Abstract
Previous studies identified individual-level socioeconomic factors as key determinants of cognitive health. This study investigated the effect of area-based socioeconomic deprivation on cognitive outcomes in midlife to early late-life New Zealanders without cognitive impairment at baseline. Data stemmed from a subsample of the New Zealand Health, Work and Retirement Study, a cohort study on ageing, who completed face-to-face interviews and were reassessed two years later. Cognitive functioning was measured using Addenbrooke’s Cognitive Examination–Revised, adapted for culturally acceptable use in Aotearoa New Zealand. Area-based socioeconomic deprivation was assessed using the New Zealand Deprivation Index (NZDep2006). Linear mixed-effects models analysed the association between area-based socioeconomic deprivation and cognitive outcomes. The analysis included 783 participants without cognitive impairment at baseline (54.7% female, mean age 62.7 years, 25.0% Māori, the Indigenous people of Aotearoa New Zealand). There was an association between higher area-based socioeconomic deprivation and lower cognitive functioning (B = -0.08, 95%CI: -0.15;-0.01; p = .050) and cognitive decline (B = -0.12, 95%CI: -0.20;-0.04, p = .013) over two years, while controlling for covariates. The findings emphasise the importance of considering neighbourhood characteristics and broader socioeconomic factors in strategies aimed at mitigating cognitive health disparities and reducing the impact of dementia in disadvantaged communities.
Similar content being viewed by others
Introduction
Cognitive functioning is a fundamental aspect of human health and well-being, integral to daily functioning, decision-making, and overall quality of life across the lifespan1. It encompasses a range of cognitive abilities, including memory, attention, language, and executive function. As individuals age, cognitive functioning changes and decline often occurs. Neuropathological processes such as neurodegeneration, neuroinflammation or ischemia can lead to cognitive impairment and dementia2,3,4. For many forms of dementia, the decline in cognitive functioning is a defining feature of the condition, progressively impairing an individual’s ability to carry out even basic activities of daily living. Thus, understanding the intricate relationship between cognitive functioning, ageing, and dementia is imperative for the development of effective strategies to mitigate cognitive decline, enhance brain health, and ultimately reduce the risk of or prevent dementia.
The role of individual-level socioeconomic factors in shaping cognitive health is well recognised in research5,6,7. From a person-centred perspective, socioeconomic status (SES) is an established multifaceted construct comprising information of an individual’s economic resources, educational attainment, and occupational status8. Studies have consistently demonstrated a strong association between individual-level SES and cognitive functioning: Lower SES has been linked to lower cognitive performance across the lifespan, with disparities emerging as early as in childhood and persisting into older age, when it becomes linked to accelerated cognitive decline and higher risk for dementia9,10. The link between SES and cognitive functioning is attributed to a combination of environmental, psychosocial, and biological mechanisms: Lower SES often results in limited access to quality education and health care, leading to less cognitive stimulation and poorer overall brain health6. Chronic stress, more prevalent in lower SES groups, can elevate cortisol levels, negatively impacting brain regions such as the hippocampus, which is critical for memory11. Additionally, lower SES is associated with poorer lifestyle and limited healthy lifestyle choices12.
More recently, studies have extended the focus, highlighting the critical role of the neighbourhood or area-based socioeconomic factors in influencing cognitive functioning and its implications for dementia above and beyond individual-level SES13. Conceptually, this is rooted in the framework on the social determinants of health (SDOH), proclaiming that the conditions in which people are born, grow, play, work, live, worship, and age have a profound impact on health across the life course14, including on cognition and risk for dementia in later life15. SDOH are non-medical factors that comprise the economic, social and community context, cultural norms, healthcare access and quality, and housing, neighbourhoods and the built environment16. To assess neighbourhood or area-based socioeconomic factors and their relationship with health outcomes, area-based socioeconomic deprivation is an established multidimensional concept to capture the relative disadvantage of a geographic area or community in terms of income, employment, educational opportunities, and housing conditions – among other factors17. It is useful for research and public health to study and address health inequities across different populations.
While there is good international evidence on links of area-based socioeconomic deprivation with adverse cognitive health outcomes, similar research is scarce in the context of Aotearoa New Zealand. Correspondingly to global trends, the number of people with dementia in Aotearoa New Zealand is expected to more than double, if not triple, in the coming decades. In the Oceanic nation home to 5 million people, the count is projected to climb from 70,000 individuals with dementia in 2020 to at least 170,000 by 2050, coupled with an escalation in total economic costs from NZ$1.9 billion to NZ$4.5 billion18. However, a recent study suggested a high dementia prevention potential in Aotearoa New Zealand19. Ma’u et al. found that 47.7% of all dementia could be attributed to modifiable risk factors highlighted in the Lancet 12 risk factor life-course model of dementia prevention20. Notably, the study revealed a marked difference in the prevention estimates regarding Aotearoa New Zealand’s main ethnic populations, ranging from 40.8% for New Zealanders of Asian (mainly Chinese and Indian) descent, 47.6% for European New Zealanders, 50.8% for Pacific Peoples, to 51.4% for Māori, the Indigenous population. The high proportion in Pacific Peoples and Māori is associated with profound structural socioeconomic disadvantages stemming from systemic discrimination and racism historically rooted in colonialism19 – stretching the importance of studying links between socioeconomic conditions and cognitive outcomes. Understanding such links is crucial for developing targeted interventions and policies aimed at reducing cognitive health and dementia disparities.
