Abstract
The phytochemical fingerprinting that add to the nutritional and nutraceutical value of the fruits during the ripening stages is beneficial for human consumption. Therefore, ripening-dependent changes in phytochemical content and antioxidant activities of mango (Mangifera indica L.) cultivar Dusehri at various ripening stages were evaluated. Bioassays for phenolic content, flavonoid content, antioxidant activities, and UHPLC/MS for phytochemical profiling was performed at five ripening stages (RSI-RSV). Total phenolic contents significantly increased from 4.25 to 13.08 µg GAE/mg extract upto stage III and non-significant decrease was observed thereafter. Flavonoid contents varied between 1.16 and 1.23 µg QE/mg extract. DPPH based free radical scavenging activity increased (41.07–52.33%) from stage I to stage V while FRSP based analysis showed decrease (53.01–27.61 µg TE/mg extract) in activity from stage I to stage V. Total antioxidant capacity and total reducing power potential of pulp extract gradually increased towards mango ripening stages. A non-significant change in amylase inhibition was observed from stage I to stage III that significantly dropped in stage IV and V. UHPLC analysis depicted that aconitic, methylisocitric, 2,4,6-Hydroxy benzoic acid and beta glucogallin, poly phenols, 1-Methylxanthine, 3-Furicacid, Heptenoic acid and many others are present at different ripening stages of dusehri mango. PCA analysis and hierarchal analysis show Stage I & II clustering while stages III-V make separate cluster. These phytochemiclas are responsible for many health benefits. The study concludes that dusehri mango have significant antioxidative capacity that are due to diverse phytochemicals.
Similar content being viewed by others
Introduction
Mango (Mangifera indica L.) belongs to family Anacardiaceaeis every green plant. Its fruit is liked by all human irrespective to age due to flavor, aroma, and nutritional value. These properties cumulatively distinguish this fruit from others therefore mango is said ‘the king of fruits”1. The evergreen mango plant grows well in tropical and subtropical climate therefore is distributed worldwide. The mango fruits are harvested when hard and green, that ripen at ambient conditions2. During ripening process, various biochemical and physiological changes occur that develop color and aroma along with other nutritional additions3. The biochemical changes include change in endogenous level of hormones, ripening agents production, change in concentrations and types of carbohydrates, phenolics, organic acids, and other organic molecules4. The biochemical changes result in development of aroma, color, sweetness, and other health beneficial effects5.
Mango is an excellent source of dietary antioxidants and bioactive compounds, such as ascorbic acid, carotenoids, provitamin A, vitamin C and especially phenolic compounds6,7, which are known to have different health-promoting properties8. The richness and diversity of nutrients and phytochemicals in mango designate it as superfruit and has significant health benefits. Due to diverse phytochemicals, mango pulp is considered effective remedy against leukemia, prostate, breast and colon cancers9,10.
Mango pulp is used as flavor ingredient in dairy and beverage industries, and also in baby food formulations due to its likeliness and taste. However phytochemical and nutritional properties of mango pulp vary depending on variety, ripening stage, growth conditions, storage, etc11. Antioxidant activities of fruits and vegetables provide nutritional and nutraceutical properties and functional qualities. It accounts for the presence of efficient oxygen radical scavengers, such as vitamin C, carotenoids, flavonoids and phenolic compounds. Such compounds either have synergistic and may also have antagonistic effects2,12. There is no precise information on the exact stage of harvest and effect of ripening treatments on the antioxidant content and antioxidant activity of mangoes. Information about the right stage for mango consumption with highest antioxidant potential will be very useful. In view of this, a study was taken up to investigate the phytochemical and antioxidantive changes during the ripening process of mango (Mangifera indica L.) variety Dusehri.
Materials and methods
Mango cultivars and ripening process
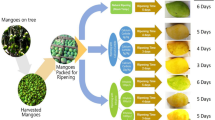
Uniform size green mature mango (Mangifera indica L.) fruits (average weight of 100–150 g) of Dusehri cultivar were harvested from Agriculture field Multan Punjab Pakistan and transported to the laboratory for evaluation. Fruits were selected based on their size, green but mature and unripen. Afterwards, fruit were sanitized with chlorinated water for 3 min and left to dry at room temperature (23–26 °C) for about 1 h. For study fruits were divided in 5 groups of 3 fruits each. The ripening process was performed as per commercial conditions to make the process simple and as per practice. The fruits were packed in cardboard boxes with holes. Approximately 0.5 g calcium carbide in paper bag was also placed inside the box. Calcium carbide produces acetylene gas that has similar effects like ethylene, the natural ripening agent. Acetylene accelerates the ripening process. Maturity was judged by visual color and texture each day. The dusehri mango does not change color from green to yellow but a little bit light green. Phytochemical evaluation was performed at five ripening stages (RS) starting from green freshly plucked state to fully ripped.
Pulp extraction and extract preparation
Mango at each ripening stage was peeled and pulp was cut into small pieces. The fresh pulp was used for proximate analysis while extracts of mango pulp were prepared by homogenization of 20 g pulp in 100 ml methanol. The mixture was left for 24 h and then filtered thereafter through Whatman filter paper No. 4. Filtrate was dried at room temperature under continuous air flow. The extract was used for phytochemical analysis and quantification of total phenolic and flavonoid contents and antioxidant activities. For assays the extract was dissolved in DMSO at 4 mg/ml.
