Abstract
The ToxiLaus study aims at evaluating the impact of environmental toxic species on health and diseases’ onset and development. Specifically, the ubiquitous presence of trace elements (TEs) in the environment urges for a better characterization of their influence on human organism. In its primary phase, the ToxiLaus study focused on measuring the urinary concentrations of 23 TEs in the baseline samples from the CoLaus|PsyCoLaus population-based cohort, using inductively coupled plasma mass spectrometry (ICP-MS). Statistical analyses were carried out on 5866 participants, investigating links between TEs concentrations and smoking status, metabolic syndrome and body mass index (BMI). Smoking status was associated with Cd, Zn, Pb, Mo and Hg (respectively OR = 3.64, 1.42, 1.20, 0.69 and 0.58) while metabolic syndrome was associated with Zn and Cd (OR = 1.81 and 1.24 respectively). Concentrations of Zn, Hg, Co, Ni, Cu, Mo, As, Sn, Tl, Fe where significantly different (p < 0.0001) between BMI groups (Normal, Overweight, Obese). Finally, this study provides an overview of the distribution of trace elements in a cohort large sample of the general population, as well as their main associations with cardiovascular risk factors. Theses relations will be further analysed in subsequent phases of the study.
Similar content being viewed by others
Introduction
With the rise of public environmental awareness, trace elements and heavy metals pollution is becoming a major and global concern. Natural phenomena like volcanic eruptions and mineral weathering but mostly anthropogenic activities including mining, vehicle exhausts, use of agricultural fertilizer and pesticides induce important releases of trace elements in the environment which end up contaminating soils, underground and surface waters as well as the atmosphere1. Their resulting ubiquitous presence leads to contamination of the food chain and ultimately accumulating in foodstuffs1,2. With diet being one of the primary routes of human trace element exposition, along with polluted air and direct skin contact3, accumulation and translocation within the entire organism is almost inevitable. When reaching important concentrations, even essential trace elements such as zinc (Zn), iron (Fe) and manganese (Mn) become toxic to the body3,4. Other non-essential trace elements such as heavy metals like arsenic (As), cadmium (Cd), lead (Pb) and mercury (Hg) are known to have deleterious effects on the body even at a low concentration5. Therefore, it is of upmost importance to better understand and characterize the impact of trace elements on the population health.
Accordingly, the ToxiLaus study is based on the measurement of multiple toxic substances present in the environment to investigate the health effects of daily exposure. It is a sub-study of the CoLaus|PsyCoLaus study6, a population-based prospective cohort in Lausanne, Switzerland. The ToxiLaus study is conducted by the department of internal medicine of the University Hospital of Lausanne (CHUV) with the collaborations of the Faculty Unit of Toxicology (UFT) and the Unit of Forensic Chemistry and Toxicology (UTCF) of the University Center of Legal Medicine, Lausanne-Geneva (CURML).
Using state of the art analytical methods, ToxiLaus assessed a wide range of toxic substances present in the environment, including trace elements in plasma and urine samples. ToxiLaus leveraged the deep-phenotyping data collected from over 6,500 individuals as part of the CoLaus|PsyColaus study and sub-studies. In the future, this initiative will help enhance our comprehension of the connections between toxic agents and various risk factors such as genetic data or cardiometabolic traits and disease outcomes.
Methodology
The CoLaus|PsyCoLaus study
The CoLaus|PsyCoLaus study is a single-centre population-based prospective cohort in Lausanne, Switzerland6. It aims at longitudinally evaluating cardiovascular risk factors prevalence in the population of Lausanne as well as identifying new genetic traits associated with those risk factors and links with factors of psychiatric disorders. Between 2003 and 2006, 6’734 inhabitants, aged 35–75 years and mainly from Caucasian origin, were recruited from a random sample of the city population for an extensive phenotyping with clinical assessment.