Against this background, the study aimed to investigate the relationship of area-based socioeconomic deprivation and cognitive functioning in a midlife to early late-life sample of New Zealanders without cognitive impairment at baseline.
Methods
Study population
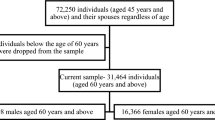
The study is part of the New Zealand Health, Work and Retirement Study (NZHWR), a longitudinal cohort study of ageing21. Biennial nationwide surveys have been conducted since 2006 with New Zealanders from mid-adulthood. Participants were recruited through the national electoral role using random sampling and over-sampling of individuals of Māori descent for adequate representation22. The sample for this study was drawn from the population-based sample of the 2010 NZHWR study wave who volunteered to participate in face-to-face cognitive assessments in addition to the postal surveys. A total of 1,001 participants completed the baseline face-to-face interviews. They were 48–84 years old, 55.8% were female and 25.8% of Māori descent. The face-to-face subsample was higher educated, under-sampled in the 45–54 age group and 75 + age group, and oversampled Māori in comparison to the general population as of the 2006 census. Two years later, follow-up assessments were completed by 873 of the baseline participants, using identical measures. Participants were visited in their homes by trained research assistants who conducted the cognitive testing. For the purpose of this study, participants were included if aged up to 75 years (as per objective to study a midlife and early late life population and the validity of the Lifestyle for Brain Health index up to 75 years) and if they were without cognitive impairment at baseline. Thus, of the 1,001 participants of the subsample study, 783 were included in this study. As per sample definition, 69 participants with cognitive impairment and 79 aged above 75 years were excluded. Among the remaining participants, further attrition was due to missing data for sociodemographic factors (n = 22), LIBRA (n = 18), net income (n = 17), social loneliness (n = 11), cognitive functioning (n = 1), and NZDep2006 (n = 1). Of the 783 baseline participants in the study sample, 699 completed the follow-up assessments two years later. Individuals who dropped out (n = 84, 10.7%) did not differ from completers regarding age (drop: M = 63.1, SD = 6.8 vs. completer: M = 62.7, SD = 6.4; p = .561), sex (women: drop: 56.0% vs. completer: 54.5%; p = .801), education (high qualification: drop: 60.8% vs. completer: 63.8%; p = .107), ethnicity (Māori: drop: 32.1% vs. completer: 24.2%, p = .111), and cognitive functioning (drop: M = 93.9, SD = 3.5 vs. completer: M = 94.6, SD = 3.3; p = .074).
Ethics
The study has been approved by the Human Ethics Committee (HEC Southern B; 09/70, 10/43) of Massey University. It adheres to ethical standards of the 1964 Declaration of Helsinki and its later amendments. All participants provided written informed consent.
Cognitive functioning
Cognitive functioning was assessed with the adapted version of the Addenbrooke’s Cognitive Examination – Revised23 for culturally appropriate use with New Zealanders (the ‘Kiwi’ ACE-R) and normative data available regarding age groups, sex, education, and ethnicity24,25. The neuropsychological test battery comprises 26 items that encompass five cognitive domains: attention and orientation (e.g., asking for location and date), memory (tasks related to short-term, long-term, anterograde, and retrograde memory), verbal fluency (naming words beginning with a specific alphabetic letter), language (e.g., writing sentences, repeating words), and visuospatial (e.g., drawing a clock and copying a pentagon). The score of each domain is summed up to form a score of global cognitive functioning ranging from 0 to 100, with higher scores indicating higher cognitive functioning. The ACE-R has been shown to be a valid and sensitive screening tool for cognitive impairment and possible dementia (cut-off 82: sensitivity = 0.84, specificity = 1.00; cut-off 88: sensitivity = 0.94, specificity = 0.98), including in older New Zealanders26. Details of the New Zealand ACE-R version have been published elsewhere26. Cognitive impairment was assigned to participants who scored 1.5 standard deviations (SD) or more below the mean total ACE-R score of the sample.
Area-based socioeconomic deprivation
The New Zealand Deprivation Index (NZDep) is a census-based index of relative socioeconomic deprivation for small areas in Aotearoa New Zealand27. The NZHWR substudy data collection in 2010 and 2012 is connected to the 2006 version of the NZDep, also referred to as NZDep200628. The index provides a deprivation score for each meshblock or a small number of agglomerated meshblocks in Aotearoa New Zealand. Meshblocks are the smallest geographical units defined by Statistics New Zealand29. In 2006, each meshblock contained a median of 87 people. NZDep2006 refers to the proportion of people in each small area regarding nine weighted aspects of deprivation calculated using principal components analysis. These are, with decreasing weight in the index: proportion of people aged 18–64 receiving a means-tested benefit, people living in equivalised households (i.e., controlled for household composition) with income below an income threshold (60% of the median equivalised disposable household income, i.e., an annual income under NZ$23,805), people not living in own home, people aged < 65 living in a single parent family, people aged 18–64 being unemployed, people aged 18–64 without any qualifications, people living in equivalised households below a bedroom occupancy threshold, people with no access to a telephone, and people with no access to a car. Details of the index development and composition have been published elsewhere28. The NZDep2006 index represents a decile scale from 1 to 10, where 1 indicates a small area in the 10% least-deprived small areas in the country and 10 indicates a small area in the 10% most-deprived small areas at the time of the 2006 census. For the purpose of descriptive analysis, area-based socioeconomic deprivation was collapsed to represent three levels: low deprivation (decile 1–3), moderate deprivation (4–7), and high deprivation (8–10). The validity of the evolving versions of the NZDep has been consistently demonstrated, particularly with regards to health measures30.