Proximate analysis of pulp
Proximate analysis i.e. moisture content, dry matter, protein content, fat, carbohydrate contents, ash content of safaid chonsa mango pulp were performed according to the Association of Official Analytical Chemists (AOAC, 2000) methods. Moisture content was calculated by taking 5 g of sample in a pre-weight aluminium moisture dish. The dishes were kept in hot air oven at 150 °C for 2 h and weighed again. The moisture content was determined as:
Moisture (%) = (Weight of fresh sample – weight of dry sample) / Weight of fresh sample × 100.
To measure ash content, 5 g of pulp in silica crucible was heated at 525 °C for 5 h in a muffle furnace. The heating was continued until the weight became constant. The weight of ash was calculated by the following formula.
Ash (%) = (Weight of fresh sample - Weight after ashing /weight of fresh sample)*100.
To determine total fat in mango pulp, 5 g pulp was weighed into fat free cellulose thimbles and placed in SocsPlus condensers. Petroleum ether (50 ml) was refluxed for 1 h over the sample. Ether was then drained and remaining by evaporation. The mass in the silica reflux cups was designated crude fat.
Crude fiber was determined by taking 2 g in beaker and was digested with 2.5 M H2SO4 followed by an equal volume of 2.5 M NaOH on a hot plate for 1 h. The sample was centrifuged and the precipitate was dried in muffle furnace at 600ºC until constant weight was obtained. Fiber (%) was calculated as follow:
Fiber (%) = ((weight of crucible − weight of crucible containing ash) ∗ 100)/ weight of sample.
Protein was determined by Kjeldahl method. Mango pulp (0.5 g) was weighed in a 50 mL Kjeldahl flask and 8 ml concentrated H2SO4 was added. 5 g copper and potassium sulfate mixture was also added as catalyst. Samples were digested until colorless residue was observed. Digested samples were distilled and vapor gas was collected in a conical flask containing mixture of 25 ml of 2% boric acid solution and indicator. The sample was titrated against 0.1 N HCl until a pink color persisted. Crude protein was calculated as.
Crude protein = ((normality of acid x volume of acid used in ml x 15 × 6.25) / weight of sample) × 100.
The total carbohydrate was calculated as.
Total carbohydrate (%) = 100 – (Moisture (%) + Protein (%) + Fat (%) + Ash (%)).
The gross energy of mango pulp was calculated as.
FE (K.Cal/100 g) = (%carbohydrate − %fiber) × 4 + (%fat x 9) + (%protein × 4).
Determination of total phenolic content
The total phenolic content in mango pulp was determined by Folin–Ciocalteu reagent with slight modifications13. 20 µl of pulp extract from 4 mg/ml DMSO stock were poured in wells of 96 well plate. 90 µl of Folin–Ciocalteu reagent was added and the plate was incubated for 5 min at room temperature. 90 µl sodium carbonate was also added in each well thereafter. Absorbance was determined at 630 nm by microplate reader (Biotech USA, microplate reader Elx 800). Gallic acid was used as standard and the results are expressed as µg gallic acid equivalent per milligram of mango pulp extract (µg GAE/mg extract).
Determination of total flavonoid content
The total flavonoid content in mango pulp was estimated by aluminum chloride colorimetric method described by Ali et al. with some modifications13. 20 µl of pulp extract from 4.0 mg/ml in DMSO stock were reacted with 10 µl each of 10% aluminum chloride and 1.0 M potassium acetate in 96 well plate. 160 µl distilled water was added in each well and plates were incubated at room temperature for 30 min. The absorbance was taken at 415 nm using microplate reader. Quercetin was used as a standard and the flavonoid content was calculated as µg equivalents of quercetin per milligram of mango pulp extract (µg QE/mg extract).
DPPH radical scavenging activity
The free radical scavenging capacity of crude mango pulp extracts was determined using 1, 1- diphenyl l- 2-picryl-hydrazil (DPPH) radical discoloration method and ascorbic acid was used as standard 14. Spectrophotometric analysis was used to measure the percent radical scavenging capacity (%RSA). To determine DPPH radical scavenging activity of mango pulp extract at different ripening stages, 180 µl of methanol solution of DPPH radical in the concentration of 9.2 mg/100 ml was added to separate wells of 96 well plate. Mango pulp extract (20 µl) was then added in to each well and incubated at room temperature for 30 min in dark. The absorbance was measured at 517 nm using microplate reader. Ascorbic acid was used as positive control. Scavenging activity in percent (%RSA) was calculated as.
DPPH scavenging effect (%) = (absorbance of negative control - absorbance of sample / absorbance of negative control) × 100.
Determination of total antioxidant capacity
Total antioxidant capacity was assessed using a modified method as described14. Activity was performed by mixing 0.1 ml mango pulp extract (4 mg/ml DMSO) with a mixture of 1 ml of reagent solution (0.6 M sulfuric acid, 28mM sodium phosphate and 4 mM ammonium molybdate). Ascorbic acid was used as positive control and DMSO was used as negative control. The tubes containing the reaction solution were then capped and incubated in a boiling water bath for 90 min at 95 °C. After incubation at high temperature samples were cooled to room temperature and absorbance of the solutions were measured at 695 nm against blank. The antioxidant activity was expressed as the µg ascorbic acid equivalent per mg of mango pulp extract i.e., µg AAE/mg extract.
Estimation of total reducing power estimation
The reducing power of the mango extract was measured by potassium ferricyanide colorimetric assay according to the method described previously14. To assess reducing power of the mango pulp extract, 200 µl of sample from 4 mg/ml in DMSO was reacted with 400 µl of 0.2 mol/l pH 6.6 phosphate buffer and 1% potassium ferricyanide [K3Fe (CN)6]. The reaction mixture was heated at 50 °C for 20 min and 400 µl of 10% trichloroacetic acid was added. The mixture was centrifuged at 3000 rpm for 10 min and 500 µl upper layer was mixed with 500 µl distilled water and 100 µl, of 0.1% FeCl3. The absorbance was measured at 700 nm. Ascorbic acid was used as positive control. The reducing power is expressed as µg ascorbic acid equivalent per milligram mango pulp extract (µg AAE/mg extract).