Prior the interview, each participant had to fill a questionnaire about demographic data, socio-economic and marital status, and several lifestyle factors namely tobacco, alcohol and caffeine consumption, physical activity and mood. During the interview, a second questionnaire was conducted with a recruiter, focused on personal and family history of disease and cardiovascular risk factors. List of personal medication was also recorded. In women, further data regarding reproductive and obstetrical history, oral contraception and hormonal replacement therapy were also collected. Clinical data including weight, height, body mass index (BMI), blood pressure and heart rate were measured. Venous blood and urine were sampled. Periodic surveys of the cohort have been carried out over the 20 years of follow-up to date, and the fourth follow-up is currently underway. Detailed methodology for cohort construction, participants’ data collection and samples analysis is explained elsewhere6.
The baseline CoLaus Study was approved by the Institutional Ethics Committee of the University of Lausanne (reference 16/03), which afterwards became the Ethics Commission of Canton Vaud (www.cer-vd.ch). The approval was renewed for the successive follow-ups and the approval for the entire CoLaus|PsyCoLaus study was confirmed in 2021 (reference PB_2018-00038, 239/09). The study was performed in agreement with the Helsinki Declaration and its former amendments, and in accordance with the Swiss legislation. All participants provided their written informed consent before entering the study.
Primary phase of ToxiLaus
This study was initially focused on the analysis of 23 trace elements from the CoLaus baseline urine samples, namely: silver (Ag), aluminium (Al), arsenic (As), beryllium (Be), bismuth (Bi), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), mercury (Hg), iodine (I), lithium (Li), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), antimony (Sb), selenium (Se), tin (Sn), thallium (Tl), vanadium (V) and zinc (Zn). Inductively coupled plasma mass spectrometry (ICP-MS), a high-throughput approach allowing to simultaneously measure the different trace element concentrations in biological matrices, was used. Samples analysed were those collected during the CoLaus study recruitment; therefore, no additional sampling was performed as part of this study. The implication of the measured trace elements was assessed using the collected data from participants, in the cases of smoking habits, metabolic syndrome and BMI. Metabolic syndrome is a combination of conditions (like abdominal obesity, hypertension, dyslipidaemia, high fasting glucose level) that together raise the risk of serious health problems such as cardiovascular diseases and diabetes.
Dataset construction
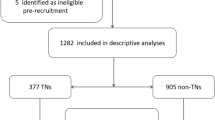
Clinical, biological, and historical data on participants were collected from the baseline CoLaus|PsyCoLaus population study database. In addition, participants’ urine samples were batch analysed for 23 different trace elements using ICP-MS. To address potential batch effects, the batch number was recorded for each observation and dataset adjustments using ComBat method were applied. To construct the dataset, participants’ information including age, gender, BMI and creatinine urinary level were retrieved from the CoLaus|PsyCoLaus database, compiling 6405 participants. Recorded medical conditions such as metabolic syndrome and diabetes status were also incorporated, in addition to the smoking status. A flowchart of participants’ selection is available in Fig. 1.
The concentrations of trace elements were measured in µg/L, while creatinine levels were measured in g/L. Consequently, the adjusted trace element concentrations, considering creatinine, were expressed as µg of trace elements per g of creatinine. BMI was defined as weight (kg) / height (m)2. For categorization, obesity was defined as BMI ≥ 30 kg/m2, overweight as BMI ≥ 25 kg/m2 and < 30 kg/m2 and normal with BMI < 25 kg/m2. The dataset contains both categorized and continuous version of the BMI. Smoking habits were classified into two categories: smoker and non-smoker. Similarly, diabetes and metabolic syndrome were binary categorized based on whether participants had the condition or not. Definition of metabolic syndrome was based on the Adult Treatment Panel III (ATP-III) criteria7.
ICP-MS analysis
An Agilent 7800 instrument (Agilent Technologies, USA) equipped with an integrated auto sampler and a quadrupole detector was used to determine trace element concentrations. Isotopes measured through a certified method were 7Li, 9Be, 27Al, 51V, 53Cr, 55Mn, 56Fe, 59Co, 60Ni, 63Cu, 66Zn, 75As, 78Se, 95Mo, 107Ag, 111Cd, 118Sn, 121Sb, 127I, 201Hg, 205Tl, 208Pb and 209Bi. Limits of detection and quantification, along with coefficients of variability for inter and intra assay are indicated in Supplementary Table S1. Sample preparation and ICP-MS acquisition were carried out by the same person.