Covariates
We included covariates that are known to be associated with cognitive functioning, and thus need to be accounted for. Sociodemographic and individual-level socioeconomic information was assessed based on a standardised self-report questionnaire and included age (years), gender (men, women), education (reflecting levels of the New Zealand educational system, ranging from no qualifications, secondary school qualification, post-secondary certificate or diploma to university degree), ethnicity (self-identified; Māori and Non-Māori), employment (employed, retired, unemployed/other), net personal annual income, and marital status (single, married/in a partnership)21.
To account for relevant health and lifestyle factors know to be associated with cognitive functioning and dementia risk, we drew on the “Lifestyle for Brain Health” (LIBRA) index31,32. LIBRA is a composite score, which was developed based on a systematic literature review and a Delphi expert panel consensus on modifiable risk and protective factors for dementia32. The LIBRA index quantifies modifiable dementia risk, or room for brain health improvement. Numerous studies have shown the utility of the score in demonstrating associations with cognitive functioning and prevalent and incident cognitive impairment, cognitive decline and incident dementia in diverse midlife and early late-life (up to 75 years of age) populations33,34,35,36,37,38, including for midlife and early late-life New Zealanders39. It contains up to 12 modifiable health and lifestyle factors33, and 8 of them were available in the NZHWR study in the 2010 wave: heart disease, kidney disease, diabetes, hypertension, moderate alcohol consumption, smoking, physical inactivity, depression; with information on obesity, hypercholesterolemia, healthy diet, and high cognitive activity not being available. A standardised weight was assigned to each factor, reflecting its relative risk for dementia retrieved from systematic literature reviews and meta-analyses32,38. Then, the natural logarithm of the relative risk for each factor was calculated. These were then standardised by taking the lowest natural logarithm as a reference value and dividing all other values by this value, thus creating the weights. The weights were summed to yield the LIBRA score. The 8-factor modified score ranged from − 1.0 to + 9.7. Higher scores indicate poorer lifestyle for brain health tied to dementia risk.
Additionally, we included a measure on social loneliness as a covariate, based on a subscale of the De Jong Gierveld Loneliness Scale (DJGLS)40. The social loneliness scale comprises five items, each scored on a 5-point Likert scale. A total score was calculated by summing the neutral and negative answers of the items, ranging from 0 to 5 with higher scores indicating higher social loneliness.
Statistical analysis
Sample characteristics were investigated by calculating means and SDs or proportions for the total sample and regarding level of area-based socioeconomic deprivation. Group differences according to level of area-based socioeconomic deprivation were assessed using Chi-squared tests and one-way analysis of variance (ANOVA), as appropriate. Bonferroni correction was applied to account for multiple comparisons. Effect sizes were calculated for significant group differences, i.e., Cramer’s V for categorical variables and Eta squared for continuous variables.
Linear mixed effects models with maximum likelihood were used to assess the longitudinal association of area-based socioeconomic deprivation with cognitive functioning and cognitive decline over the two years of observation. The association between area-based socioeconomic deprivation and cognitive decline was estimated by including a two-way interaction term of time and area-based socioeconomic deprivation, which represents the rate of change in cognitive functioning as a function of area-based socioeconomic deprivation. Three models were inspected: unadjusted, base-adjusted for age, sex, education and ethnicity, and adjusted for all above named covariates, i.e., sociodemographic factors (age, sex, education, ethnicity, marital status), lifestyle and health factors (LIBRA index, social loneliness), and individual-level socioeconomic factors (employment status, net personal annual income). No centering was applied on covariates. Following likelihood ratio tests and inspection of fit criteria (Akaike Information Criterion [AIC], Bayesian Information Criteria [BIC], the models included an unstructured covariance matrix, a random intercept, but not a random slope. The adjusted models included a quadratic term for age to account for the non-linear relationship with cognitive functioning. Predictive margins were calculated to visualize associations for the adjusted analysis. To inspect model fit, AIC, BIC, and Intraclass Correlation Coefficient (ICC) were calculated. A level of statistical significance of p < .05 was assumed. Analyses were performed with STATA/SE 17.0 (StataCorp, Texas).
Results
Sample characteristics
The analytical sample had a mean age of 62.7 (SD = 6.4, range = 48–75) years, 54.7% were female, and 25.0% identified as Māori. Almost two thirds (63.4%) of the sample reported a high level of education, having obtained either a post-secondary or university degree.
