Abstract
Chronic obstructive pulmonary disease (COPD) is a common condition that complicates major surgeries like coronary artery bypass grafting (CABG). This study aims to evaluate the impact of COPD on the outcome of CABG. A registry-based retrospective cohort study included individuals who received CABG between 2009 and 2016. Data were collected on patient demographics, intraoperative factors, and postoperative outcomes. Cox proportional hazard with inverse probability weighting (IPW) and propensity score matching (PSM) were conducted to assess the adjusted effect of COPD on 30-day and long-term mortality and major adverse cardiac and cerebrovascular events (MACCE). Moreover, the impact of COPD in smokers and non-smokers on short/long-term outcomes was assessed. Sensitivity analysis was conducted using multiple imputations. In the present investigation, 17,315 patients including 629 with COPD (mean age 69 ± 9.74), were followed up for a median duration of 8.25 years. Although COPD did not increase 30-day mortality and MACCE risk, the models showed that patients with COPD are at a significantly higher risk of long-term mortality and MACCE after CABG (IPW: HR for mortality: 1.53, 95% CI: 1.31–1.79; HR for MACCE: 1.29, 95% CI: 1.12–1.47). After multiple imputations, the mortality and MACCE hazard ratio in IPW analysis remained statistically significant. COPD significantly increases long-term mortality and MACCE following CABG, independent of smoking status.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD), depicted by airflow obstruction, emphysema, and chronic bronchitis, is the third leading cause of mortality globally with the majority of deaths occurring in low- and middle-income countries1. COPD is a significant risk factor for worse outcomes after cardiac revascularization surgery i.e. coronary artery bypass graft (CABG) and percutaneous coronary intervention (PCI), including higher incidence of postoperative pulmonary complications and reduced long-term survival2. However, according to a meta-analysis, COPD did not increase the risk of early postoperative mortality3.
There is a discrepancy in the literature about the exact role of COPD regarding the short-term outcome of patients undergoing CABG. Some studies suggest that COPD is not a risk factor for short-term mortality3,4,5,6 while others found a significant association between COPD and higher short-term mortality risk7,8. Moreover, It is well understood that COPD is associated with a higher risk of respiratory failure, renal failure, pneumonia, stroke, and wound infection after CABG3. Also, studies were consistent in that COPD predicted higher mortality in the long term2,9.
Despite these findings, the applicability of existing evidence to developing countries like Iran is limited. Iran’s unique healthcare system, demographic characteristics, and prevalence of risk factors, such as smoking and air pollution, may contribute to distinct patterns of COPD and its associated complications and may alter the interplay between COPD and CABG outcomes. For instance, Iran’s high rates of air pollution—a leading risk factor for COPD10—combined with limited access to specialized postoperative care11, might exacerbate the disease’s impact on surgical outcomes. Moreover, differences in surgical techniques, postoperative care, and patient comorbidities between developed and developing countries can influence patient outcomes. In this regard, healthcare disparities in rural versus urban areas and resource limitations in managing chronic conditions pose additional challenges. These factors underscore the necessity of region-specific data to inform clinical practice and healthcare policies in Iran.
This study addresses a significant gap in the literature by focusing on the Iranian population, a context often underrepresented in global research on COPD and CABG outcomes. By leveraging advanced statistical methods such as inverse probability weighting and propensity score matching, we aim to provide robust and reliable estimates of the impact of COPD on surgical outcomes. These findings will enhance our understanding of patients’ unique challenges in developing countries and inform evidence-based strategies for improving care. Ultimately, this research contributes to advancing clinical decision-making and optimizing outcomes for COPD patients undergoing CABG in resource-constrained settings.
Methods
Study design and population
We conducted a retrospective cohort study using the cardiac surgery registry at Tehran Heart Center. The study included consecutive patients who underwent isolated CABG between March 2009 and March 2016 and were followed up long-term. The ethics committee of Tehran University of Medical Sciences approved the study (IR.TUMS.THC.REC.1403.016).