Each batch (≈ 100 urine samples) was processed with a 6-point calibration curve (LabKings, The Netherlands) and certified reference materials (ClinChek® Urine Controls, RECIPE, Germany)8. Urine samples, kept at -80 °C prior to analysis, were prepared by dilution 1/10 (v/v) with a solution containing HNO3 (1%), N-butanol (0.5%), Triton X-100 (0.1%) as well as rhodium (Rh) and indium (In) (10ng/mL each). To prepare this solution, 18.2 mΩ ultrapure Milli-Q® water was used along with nitric acid Suprapur® 65% and Triton™ X-100 purchased from Merck (Germany), N-butanol GPR Rectapur® from VWR Chemicals (France) and internal standards (Rh and In) bought from LabKings (The Netherlands).
Statistical analysis
Participants that had missing data regarding diabetes status, metabolic syndrome and recorded BMI were excluded from the statistical analysis. For each participant, trace element concentrations were divided by the measured urinary creatinine level to avoid the effect of urine concentration.
Participants’ characteristics were expressed as median with 5th and 95th percentiles or as number of subjects / total subjects and (percentage) for continuous and categorical variables respectively. Comparisons were assessed using Wilcoxon rank test for continuous variables and Pearson’s chi-square test for categorical variables. Statistical significance was assessed for p < 0.05.
To further evaluate differences in trace element distribution between cohort subgroups like smoking status, metabolic syndrome and BMI categories, Kruskal-Wallis tests were conducted. In the case where more than two groups were compared, post hoc Dunn’s test was performed to assess each pair’s comparison.
Logistic regression models were performed to evaluate how well smoking status and metabolic syndrome can be predicted by the different trace element concentrations. To mitigate the impact of the differences in order of magnitude among trace element concentrations, standardization was applied by dividing each value by its respective standard deviation. Regression models for both smoking status and metabolic syndrome were constructed with the same predictors, namely age, gender, BMI (continuous) and all 23 trace element concentrations. Produced regression tables contain odds ratio (OR), 95% confidence intervals (CI), p-values and q-values. These q-values were calculated using Bonferroni’s method, to account for false discovery rates. All statistical analyses and table construction were performed using RStudio (version 2023.06.1 + 524).
Results
The resulting dataset for the primary phase of the ToxiLaus study included 5866 participants with complete information for each variable of interest. Links between trace element concentrations and smoking status, metabolic syndrome or BMI were investigated.
Smoking status
Significant statistical differences between smokers and non-smokers were observed in all trace element levels (Table 1). Concentrations of Li, Be, Al, Cr, Mn, Ni, Cu, As, Se, Mo, Ag, Sn, I, Ag, Tl and Bi were higher in non-smoking participants, while concentrations of V, Fe, Co, Zn, Cd, Sb and Pb were statistically higher in smoking participants. Results of Kruskal-Wallis test (Supplementary Table S2) highlighted a preponderant difference for Cd concentrations (p ≈ 10− 97), followed by Pb (p ≈ 10− 21), Sb (p ≈ 10− 19) and Hg (p ≈ 10− 16) concentrations. Results of the regression model (Table 2) showed that smoking status was associated with higher Cd, Zn and Pb concentrations (respectively OR = 3.64, OR = 1.42, OR = 1.20) and lower Mo and Hg concentrations (OR = 0.69 and OR = 0.58 respectively).
Metabolic syndrome
Significant statistical differences between participants with or without metabolic syndrome were observed for V, Fe, Co, Ni, Cu, Zn, As, Mo, Cd, Sb, I, Hg, Tl, and Pb levels (Table 3). Concentrations of Co, Ni, As, Mo, Hg and Tl were statistically higher in participants without metabolic syndrome, while concentrations of V, Fe, Cu, Zn, Cd, Sb, I, Pb were statistically higher in participants with metabolic syndrome. Kruskal Wallis test revealed a major difference between participants with and without metabolic syndrome regarding Zn concentrations (p ≈ 10− 57), followed by Hg (p ≈ 10− 21) and Cu (p ≈ 10− 16) concentrations (Supplementary Table S3). Metabolic syndrome was associated with higher Zn and Cd concentrations (respectively OR = 1.81 and OR = 1.24) according to results of the regression model (Table 4).