Regarding area-based socioeconomic deprivation, 311 (39.7%) of the participants lived in low deprivation, 305 (39.0%) in moderate deprivation, and 167 (21.3%) in high deprivation. The age and gender distribution did not differ between levels of area-based socioeconomic deprivation. Lower levels of education, being of Māori descent, being single, and being unemployed were more frequent among high levels of deprivation. Detailed results are presented in Table 1. Bonferroni tests for multiple comparisons showed that group differences for LIBRA scores and cognitive functioning were between the low and high level of deprivation, but not between low and moderate, and moderate and high, except for net personal annual income, which was also different between low and moderate level of deprivation (results not further shown). There were no group differences regarding social loneliness.
At follow-up after two years, 57 (8.2%) obtained an ACE-R score that indicated incident cognitive impairment.
Associations of area-based socioeconomic deprivation and cognition
In the unadjusted model, higher area-based socioeconomic deprivation was associated with lower cognitive functioning (B = -0.16, 95%CI = -0.24; -0.08; p < .001) and cognitive decline (B = -0.12, 95%CI = -0.22; -0.02; p = .015). Results are further detailed in Table 2.
Similar results were found in the base-adjusted model accounting for age, age², sex, education, and ethnicity: Higher area-based socioeconomic deprivation was associated with lower cognitive functioning (B = -0.10, 95%CI = -0.17; -0.02; p = .018) and cognitive decline (B = -0.12, 95%CI = -0.22; -0.02; p = .017). Further results are presented in Table 3.
Likewise, in the adjusted model, higher area-based socioeconomic deprivation was associated with lower cognitive functioning (B = -0.08, 95%CI = -0.17; -0.01; p = .050) and cognitive decline (B = -0.12, 95%CI = -0.22; -0.03; p = .013). In detail, a one-unit change in area-based socioeconomic deprivation, i.e. a difference of one decile, had an adjusted average marginal effect on the ACE-R score of -0.08 (95%CI = -0.17; -0.01; p = .050) at baseline and − 0.21 (95%CI = -0.32; -0.10; p < .001) at follow-up after two years (Figs. 1 and 2). Detailed results are presented in Table 4.
Adjusted average predicted cognitive functioning (ACE-R scores) according to decile of are-based socioeconomic deprivation (NZDep) at baseline and at 2-year follow-up. Higher scores on the Y-axis indicate better cognitive functioning. Higher scores on the X-axis indicate higher area-based socioeconomic deprivation.
Discussion
We investigated area-based socioeconomic deprivation in relation to cognitive functioning and cognitive decline in midlife to early late-life New Zealanders without cognitive impairment at baseline. In the sample of 783 adults aged 48 to 75 years, higher area-based socioeconomic deprivation was associated with lower cognitive functioning and predicted cognitive decline over two years, accounting for sociodemographic, individual-level socioeconomic as well as health and lifestyle factors.
Our results are in line with the growing body of literature that underlines the relevance of living conditions for cognitive health and the importance to consider them in strategies targeted at improving brain health and reducing the risk of cognitive decline and dementia41,42,43,44,45,46. A recent study from Aotearoa New Zealand, the first of its kind, found that people residing in disadvantaged neighbourhoods were at greater risk for dementia and showed deficits in brain structure as well as cognitive difficulties decades before dementia-related outcomes may occur47 – our study corroborates these findings. Notably, a study in various Latin American populations suggested that social and environmental factors likely play a more crucial role than genetic ancestry in predicting dementia, and individuals with higher proportions of Native American (> 70%) and African American (> 70%) ancestry were more likely to exhibit factors contributing to worse SDOH48. Overall, results from diverse older populations from varying geographical regions seem to rather consistently point towards an association of area-based socioeconomic deprivation and poorer cognitive outcomes in later life, independent of individual-level socioeconomic factors such as education and income. This is also despite notable variety in the characteristics of indices quantifying area-based or neighbourhood socioeconomic deprivation17. Thus, area-based socioeconomic deprivation constitutes a risk factor for cognitive decline and dementia – but a modifiable one that can be addressed in strategies aimed at risk reduction.
