Abstract
Thyroid dysfunctions are common in type 1 diabetes mellitus (T1DM) pregnancies, impacting embryogenesis and fetal neurodevelopment. This study investigates the effects of subclinical hypothyroidism and BDNF (Brain-derived neurotrophic factor) telomere length in T1DM mothers and their newborns. In a recent study, researchers found an inverse relationship between TSH (thyroid-stimulating hormone) levels and telomere length in the cord blood of newborns. This was prospective cohort analysis of 70 mothers and their newborns with T1DM. The study measured leukocyte telomere length (LTL) in maternal and neonatal samples. Subclinical hypothyroidism during the first trimester was characterized by TSH levels ranging from 2.5 to 5.0 mIU/L alongside normal free thyroxine (FT4) concentrations. In this study, we proved that maternal telomere length predicts telomere length in the newborn. Furthermore, we investigated the influence of maternal hypothyroidism on telomere length in the newborn. Maternal hypothyroidism in the first trimester of pregnancy has a strong influence on the shortening of newborn telomeres. BDNF has a positive effect on maternal and newborn telomere length. These results can have an important impact on the subsequent development of a child born to a diabetic mother. Health and disease associated with telomere length later in life may be programmed at birth.
Similar content being viewed by others
Introduction
Telomeres, the protective complexes at the termini of chromosomes, are instrumental in preserving cellular integrity and have been implicated in various human diseases1. The length of telomeres, influenced by genetics, environmental factors, diet, and ageing, is crucial for cellular ageing and susceptibility to disease2. In this context, Type 1 diabetes mellitus (T1DM) is an autoimmune condition characterized by the selective destruction of β-cells and consequent insulin secretion anomalies3. Pregnancy in women with T1DM introduces significant risks, impacting both maternal and neonatal health, with emerging evidence suggesting that diabetic pregnancies may predispose offspring to long-term metabolic disturbances, thereby highlighting the developmental origins of health and disease paradigm4,5.
Thyroid hormones (THs) are essential for embryonic development, with maternal thyroxine (T4) transfer to the embryo playing a crucial role, especially during early gestation6. THs are integral for regulating metabolic processes, including glucose and lipid metabolism and calcium homeostasis in the mother and fetus, underpinning normal fetal growth and development7. Pregnant women with subclinical hypothyroidism exhibit a heightened susceptibility to metabolic dysregulation, underscoring the importance of monitoring thyroid hormones and thyroid peroxidase antibodies (TPO) in T1DM pregnancies due to the frequent co-occurrence of thyroid disorders and diabetes8.
Subclinical hypothyroidism during pregnancy is associated with increased risks of adverse outcomes and potential neurocognitive impairments in offspring9. Insufficient maternal T4 production early in pregnancy is linked to an elevated risk of neurological impairments in children10.
Brain-derived neurotrophic factor (BDNF), a member of the nerve growth factor family, plays a crucial role in neurogenesis and synaptic transmission regulation, maintains adult synapses in the CNS, and enhances cognitive function11,12. Synthesized in the CNS during fetal development, BDNF significantly influences brain development, glucose and lipid metabolism, angiogenesis, placental development, and fetal growth12. In both the brain and periphery, BDNF also plays a crucial role in regulating glucose and energy metabolism12,13.
The study aimed to investigate the effect of subclinical hypothyroidism and BDNF on telomere length in T1DM mothers and their newborns.
Methods
Study design
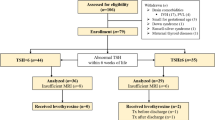
In this prospective cohort study, we consecutively included 84 women with type-1 diabetes mellitus before completing ten gestational weeks and had a single living fetus. Among these, 14 were excluded due to various reasons: withdrawal of participation (n = 2), delivery at another facility (n = 3), miscarriage (n = 3), and inability to accurately measure levels of thyroid hormones, BDNF, or telomeres (n = 6). The remaining 70 participants were stratified based on thyroid-stimulating hormone (TSH) levels into the subclinical hypothyroid group (SHG, n = 35) and the euthyroid group (ETG, n = 35). All participants underwent cesarean section for delivery. This study started on 1 February 2019 and ended on 31 January 2021 and was conducted at the Referral Center Ministry of Health, Department of Obstetrics and Gynecology, Clinical Hospital Centre Zagreb, School of Medicine, University of Zagreb.
Inclusion criteria
We included 70 pregnant women with T1DM and singleton pregnancies who received insulin therapy for at least two years. At pregnancy confirmation, the HbA1c was ≤ 8% (64 mmol/mol). All pregnant women received intensified insulin therapy with fast-acting insulin and long-acting insulin.
Exclusion criteria
Pregnant women with T1DM who had proliferative retinopathy, nephropathy, and chronic hypertension were excluded from the study. Additionally, women under the age of 18 years and those with major fetal defects observed on the 11 to 13 weeks of gestation ultrasound scan were excluded.