Body-mass index
Significant statistical differences between BMI groups (normal, overweight, obese) were observed for most TEs, above all when comparing normal subjects to overweight or obese ones (Table 5). Results of Kruskal Wallis and Dunn’s tests showed strong associations of BMI with Zn (p ≈ 10− 30) and Hg (p ≈ 10− 27) concentrations (Supplementary Tables S4, S5). In particular, urinary Zn levels were statistically higher in individuals who are obese compared to those who are overweight and normal weight while urinary Hg demonstrated a reversed trend, with higher concentrations in the normal group compared to the overweight and obese groups (Table 5).
Combination of factors
After categorizing the samples into four groups presenting 0 to 3 of the conditions previously described (smoking, metabolic syndrome, overweight/obesity), results of Kruskal-Wallis test highlighted differences of TE concentrations between groups, especially for Cu, Zn, Cd, Hg and Pb (Table 6). An increase in Cu, Zn, Cd and Pb excretion and a decrease in Hg excretion were reported with accumulation of conditions.
Discussion
Thanks to the large population-based CoLaus|PsyCoLaus cohort, the primary phase of the ToxiLaus study was conducted on 5866 individuals. Smoking was particularly associated with urinary Cd, Pb, Zn, Mo, Sb and Hg concentrations whereas Zn, Cd, Hg and Cu were associated with metabolic syndrome. Urinary Zn and Hg concentrations were associated with BMI, obese participants showing higher Zn and lower Hg levels than normal weight participants.
Smoking status and trace elements
Association of tobacco consumption with higher Cd blood and urine concentrations in smokers had been extensively described in the literature9. In addition, we reported an association of smoking with higher Zn and Pb urinary levels, which had also been mentioned in other studies10,11,12 and can be explained by their concomitant presence in cigarettes, both in filters and tobacco13. Higher excretion of Zn could also be explained by zinc transporters upregulation, induced by higher Cd levels, resulting in homeostasis dysregulation14.
Metabolic syndrome and trace elements
Metabolic syndrome appeared to be associated with higher Zn and Cd excretion. Indeed, zinc has a key role in insulin secretion as well as a coeffect with insulin on glucose metabolism regulation and lipogenesis. Observations in diabetic patients revealed lower Zn plasma and higher Zn urinary levels, suggesting a link between diabetes and disrupted Zn homeostasis15. Although the direction of influence is not completely understood, hyperglycaemia is likely to influence Zn urinary loss more than other primary lesions associated with diabetes15. Regarding cadmium, multiple studies linked higher Cd urine and blood levels in smokers to cardiovascular disease such as peripheral artery disease and hypertension9. Altogether, these results help linking clinical conditions related to metabolic syndrome with the observed trace element levels. In addition, a recent study carried out on 3748 Chinese adults and focused on the effects -both individually and as a mixture - of 21 trace element urinary concentrations on metabolic syndrome also concluded on the positive association between Zn urinary levels and metabolic syndrome16. Moreover, consistent with our results, they reported higher Cd and Cu concentrations in participants with metabolic syndrome compared to those without.
Obesity and trace elements
Alteration of trace element levels in obese subjects compared to normal weight ones had already been mentioned in other studies17,18. In particular, higher urinary Zn concentrations were reported in groups of obese children and adolescents compared to controls19,20. Zinc is known to be implicated in regulation of adipogenesis21, a perturbed mechanism in case of obesity. Therefore, its association with BMI in this study is not surprising. Mercury is also thought to be linked with obesity pathogenesis as it is an important endocrine disruptor, inducer of oxidative stress and endoplasmic reticulum stress22. In a study evaluating the risk of cumulative exposure to multiple correlated trace elements with obesity, Hg urinary levels were negatively linked with environmental risk score23. The latter was associated with higher waist circumference, higher prevalence of obesity and an increase of related comorbidities like elevated systolic blood pressure, hypertension and type 2 diabetes mellitus. Therefore, our results of statistically lower Hg urinary concentrations in overweight and obese people are consistent with these conclusions. In addition, in another study focusing on metabolic syndrome, obese individuals also showed lower mercury excretion rates than overweight and normal participants, without substantial differences between the overweight and normal groups24. Interestingly, more studies focused on the relationship with Hg levels in blood rather than those in urine. However, results were rather inconsistent showing positive25 and negative26 associations with obesity and BMI.