Variables and definitions
Baseline characteristics were gathered as routine in the cardiac surgery data registry, including demographics and preprocedural variables. Patient characteristics were age, sex, body mass index (BMI), diabetes (DM), hypertension (HTN), dyslipidemia, cigarette smoking, opium use, previous myocardial infarction (MI), peripheral vascular disease (PVD), glomerular filtration rate (GFR), history of cerebrovascular accident (CVA) or transient ischemic attack (TIA), and ejection fraction (EF). Medication use included diuretics, calcium channel blockers, nitrates, and angiotensin-converting enzyme inhibitors. Off-pump CABG and intra or post-operative blood transfusion were also recorded.
Iran has the world’s highest rate of opium abuse12 and is considered an alarming public and cultural health concern, especially among older generations13. Additionally, opium use has been associated with higher mortality after CABG14 and PCI15. Therefore, Its inclusion as a variable in this study is particularly relevant due to its potential impact on CABG outcomes of COPD patients.
COPD, as defined by the American Thoracic Society16, is characterized by persistent respiratory symptoms such as cough, dyspnea, expectoration, or exacerbations. The condition is caused by airway (bronchitis, bronchiolitis) or alveoli (emphysema) abnormalities often resulting in progressive airflow obstruction. COPD was diagnosed after consulting a pulmonologist based on clinical presentations and spirometry indicating FEV1/FVC < 0.7 after bronchodilator.
The main outcomes measured in the study were all-cause mortality and major adverse cardiac and cerebrovascular events (MACCE). MACCE was a composite of all-cause death, acute coronary syndrome, stroke or transient ischemic attack (TIA), and revascularization. The occurrences of MACCE components were recorded based on whichever happened first.
Statistical analysis
The continuous variables age and BMI were presented as mean with standard deviation (SD); and were compared between the COPD and Non-COPD groups applying independent samples t-test. categorical variables were expressed as frequency and percentages and were compared between the COPD groups using the chi-squared test. The patients with missing data at least in one of the variables including completely lost to follow-up were excluded from the final analysis.
To investigate the specific effect of COPD on all-cause mortality and MACCE, stabilized inverse probability weighting (IPW) based on the propensity scores, and 1 to 4 propensity score matching (PSM) with nearest neighbor matching method techniques were applied to balance the distribution of potential confounders between the COPD and Non-COPD groups. The variables age, gender, BMI, hypertension, diabetes, dyslipidemia, cigarette smoking, opium consumption, history of PVD, MI, renal failure, CVA, EF, off-pump surgery, intra-operative blood transfusion, using angiotensin-converting enzyme inhibitors, calcium channel blockers, diuretics, and nitrates were considered in the IPW and PSM methods.
We used both PSM and IPW methods in our analysis to ensure the robustness and credibility of our findings. PSM creates a balanced matched sample for direct comparison, while IPW utilizes the entire dataset to construct a pseudo-population that adjusts for confounding. Reporting results from both methods demonstrates that the observed effects are consistent across different statistical approaches, adding confidence in the validity of the results.
A Cox proportional hazards (PH) model was utilized to assess COPD’s unadjusted and adjusted effects on outcomes. The effects were reported via hazard ratio (HR) with 95% confidence intervals (CI). The IPW weights were considered in the Cox PH model to obtain the adjusted effect of COPD on outcomes. Also, the matching clusters were indicated in the Cox PH model to achieve a robust standard error (SE) of the effect.
In this study, covariate balance before and after matching or weighting was primarily assessed using standardized mean differences (SMD), as this metric provides a more meaningful measure of the magnitude of differences between groups, independent of sample size. SMD values less than 0.1 were considered indicative of adequate balance. SMD plots were included in the “Results” section to visually illustrate the balance achieved, providing a clear comparison of covariate distributions before and after adjustment.
To ensure the separation of the effect of cigarette smoking and COPD, the impact of COPD on outcomes was investigated in two groups of cigarette smokers and non-smokers.