Research has identified a variety of mechanisms that likely underlie the link between area-based socioeconomic deprivation and poorer cognitive functioning. A well-established pathway is limited access to healthcare services, often entangled with lower health literacy and financial barriers, which can lead to no or late diagnoses and thus missed opportunities for intervention49,50. Socioeconomic deprivation drives poor health behaviours and lifestyles known to be associated with cognitive decline and dementia, such as more smoking and alcohol consumption, unhealthy diets, less physical, social and cognitive stimulation12,51,52. In a previous study, we have found that area-based socioeconomic deprivation was linked to such modifiable health and lifestyle factors in Māori, but not in Non-Māori53. This is often coupled with restricted resources to make good lifestyle choices in deprived areas, such as lack of availability of nutritious food and opportunities for physical activity, sometimes referred to as obesogenic environments54 – an issue that is specifically prevalent in NZ55. Living in deprived areas can expose residents to chronic stress, known to affect cognitive functioning and brain structure56. Specific environmental stressors may be more present in deprived urban areas, such as air and noise pollution57,58, or lack of access to green and blue space59,60 – both has been shown for the Auckland region in NZ61,62. Poor housing and low neighbourhood satisfaction as well as low neighbourhood social cohesion have been identified as environmental stressors in NZ63. Moreover, unhealthy environments are linked to higher psychological distress and a higher prevalence of mental health conditions, such as depression and anxiety64,65, which in turn are risk factors for cognitive decline and dementia65,66. Studies that investigate mechanisms underlying the link between NZDep and cognitive functioning, specifically, are needed for the development and implementation of public health strategies that address the multifaceted modifiable risks for cognitive decline and dementia67. Current such strategies tend to focus on individual-level factors, aiming at improving lifestyle behaviours and the management of health conditions68, often leaving the neighbourhood and the wider environmental context out of the picture. A more comprehensive strategy that considers change at the structural level, and not only on the individual level, can create better living conditions for better brain health outcomes for whole populations over generations to come. Walsh et al. defined such a population-level approach to dementia risk reduction as “measures applied to populations, groups, areas, jurisdictions, or institutions with the aim of changing the social, physical, economic, or legislative environments to make them less conducive to the development or maintenance of dementia and its modifiable life course risk factors”69. The core idea is to make healthy lifestyle choices the default options. Policy examples include public smoking bans, bans of high-emission cars in city centres, or legislations for healthy housing standards. In addition to area-based socioeconomic factors, it has been argued that other environmental factors such as the natural environment (green and blue spaces) and the design of the built environment can promote (brain) health and be utilised to reduce the risk and prevent cognitive ill-health in later life70. Working towards positive change in deprived neighbourhoods specifically might be more effective than trying to change the health behaviours of people living in areas of limited resources and opportunities that hinder unfolding their brain health potentials in the first place.
Strengths and limitations
The study drew on a large, well-phenotyped sample of midlife and early late-life New Zealanders. Strengths of the study include the longitudinal design and the use of culturally acceptable measures that allowed for providing insights into links of area-based socioeconomic deprivation and cognitive functioning in a geographical area with little prior knowledge.
Regarding limitations, our findings may not be generalised to New Zealand’s midlife and early late-life population as the volunteer sample deviated from sociodemographic distributions among the general population. The sample was more highly educated, and thus may point towards better socioeconomic circumstances, potentially leading to an underestimation of the association between area-based socioeconomic deprivation and cognitive functioning.
Given the short observation period, we did not inspect changes in participants’ residence. However, it needs to be noted that studies have shown that the dynamics of internal mobility and migration in Aotearoa New Zealand tend to perpetuate rather than counter the pre-existing level of area-based deprivation71. The observation period of two years is also rather short in terms of observing cognitive changes and may explain why effects found are rather small.
The LIBRA index computed for this study was based on information for 8 out of 12 modifiable health and lifestyle factors for dementia due to limited data availability in the 2010 NZHWR wave. The subsequent narrower range of the LIBRA scores may have led to an underestimation of covariate association with cognitive outcomes. This assumption is supported by studies that suggested including more modifiable risk factors for dementia leads to more informative results72. Lastly, the operationalisation of area-based socioeconomic deprivation (NZDep) and lifestyle for brain health (LIBRA index) differed from other studies, which compromises comparability.
Conclusions
Higher area-based socioeconomic deprivation was associated with lower cognitive functioning and predicted cognitive decline independent of individual-level socioeconomic factors in midlife to early late-life New Zealanders. Our results add to a growing body of literature that highlights the importance of addressing neighbourhood contexts in public heath agendas targeting risk reduction and prevention of cognitive decline and dementia in later life. Population-based approaches aimed at improving living conditions may be particularly beneficial to address cognitive health disparities and reduce the impact and scale of dementia in disadvantaged communities.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Glisky, E. L. Changes in cognitive function in human aging. In Brain Aging 3–20 (CRC (2007).
Desmond, D. W., Moroney, J. T., Sano, M. & Stern, Y. Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke. 33, 2254–2260. https://doi.org/10.1161/01.STR.0000028235.91778.95 (2002).
Heneka, M. T., Golenbock, D. T. & Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 16, 229–236. https://doi.org/10.1038/ni.3102 (2015).
Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608. https://doi.org/10.15252/emmm.201606210 (2016).
Ibáñez, A., Legaz, A. & Ruiz-Adame, M. Addressing the gaps between socioeconomic disparities and biological models of dementia. Brain. 146, 3561–3564. https://doi.org/10.1093/brain/awad236 (2023).
Hackman, D. A., Farah, M. J. & Meaney, M. J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 11, 651–659. https://doi.org/10.1038/nrn2897 (2010).
Farah, M. J. Socioeconomic status and the brain: prospects for neuroscience-informed policy. Nat. Rev. Neurosci. 19, 428–438. https://doi.org/10.1038/s41583-018-0023-2 (2018).
Oakes, J. M. & Rossi, P. H. The measurement of SES in health research: current practice and steps toward a new approach. Soc. Sci. Med. 56, 769–784 (2003). -9536(02)00073 – 4.