Trace elements multicollinearity
Within a broader scope, inconsistencies regarding associations between heavy metals and obesity or related outcomes were reported for different elements27. In addition, within constructed models, slight multicollinearity was observed for certain trace elements - namely Zn, Mo, Se, Hg, Tl, and Cd – with VIFs ranging up to 6.8 as well as correlation between them (Data not shown). However, since the VIF values are below 8, it is not impacting the reliability of the models. These observations might be explained, as heavy metals are known to interact with essentials trace elements through multiple pathways and cellular transporters. Rahman et al.28 reviewed the role of Se and Zn homeostasis in heavy metals detoxification. Described mechanisms included activation metallothionein synthesis, interactions with metal binding proteins and chelation preventing metal dispersion in the organism and facilitating excretion29. Solute Carrier Family transporters (SLC) are also known to play a role in trace element and heavy metal homeostasis. Zn efflux and influx transporter SLC30A and SLC39A have been shown to be upregulated in presence of Cd as well as having a particular affinity with Cd ions14. Hence, additional studies are required to gain a deeper understanding of their underlying relationships. Leveraging the extensive ToxiLaus data could considerably contribute to addressing this matter.
Limitations
ToxiLaus has some limitations. First, being a geographically limited, monocentric study focused on people aged 35 to 75 from Caucasian origins principally could limit the application of results to other populations. Moreover, due to missing values for certain variables of interest, 539 (8.4%) participants were excluded from the analysis. However, the large cohort number and its construction method allowed producing results similar with previous studies. In addition, when conducted with the entire dataset, descriptive analyses showed marginal differences in trace element distributions among assessed stratifications. Finally, it would also have been interesting to carry out speciation analysis of arsenic and mercury, to differentiate inorganic concentrations from organic compounds - mostly due to seafood consumption. Nevertheless, this type of analysis is considerably longer than common ICP-MS quantification because of previous chromatographic separation and, therefore, more complicated and more expensive to implement for a cohort of this proportion.
Strengths
To our knowledge, this is the largest and most comprehensive study on urinary trace elements worldwide, bigger than studies conducted in the USA30 or China31. This allows a higher statistical power, enabling the assessment of newer associations or the confirmation of previous ones.
With the extensive analysis of toxic species and trace elements measured, the ToxiLaus study enables to broaden the scope of prospective research already possible due to the range of data collected in CoLaus|PsyCoLaus and its sub-studies. The utilization of ICP-MS for analysis allows to accurately and sensitively measuring trace element concentrations not only in urine but also in other biological matrices such as blood. This would also allow us studying the collected blood samples available from CoLaus|PsyCoLaus in future phases of the study. Additionally, the cohort’s longitudinal follow-up enables tracking changes in trace element levels over time within the population and exploring potential associations with health outcomes. Overall, it will allow establishing new links with potential risk factors of cardiovascular diseases as well as better understanding the extent of the exposure impact to toxic species, such as trace elements.
Conclusion
This study highlighted associations between trace elements – especially Zn, Cd and Hg – and BMI, metabolic syndrome and smoking status. These results were consistent with existing literature. In addition, this study allowed to gain insight into the distribution of trace element concentrations in large sample of the general population. Deeper analyses, considering factors like medical treatments, diet and lifestyle will be explored in future phases of the study and may provide a better understanding of the data. Ultimately, it will pave the way for establishing potential prevention strategies and for better assessing diseases concerning toxic species exposition.