For the sensitivity analysis, multiple imputations using the chained rule method were conducted on the applied variables in the complete cases analysis. The effects of COPD on outcomes were re-estimated after missing imputation to confirm the reported effects. Statistical analyses were conducted using the R statistical language (version 4.3.2; R Core Team, 2023), using the packages survminer (version 0.4.9)17, survival (version 3.5.7)18,19, ggplot2 (version 3.4.4)20 and SurvMetrics (version 0.5.0)21, cobalt (version 4.5.5)22, MatchIt (version 4.5.5)23, and Mice (version 3.16.0)24.
Results
Of 17,315 included patients undergoing CABG, 629 (3.6%) patients had COPD. The median follow-up was 8.25 years (95% CI: 8.17–8.33). The flow diagram of patient selection is shown in Fig. 1. The mean age of COPD patients was 69.08 (SD = 9.74). The majority of patients were men (73.3%). Before PSM, the descriptive of patient’s characteristics at baseline showed a significant difference between patients with and without COPD in terms of age (69.08 ± 9.74 in COPD patients vs. 67.52 ± 9.67 in non-COPD; P < 0.001), cigarette smoking (26.9% vs. 17.4%; P < 0.001), opium use (24.2% vs. 16.0%; P < 0.001), PVD (3.0% vs. 1.9%; P = 0.043), renal failure-GFR < 60 (27.0% vs. 22.7%; P = 0.011), and previous CVA/TIA (10.0% vs. 6.7%; P = 0.001). A higher percentage of patients with COPD, compared to non-COPD, required intra/post-CABG blood transfusion (55.6% vs. 48.4%; P < 0.001). Moreover, patients with COPD received significantly more CCB (20.8% vs. 13.3%; P < 0.001) and diuretics (26.7% vs. 18.7%; P < 0.001). The characteristics of patients have been described and compared before and after PSM as shown in Table 1.
Before matching, notable imbalances were observed in the covariates, as indicated by high SMD. After applying IPW and PSM, the covariates achieved improved balance, with SMD values below 0.1, as shown in Supplementary Fig. 1A and B, respectively. These results demonstrate the effectiveness of the matching and weighting approaches in reducing covariate differences. Furthermore, the coverage plots in Supplementary Fig. 2A confirm the balanced distribution of variables between COPD and non-COPD patients, reinforcing the robustness of the adjustment methods.
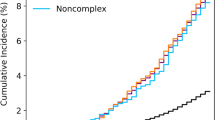
The cumulative mortality hazard was higher in patients with COPD. The Kaplan–Meyer curves and table of at-risk patients and events in unadjusted, IPW, and PSM analysis for all-cause mortality and MACCE are shown in Figs. 2 and 3 respectively.
Our study showed that COPD is not associated with higher 30-day mortality (IPW: HR: 1.16, 95% CI: 0.63–2.15, P = 0.622) and MACCE (HR: 1.43, 95% CI: 0.83–2.45, P = 0.191) in the unadjusted model and after matching. Table 2 presents the effect of COPD on the risk of 30-day all-cause mortality and MACCE. The effect of COPD on long-term all-cause mortality/MACCE is shown in Table 3. All models showed that patients with COPD have a higher risk of all-cause mortality (IPW: HR: 1.53, 95% CI: 1.31–1.79) and MACCE (IPW: HR: 1.29, 95% CI: 1.12–1.47) after CABG.