Lyu, J. & Burr, J. A. Socioeconomic status across the life course and cognitive function among older adults: an examination of the latency, pathways, and Accumulation hypotheses. J. Aging Health. 28, 40–67. https://doi.org/10.1177/0898264315585504 (2016).
Luo, Y. & Waite, L. J. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J. Gerontol. B Psychol. Sci. Soc. Sci. 60, S93–S101. https://doi.org/10.1093/geronb/60.2.S93 (2005).
Baum, A., Garofalo, J. P. & Yali, A. M. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann. N. Y. Acad. Sci. 896, 131–144. https://doi.org/10.1111/j.1749-6632.1999.tb08111.x (1999).
Röhr, S. et al. Social determinants and lifestyle factors for brain health: implications for risk reduction of cognitive decline and dementia. Sci. Rep. 12 https://doi.org/10.1038/s41598-022-16771-6 (2022).
Wu, Y. T., Prina, A. M. & Brayne, C. The association between community environment and cognitive function: a systematic review. Soc. Psychiatry Psychiatr Epidemiol. 50, 351–362. https://doi.org/10.1007/s00127-014-0945-6 (2015).
Marmot, M. Social determinants of health inequalities. Lancet. 365, 1099–1104 (2005).
Majoka, M. A. & Schimming, C. Effect of Social Determinants of Health on Cognition and Risk of Alzheimer Disease and related dementias. Clin. Ther. 43, 922–929. https://doi.org/10.1016/j.clinthera.2021.05.005 (2021).
Solar, O. & Irwin, A. A Conceptual Framework for Action on the Social Determinants of Health (WHO Document Production Services, 2010).
Trinidad, S. et al. Use of Area-based socioeconomic deprivation indices: a scoping review and qualitative analysis. Health Aff. 41, 1804–1811. https://doi.org/10.1377/hlthaff.2022.00482 (2022).
Alzheimers New Zealand. Dementia economic impact report. Alzheimers New Zealand, (2008).
Ma’u, E., Cullum, S., Cheung, G., Livingston, G. & Mukadam, N. Differences in the potential for dementia prevention between major ethnic groups within one country: a cross sectional analysis of population attributable fraction of potentially modifiable risk factors in New Zealand. Lancet Reg. Health - Western Pac. 13, 100191. https://doi.org/10.1016/j.lanwpc.2021.100191 (2021).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 396, 413–446. https://doi.org/10.1016/S0140-6736(20)30367-6 (2020).
Allen, J. et al. The health, work, and retirement study: representing experiences of later life in Aotearoa New Zealand. J. Royal Soc. New. Z. 1–16. https://doi.org/10.1080/03036758.2022.2099911 (2022).
Alpass, F. et al. Independence, well-being, and social participation in an aging population. Ann. N. Y. Acad. Sci. 1114, 241–250. https://doi.org/10.1196/annals.1396.009 (2007).
Mioshi, E., Dawson, K., Mitchell, J., Arnold, R. & Hodges, J. R. The Addenbrooke’s cognitive examination revised (ACE-R): a brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry. 21, 1078–1085. https://doi.org/10.1002/gps.1610 (2006).
Callow, L., Alpass, F., Leathem, J. & Stephens, C. Normative data for older New zealanders on the Addenbrooke’s cognitive examination-revised. New. Z. J. Psychol. 44, 29–41 (2015).
Taylor, Addenbrooke’s Cognitive Examination - ACE-R & REvised Version, A. - NZ Adaptation. (2004). http://www.ftdrg.org/wp-content/uploads/New-Zealand-ACE-R-version-Av1f.pdf (2008).
Cheung, G. et al. Performance of three cognitive screening tools in a sample of older New zealanders. Int. Psychogeriatr. 27, 981–989. https://doi.org/10.1017/S1041610214002889 (2015).
Crampton, P. R., Sutton, F. & Salmond, C. E. NZDep91: Index of Deprivation (Health Services Research Centre, 1997).
Salmond, C. E., Crampton, P. & Atkinson, J. NZDep2006 Index of Deprivation (Department of Public Health, University of Otago Wellington, 2007).
Statistics New Zealand. Statistical Standard for Meshblock (Statistics New Zealand, 2016).
Salmond, C. E. & Crampton, P. Development of New Zealand’s deprivation index (NZDep) and its uptake as a national policy tool. Can. J. Public. Health = Revue canadienne de sante Publique, S7–S11 (2012).
Deckers, K. et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int. J. Geriatr. Psychiatry. 30, 234–246. https://doi.org/10.1002/gps.4245 (2015).
Schiepers, O. J. G. et al. Lifestyle for Brain Health (LIBRA): a new model for dementia prevention. Int. J. Geriatr. Psychiatry. 33, 167–175. https://doi.org/10.1002/gps.4700 (2018).
Vos, S. J. B. et al. Modifiable Risk Factors for Prevention of Dementia in Midlife, late life and the Oldest-Old: validation of the LIBRA Index. J. Alzheimers Dis. 58, 537–547. https://doi.org/10.3233/JAD-161208 (2017).