Determination of metal chelating ability
The protocol reported by Ali et al., was followed to determine metal chelating ability of samples13. 20 µl of pulp extract was reacted with 50 µl of 2mM FeCl2 in 96 well plate. After incubation for 10 min in dark, 20 µl of 5 mM ferrozine was poured into each well and incubated again for 5–10 min. Absorbance was measured at 562 nm. EDTA was used as positive controland calculated as.
MC ability % = [(Absorbance of Control – Absorbance of sample)/ Absorbance of Control]* 100.
Determination of ABTS radical scavenging potential
The mixture of 7mM ABTS and 2.45mM potassium per sulphate (1:1) was kept in dark for 12–18 h and diluted at 1:2 thereafter. The absorbance was adjusted at 0.7 ± 0.02 at absorbance 540 nm. To perform assay, 10 µl of samples was reacted with 100 µl above reagent in 96 well micro plate. The plates were incubated in dark at room temperature for 10 min and final absorbance of the reaction mixture was measured at 540 nm13.
α-Amylase inhibition assay
The assay was performed following reported protocol12. 15 µL phosphate buffer (pH 6.8), 25 µL of α-amylase enzyme (0.14 U/mL), 10 µL of extract (4 mg/mL in DMSO) and 40 µL starch solution (2 mg/mL in potassium phosphate buffer) were periodically added into each well of 96 well plate. The plates were incubated for 30 min at 50˚C and 20 µL of 1 M HCl, 90 µL iodine reagent were added into each well. The optical density (OD) was taken at 540 nm. Acarbose was used as positive control at 5–200 µg/mL. The percent α-amylase inhibition was calculated as.
Enzyme Inhibition (%) = (OD of Control – OD of sample / OD of control) x 100.
Secondary metabolite profiling by UHPLC/MS
Pytochemical profiling of mango extracts was evaluated through UHPLC (Agilent Technologies, Santa Clara, CA, USA) and Agilent 6520 Q-TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA) via an electrospray ionisation source (ESI) was used for the tentative identification and characterization of the compounds. Agilent Zorbax xdb-C18 at 25 °C was used for analysis. Mobile phases 0.1% formic acid in water (A) and acetonitrile (B) at flow rate 0.5 ml/min was used. Injection volume was 10 µL with run time and post run time 25 min and 5 min, respectively. The scan was performed from 100 to 1000 m/z. Peak identification was performed in both negative and positive modes. The mass spectrometry conditions were set, as follows: nitrogen gas temperature 350 °C with the flow rate 300 L/hr, sheath gas temperature 250 °C with the flow rate 660 L/h, and nebulizer gas pressure 45 psi. The capillary and nozzle voltage were set at 3.5 kV and 500 V, respectively. The fragmentation voltage was optimized to 125 V. Analysis was performed with a capillary voltage of 3500 V. Data acquisition and analysis was performed using Agilent LC-MS-QTOF Mass Hunter Data Acquisition Software Version B.03.01 (Agilent Technologies, Santa Clara, CA, USA).
Statistical analysis
All the assays were performed in triplicate. The results are reported as mean ± standard error. Moreover, the results were analyzed statistically through analysis variance (ANOVA) and the means were analyzed by LSD at 0.05% probability. The chromatographic data was further analyzed for principal component analysis (PCA) in order to detect the phytocomponents able to differentiate the dusehri mango samples of different ripening stages. PCA is multivariate method that is used for visualization of hidden trends in a data matrix among the variables. Cases plotted in the PCs explain the differences/similarities between the mango ripening stages. The PCA statistical analysis on phytochemicals was performed by using the OriginPro 9.0 software.
Results and discussion
Mango pulp is a source of carbohydrates, lipid and fatty acids, protein, organic acids, vitamins and many other phytochemicals. The energy value for 100 g of the pulp increased from 94.55 at RSI to 114.05 kcal at RSV (Table 1). The dry matter increased from 26.31 to 29.05% from RSI to RSIV and then decreased at RS V. Protein content continued to increase from 1.49 to 1.7% from RS I to RSV. However fats and fiber did not changed at significant level from stage I to stage V. During the ripening process there was significant increase of carbohydrate, 29.14% at stage I to 31.37% at stage III and then slight decrease in carbohydrate content was observed in next two stages (Table 1). The nutritional, solid content, and water content of mango fruit change depending upon cultivar and preharvest and postharvest factors4. The proximate analysis of Haden, Kent, Keitt, Tommy Atkins pulp15, Malgoa, Totapuri, Benishan, Sundari, and Neelam16, Alphonso, Pairi, and Kent17, Harumanis, Kalabau, Stam Panjang, African Bush, Fazil, and Kanchamithia varieties18has also been reported with variation based on cultivar type, environmental condition, processing stratigy, and many other factors19.
Dry matter content ranged from 16.38 to 20.52%. Keitt (20.52%) had the highest dry matter Results were also comparable to the reports of Kansci et al.20 and Saranwong et al.21who had reported variation in different parameters in different varieties. The nutritional properties and non-nutritional contents i.e. fiber offers the potential for its use in human diet, nutraceuticals, and other industrial products22.