We further analyzed the association between COPD in smokers and non-smokers with the risk of all-cause mortality and major adverse cardiac and cerebrovascular events (MACCE), presented in Table 4. In 30 days, both unadjusted and IPW showed that COPD in smokers and non-smokers was not significantly associated with all-cause death. COPD in non-smokers was associated with a higher risk of 30-day MACCE in unadjusted analysis (HR: 1.79, 95% CI: 1.081–2.964, P = 0.024), however, this effect was not observed after IPW (HR: 1.75, 95% CI: 0.990–3.093, P = 0.054). In the long term, COPD in smokers and non-smokers was significantly associated with a higher risk of all-cause and mortality in unadjusted analysis. Smokers indicated higher HR in unadjusted analysis (2.31, 95% CI: 1.77–3.02, P < 0.001) compared to non-smokers (HR: 1.84, 95% CI: 1.56–2.17, P-value < 0.001). After IPW, the impact of COPD on the outcome remained higher in smokers (HR: 1.75, 95% CI: 1.31–2.34, P < 0.001) compared to non-smokers (HR: 1.50, 95% CI: 1.25–1.80, P < 0.001). The hazard ratios of all-cause mortality remained significant after IPW, however, COPD was not associated with a higher risk of MACCE in smokers.
The sensitivity analysis showed that after multiple imputations, the hazard ratio for 30-day mortality and MACCE remained statistically insignificant. In addition, the sensitivity analysis for long-term all-cause mortality and MACCE in IPW analysis demonstrated hazard ratios of 1.53 (95% CI: 1.31–1.78) and 1.31 (95% CI: 1.15–1.50) respectively, indicating that missing values were at random and did not significantly affect the association of COPD and outcomes (Table 3). The SMD plots after multiple imputations are shown in Supplementary Fig. 1C and D. Moreover, the coverage plots after multiple imputations are demonstrated in Supplementary Fig. 2B.
Discussion
This study, involving 17,315 Iranian adults undergoing coronary artery bypass grafting (CABG), provides valuable insights into the long-term outcomes associated with COPD, with a median follow-up of 8.25 years. Leveraging a large cohort from a developing country, this research offers a unique perspective on a population with distinct demographic, clinical, and healthcare characteristics, enhancing the understanding of CABG outcomes in underrepresented settings. Our analysis demonstrated that COPD patients had a significantly higher risk of long-term mortality and MACE after CABG compared to patients without COPD, however, this association was not observed in short-term 30-day outcomes. Moreover, we found that irrespective of smoking status, COPD was not associated with 30-day mortality but was a significant predictor of a higher risk of long-term all-cause mortality. Smokers with COPD exhibited a higher hazard of adverse outcomes compared to non-smokers, underscoring the compounded impact of smoking and COPD in this population.
We are the first study in the literature that utilized inverse probability weighting (IPW) and propensity score matching (PSM) to comprehensively evaluate the association between COPD and adverse outcomes in patients undergoing CABG. Using IPW and PSM can help address confounding factors and estimate the true effect of COPD on 30-day and long-term outcomes, by adjusting for the differences in the distribution of confounding variables between COPD and non-COPD patients. These results were consistent across IPW and PSM methodologies, strengthening the study’s credibility. Moreover, the consistency of findings following multiple imputations further validated the conclusions’ robustness, demonstrating that they remained reliable even when accounting for potentially missing data. This underscores the importance of COPD as a predictor of long-term outcomes post-CABG in this cohort.
Long-term outcome
We found that patients with COPD have a 53% higher long-term mortality risk and a 30% higher MACCE risk. Comparable to our study, Leavit et al. discovered an 80% higher death risk in patients with COPD compared to non-COPD9. Similarly, Andell et al. in the SWEDEHEART registry showed that COPD was linked to a significantly higher 5-year mortality rate (HR: 1.52, 95% CI: 1.25–1.86)25. A study by Wang et al. found that patients with COPD had a significantly higher risk of mortality at five and ten years (HR: 2.15 and 2.03, respectively) and higher MACCE (HR: 1.42, P = 0.010)2. Another study by Angouras et al. that used PSM and Cox analysis demonstrated that after adjustment for preoperative factors, the hazard ratio of long-term mortality for COPD patients was 1.28 (95% CI: 1.11–1.47)4, nevertheless, no significant impact of COPD on in-hospital mortality and morbidity was identified, which is comparable to our study indicating no association between COPD and 30-day mortality.