Pons, A. et al. Utility of the LIBRA Index in Relation to Cognitive Functioning in a Clinical Health seeking sample. J. Alzheimers Dis. 62, 373–384. https://doi.org/10.3233/JAD-170731 (2018).
Deckers, K. et al. Long-term dementia risk prediction by the LIBRA score: a 30-year follow-up of the CAIDE study. Int. J. Geriatr. Psychiatry. 35, 195–203. https://doi.org/10.1002/gps.5235 (2020).
Deckers, K. et al. Gender and Educational differences in the Association between Lifestyle and Cognitive decline over 10 years: the Doetinchem Cohort Study. J. Alzheimers Dis. 70 (41), S31–S. https://doi.org/10.3233/JAD-180492 (2019).
Deckers, K. et al. Lack of associations between modifiable risk factors and dementia in the very old: findings from the Cambridge City over-75s cohort study. Aging Ment. Health. 22, 1272–1278. https://doi.org/10.1080/13607863.2017.1280767 (2018).
Heger, I. S. et al. Associations of the Lifestyle for Brain Health Index with Structural Brain Changes and Cognition: results from the Maastricht Study. Neurology. 97, e1300–e1312. https://doi.org/10.1212/WNL.0000000000012572 (2021).
Röhr S, Stephens C, Alpass F. Lifestyle for brain health and cognitive functioning in midlife to early late-life New Zealanders: utility of the LIBRA index. Int J Geriatr Psychiatry. 2024;e6091. https://doi.org/10.1002/gps.6091
de Jong-Gierveld, J. & Kamphuls, F. The development of a rasch-type loneliness scale. Appl. Psychol. Meas. 9, 289–299 (1985).
Kucharska-Newton, A. M. et al. Association of Childhood and Midlife Neighborhood Socioeconomic Position With Cognitive Decline. JAMA network open 6, e2327421; (2023). https://doi.org/10.1001/jamanetworkopen.2023.27421
Vassilaki, M. et al. Association of neighborhood socioeconomic disadvantage and cognitive impairment. Alzheimer’s Dement. https://doi.org/10.1002/alz.12702 (2022).
Hofbauer, L. M. & Rodriguez, F. S. Association of social deprivation with cognitive status and decline in older adults. Int. J. Geriatr. Psychiatry (2021).
McCann, A. et al. Effect of area-level socioeconomic deprivation on risk of cognitive dysfunction in older adults. J. Am. Geriatr. Soc. 66, 1269–1275. https://doi.org/10.1111/jgs.15258 (2018).
Wee, L. E. et al. Individual and Area Level Socioeconomic Status and its association with cognitive function and cognitive impairment (low MMSE) among Community-Dwelling Elderly in Singapore. Dement. Geriatric Cogn. Disorders Extra. 2, 529–542. https://doi.org/10.1159/000345036 (2012).
Wu, F. et al. Social-Economic Status and Cognitive performance among Chinese aged 50 years and older. PloS One. 11, e0166986. https://doi.org/10.1371/journal.pone.0166986 (2016).
Reuben, A. et al. Dementia, dementia’s risk factors and premorbid brain structure are concentrated in disadvantaged areas: national register and birth-cohort geographic analyses. Alzheimer’s Dement. https://doi.org/10.1002/alz.13727 (2024).
Llibre-Guerra, J. J. et al. Social determinants of health but not global genetic ancestry predict dementia prevalence in Latin America. Alzheimer’s Dement. 20, 4828–4840. https://doi.org/10.1002/alz.14041 (2024).
Sudore, R. L. et al. Limited literacy in older people and disparities in health and healthcare access. J. Am. Geriatr. Soc. 54, 770–776. https://doi.org/10.1111/j.1532-5415.2006.00691.x (2006).
Stewart, C. C., Yu, L., Wilson, R. S., Bennett, D. A. & Boyle, P. A. Healthcare and Financial decision making and incident adverse cognitive outcomes among older adults. J. Am. Geriatr. Soc. 67, 1590–1595. https://doi.org/10.1111/jgs.15880 (2019).
Röhr, S. et al. Socioeconomic inequalities in cognitive functioning only to a small extent attributable to Modifiable Health and Lifestyle factors in individuals without dementia. J. Alzheimers Dis. https://doi.org/10.3233/JAD-220474 (2022).
Deckers, K. et al. Modifiable risk factors explain socioeconomic inequalities in Dementia Risk: evidence from a Population-based prospective cohort study. J. Alzheimers Dis. 71, 549–557. https://doi.org/10.3233/JAD-190541 (2019).
Röhr, S., Gibson, R., Alpass, F. & Stephens, C. Social determinants of modifiable dementia risk in Māori and Non-Māori: results of the New Zealand Health, Work and Retirement study. Alzheimer’s Dement. 19 https://doi.org/10.1002/alz.074406 (2023).
Giskes, K., van Lenthe, F., Avendano-Pabon, M. & Brug, J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obes. Rev. 12, e95–e106. https://doi.org/10.1111/j.1467-789X.2010.00769.x (2011).
Vandevijvere, S., Mackay, S., D’Souza, E. & Swinburn, B. How Healthy are New Zealand food Environments? A Comprehensive Assessment 2014–2017. University of Auckland.