Antioxidative capacity, total phenolics and flavonoid content
Phenolic class of phytochemical and its sub-classes are well characterized due to their antioxidative properties and free radical scavenging capabilities23. Total phenolic content (TPC) of Dusehri mango varieties increased significantly with advances in ripening stages from RSI (4.25 ± 0.38 µg GAE/mg extract) to RSIII (13.08 ± 0.58 ug GAE/mg extract) but after RSIII phenolic content remained constant up till RS5 which was last stage of ripening (Fig. 1). However non-significant variation in total flavonoid contents was observed during fruit ripening stages with TFC range from 1.16 to 1.23 ug QE/mg extract. Total polyphenol content in fully ripe mango flesh is lower than green mature mango flesh. However TPC were lower in Dosehri cultivar as reported in other cultivars24,25. This might be due to differences in the cultivar, sources of the materials, ripening stage, soil and climate conditions, etc. The ripening process generates heat that results in decrease of phenolic contents in mango pulp26,27. While Gil et al.28, reported increase in soluble phenolics in mangoes because starch converts to simple sugars by amylase and it breakdown the conjugative molecules. However ripening process did not change flavonoids contents of mango pulp. This has also been reported by others29,30. The total phenolic contents in dosehri pulp analyzed in this study are in agreement with reported earlier in different varieties of mango, such as Haden, Mallika, Tommy Atkins, Pica, Ataulfo, and Pica mango varieties from different countries31,32,33,34,35,36.
Several methods are used to determine total antioxidant capacity, and each has some limitations36. Therefore different methods are opted for analysis of antioxidant activity. The total antioxidant capacity (TAC) is based on reduction of Mo (VI) to Mo (V) that produces green color and is consequence of antioxidants. Maximum TAC was displayed by Dusehri pulp extract at last ripening stage (343.17 µg AAE/mg extract). However non-significant variation was observed to the adjunct ripening stage that shows gradual variation of antioxidative property of mango pulp (Fig. 1). Total reducing power of mango pulp also increased as mango pulp ripened. TRP was 340.75–356.83 µg AAE/mg extract from stage I to stage III and then significantly increased at stage IV (392.06 µg AAE/mg extract) and stage V (452.86 µg AAE/mg extract). Free radical scavenging activity was performed by DPPH, FRSP, and metal chelating assays. DPPH assay showed 41.07% activity at stage I that increased up to 52.33% at stage V (Fig. 1). Inverse values were observed in case of FRSP activity where values decreased along with the ripening stages; 53.01 µg TE/mg extract at stage I to 27.61 µg TE/mg extract at stage V. The pulp extract showed minor metal chelating activity (6 µg EDTA/mg extract) at stage I and II that increased up to 20–21 µg EDTA/mg at stage III and IV, and then decreased at stage V. In biological system, oxidation is a natural phenomenon that produces highly reactive hydroxyl and peroxyl radicals. The antioxidants reduce these radicals. If not reduced, excess of these free radicals may cause damage to DNA, proteins and fatty acid, phospholipids Damage in cellular components may lead to diseases and cancer37,38,39.
DPPH converts into the stable molecule when capture an electron or hydrogen radical. Therefore it is frequently used to investigate free radical scavenging activity of plant extracts40. The DPPH method to determine free radical scavenging potential has been used by many researchers in many mango varieties such as Manila, Ataulfo, Tommy Atkins, Kent and it was found that percent radical scavenging potential varies among the mango cultivars26,41,42,43. DPPH results correlate with the total phenolic and flavonoid contents in the sample and there is a linear correlation between these activities25,38. The values of oxygen radical absorbance capacity in mango pulp are comparable to others findings5,33,42. There is direct correlation between estimation of phenolics and flavonoids with antioxidant and radical scavenging activities. Therefore variation in activity was observed according to the ripening stage44.
Total phenolic content (TPC µg GAE/mg extract), total flavonoid content (TFC µg QE/mg extract), antioxidative response (total antioxidant capacity (TAC µg AAE/mg extract) and total reducing power (TRP µg AAE/mg extract)), and free radical scavenging activity (% inhibition) of pulp extracts of dusehri mango at five ripening stages. The values are mean of triplicates. The small alphabets on bars represent significant difference between the mean by LSD at p < 0.05.
Amylase inhibition of pulp extracts
The results show that phytochemicals in mango pulp inhibit the amylase activity at different stages of ripening. Amylase inhibition was non-significantly different in between RSI to RSIII (27 − 24%) and then significantly dropped at RSIV (9.00%) and RSV (2%) (Fig. 2). The activity of amylase indicates the role played in the onset of fruit ripening process as reported for Ashwina hybrid variety of mango45. The physiological and biochemical variations during fruit ripening are due to expression of fruit ripening-related genes. The main role is played by enzymes that are responsible for texture, taste, aroma, ad softening of fruits. Carbohydrate hydrolyzing enzymes as amylase are critical in this process11,46. The results show that at the initial stage amylase is inhibited and as maturity of mango proceed, the amylase inhibition reduced therefore the mango turn more sweet with the ripening process.
Phytochemical profiling
UHPLC analysis was performed to determine presence and change in phytochemicals during the mango ripening process. The biochemical changes that occur during repining process include variation in color, aroma, taste, and others. The conversion of green to yellow color is due to Carotenoids synthesis while aroma and flavor variations are due to volatile compounds such as esters, terpenes, lactones, aldehydes, etc47,48,49. RSI, the unripen mango analysis demonstrated 24 compounds while RSII demonstrated 22 phytochemicals (Tables 1 and 2). While RSIII, RSIV and RSV analyses presented 8, 12, and 14 compounds, respectively (Table 1).