Data availability
The data of CoLaus|PsyCoLaus study used in this article cannot be fully shared as they contain potentially sensitive personal information on participants. According to the Ethics Committee for Research of the Canton of Vaud, sharing these data would be a violation of the Swiss legislation with respect to privacy protection. However, coded individual-level data that do not allow researchers to identify participants are available upon request to researchers who meet the criteria for data sharing of the CoLaus|PsyCoLaus Datacenter (CHUV, Lausanne, Switzerland). Any researcher affiliated to a public or private research institution who complies with the CoLaus|PsyCoLaus standards can submit a research application to research.colaus@chuv.ch or research.psycolaus@chuv.ch. Proposals will be evaluated by the Scientific Committee (SC) of the CoLaus|PsyCoLaus study. Detailed instructions for gaining access to the CoLaus|PsyCoLaus data used in this study are available at www.colaus-psycolaus.ch/professionals/how-to-collaborate/. If necessary, please contact Pr Pedro Marques-Vidal (Pedro-Manuel.Marques-Vidal@chuv.ch), data manager of the CoLaus study and supervisor of the ToxiLaus data analysis.
References
Gupta, N. et al. Trace elements in soil-vegetables interface: translocation, bioaccumulation, toxicity and amelioration - a review. Sci. Total Environ. 651, 2927–2942 (2019).
El Youssfi, M. et al. Trace elements in foodstuffs from the mediterranean basin—occurrence, risk assessment, regulations, and prevention strategies: a review. Biol. Trace Elem. Res. 201 (5), 2597–2626 (2022).
Masindi, V. & Muedi, K. L. Environmental contamination by heavy metals., in Heavy Metals,(H.E.-D.M. Saleh and R.F. Aglan, Editors). (2018).
Tam, M., Gómez, S., González-Gross, M. & Marcos, A. Possible roles of magnesium on the immune system. Eur. J. Clin. Nutr. 57 (10), 1193–1197 (2003).
Rehman, K., Fatima, F., Waheed, I. & Akash, M. S. H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 119 (1), 157–184 (2017).
Firmann, M. et al., The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc. Disord. 8, (1), 6 (2008).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112 (17), 2735–2752 (2005).
Perrais, M., Thomas, A., Augsburger, M. & Lenglet, S. Comparison of dried blood spots and Microtubes techniques for traces elements quantification by ICP-MS. J. Anal. Toxicol. 47(2), 175-81 (2022).
Richter, P., Faroon, O. & Pappas, R. S. Cadmium and cadmium/zinc ratios and tobacco-related morbidities. Int. J. Environ. Res. Public. Health 14, (10) 1154 (2017).
Hoet, P., Jacquerye, C., Deumer, G., Lison, D. & Haufroid, V. Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clin. Chem. Lab. Med. 51 (4), 839–849 (2013).
Afridi, H. I. et al. Evaluation of cadmium, lead, nickel and zinc status in biological samples of smokers and nonsmokers hypertensive patients. J. Hum. Hypertens. 24 (1), 34–43 (2009).
Watanabe, T., Nakatsuka, H., Kasahara, M. & Ikeda, M. Urinary lead levels among farmers in non-polluted areas in Japan. Toxicol. Lett. 37 (1), 69–78 (1987).
Benson, N. U., Anake, W. U., Adedapo, A. E., Fred-Ahmadu, O. H. & Ayejuyo, O. O. Toxic metals in cigarettes and human health risk assessment associated with inhalation exposure. Environ. Monit. Assess. 189 (12), 619 (2017).
Gasser, M. et al. Cadmium acute exposure induces metabolic and transcriptomic perturbations in human mature adipocytes. Toxicology 470, 153153 (2022).
Miao, X., Sun, W., Fu, Y., Miao, L. & Cai, L. Zinc homeostasis in the metabolic syndrome and diabetes. Front. Med. 7 (1), 31–52 (2013).
Liu, L. et al. Individual and joint effects of metal exposure on metabolic syndrome among Chinese adults. Chemosphere 287 (Pt 3), 132295 (2022).
