Abstract
Worldwide, in Africa and Ethiopia prevalence of Benign Prostatic Hyperplasia among patients with lower urinary tract symptoms was 26.2%, 44.2%, and 33.4% respectively. However, there is limited evidence regarding Benign Prostatic Hyperplasia in southern Ethiopia and it was not well explored. Therefore, this study aimed to assess the magnitude and associated factors of Benign Prostatic Hyperplasia among adult male patients visiting Wolaita Sodo University Comprehensive Specialized Hospital, southern Ethiopia 2022. A hospital-based cross-sectional study was conducted from July to September 2022. A systematic random sample technique was employed to select 376 participants. Data were gathered using a structured questionnaire and entered, cleaned, coded, and analyzed using SPSS Version 25.0. To describe the study respondents, descriptive statistics were used. Bivariate and multivariate analyses were conducted and an adjusted odd ratio with 95% Confidence Interval (CI) was utilized to quantify the degree of association, and statistical significance was declared at p-value < 0.05. A total of 376 male patients admitted to the surgical department of the Urology ward were included in this study and the magnitude of Prostatic Hyperplasia was 21.3%; 95% CI: 17.3, 25.8. The vegetable consumption, fruit consumption, physical activity, sleeping time and sexual dysfunction [(AOR = 7.57, 95% CI: (2.78, 20.60)], [(AOR = 21.06,95% CI: (7.06,27.53)], [(AOR = 0.57,95% CI: (0.19, 0.67)], [(AOR = 3.23, 95% CI: (1.18,8.79)] and [(AOR = 17.05, 95% CI: (4.82,60.28)] were factors associated with Benign Prostatic Hyperplasia respectively. The benign prostatic hyperplasia is a prevalent disease among men in this study. Sexual dysfunction, consumption of vegetables, consumption of fruits, practice of physical activity and sleeping time were factors associated with BPH. Hence, screening programs for higher-risk people to ensure the early presentation of benign prostatic hyperplasia and practicing eating balanced diets are vital areas to reduce the prevalence.
Similar content being viewed by others
Introduction
The prostate is a chestnut-shaped auxiliary genital gland located in the pelvis, below the bladder, surrounding the prostatic portion of the urethra1.
Benign prostatic hyperplasia (BPH), affecting the center of the per urethral glandular tissue2, is a chronic and complex disease that is progressive in many men3. It has been connected to unchecked glandular epithelium, smooth muscle, and connective tissue growth in the prostatic transition zone. The development of BPH glandular nodules initially begins to form in the periurethral region followed by an increment in size1.
The burden of BPH has been increasing from time to time, predominantly in low-income and middle-income countries4. BPH affects about 210 million males worldwide with the estimated risk of developing it over the next 30 years is 45%5 and 379,000 men over the age of 55 annually which made it the main reason for major surgery according to studies done in the United States6.
In adult men in the Sub-Saharan African region, urinary bladder outlet obstruction due to BPH is a common urological condition2. The incidence of BPH is increasing from 0.3% of men aged 45–49 years to 0.38% of men aged between 75 and 79 years with the respective prevalence rates of 2.7% and 24%7. The study in Japan revealed the prevalence of BPH in participants aged 70 and above as 25.2%5. Another study indicated prevalence of LUTS for ages between 50 and 79 as 56%, 80 to 89 years old as 70%, and men aged 90 or beyond as 90%6. Similarly, the prevalence of BPH is stated as 62.3% in western Africa8, 42.0% in Egypt1and 20% in Gondar Ethiopia9.
A growing body of evidence suggests that modifiable factors such as increasing prostate volume, obesity, diet, dyslipidemia, hormonal imbalance, hypertension, metabolic syndrome, alcohol, smoking, and aging contribute to the development of BPH6.
Evidence-based data is important to determine the magnitude of BPH and its associated factors. Finding detailed and up-to-date estimates of the illness burden of BPH in a particular study region is beneficial. Therefore, the current study aimed to assess the prevalence of BPH and associated factors among men visiting Wolaita Sodo University Comprehensive Specialized Hospital (WSUCSH).