The analyses show that during the ripening stages (RSI-RSV); the identification of phytochemicals was diverse. Some classes were present at all the stages with varying concentration of metabolites, 4-O-Methyl-gallate, a polyphenol was detected at all stages with maximum value at RSI that gradually decreased in lateral stages. The retention time of 6.6 min indicates the time it took for 4-O-Methyl-gallate to elute from the chromatography column. The observed mass-to-charge ratio (m/z) of 183.03 corresponds to the molecular weight of 4-O-Methyl-gallate 184.037 (Table 2). This value is consistent with the expected mass of the compound, further confirming its presence in the sample. The high value of DB and MFG score reinforces the accurate identification and high match quality of the compound. The presence o ions refer to the different ion fragments detected during the mass spectrometry analysis of 4-O-Methyl-gallate.
Tricarboxylic acid such as aconitic acid and methylisocitric acid were also detected at all stages of mango ripening stages while citric acid was only detected at stage II and stage V (Table 2). The UHPLC results showed aconitic acid and methylisocitric acid with a retention time of 1.6 and 1.5 min, respectively depicted m/z ratio 173.0 and 205.03 and molecular mass of 174.01 and 206.04, respectively and high confidence scores strongly confirm the presence of aconitic acid and methylisocitric acid at all ripening stages. 2,4,6-Hydroxy benzoic acid and beta glucogallin, the component of hydroxyl benzoic acid were also observed at all stages. Theobromine was also detected at all stages that represent xanthines class. 1-Methylxanthine and purine of same class were also detected at RSI and II. 3-Furicacid (carboxylic acid) is also a phytochemicals that occurs during ripening of the mango. The m/s ration 111.008 retained at 0.87–1.65 min with molecular mass 112.06 was predicted as 3-furoic acid that was observed at all ripening stages. Marchantin A, a phenylpropionide also dominate in phytochemicals at all ripening stages of mango. The UHPLC results for (3R,5 S,6E)-rel-7-[3-(4-fluorophenyl)−1 H-indol-2-yl]−3,5-dihydroxy-6-Heptenoic acid with a retention time of 0.65 min depict m/z ratio 404.108 and molecular mass of 369.138, and high confidence scores strongly confirm the presence of Heptenoic acid derivative at all ripening stages. The compound was significantly at high concentration assumed by height volume ratio. Other than these fatty acids (xylonate derivative), carbohydrates (rhamnose), phenols, phenylpyrroles (heptenoic acid), phenolic glycosides (glucopyranoside), quebrachitol, derivatives and conjugates were also detected at different stages. The presence of different classes of phytochemicals in mango pulp have been reported though type and concentration of such classes depends on mango variety, cultivation conditions, ripening environment, and others7,8,50,51.
Phenolic acids either alone or conjugated with esters are commonly present in fruits that contribute to taste, color, nutritional value, and health benefits. The bioavailability of phenolic acids depends on the presence of free or conjugated forms52. These compounds are well-known for their potent antioxidant properties53, aiding in the prevention of diseases related to oxidative stress, such as cardiovascular and neurodegenerative diseases, and cancer54. Additionally, many phenolic acids and their derivatives are recognized for their anti-inflammatory and antimicrobial activities53,55. Xanthines and its derivatives significantly contribute to the prevention of chronic diseases. The improve mood, physical performance, brain efficiency, and overall well being by functioning as antagonists of adenosine receptors56,57,58.
It has been reported that phenolic acid and its derivatives are present in different varieties of mango at varying concentrations43,59. These components have different health benefits and have the capability to overcome oxidative stress by neutralizing oxidants produced inside the cell60,61. Furthermore, the synergistic effects of complex phytochemicals present in mango pulp are more beneficial. Polyphenolic compounds are important anti-radical, anti-mutagenic, and anti-carcinogenic agents62,63,64. These compounds also reduce the risk of chronic diseases where hydroxyl groups have integrative role in biological activities. Phenolic as antioxidative molecules i.e. hydroxyl benzoic acid and its derivatives prevent DNA damage and tumor promotion due to quenching of free radicals. Phenolic acids are predominant compounds in mango pulp62,65. Consuming ripened mango contains significant amount of phenolic acids that play a significant role in neutralizing free radicals and improving consumer health.
Ferulic acid, an important phenolic compound produced from the metabolism of phenylalanine and tyrosine, effectively scavenges free radicals and suppresses radiation-induced oxidative reactions. It maintains physiological integrity of the cell and inhibits inflammatory diseases66,67. Chlorogenic acid is also an abundant polyphenols in the human diet. Chlorogenic acid has anti-nociceptive, anti-carcinogenic, anti-edematogenic, antioxidative properties68.
The output of a PCA is a combination of two plots, a loading plot and a score (scree) plot. The loading plot identifies key variables responsible for variances. While the score plot shows relationship of samples and gives a quantitative value for variance among the samples. The plot shows how the phytochemicals are distributed onto the calculated PCs. This shows that there is diversity of phytochemicals and there is variation in correlation in between the stages and concentration (volume) of phytochemicals (Fig. 3). A number of components that were not detected in between the stages or they are scattered according to the positive or negative relation. Figure also depicts that concentration of compounds mostly downreglate or upregulate during the ripening process. Principal component analysis (PCA) is often the first choice in analyses for exploring grouping relationships in samples69,70. The plot describes there is change of phytochemicals during the ripening stages of dusehri mangoes. The stage I (RSI) and stage II (RSII) have strong interaction while RS III, IV, and V interact with each other (Fig. 4). A number of components are present at all ripening stages however the correlation among them varies. Negative correlation describes that the components have difference in concentration as the ripening stage varies. This shows that although numbers of phytochemicals are present in mango, their correlation with the stage varies depending upon the concentration/presence of that component. The hierarchal analysis between antioxidative response (Fig. 5A) and distribution of phytochemical in between the ripening stages (Fig. 5B) also shows that there are step by step variations in both antioxidative response and phytochemicals in between the ripening stages. Antioxidative bioassays (TAC, TRP, DPPH, FRSP, and MC) along with total phenolics and flavonoids also support the hierarchal clustering analysis that there are variations in metabolites at ripening stages. Furthermore the presence of compounds and their characteristics also favor clustering of the ripening stages in coordinated groups.