Short-term outcome
Although the literature consistently states that COPD could negatively impact long-term outcomes, its effect on short-term outcomes remains a topic of debate. Ho et al., in a propensity score-matched case-control study, found that COPD does not predict higher 30-day mortality in patients undergoing CABG26. On the other hand, the pooled effect of COPD in revascularization methods i.e. PCI and CABG, was recently studied by Li et al. in a systematic review and showed that COPD independently predicted a higher risk of short-term mortality, long-term mortality, and long-term MI7. Still, the high level of bias introduced to the meta-analysis when pooling estimates from completely different revascularization strategies should be considered. In addition, the severity of COPD could significantly affect its association with short-term mortality27. In this regard, Saleh et al. demonstrated that severe, but not moderate COPD, is linked to higher early mortality (adjusted OR: 2.31; 95% CI: 1.23–4.36; P = 0.01)8. Conversely, Manganas et al. demonstrated no association between the severity of airflow obstruction and postoperative outcomes in COPD patients5. The heterogeneity in study designs and populations could be responsible for the conflicting role of COPD in short-term outcomes.
Cigarette smoking
We found that COPD in non-smokers could increase the risk of 30-day and long-term MACCE. Similarly, Kang et al. showed that non-smokers with COPD had a higher risk of long-term cardiocerebrovascular disease-related mortality compared to non-smokers without COPD28. In addition, smokers with COPD had a higher risk of long-term all-cause mortality and respiratory disease-related mortality compared to non-smokers with COPD. Equivalent to our findings, Saxena et al. found no association between smoking status and early mortality after isolated CABG29. Our results indicated that the negative impact of COPD on the surgical outcome is irrespective of smoking status. However, COPD and concomitant smoking can additively aggravate systemic inflammation30 and outcomes after CABG.
Several factors likely contribute to the poorer outcomes in COPD patients undergoing CABG. COPD is characterized by progressive airflow limitation, air trapping, and lung hyperinflation, which can reduce respiratory reserve and increase the risk of postoperative pulmonary complications, such as pneumonia, respiratory failure, and acute exacerbations3. Additionally, lung hyperinflation could contribute to increased intrathoracic pressure, thereby impairing venous return, preload, and consequently cardiac output during or after surgery31. Chronic hypoxia, on the other hand, might amplify oxidative stress, worsening tissue healing and increasing susceptibility to complications such as infections or arrhythmias postoperatively32. Studies also suggest chronic hypoxia contributes to systemic inflammation, an essential factor in atherosclerotic plaque formation and progression33,34. Moreover, COPD is not only culpable for plaque growth but also for the vulnerability of the plaques leading to rupture35,36. Additionally, COPD is also often accompanied by other comorbidities, such as heart failure and arrhythmias, which can further compromise the patient’s ability to withstand the physiological stress of CABG9.
Our findings emphasize the importance of careful management optimization of COPD patients undergoing CABG. In this regard, preoperative pulmonary rehabilitation, bronchodilator therapy, and oxygen supplementation could play a critical role in mitigating the detrimental risk of lung hyperinflation and chronic hypoxia37. Strategies to mitigate the elevated risks in this high-risk population may include inhaled triple combination therapy (long-acting muscarinic antagonists, long-acting β2-agonists, and inhaled corticosteroid)38, and using the left internal mammary artery39, which improves long-term survival in COPD patients. Studies demonstrated that inhaled corticosteroids (ICS) could diminish the risk of coronary artery disease in COPD patients40,41 subsequently reducing the mortality risk42.
Limitations
Despite the strength of sophisticated statistical analysis, this study has some limitations that should be considered when interpreting the results. First, as a retrospective analysis, it is subject to the inherent biases and potential unmeasured confounding factors associated with observational studies. Second, due to administrative issues, the patients were not included after 2016, therefore advancements in perioperative management and COPD treatment strategies may have impacted the observed outcomes.
Additionally, although the COPD diagnosis was based on spirometric results, detailed data on the pulmonary function test results or disease severity metrics was unavailable in the data registry, which could have provided a more nuanced understanding of the impact of COPD on CABG outcomes. The study population was also limited to a single healthcare center, which may limit the generalizability of the findings to other settings. Therefore, prospective, multicenter studies with comprehensive clinical data collection would be valuable for confirmation of the current findings.