Tinkov, A. A. et al. Selenium, Zinc, Chromium, and Vanadium levels in serum, hair, and urine samples of obese adults assessed by inductively coupled plasma Mass Spectrometry. Biol. Trace Elem. Res. 199 (2), 490–499 (2021).
Tinkov, A. A. et al., Trace Element and Mineral Levels in Serum, Hair, and Urine of Obese Women in Relation to Body Composition, Blood Pressure, Lipid Profile, and Insulin Resistance. Biomolecules 11 (5), 689 (2021).
Marreiro, D. N., Fisberg, M. & Cozzolino, S. M. F. Zinc nutritional status and its relationships with hyperinsulinemia in obese children and adolescents. Biol. Trace Elem. Res. 100 (2), 137–150 (2004).
Nasab, H. et al., Association of As, Pb, Cr, and Zn urinary heavy metals levels with predictive indicators of cardiovascular disease and obesity in children and adolescents. Chemosphere 294, 133664 (2022).
Skalny, A. V., Aschner, M. & Tinkov, A. A. Zinc, in Advances in Food and Nutrition Research. 251–310. (2021).
Tinkov, A. A. et al. Mercury and metabolic syndrome: a review of experimental and clinical observations. BioMetals 28 (2), 231–254 (2015).
Wang, X., Mukherjee, B. & Park, S. K. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ. Int. 121, 683–694 (2018).
Bulka, C. M., Persky, V. W., Daviglus, M. L., Durazo-Arvizu, R. A. & Argos, M. Multiple metal exposures and metabolic syndrome: a cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res. 168, 397–405 (2019).
Shin, Y. Y., Ryu, I. K., Park, M. J. & Kim, S. H. The association of total blood mercury levels and overweight among Korean adolescents: analysis of the Korean National Health and Nutrition Examination Survey (KNHANES) 2010–2013. Korean J. Pediatr. 61 (4), 121 (2018).
Rothenberg, S. E., Korrick, S. A. & Fayad, R. The influence of obesity on blood mercury levels for U.S. non-pregnant adults and children: NHANES 2007–2010. Environ. Res. 138, 173–180 (2015).
Moon, M. K. et al., Lead, mercury, and cadmium exposures are associated with obesity but not with diabetes mellitus: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Res. 204, 111888 (2022).
Rahman, M. M. et al. Selenium and zinc protections against metal-(loids)-induced toxicity and disease manifestations: a review. Ecotoxicol. Environ. Saf. 168, 146–163 (2019).
Spiller, H. A. Rethinking mercury: the role of selenium in the pathophysiology of mercury toxicity. Clin. Toxicol. (Phila). 56 (5), 313–326 (2017).
Centers for Disease Control and Prevention, Fourth National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services. (2021).
Yang, F. et al. Association of plasma and urine metals levels with kidney function: a population-based cross-sectional study in China. Chemosphere 226, 321–328 (2019).
Acknowledgements
The authors would like to thank all the participants of the CoLaus|PsyCoLaus cohort as well as the investigators who collaborated in recruiting participants and collecting their samples. Authors also addressed sincere thanks for grants attributions to the CoLaus|PsyCoLaus cohort, and especially to the Fondation pour la recherche sur le diabète (https://fondation-diabete.ch) for its research grant (Prix de la Fondation 2020) for the ToxiLaus study.The CoLaus|PsyCoLaus study was supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne, the Swiss National Science Foundation (grants 3200B0–105993, 3200B0-118308, 33CSCO-122661, 33CS30-139468, 33CS30-148401, 33CS30-177535, 31003 A-182420, 3247730-204523 and 320030-220190) and the Swiss Personalized Health Network (grant 2018DRI01). The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
IM and MP share first authorship. AT and JV share last authorship. Study conception and design: AT and JV. Data collection: MP. Analysis and interpretation of results: IM and PMV. Manuscript preparation: IM and MP. Manuscript review and editing: PMV, AT and JV.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mouti, I., Perrais, M., Marques-Vidal, P. et al. Investigation of the impact of exposure to trace elements on health and disease from the ToxiLaus study. Sci Rep 14, 29725 (2024). https://doi.org/10.1038/s41598-024-81544-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81544-2