Conclusion
The present study shows that the levels of total phenolic and flavonoid content, and antioxidant activities are significantly affected by ripening stage of mango cultivar dusehri. Dusehri exhibited relatively higher levels of total phenols and flavonoids, and antioxidant activities, which may offer potential health benefits of respective cultivar. The antioxidant activities such as DPPH free radical scavenging activity, total antioxidant capacity and total reducing power were also higher at ripening stage. The variations in the activities are due to change of composition of phytochemicals in the mango pulp. Different classes of phytochemicals at different ripening stages show that the nutritional and nutraceutical value of mango are due to presence of phytochemicals. Hierarchal analysis also shows that there is gradual variation in the activities of mango with the ripening stages.
Data availability
All the relevant data are reported in the manuscript.
References
Bompard, J. Taxonomy and Systematics. The Mango: Botany, Production and uses19–41 (CAB International, 2009).
Suleman, M., Ali, J. S., Nisa, S. & Zia, M. Antioxidative, protein kinase inhibition and antibacterial potential of seven mango varieties cultivated in Pakistan. Pak. J. Pharm. Sci., 32(4). (2019).
Rahman, K. S. et al. Prediction of mango quality during ripening stage using MQ-based electronic nose and multiple linear regression. Smart Agricultural Technol. 9, 100558 (2024).
Hussain, A. et al. Physiological and biochemical variations of naturally ripened mango (Mangifera Indica L.) with synthetic calcium carbide and ethylene. Sci. Rep. 14 (1), 2121 (2024).
Saikaew, K., Siripornadulsil, W. & Siripornadulsil, S. Improvements in the color, phytochemical, and antioxidant properties of frozen ripe mango pieces using calcium chloride dipping and chitosan coating. J. Food Sci. 88 (8), 3239–3254 (2023).
Nguyen, N. X. et al. Methyl salicylate induces endogenous jasmonic acid and salicylic acid in’Nam Dok Mai’mango to maintain postharvest ripening and quality. J. Plant Physiol. 303, 154356 (2024).
Rocha, R. et al. Phytochemicals, antioxidant activity and nutritional profile of pulp, peel and peel fiber of mango (Mangifera indica l.) cultivar ataulfo. Funct. Foods Health Disease. 14 (10), 713–727 (2024).
Ghosh, S., Avinashe, H. & Dubey, N. Nutritional and phytochemical changes during ripening in Mango: a review. Curr. Nutr. Food Sci. 19 (5), 519–528 (2023).
Saroj, N. & Prasad, K. Assessment of Himalayan plain mango genotypes for phytochemicals, biochemical-nutraceutical characterisation and quality change during storage life. Int. J. Food Sci. Technol. 58 (7), 3781–3799 (2023).
Luthria, D. L. Optimization of extraction of phenolic acids from a vegetable waste product using a pressurized liquid extractor. J. Funct. Foods. 4, 842–850 (2012).
Pérez-Meza, N. B. et al. The Nutritional, Mineral, and Nutraceutical Quality is differentially affected by the Mango Cultivar. Horticulturae 10 (10), 1082 (2024).
Dar, A. N. et al. Bioassay guided triterpene isolation and its biological evaluation using branches extract of a significant medicinal plant; Monotheca Buxifolia. Pharmacol. Research-Natural Prod. 3, 100026 (2024).
Ali, J. S., Riaz, N., Mannan, A., Tabassum, S. & Zia, M. Antioxidative-, antimicrobial-, enzyme inhibition-, and cytotoxicity-based fractionation and isolation of active components from Monotheca Buxifolia (Falc.) A. DC. Stem extracts. ACS Omega. 7 (4), 3407–3423 (2022a).
Ali, J. S., Riaz, N., Mannan, A., Latif, M. & Zia, M. Antioxidant, antimicrobial, enzyme inhibition, and cytotoxicity guided investigation of Sideroxylon mascatense (A. DC.) TD Penn. leaves extracts. Nat. Prod. Res. 36 (16), 4227–4230 (2022b).
Corrales-Bernal, A., Jaramillo, G., Rodríguez, B., Kazuz, E. Y. & Maldonado-Celis, M. E. Mango (Mangifera indica cv. Azúcar) antiinflammatory and chemopreventive role during colorectal carcinogenesis. Emirates J. Food Agric. (EJFA), 28(10). (2016).
Pathak, S. R. & Sarada, R. (1974). Lipids of mango (Mangifera indica).
Deshpande, A. B. et al. Transcriptional transitions in Alphonso mango (Mangifera indica L.) during fruit development and ripening explain its distinct aroma and shelf life characteristics. Sci. Rep. 7 (1), 8711 (2017).
Dar, M. S. et al. Nutrient and flavor content of mango (Mangifera indica L.) cultivars: an appurtenance to the list of staple foods. In Nutritional Composition of Fruit Cultivars (445–467). Academic. (2016).
Majumdar, A. D., Kamboj, U. & Munjal, N. Chemical composition of mango pulp and the comparison of two vibrational spectra. In AIP Conference Proceedings (Vol. 2800, No. 1). AIP Publishing. (2023), September.