Method and materials
Study setting
The study was conducted at WSUCSH in Wolaita Sodo town, located in Wolaita Zone, 154 km from Hawassa, the capital of Southern Ethiopia, and 329 km away from Addis Ababa, the capital of Ethiopia. The hospital provides general outpatient and inpatient services, including medical, surgical, pediatric, psychiatric, ophthalmic, emergency, gynecology, and obstetrics care. It serves approximately 3.5–5 million patients annually in Wolaita and the neighboring zones like Dawuro, Gamo, Gofa, and Kambata-Tambaro.
Study design and period
An institutional based cross-section design study was conducted from July 1 through August 31, 2022.
Source and study population
All male patients presented at Wolaita Sodo University Compressive Specialized Hospital were the source population. All male patients admitted to the urology ward with LUTS who fulfilled the inclusion criteria were the study population.
Eligibility criteria
All male patients aged 30 and above admitted to the urology department with LUTS were included in this study and Patients with confirmed cases of prostatic cancer, unconscious and unable to speak and those patients with confirmed mental disorders were excluded.
Sample size determination and sampling procedure
The sample size was calculated using a single population proportion formula considering the following assumptions: 95% confidence level with a margin of error (5%), using the anticipated population proportion of prevalence of BPH from the previous study conducted in Mekelle Hospital, Northern Ethiopia 33.4%10. The final sample size calculated after considering a 10% non-response rate was 376.
Hence, n = Z (1- α/2) 2 p (1-p).
d2.
n = (1.96)2 (0.334) (0.66) = (3.8416) (0.2224) = 342.
(0.05)2 0.0025.
Where n = required sample size.
Z = critical value for normal distribution at 95% confidence level which equals to 1.96 (z value at α = 0.05, two tailed).
Sample size = 342.
Expecting a 10% non-response rate, the final sample size calculated was 376.
Sampling procedure
Systematic random sampling was employed to select study participants. The total number of men patients who visited urology units (N) was estimated by considering the past 5 months’ case flow and reviewing the records which yielded a total of 1002 clients. Then the number of sampling intervals was determined by dividing the number of clients (N = 1002) by the estimated sample size (n = 376), and hence the sampling interval (k) was 3. The first sampling unit was selected by the lottery method, and then every 3rd unit was taken. To control the second visit of participants, the charts of those with appointments were kept in urology OPD separately.
Study variables
Dependent variables
Presence or absence of BPH.
Independent variables
Socio-demographic, Family history, and Metabolic syndrome factors and Behavioral and psychological related factors were used as predictors.
Operational definitions
BPH
diagnosed when a digital rectal examination revealed symmetrically enlarged, smooth, rubbery, movable rectal mucosa and a normal median sulcus detected8.
LUTS
refers to the typical aging male voiding and/or storage issues. Daytime frequency and nocturia are two examples of storage symptoms that occur during the storage phase of the bladder, while voiding symptoms occur during the voiding phase11.
International Prostate Symptom Score (I-PSS)
Questionnaires were utilized to rate the intensity of three storage symptoms (frequency, nocturia, and urgency) and four voiding symptoms (feeling of incomplete emptying, intermittency, straining, and a weak stream)12. Men with an IPSS score of 0 were classified as asymptomatic, those with values 1–7 as mildly symptomatic, and those with scores 8–35 as severely symptomatic8.
Bladder Outlet Obstruction (BOO)
is the generic term for all forms of obstruction to the bladder outlet (e.g., urethral stricture), including BPO12.
Hypertension
Men were regarded as having a history of hypertension if they were actively using medication to treat their hypertension or if their doctor had diagnosed them with the condition13.
Diabetes mellitus
Men are considered to have a history of diabetes mellitus if they are currently taking medicine to treat it or have been told by their doctor that they have diabetes, high blood sugar, or both13.
