells. J. Cell Biochem. 90, 1276–1286 (2003).
Othman, A. I. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol. Ind. Health. 32, 1537–1549 (2016).
Olukole, S. G., Ola-Davies, E. O., Lanipekun, D. O. & Oke, B. O. Chronic exposure of adult male Wistar rats to bisphenol A causes testicular oxidative stress: Role of gallic acid. Endocr. Regul. 54, 14–21 (2020).
Lawrence, B. M., O’Donnell, L., Smith, L. B. & Rebourcet, D. New insights into testosterone biosynthesis: Novel observations from HSD17B3 deficient mice. Int. J. Mol. Sci. 23, 15555 (2022).
Li, X. et al. Bisphenols and Leydig cell development and function. Front. Endocrinol. 11, 447 (2020).
Ye, L., Zhao, B., Hu, G., Chu, Y. & Ge, R. S. Inhibition of human and rat testicular steroidogenic enzyme activities by bisphenol A. Toxicol. Lett. 207, 137–142 (2011).
Kamel, M. E., Mohammad, H. M., Maurice, C. & Hagras, M. M. Ginseng nanoparticles protect against methotrexate-induced testicular toxicity in rats. Egypt J. Basic Clin. Pharmacol. 9, 101397 (2019).
Linjawi, S. A. Evaluation of the protective effect of Panax ginseng nanoparticles against nicotine-induced reproductive disorders in male rats. J. Pharm. Sci. Rev. Res. 32, 38–45 (2015).
Tambi, M. I. B. M., Imran, M. K. & Henkel, R. R. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism?. Andrologia 44, 226–230 (2012).
Akram, H., Pakdel, F. G., Ahmadi, A. & Zare, S. Beneficial effects of american ginseng on epididymal sperm analyses in cyclophosphamide treated rats. Cell J. 14, 116 (2012).
Rehab, M. E. & Ashraf, M. E. The protective effect of Panax ginseng against chromium picolonate induced testicular changes. Afr. J. Pharm. Pharmacol. 8, 346–355 (2014).
Gavazza, M. & Catalá, A. The effect of α-tocopherol on the lipid peroxidation of mitochondria and microsomes obtained from rat liver and testis. Mol. Cell Biochem. 225, 121–128 (2001).
Aydilek, N., Aksakal, M. & Karakılçık, A. Z. Effects of testosterone and vitamin E on the antioxidant system in rabbit testis. Andrologia 36, 277–281 (2004).
Mittal, M. & Flora, S. J. S. Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem. Toxicol. 30, 263–281 (2007).
Umesiobi, D. O. The effect of Vit. E supplementation on the libido and reproductive capacity of Large White boars. S. Afr. J. Anim. Sci. 42, 559–563 (2012).
Zhang, C. et al. Protective effects of Lycium barbarum polysaccharides on testis spermatogenic injury induced by bisphenol A in mice. Evid. Based Complement. Alternat. Med. 2013, 690808 (2013).
El Henafy, H. M., Ibrahim, M. A., Abd El Aziz, S. A., & Gouda, E. M. Oxidative Stress and DNA methylation in male rat pups provoked by the transplacental and translactational exposure to bisphenol A. Environ. Sci. Pollut. Res. Int. 27, 4513–4519 (2020).
Aydoğan, M., Korkmaz, A., Barlas, N. & Kolankaya, D. Pro-oxidant effect of vitamin C coadministration with bisphenol A, nonylphenol, and octylphenol on the reproductive tract of male rats. Drug Chem. Toxicol. 33, 193–203 (2010).
Hammami, K. Z., Fki, H., Damak, J. & Bahloul, A. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch. Androl. 49, 83–94 (2003).
He, B. et al. Oxidative stress and ginsenosides: An update on the molecular mechanisms. Oxid. Med. Cell Longev. 2022, 9299574 (2022).
Morshed, M. N. et al. Antioxidant activity of panax ginseng to regulate ROS in various chronic diseases. Appl. Sci. 13, 2893 (2023).
Tiwari, D. et al. Clastogenic and mutagenic effects of bisphenol A: An endocrine disruptor. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 743, 83–90 (2012).
Omari Shekaftik, S. & Nasirzadeh, N. 8-Hydroxy-2′-deoxyguanosine (8-OHdG) as a biomarker of oxidative DNA damage induced by occupational exposure to nanomaterials: A systematic review. Nanotoxicology 15, 850–864 (2021).
Guo, C. et al. Association between oxidative DNA damage and risk of colorectal cancer: Sensitive determination of urinary 8-hydroxy-2′-deoxyguanosine by UPLC-MS/MS analysis. Sci. Rep. 6, 32581 (2016).
Dani, I. C., Rugian, C. F. O., & Handayani, S. In Vivo Study of DNA Adduct (8-OHdG) Formation of Rattus novergicus Using Bisphenol a through Fenton-Like Reaction and Nickel (II) as Cancer Risk Biomarker. In Bisphenols (ed. Erkekoğlu, P.) (IntechOpen, 2022).
Kitts, D. D., Wijewickreme, A. N. & Hu, C. Antioxidant properties of a North American ginseng extract. Mol. Cell Biochem. 203, 1–10 (2000).
El-Banna, M. A., Hendawy, O. M., El-Nekeety, A. A. & Abdel-Wahhab, M. A. Efficacy of ginsenoside Rg3 nanoparticles against Ehrlich solid tumor growth in mice. Environ. Sci. Pollut. Res. Int. 29, 43814–43825 (2022).