Kansci, G., Koubala, B. B. & Mbome, I. L. Biochemical and physicochemical properties of four mango varieties and some quality characteristics of their jams. J. Food Process. Preserv. 32 (4), 644–655 (2008).
Saranwong, S., Sornsrivichai, J. & Kawano, S. Prediction of ripe-stage eating quality of mango fruit from its harvest quality measured nondestructively by near infrared spectroscopy. Postharvest Biol. Technol. 31 (2), 137–145 (2004).
Alabi, M. A., Aremu, M. O. & Akpomie, T. M. Phytochemical, antioxidant, and nutritional evaluation of kernel and pulp of Bush mango (Irvingia gabonesis). Hum. Health Halal Metrics, 5(1). (2024).
Petti, S. & Scully, C. Polyphenols, oral health and disease: a review. J. Dent. 37 (6), 413–423 (2009).
Klepacka, J., Gujska, E. & Michalak, J. Phenolic compounds as cultivar-and variety-distinguishing factors in some plant products. Plant Foods Hum. Nutr. 66 (1), 64–69 (2011).
Awan, M. S. et al. Assessment of physicochemical parameters, and antioxidant properties of mango concentrate during different storage intervals. Int. J. Food Prop. 27 (1), 71–87 (2024).
Yahia, E. M., de Jesús Ornelas-Paz, J., Brecht, J. K., García-Solís, P. & Celis, M. E. M. The contribution of mango fruit (Mangifera indica L.) to human nutrition and health. Arab. J. Chem. 16 (7), 104860 (2023).
Robles-Sánchez, R. M. et al. Quality index, consumer acceptability, bioactive compounds, and antioxidant activity of fresh cut ataulfo mangoes (Mangiferaindica L.) as affected by low temperature storage. J. Food Sci. 74, S126–S134 (2009).
Gil, A. M. et al. Study of the compositional changes of mango during ripening by use of nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 48 (5), 1524–1536 (2000).
Pinsirodom, P., Taprap, R. & Parinyapatthanaboot, T. Antioxidant activity and phenolic acid composition in different parts of selected cultivars of mangoes in Thailand. Int. Food Res. J. 25(4). (2018).
Akther, S. et al. Drying methods effect on bioactive compounds, phenolic profile, and antioxidant capacity of mango powder. J. King Saud University-Science. 35 (1), 102370 (2023).
Ramirez, J. E., Zambrano, R., Sepúlveda, B. & Simirgiotis, M. J. Antioxidant properties and hyphenated HPLC-PDA-MS profiling of Chilean Pica mango fruits (Mangifera indica L. Cv. piqueño). Molecule 19, 438–458 (2014).
Siddiq, M., Sogi, D. S. & Dolan, K. D. Antioxidant properties, total phenolics, and quality of fresh-cut Tommy Atkins mangoes as affected by different pre-treatments. LWT Food Sci. Technol. 53, 156–162 (2013).
Sogi, D. S., Siddiq, M., Greiby, I. & Dolan, K. D. Total phenolics, antioxidant activity, and functional properties of Tommy Atkins mango peel and kernel as affected by drying methods. Food Chem. 141, 2649–2655 (2013).
Manthey, J. A. & Perkins-Veazie, P. Influences of harvest date and location on the levels of β-carotene, ascorbic acid, total phenols, the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera indica L). J. Agric. Food Chem. 57, 10825–10830 (2009).
Simirgiotis, M. J., Theoduloz, C., Caligari, P. D. S. & Schmeda-Hirschmann, G. Comparison of phenolic composition and antioxidant properties of two native Chilean and one domestic strawberry genotypes. Food Chem. 113, 377–385 (2009).
Robles-Sánchez, R. M., Rojas-Graü, M. A., Odriozola-Serrano, I., González-Aguilar, G. A. & Martín-Belloso, O. Effect of minimal processing on bioactive compounds and antioxidant activity of fresh-cut ‘Kent’mango (Mangifera indica L). Postharvest Biol. Technol. 51 (3), 384–390 (2009).
Rahaman, M. M., Hossain, R., Herrera-Bravo, J., Islam, M. T., Atolani, O., Adeyemi, O. S., … Sharifi‐Rad, J. (2023). Natural antioxidants from some fruits, seeds, foods, natural products,and associated health benefits: An update. Food science & nutrition, 11(4), 1657–1670.
Fatima, H. et al. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: an in vitro biological and phytochemical investigation. BMC Complement. Altern. Med. 15 (1), 376 (2015).
Muscolo, A., Mariateresa, O., Giulio, T. & Mariateresa, R. Oxidative stress: the role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 25 (6), 3264 (2024).
Laophongphit, A., Siripornadulsil, S. & Siripornadulsil, W. Improvements in the functions of probiotic-based mango pulp rich in phenolic and proline antioxidants by treatment with pectinase and fermentation with lactic acid bacteria. LWT 181, 114756 (2023).
Ribeiro, S. M. R., Queiroz, J. H., de Queiroz, M. E. L. R., Campos, F. M. & Sant’Ana, H. M. P. Antioxidant in mango (Mangifera indica L.) pulp. Plant Foods Hum. Nutr. 62 (1), 13–17 (2007).
Ma, X. et al. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hort. 129 (1), 102–107 (2011).
Robles-Sánchez, R. et al. Quality index, consumer acceptability, bioactive compounds, and antioxidant activity of fresh‐cut ataulfo mangoes (Mangifera indica L.) as affected by low‐temperature storage. J. Food Sci. 74 (3), S126–S134 (2009).