Body mass index (BMI)
defined as the body weight in kilograms divided by the height in square meters and categorized as normal weight (18.5–22.9 kg/m2), overweight (23–24.9 kg/m2), and obesity (> 25 kg/m2)9.
Smoking
was assessed during the interview, and men were classified according to their smoking habits as current, former, or never smokers14. A never smoker is someone who has never smoked in his life; a former smoker is someone who has been smoke-free for the past one month and a person who smokes every day or some days is classified as a current smoker15.
Alcohol intake
an individual who has never drunk alcohol in his life was considered as a never drinker, consumed two or more glasses weekly was considered as a current drinker, and has been alcohol-free for the past one month was considered as former drinker12.
Physical activity
Information about home, work, and leisure activities were combined to create the Psoriatic Arthritic Screening Evaluation (PASE) scores. The aggregate of all activities was used to calculate the total PASE score by multiplying either the amount of time (in hours per week) or involvement (i.e., yes or no) in each activity by experimentally obtained item weights. The PASE score might range from 0 to 400 or higher overall. The PASE score was divided into tertiles 0 to 40 (sedentary), 41 to 90 (mild physical activity), and more than 90 (moderate to intense activity)16.
Sexual dysfunction
The Arizona Sexual Experience Questionnaire (ASEX) is a 5-item rating scale that analyzes the four stages of sexual functioning, including sexual drive, arousal with sexual stimuli, penile erection, the ability to reach orgasm, and orgasm satisfaction. The total score goes from 5 to 30, with a score of 19 or higher suggesting sexual dysfunction. Scores of 4 or 5 were regarded as having no sexual dysfunction in that category for each domain17.
Sleeping time
defined as the total amount of time a person sleeps in 24 h. A person who sleeps 0–6 h per day is considered to have inadequate sleeping time, and 7 or more hours per day is considered to have adequate sleeping time18.
Fruit and vegetable consumption
the total amount of fruit and vegetables an individual consumes. Consumption of four servings per day is considered adequate, while consumption of less than four servings per day is considered inadequate19.
Co-morbidity
a medical term that describes the long-term or chronic existence of more than one disease or condition within the body at the same time20.
Data collection procedure and collection instrument
Data was collected using ODK collect survey tool through interviewer-administered questionnaire which was adapted from previous literatures and modified according to the current study objectives. The pre-tested questionnaires were translated into Amharic and back to English to ensure consistency in meaning. The questionnaire is divided into four sections. Part I: socio-demographic factors; Part II: family history and metabolic syndrome factors; Part III: behavioral and psychological-related factors; and Part IV: a digital rectal examination checklist.
Two data collectors and one supervisor were recruited for data collection and supervision respectively.
Data processing and analysis
The collected data was transferred from Open Data Kit (ODK) to Microsoft Excel 2010 and then completeness of data and cleaning were done and any error identified was corrected. Then the data was exported to SPSS version 25 for further analysis. The binary and multivariable logistic regressions were used. All variables with a p-value < 0.25 were taken into the multivariable model. Finally, the results of multivariable logistic regression analysis were presented to identify the independent effects of predictors on outcome variables. The factors with P < 0.05 were considered as having significant association with outcome variable and adjusted odd ratios with 95% Confidence Interval (CI) were used to measure a degree of association. A Hosmer - Lemeshow test was done to check for model fitness.
Data quality assurance
To maintain the quality of data, two data collectors and one supervisor were trained for three days about the purpose of the study, data collection tools, and ethical procedures. A pretest was conducted on 5% of the study participants out of the study area with similar population to assess the validity of the instrument. Each study subject was identified only by code and the collected data was kept secure by the principal investigator. Following data collection, data was stored in a secure location to maintain confidentiality. To maintain the quality of data senior medical doctors were participated to diagnosis BPH by digital rectal examination. Moreover, all the study participants were informed orally about the purpose and benefit of the study, along with their right to refuse or decline participation in the study at any time.