Conclusion
This study, using IPW and PSM, consistently demonstrated that patients with COPD undergoing CABG have a significantly higher risk of long-term mortality and major adverse cardiac and cerebrovascular events compared to CABG patients without COPD. However, no association was observed between COPD and 30-day mortality. These findings underscore the importance of vigilant management optimization in COPD patients. Future research should focus on identifying the most effective strategies to mitigate the elevated risks associated with COPD in the setting of cardiac surgery.
Data availability
The dataset of the present study is available upon reasonable request from the corresponding author.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass graft
- CCB:
-
Calcium channel blocker
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- CVA:
-
Cerebral vascular accident
- DM:
-
Diabetes mellitus
- EF:
-
Ejection fraction
- FEV1:
-
Forced expiratory volume
- FVC:
-
Forced vital capacity
- GFR:
-
Glomerular filtration rate
- HR:
-
Hazard ratio
- HTN:
-
Hypertension
- ICS:
-
Inhaled corticosteroids
- IPW:
-
Inverse probability weighting
- MACCE:
-
Major adverse cardiac and cerebrovascular events
- MI:
-
Myocardial infarction
- OR:
-
Odds ratio
- PCI:
-
Percutaneous coronary intervention
- PH:
-
Proportional hazards
- PSM:
-
Propensity score matching
- PVD:
-
Peripheral vascular disease
- SD:
-
Standard deviations
- SMD:
-
Standard mean difference
- TIA:
-
Transient ischemic attack
References
WHO. Chronic Obstructive Pulmonary Disease (COPD). https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
Wang, R. et al. Impact of chronic obstructive pulmonary disease on 10-year mortality after percutaneous coronary intervention and bypass surgery for complex coronary artery disease: Insights from the SYNTAX extended survival study. Clin. Res. Cardiol. 110(7), 1083–1095. https://doi.org/10.1007/s00392-021-01833-y (2021).
Zhao, H. et al. Postoperative outcomes of patients with chronic obstructive pulmonary disease undergoing coronary artery bypass grafting surgery: A meta-analysis. Medicine 98(6), e14388 (2019).
Angouras, D. C. et al. Postoperative and long-term outcome of patients with chronic obstructive pulmonary disease undergoing coronary artery bypass grafting. Ann. Thorac. Surg. 89(4), 1112–1118. https://doi.org/10.1016/j.athoracsur.2010.01.009 (2010).
Manganas, H. et al. Postoperative outcome after coronary artery bypass grafting in chronic obstructive pulmonary disease. Can. Respir. J. 14(1), 19–24. https://doi.org/10.1155/2007/378963 (2007).
Ovalı, C. & Şahin, A. Chronic obstructive pulmonary disease and off-pump coronary surgery. Ann. Thorac. Cardiovasc. Surg. 24(4), 193–199. https://doi.org/10.5761/atcs.oa.17-00231 (2018).
Li, Y. et al. The impact of chronic obstructive pulmonary disease on the prognosis outcomes of patients with percutaneous coronary intervention or coronary artery bypass grafting: A meta-analysis. Heart Lung 60, 8–14. https://doi.org/10.1016/j.hrtlng.2023.02.017 (2023).
Saleh, H. Z. et al. Impact of chronic obstructive pulmonary disease severity on surgical outcomes in patients undergoing non-emergent coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 42(1), 108–113. https://doi.org/10.1093/ejcts/ezr271 (2012) (discussion 113).
Leavitt, B. J. et al. Long-term survival of patients with chronic obstructive pulmonary disease undergoing coronary artery bypass surgery. Circulation 114(1_supplement), I-430–I−4434. https://doi.org/10.1161/CIRCULATIONAHA.105.000943 (2006).