Luo, J., Beam, C. R. & Gatz, M. Is stress an overlooked risk factor for Dementia? A systematic review from a Lifespan Developmental Perspective. Prev. Science: Official J. Soc. Prev. Res. 24, 936–949. https://doi.org/10.1007/s11121-022-01385-1 (2023).
Hahad, O. et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 21, 4306 (2020).
Paul, K. C., Haan, M., Mayeda, E. R. & Ritz, B. R. Ambient air Pollution, noise, and late-life cognitive decline and Dementia Risk. Annu. Rev. Public Health. 40, 203–220. https://doi.org/10.1146/annurev-publhealth-040218-044058 (2019).
Astell-Burt, T., Navakatikyan, M. A. & Feng, X. Urban green space, tree canopy and 11-year risk of dementia in a cohort of 109,688 australians. Environ. Int. 145, 106102 (2020).
Georgiou, M., Morison, G., Smith, N., Tieges, Z. & Chastin, S. Mechanisms of Impact of Blue Spaces on Human Health: a systematic literature review and Meta-analysis. Int. J. Environ. Res. Public Health. 18 https://doi.org/10.3390/ijerph18052486 (2021).
Wrightson, S. Associations between Population Density, Air Pollution Exposure and Related Health Outcomes in Auckland, NZ. Associations between Population Density, Air Pollution Exposure and Related Health Outcomes in Auckland, NZ. ResearchSpace@Auckland.
Zhang, Y., Zhao, J., Mavoa, S. & Smith, M. Inequalities in urban green space distribution across priority population groups: evidence from Tāmaki Makaurau Auckland, Aotearoa New Zealand. Cities. 149, 104972. https://doi.org/10.1016/j.cities.2024.104972 (2024).
Stephens, C., Szabó, Á., Allen, J. & Alpass, F. Livable environments and the quality of life of older people: an ecological perspective. Gerontologist. 59, 675–685. https://doi.org/10.1093/geront/gny043 (2019).
Hobbs, M., Kingham, S., Wiki, J., Marek, L. & Campbell, M. Unhealthy environments are associated with adverse mental health and psychological distress: cross-sectional evidence from nationally representative data in New Zealand. Prev. Med. 145, 106416. https://doi.org/10.1016/j.ypmed.2020.106416 (2021).
Carles, S. et al. A cross-national study of depression in preclinical dementia: a COSMIC collaboration study. Alzheimer’s Dementia: J. Alzheimer’s Association. https://doi.org/10.1002/alz.12149 (2020).
Becker, E. et al. Anxiety as a risk factor of Alzheimer’s disease and vascular dementia. Br. J. Psychiatry: J. Mental Sci. 213, 654–660. https://doi.org/10.1192/bjp.2018.173 (2018).
Röhr, S. Social determinants of brain health need to be addressed in risk reduction of cognitive decline and dementia. Int. Psychogeriatr. 33, 1249–1251. https://doi.org/10.1017/S104161022100260X (2021).
World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines. 92415505 (2019).
Walsh, S. et al. What would a population-level approach to dementia risk reduction look like, and how would it work? Alzheimer’s Dement. 19, 3203–3209. https://doi.org/10.1002/alz.12985 (2023).
Röhr, S. et al. How can urban environments support dementia risk reduction? A qualitative study. Int. J. Geriatr. Psychiatry. 37 https://doi.org/10.1002/gps.5626 (2022).
Morrison, P. S. & Nissen, K. Moving in and out of areas of deprivation: evidence from the New Zealand census. New. Z. Popul. Rev. 36, 55 (2010).
Huque, M. H. et al. ANU-ADRI, CAIDE, and LIBRA Risk scores for estimating dementia risk. JAMA Netw. open. 6, e2331460–e2331460. https://doi.org/10.1001/jamanetworkopen.2023.31460 (2023). CogDrisk.
Acknowledgements
We thank past and present participants in the NZHWR study for their involvement. We thank Health and Ageing Research Team staff and research collaborators, as well as current and past members of the Health and Ageing Research Team Māori Advisory Group.
Funding
The New Zealand Health, Work and Retirement study in 2010 and 2012 has been supported by the New Zealand Foundation for Research, Science, and Technology (grant number MAUX0606). SR is a Global Atlantic Fellow for Equity in Brain Health and is supported by the Global Brain Health Institute (GBHI). This study is further supported by the Hans and Ilse Breuer Foundation. Open Access publishing was supported by the Health Research Council (HRC) of New Zealand (23/598).
Author information
Authors and Affiliations
Contributions
S .R. designed the study, conducted the statistical analysis, and wrote the paper. R .G. supported data interpretation and provided important intellectual content. F.A. acquired funding, designed the cohort study, oversaw data collection, supported data interpretation, and provided important intellectual content. All authors have reviewed and agreed with the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Röhr, S., Gibson, R.H. & Alpass, F.M. Higher socioeconomic deprivation in areas predicts cognitive decline in New Zealanders without cognitive impairment. Sci Rep 14, 28314 (2024). https://doi.org/10.1038/s41598-024-79583-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79583-w