Results
Socio demographic characteristics of the participants
A total of 376 patients were interviewed in this study, with a response rate of 100%. One hundred twenty-six (33.5%) were aged between 50 and 59 years, and the mean age (SD) of the respondents was 55.06 ± 9.95 years. Protestants were predominant (51.3%) in religion and the majority of the respondents, 253 (67.3%) were Wolaita in ethnicity. Three hundred two (80.3%) of the respondents were married and in terms of educational attainment, 109 (29.0%) had completed college or higher, and 116 (30.9%) were government employees (Table 1).
Family history and metabolic syndrome-related factors
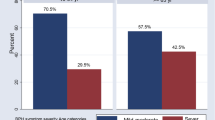
Among all study participants, 156 (41.5%) had no family history of BPH, and 108(31.5%) did not know about their family history of BPH. One hundred sixty-eight, (44.6%) of participants had normal Body Mass Index whereas overweight and obese participants were 115(30.7%) and 93 (24.7%) respectively. Of 119 (31.6%) of the respondents diagnosed with a chronic illness, 50 (42.8%) were hypertensive patients (Fig. 1).
Behavioral and psychological-related factors
Nearly half of the participants (49.2%) were never drinkers, and 255 (67.82%) were never smokers. The majority of the respondents, 319 (84.8%) eat fruits with 242 (68.75%) having adequate fruit consumption. Regarding sleeping time, physical activity, and sexual dysfunction, two hundred forty-eight (65.95%) of the respondents had adequate sleeping time, 257 (68.4%) did moderate-to-intense physical activity and about 313 (83.2%) of the respondents had no sexual dysfunction (Table 2).
The magnitude of benign prostatic hyperplasia
The magnitude of Benign Prostatic Hyperplasia among 376 patients was found to be 80 (21.3%; 95% CI: 17.3, 25.8) with 46 (57.5%) had severe BPH (Fig. 2).
Factors associated with benign prostatic hyperplasia
In bivariate logistic regression, fruit consumption, vegetable consumption, physical activity, sleeping time, depression, BMI of respondents, sexual dysfunction, and family history of BPH were with p < 0.25. However, in multivariable logistic regression, vegetable consumption, fruit consumption, physical activity, sleeping time, and sexual dysfunction were significantly associated with BPH with p < 0.05.
Participants who had inadequate fruit consumption were twenty-one times more likely to develop BPH than participants who had adequate fruit consumption [(AOR = 21.06, 95% CI: (7.06, 27.53)]. Likewise, the odds of BPH among participants with inadequate vegetable consumption were seven times more likely to develop BPH than participants who had adequate vegetable consumption [(AOR = 7.57, 95% CI: (2.78, 20.60)]. Those participants who did moderate to intense [(AOR = 0.57, 95% CI: (0.19, 0.67)] and light physical activity [(AOR = 0.05, 95% CI: (0.01, 0.56)] were 43% and 95% less likely to develop BPH than who did sedentary physical activity respectively.
The odds of developing BPH increased by three times for the participants who had inadequate sleeping time than those who had adequate sleeping time [(AOR = 3.23, 95% CI: (1.18, 8.79)]. Similarly, the odds of developing BPH increased by seventeen times for the participants who had sexual dysfunction than those who had not [(AOR = 17.05, 95% CI: (4.82, 60.28)] (Table 3).
Discussion
The magnitude of benign prostatic hyperplasia in this study was found to be 21.3% (95% CI: 17.3, 25.8). This study finding is higher than previous studies done in China, 10.66%)21, and Turkey, 13.84%6.This might be due to the difference in living standards, socioeconomic status, and quality of health service provision. On the contrary, it is lower than the study done in Egypt, where the overall prevalence of benign prostatic hyperplasia using the IPSS questionnaire is 42.0%1, 62.3%8in West Africa, and 33.4%10 in Ethiopia. This might be due to the difference in sample size and the difference in the tools used to diagnose BPH.