Khoshakhlagh, A. H., Mohammadzadeh, M. & Morais, S. Air quality in Tehran, Iran: Spatio-temporal characteristics, human health effects, economic costs and recommendations for good practice. Atmos. Environ. X 19, 100222. https://doi.org/10.1016/j.aeaoa.2023.100222 (2023).
Doshmangir, L., Shirjang, A., Assan, A. & Gordeev, V. S. Iranian primary health care network: Challenges and ways forward. Prim. Health Care Res. Dev. 24, e1. https://doi.org/10.1017/s1463423622000354 (2023).
Moradinazar, M. et al. Prevalence of drug use, alcohol consumption, cigarette smoking and measure of socioeconomic-related inequalities of drug use among Iranian people: Findings from a national survey. Subst. Abuse Treat. Prev. Policy 15(1), 39. https://doi.org/10.1186/s13011-020-00279-1 (2020).
Davoodi, S. et al. Coronary artery bypass grafting in octogenarians: A nomogram for predicting all-cause mortality. J. Cardiothorac. Surg. 19(1), 586. https://doi.org/10.1186/s13019-024-03054-6 (2024).
Sheikhy, A. et al. Opium consumption and long-term outcomes of CABG surgery in patients without modifiable risk factors. Front. Surg. 10, 1047807. https://doi.org/10.3389/fsurg.2023.1047807 (2023).
Amoli, A. I. et al. Long-term effects of opium consumption following percutaneous coronary intervention: A 10-year follow-up study. Glob. Heart 19(1), 38. https://doi.org/10.5334/gh.1315 (2024).
Agustí, A. et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Am. J. Respir. Crit. Care Med. 207(7), 819–837. https://doi.org/10.1164/rccm.202301-0106PP (2023).
Survminer: Drawing Survival Curves Using ‘ggplot2’ (2021).
Therneau T. M. A Package for Survival Analysis in R (2023).
Therneau, T. M. & Grambsch, P. M. Modeling Survival Data: Extending the {C}ox Model (Springer, 2000).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Zhang, H., Cheng, X., Wang, S., Zou, Y. & Wang, H. SurvMetrics: Predictive Evaluation Metrics in Survival Analysis (2022).
Greifer, N. Covariate balance tables and plots: A guide to the cobalt package. Accessed March. 10, 2020 (2020).
Randolph, J. J. & Falbe, K. A step-by-step guide to propensity score matching in R. Prac. Assess. Res. Eval. 19, 1–7 (2014).
van Buuren, S. & Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45(3), 1–67. https://doi.org/10.18637/jss.v045.i03 (2011).
Andell, P., Sjögren, J., Batra, G., Szummer, K. & Koul, S. Outcome of patients with chronic obstructive pulmonary disease and severe coronary artery disease who had a coronary artery bypass graft or a percutaneous coronary intervention. Eur. J. Cardiothorac. Surg. 52(5), 930–936. https://doi.org/10.1093/ejcts/ezx219 (2017).
Ho, C. H., Chen, Y. C., Chu, C. C., Wang, J. J. & Liao, K. M. Postoperative complications after coronary artery bypass grafting in patients with chronic obstructive pulmonary disease. Medicine (Baltimore) 95(8), e2926. https://doi.org/10.1097/md.0000000000002926 (2016).
Fuster, R. G. et al. Prognostic value of chronic obstructive pulmonary disease in coronary artery bypass grafting✩. Eur. J. Cardio-Thorac. Surg. 29(2), 202–209. https://doi.org/10.1016/j.ejcts.2005.11.015 (2006).
Kang, H. R., Kim, S. J., Nam, J. G., Park, Y. S. & Lee, C. H. Impact of smoking and chronic obstructive pulmonary disease on all-cause, respiratory, and cardio-cerebrovascular mortality. Int. J. Chron. Obstruct. Pulmon. Dis. 19, 1261–1272. https://doi.org/10.2147/copd.S458356 (2024).
Saxena, A. et al. Impact of smoking status on early and late outcomes after isolated coronary artery bypass graft surgery. J. Cardiol. 61(5), 336–341. https://doi.org/10.1016/j.jjcc.2013.01.002 (2013).