Hoang, B. Q., Nguyen, T. T. & Duong, D. N. T. Mango juice: behavior of physicochemical properties and antioxidant activity during lactic acid fermentation. Nutrire 48 (2), 35 (2023).
Hossain, M. A., Rana, M. M., Kimura, Y. & Roslan, H. A. Changes in biochemical characteristics and activities of ripening associated enzymes in mango fruit during the storage at different temperatures. Biomed. Res. Int. 2014 (1), 232969 (2014).
Goyal, H., Gill, M. S., Gill, P. S., Jawandha, S. K. & Singh, N. Changes in physicochemical and enzymatic activities of mango hybrids during fruit ripening. Erwerbs-obstbau 65 (2), 355–362 (2023).
Medlicott, A. P. & Thompson, A. K. Analysis of sugars and organic acids in ripening mangoes (Mangifera indica L. var Keitt) by high performance liquid chromatography. J. Sci. Food. Agric. 36 (7), 561–566 (1985).
Ornelas-Paz, J. D. J., Yahia, E. M. & Gardea, A. Changes in external and internal color during postharvest ripening of ‘Manila’ and ‘Ataulfo’ mango fruit and relationship with carotenoid content determined by liquid chromatography–APcI+-time-of-flight mass spectrometry. Postharvest Biol. Technol. 50 (2), 145–152 (2008).
Masibo, M. & He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 7, 309–319 (2008).
Ristow, M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat. Med. 20 (7), 709–711 (2014).
Xing, Y., Huang, M., Olovo, C. V., Mgbechidinma, C. L., Yang, Y., Liu, J., … Akan,O. D. (2023). Traditional fermented foods: challenges, sources, and health benefits of fatty acids. Fermentation, 9(2), 110.
Bento-Silva, A. et al. Factors affecting intake, metabolism and health benefits of phenolic acids: do we understand individual variability? Eur. J. Nutr. 59, 1275–1293 (2020).
Sova, M. & Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 12 (8), 2190 (2020).
Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid. Med. Cell. Longev. 2016, 3571614 (2016).
Nagasaka, R. et al. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem. Biophys. Res. Commun. 358, 615–619 (2007).
Briz, M. R. M., Ruiz, B. S. & Bravo-Clemente, L. Methylxanthines: Dietary Sources, Bioavailability, and Health Benefits. In Fruit and Vegetable Phytochemicals, E.M. Yahia (Ed.). John Wiley & Sons, pp. 183–198; (2017).
Fusco, R., Di Paola, R., Cuzzocrea, S., Matera, M. G. & Page, C. The cardiovascular effects of xanthines and selective PDE inhibitors: A risk–benefit analysis. Martínez-García MA, Pépin JL, Cazzola M. Cardiovascular Complications of Respiratory Disorders (ERS Monograph). Sheffield, European Respiratory Society, 279–286; (2020).
Majhi, S. Recent developments in the synthesis and anti-cancer activity of acridine and xanthine-based molecules. Phys. Sci. Reviews. 8 (9), 2405–2439 (2023).
Palafox-Carlos, H., Yahia, E. M. & González-Aguilar, G. A. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC-DADMS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 135, 105–111 (2012).
Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: a review. LWT Food Sci. Technol. 40, 1–11 (2007).
Rehman, M. M., Khiyanagi, T., Komiyana, T., Sato, S. & Konishi, T. Effect of anthocyanins on psychologcal stress-induced oxidative stress and neuro-transmitter status. J. Agric. Food Chem. 56, 7545–7550 (2008).
Matsumura, Y., Kitabatake, M., Kayano, S. I. & Ito, T. Dietary phenolic compounds: their health benefits and association with the gut microbiota. Antioxidants 12 (4), 880 (2023).
Abd Elgadir, M., Chigurupati, S. & Mariod, A. A. Selected potential pharmaceutical and medical benefits of phenolic compounds: recent advances. Funct. Food Science-Online ISSN: 2767–3146. 3 (7), 108–128 (2023).
El-Saadony, M. T., Zabermawi, N. M., Zabermawi, N. M., Burollus, M. A., Shafi, M.E., Alagawany, M., … Abd El-Hack, M. E. (2023). Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Reviews International, 39(4), 2138–2160.
Abd-Elgadir, M., Chigurupati, S. & Mariod, A. A. Selected potential pharmaceutical and medical benefits of phenolic compounds: Recent advances. Functional Food Science-Online ISSN: 2767–3146, 3(7), 108–128; (2023).
Pyrzynska, K. Ferulic acid—a brief review of its extraction, bioavailability and biological activity. Separations 11 (7), 204 (2024).
Karademir, Y., Mackie, A., Tuohy, K. & Dye, L. Effects of Ferulic Acid on cognitive function: a systematic review. Mol. Nutr. Food Res., 2300526. (2024).
Zhang, Y. et al. In vitro and in silico studies of the structure and functional properties of the lactoferrin-chlorogenic acid complex. Food Hydrocoll. 144, 109051 (2023).
Lv, H. & Guo, S. Comparative analysis of flavonoid metabolites from different parts of Hemerocallis citrina. BMC Plant Biol. 23 (1), 491 (2023).
Kisiel, A., Krzemińska, A., Cembrowska-Lech, D. & Miller, T. Data science and plant metabolomics. Metabolites 13 (3), 454 (2023).
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
Aniqa performed the experiments, wrote the manuscript. ZFR supervised the work, and proofread the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Manuscript does not contain human-related data therefore consent to participate is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aniqa, Rizvi, Z.F. Ripening associated antioxidant and phytochemical changes in mango (Mangifera indica) cultivar Dusehri. Sci Rep 15, 410 (2025). https://doi.org/10.1038/s41598-024-84426-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84426-9