Patients with inadequate sleep time were more likely to develop BPH compared to those with adequate sleep time. This is consistent with studies conducted in China22,23. This might be explained in terms of the Production of melatonin (sleep regulator hormone) which generally decreases with age. The older individuals show an increased lag from sunset to the onset of melatonin pulse and the middle of the sleep period 24 and this could be the reason why BPH presence was high among respondents with inadequate sleeping time.
Regarding sexual function, in the current study, prostate enlargement cases with sexual dysfunction were more frequent among BPH patients than those with normal sexual function. This evidence was supported with previous study findings in Ethiopia, Germany, and the United States of America on the relationship between sexual dysfunction and prostate enlargements25,26,27. There is a high relative risk of sexual dysfunction in individuals with BPH28. This might be due to the similarity of most studied participants were elderly patients and thus the amount of the hormone testosterone which controls sexual activity declines with age and may have an impact on prostatic growth27. Emerging research has suggested lack of testosterone may be a risk factor for BPH26.
The findings of the present study revealed that the practice of moderate to intense and light physical activities were factors that significantly decreased the risk of BPH. This result is similar to other cross-sectional studies done in Europe30,31,32,33. Patients who do moderate to intense and light physical activities were less likely to develop BPH as compared with those who did sedentary physical activity which is supported by the previous study conducted in Saudi Arabia.1 Besides, prostate enlargement is related to serum testosterone levels, which believed to fall with less physical activity1. Increasing physical activity to moderate levels reduces sympathetic nervous system activity at rest which generally controls prostate growth34. Thus, physical activity has a beneficial effect on BPH, which is in part mediated by diminished systemic sympathetic nervous system activity.
According to prior studies in Europe25,28and Mekelle, North Ethiopia10, inadequate intake of fruits and vegetables increased the risks of BPH, which is in line with the current study findings. This could be justified as fruits and vegetables contain significant amounts of antioxidants, polyphenols, vitamins, minerals, and fiber which might have a significant impact on the inflammatory pathways involved in the pathophysiology of benign prostatic hyperplasia and reduce prostate hyperplasia28.
Limitations of the study
Since the study was conducted in one hospital, the findings of the current study may not be generalized to the larger population and the study did not use ultrasound (imaging) which made it difficult to know the exact volume of the prostate and PSA test was not conducted to assess the possible risk of prostatic cancer. Since it is a cross sectional study design causality cannot be determined between outcome and independent variables.
Conclusion and recommendation
In this study, benign prostatic hyperplasia was found to be a highly prevalent disease among men. Sexual dysfunction, consumption of vegetables, consumption of fruits, practice of physical activity, and sleeping time were factors significantly associated with BPH.
Healthcare providers should do screening programs for all people in a higher-risk group to ensure the early presentation of benign prostatic hyperplasia and It is recommended that scholars to conduct further studies about benign prostatic hyperplasia. For the community, it is recommended to serve a high amount of fresh fruit and vegetables and do physical activity regularly. To determine causality between dependent and independent variables cohort or follow up study design is highly recommended.
Data availability
Used and analyzed datasets are available from the corresponding author upon reasonable request.
References
Yasein, Y. Prevalence And Determinants of Benign Prostatic Hyperplasia Among Males Attending Primary Health Care Clinics At KFMMC, Dhahran, Eastern Region, KSA. IOSR J. Dent. Med. Sci. 2017;Volume 16:PP 63–72 .
Ugwumba, F. O., Ozoemena, O. F., Okoh, A. D., Echetabu, K. N. & Mbadiwe, O. M. Transvesical prostatectomy in the management of benign prostatic hyperplasia in a developing country. Niger J. Clin. Pract. 17 (6), 797–801 (2014).
Emberton, M. et al. Benign prostatic hyperplasia as a progressive disease: a guide to the risk factors and options for medical management. Int. J. Clin. Pract. 62 (7), 1076–1086 (2008).