Nielsen, A. O. et al. COPD and smoking status—It does matter: Characteristics and prognosis of COPD according to smoking status. Chronic. Obstr. Pulm. Dis. 11(1), 56–67. https://doi.org/10.15326/jcopdf.2023.0433 (2024).
O’Donnell, D. E., Webb, K. A. & Neder, J. A. Lung hyperinflation in COPD: Applying physiology to clinical practice. COPD Res. Pract. 1(1), 4. https://doi.org/10.1186/s40749-015-0008-8 (2015).
Geçmen, Ç. et al. SYNTAX score predicts postoperative atrial fibrillation in patients undergoing on-pump isolated coronary artery bypass grafting surgery. Anatol. J. Cardiol. 16(9), 655–661. https://doi.org/10.5152/AnatolJCardiol.2015.6483 (2016).
Rabe, K. F., Hurst, J. R. & Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons?. Eur. Respir. Rev. 27(149), 180057. https://doi.org/10.1183/16000617.0057-2018 (2018).
Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 117(13), 2525–2536. https://doi.org/10.1093/cvr/cvab303 (2021).
Russo, M. et al. Atherosclerotic coronary plaque features in patients with chronic obstructive pulmonary disease and acute coronary syndrome. Am. J. Cardiol. 224, 36–45. https://doi.org/10.1016/j.amjcard.2024.06.005 (2024).
Kumagai, S. et al. Impact of chronic obstructive pulmonary disease on composition of left main coronary artery plaque with intermediate stenosis. Int. J. Cardiol. 174(3), 865–866. https://doi.org/10.1016/j.ijcard.2014.04.223 (2014).
ZuWallack, R. L. The roles of bronchodilators, supplemental oxygen, and ventilatory assistance in the pulmonary rehabilitation of patients with chronic obstructive pulmonary disease. Respir. Care 53(9), 1190–1195 (2008).
Martinez, F. J. et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am. J. Respir. Crit. Care Med. 203(5), 553–564. https://doi.org/10.1164/rccm.202006-2618OC (2021).
O’Boyle, F. et al. Long-term survival of patients with pulmonary disease undergoing coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 43(4), 697–703. https://doi.org/10.1093/ejcts/ezs454 (2013).
Gadhvi, K. et al. Inhaled corticosteroids and risk of cardiovascular disease in chronic obstructive pulmonary disease: A systematic review and meta-regression. Chronic. Obstr. Pulm. Dis. 10(3), 317–327. https://doi.org/10.15326/jcopdf.2022.0386 (2023).
Shin, J., Yoon, H.-Y., Lee, Y. M., Ha, E. & Lee, J. H. Inhaled corticosteroids in COPD and the risk for coronary heart disease: A nationwide cohort study. Sci. Rep. 10(1), 18973. https://doi.org/10.1038/s41598-020-74854-8 (2020).
Malo de Molina, R. et al. Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalised with pneumonia. Eur. Respir. J. 36(4), 751. https://doi.org/10.1183/09031936.00077509 (2010).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to this work. RA and AJ hypothesized this work. AJ designed the study. MSN, MD, and AJ wrote the main manuscript. AJ, ZK, and MSN prepared the figures. SHAT, SD, KM, and MD contributed to the data collection AJ and ZK cleaned the data and did the statistical analysis. RA, AJ, and MSN revised the manuscript. RA, AJ, and MSN supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Tehran University of Medical Sciences ethics committee with the code IR.TUMS.THC.REC.1403.016. All participants were provided written informed consent at the start of the study. The study was carried out according to the Helsinki Declaration.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Najafi, M., Jalali, A., Karimi, Z. et al. Prognostic impact of chronic obstructive pulmonary disease on short-term and long-term outcomes following coronary artery bypass grafting. Sci Rep 15, 1865 (2025). https://doi.org/10.1038/s41598-024-83860-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83860-z