Tsai, W. C., Li, W. C., Yin, H. Y., Yu, M. C. & Wen, H. W. Constructing liposomal nanovesicles of ginseng extract against hydrogen peroxide-induced oxidative damage to L929 cells. Food chem. 132, 744–751 (2012).
Ullah, H. A. et al. Red ginseng oil attenuates oxidative stress and offers protection against ultraviolet-induced photo toxicity. Oxid. Med. Cell Longev. 2021, 5538470 (2021).
Zhou, D. X., Qiu, S. D., Zhang, J., Tian, H. & Wang, H. X. The protective effect of vitamin E against oxidative damage caused by formaldehyde in the testes of adult rats. Asian. J. Androl. 8, 584–588 (2006).
Hassanien, R. T. et al. Vitamin E improves doxorubicin induced nephrotoxicity; possible underlying mechanisms. Med. J. Cairo Univ. 86, 651–657 (2018).
Oblette, A. et al. Assessment of sperm nuclear quality after in vitro maturation of fresh or frozen/thawed mouse pre-pubertal testes. Mol. Hum. Reprod. 23, 674–684 (2017).
Omolaoye, T. S. & du Plessis, S. S. Male infertility: A proximate look at the advanced glycation end products. Reprod. Toxicol. 93, 169–177 (2020).
Chen, M. C., Lin, J. A., Lin, H. T., Chen, S. Y. & Yen, G. C. Potential effect of advanced glycation end products (AGEs) on spermatogenesis and sperm quality in rodents. Food Funct. 10, 3324–3333 (2019).
Zhou, M. et al. Activation and Modulation of the AGEs-RAGE Axis: Implications for inflammatory pathologies and therapeutic interventions-a review. Pharmacol. Res. 206, 107282 (2024).
Chuong, C., Katz, J., Pauley, K. M., Bulosan, M. & Cha, S. RAGE expression and NF-κB activation attenuated by extracellular domain of RAGE in human salivary gland cell line. J. Cell Physiol. 221, 430–434 (2009).
Tobon-Velasco, J., Cuevas, E., & A Torres-Ramos, M. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. Drug Targets. 13, 1615–1626 (2014).
Tekin, S. & Çelebi, F. Investigation of the effect of hesperidin on some reproductive parameters in testicular toxicity induced by Bisphenol A. Andrologia 54, e14562 (2022).
Ali, M. Y., Jannat, S. & Rahman, M. M. Ginsenoside derivatives inhibit advanced glycation end-product formation and glucose–fructose mediated protein glycation in vitro via a specific structure–activity relationship. Bioorg. Chem. 111, 104844 (2021).
Tan, X. et al. Ginseng improves cognitive deficit via the RAGE/NF-κB pathway in advanced glycation end product-induced rats. J. Ginseng Res. 39, 116–124 (2015).
Kim, D. H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J. Ginseng Res. 42, 255–263 (2018).
Reckelhoff, J. F. et al. Vitamin E ameliorates enhanced renal lipid peroxidation and accumulation of F2-isoprostanes in aging kidneys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 274, R767–R774 (1998).
Glauert, H. P. Vitamin E and NF-κB Activation: A Review. Vitam. Horm. 76, 135–153 (2007).
Eid, R. A. & Ahmed, M. A. A. S. Effect of bisphenol-A on the testis structure of the adult male albino rats. Bothlia J. 43, 69–86 (2013).
Kim, Y. H., Kim, G. H., Shin, J. H., Kim, K. S. & Lim, J. S. Effect of korean red ginseng on testicular tissue injury after torsion and detorsion. Korean J. Urol. 51, 794–799 (2010).
Ali, S. A. & Al-Derawi, K. H. Testicular toxic effect of lead acetate on adult male rats and the potential protective role of alcoholic extract of ginseng (histological, histomorphometrical and physiological). Sci. J. Med. Res. 2, 87–92 (2018).
El Gharabawy, G. S., Abd Allah, E. E. D. E., Amr, I. M. & Elmitwalli, M. Histological and immunohistochemical study of the effect of cyclophosphamide on testis of male adult albino rats and the possible protective role of Vit. E. Egypt J. Hosp. Med. 77, 5930–5946 (2019).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Basma I. El-Shimi: Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Investigation, Writing- Reviewing and Editing. Rafat M. Mohareb: Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Supervision, Investigation, Writing- Reviewing and Editing. Hanaa H. Ahmed: Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Supervision, Investigation, Writing- Reviewing and Editing. Rehab S. Abohashem: Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Supervision, Investigation, Writing- Reviewing and Editing. Khaled F. Mahmoud: Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Supervision, Investigation, Writing- Reviewing and Editing. Demiana H. Hanna: Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Supervision, Investigation, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical Committee for Medical Research of the NRC, Egypt has been granted the final approval for the study under registration number of 13104112022 and all methods were performed in accordance with relevant regulations and guidelines including the ARRIVE guideline.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in two equations in the Methods section. In addition, the legend of Figure 7 was incorrect. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Shimi, B.I., Mohareb, R.M., Ahmed, H.H. et al. Panax ginseng nanoemulsion for counteracting male infertility via modulating sex hormones and oxidative stress in a rat model. Sci Rep 14, 29239 (2024). https://doi.org/10.1038/s41598-024-79388-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79388-x