Anegagregn, A. et al. Magnitude and associated risk factors of benign prostatic hyperplasia among patients admitted to the surgical department of urology ward in Hawassa University Comprehensive Specialized Hospital, Sidama region, Ethiopia. Ethiop. J. Med. Health Sci. 2 (2), 127–135 (2022).
Roberts, R. O. et al. Association between cigarette smoking and prostatism in a Japanese community. Prostate 30 (3), 154–159 (1997).
Calogero, A. E., Burgio, G., Condorelli, R. A., Cannarella, R. & La Vignera, S. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male Off J. Int. Soc. Study Aging Male. 22 (1), 12–19 (2019).
Mohamed, E., lsayed, A., Guirguis Ragheb, S. & Abo Bakr, M. Quality of Life among Clients with Benign Prostatic Hyperplasia. Egypt. J. Health Care. 9 (4), 206–220 (2018).
Chokkalingam, A. P. et al. Prevalence of BPH and lower urinary tract symptoms in West Africans. Prostate Cancer Prostatic Dis. 15 (2), 170–176 (2012).
Messele Getahun, G. & Getachew Kebede, A. Comparison of prostatic volume measured with abdominal ultrasound and prostatic weight determined after open enucleation performed in Gondar University Hospital, Ethiopia. Afr. J. Urol. 14 (2), 86–89 (2008).
View of the Magnitude. and Treatment modalities of urologic diseases in patients admitted in mekelle hospital, Ethiopia. | Ethiopian Medical Journal [Internet]. [cited 2024 Jan 1]. https://emjema.org/index.php/EMJ/article/view/387/pdf_130
Maharajh, S., Abdel Goad, E., Ramklass, S. & Conradie, M. Lower urinary tract symptoms (LUTS) in males: A review of pathophysiology. South. Afr. Fam Pract. 57, 1–5 (2015).
Klotz, L. et al. Optimization of prostate biopsy - Micro-Ultrasound versus MRI (OPTIMUM): A 3-arm randomized controlled trial evaluating the role of 29 MHz micro-ultrasound in guiding prostate biopsy in men with clinical suspicion of prostate cancer. Contemp Clin Trials [Internet]. Jan [cited 2024 Jan 1];112(106618). (2022). http://www.scopus.com/inward/record.url?scp=85121255955&partnerID=8YFLogxK
07_IPFD_V3N2_PP_49_51. pdf [Internet]. [cited 2024 Jan 1]. http://www.tcs.org.tw/issue/Folder/3_2/07_IPFD_V3N2_PP_49_51.pdf
Kang, D. et al. Risk behaviours and benign prostatic hyperplasia. BJU Int. 93 (9), 1241–1245 (2004).
Parsons, J. K. Benign Prostatic Hyperplasia and Male Lower Urinary Tract Symptoms: Epidemiology and Risk Factors. Curr. Bladder Dysfunct. Rep. 5 (4), 212–218 (2010).
Husni, M. E., Meyer, K. H., Cohen, D. S., Mody, E. & Qureshi, A. A. The PASE questionnaire: pilot-testing a psoriatic arthritis screening and evaluation tool. J. Am. Acad. Dermatol. 57 (4), 581–587 (2007).
Venkatesh, K., Mattoo, S. K. & Grover, S. Sexual dysfunction in men seeking treatment for opioid dependence: a study from India. J. Sex. Med. 11 (8), 2055–2064 (2014).
Liu, Y. et al. Prevalence of Healthy Sleep Duration among Adults–United States, 2014. MMWR Morb Mortal. Wkly. Rep. 65 (6), 137–141 (2016).
Peltzer, K. & Phaswana-Mafuya, N. Fruit and vegetable intake and associated factors in older adults in South Africa. Glob Health Action. 5 https://doi.org/10.3402/gha.v5i0.18668 (2012).
de Groot, V., Beckerman, H., Lankhorst, G. J. & Bouter, L. M. How to measure comorbidity. a critical review of available methods. J. Clin. Epidemiol. 56 (3), 221–229 (2003).
Zhang, W. et al. Prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH) in China: results from the China Health and Retirement Longitudinal Study. BMJ Open. 9 (6), e022792 (2019).
Ekman, P. BPH epidemiology and risk factors. Prostate 15 (S2), 23–31 (1989).
Xiong, Y. et al. Reduced sleep duration increases the risk of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in middle-aged and elderly males: a national cross-sectional study. Aging Male Off J. Int. Soc. Study Aging Male. 25 (1), 159–166 (2022).
Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 175 (16), 3190–3199 (2018).
Parsons, J. K. et al. Metabolic factors associated with benign prostatic hyperplasia. J. Clin. Endocrinol. Metab. 91 (7), 2562–2568 (2006).
Braun, M. H. et al. Lower Urinary Tract Symptoms and Erectile Dysfunction: Co-Morbidity or Typical Aging Male Symptoms? Results of the Cologne Male Survey. Eur. Urol. 44 (5), 588–594 (2003).
Rosen, R. C. et al. Association of sexual dysfunction with lower urinary tract symptoms of BPH and BPH medical therapies: results from the BPH Registry. Urology 73 (3), 562–566 (2009).
Rosen, R. C. et al. Lower urinary tract symptoms and sexual health: the role of gender, lifestyle and medical comorbidities. BJU Int. 103 (Suppl 3), 42–47 (2009).
Roberts, R. O. et al. Serum sex hormones and measures of benign prostatic hyperplasia. Prostate 61 (2), 124–131 (2004).
Blanker, M. H. et al. Correlates for erectile and ejaculatory dysfunction in older Dutch men: a community-based study. J. Am. Geriatr. Soc. 49 (4), 436–442 (2001).
Camacho, M. E. & Reyes-Ortiz, C. A. Sexual dysfunction in the elderly: age or disease? Int. J. Impot. Res. 17 (Suppl 1), S52–56 (2005).
Parsons, J. K. & Kashefi, C. Physical activity, benign prostatic hyperplasia, and lower urinary tract symptoms. Eur. Urol. 53 (6), 1228–1235 (2008).
Sea, J., Poon, K. S. & McVary, K. T. Review of exercise and the risk of benign prostatic hyperplasia. Phys. Sportsmed. 37 (4), 75–83 (2009).
Platz, E. A. et al. Physical activity and benign prostatic hyperplasia. Arch. Intern. Med. 158 (21), 2349–2356 (1998).
Acknowledgements
We would like to express our gratitude to Arba Minch University, College of Medicine and Health Science for supporting this study. The authors like to extend their deepest gratitude to all respondents, data collectors, and supervisors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
TT, TG, and FG made substantial contributions to the conception and design; execution and acquisition of data; analysis and interpretation of data. TE and SM took part in drafting the manuscript and revising it critically for important intellectual content. All agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethical approval and informed consent
>Ethical clearance was obtained from the AMU College of Medicine and Health Sciences Ethical Review Board. An official letter was obtained from the IRB. Accordingly, a letter of cooperation was obtained from the concerned administrative body to the corresponding urological unit. Written informed consent was obtained from all participants. To ensure participants’ anonymity and privacy during interviews, private areas were used and kept confidential. Each study participant was identified only by code. Moreover, all the study participants were informed orally about the purpose and benefit of the study, along with their right to refuse or decline participation in the study at any time. This study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tiruneh, T., Getachew, T., Getahun, F. et al. Magnitude and factors associated with benign prostatic hyperplasia among adult male patients visiting Wolaita Sodo University comprehensive specialized hospital southern Ethiopia. Sci Rep 14, 31556 (2024). https://doi.org/10.1038/s41598-024-83483-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83483-4
Keywords
This article is cited by
-
Global, regional, and national lifetime risks of developing benign prostatic hyperplasia in men aged over 40: a population-based cross-sectional study from 1990 to 2021
Tropical Medicine and Health (2025)
-
Predictors and predictive performance of immune–inflammation indices for symptom severity in benign prostatic hyperplasia
Scientific Reports (2025)