Data collection
Pregnancy information, gestational weight gain expressed as the difference in weight before pregnancy (self-reported) and at the time of delivery, and pre-pregnancy body mass index (kg/m2) were calculated from pre-pregnancy values and collected for each participant. Neonatal macrosomia was defined as a birth weight of ≥ 4000 g. Large for gestational age (LGA) was defined if birth weight was ≥ 90th percentile for gestation weight and sex. Ponderal index was calculated according to the formula: PI = weight (g)/height (cm)3. The prospective study included newborns of T1DM mothers who were evaluated for their clinical condition immediately after birth. Anthropometric measurements (body mass and length) were taken upon birth. Maternal venous blood samples were collected both the pregnancy trimester and cesarean section, with analyses of glucose, BDNF, thyroid hormones (TSH, FT3, and FT4), and HbA1c percentage. Umbilical vein blood samples were obtained immediately after birth, but before removing the placenta, for glucose, C-peptide, BDNF, and thyroid hormones (TSH, FT3, FT4). Blood was collected from an umbilical vein on the placenta’s side after clamping the umbilical cord. At delivery, whole blood samples from the mother and umbilical vein were collected for telomere length.
Analysis of blood sample
The quantification of glucose was carried out using hexokinase method on a Cobas C301 analyser, utilizing from the same manufacturer, (Roche, Basel, Switzerland). Measurement of HbA1c levels in whole blood was conducted through turbimetric inhibition immunoassays on a Cobas C501 instrument, also from Roche (Basel, Switzerland). The serum C-peptide concentration was evaluated by electrochemiluminescence immunoassays (ECLIAs) with Elecsys immunoassays from Roche Diagnostics (Switzerland), with the lower detection limit of 0·003 nmol/L. Neonatal insulin resistance was calculated based on a homeostasis model available at https://homa-calculator.informer.com/2.2/, accessed on 23 January 2023. To determine subclinical hypothyroidism, we relied on TSH values. Euthyroidism was considered for the first trimester when the TSH value was less than 2.5 mIU/L. For the quantitative determination of human thyroid stimulating hormone (TSH), we used the Architect TSH, FT3, and FT4, employing a chemiluminescent microparticle immunoassay (CMIA) from Abbott Longford Co. (Longford Ireland). The definition of subclinical hypothyroidism was based on the TSH value.
Serum concentration of Brain-Derived-Neurotropic Factor (BDNF) was determined using a Sandwich ELISA Kit from ChemiKine, Merck KGaA, Darmstadt, Germany (No. Cyt306), with a test sensitivity of 15 pg/mL. The Leptin serum concentration was determined with a sandwich Kit from Tecan, IBL International, Hamburg, Germany (Cat. No. MD53001).
DNA isolation, quantification, and dilution
Blood samples (3 mL each) were collected from 70 mothers and 70 umbilical veins, all stored in EDTA tubes. The samples were kept at -80 °C before DNA extraction to enable the laboratory procedure of telomere length determination in the Laboratory for Epigenetics and Molecular Medicine, Department of Medical Biology, Faculty of Medicine, University of Zagreb. DNA was isolated from 3 mL of whole blood using a protocol based on protein precipitation at high salt concentrations14. The resulting DNA was further purified with the NucleoSpin gDNA Clean-up kit (Machery-Nagel, Düren, Germany) according to the manufacturer’s instructions to ensure high-quality, high-purity DNA suitable for downstream analysis of telomere length. DNA quality and concentration were measured with a NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), yielding concentrations suitable for subsequent analysis. Each DNA sample was then diluted to a working concentration of 20 ng/µL, aliquoted into 1.5 mL Eppendorf tubes (Eppendorf, Hamburg, Germany), and stored at − 20 °C until analysis.
qPCR analysis
We used human β-globin as a single-copy reference gene in telomere length studies because of its stability across cell types and low variability between samples. This stability allows for more accurate normalization in qPCR-based measurements of telomere length, as variations in telomere signals can be better assessed compared to a consistent internal control. Previous studies, such as those conducted by Cawthon15, and Joglekar et al.16, successfully used β-globin for telomere quantification, establishing it as a reliable standard in telomere research.
The annealing temperature was optimized at 58°C for both telomere and β-globin analysis. qPCR reactions were performed in triplicate, using 1 µL of isolated DNA (concentration: 20 ng/µL), Fast SYBR Green master mix ThermoFisher Scientific, Waltham, MA, USA, and 10 µM forward and reverse primers. Each plate contained triplicates of no sample control (NTC), control DNA (Promega, Madison, WI, USA), and DNA samples with telomere primers and β-globin primers. Amplification and real-time PCR analysis were performed using the CFX96 Touch real-time PCR detection system from Bio-Rad Laboratories, Hercules, CA, USA, and CFX Maestro software from Bio-Rad Laboratories, Hercules, CA, USA, following the established protocol of Joglekar et al. in 202016.
Primer sequences
Primer sequences used in this study are based on those from Joglekar et al., 2020, specifically validated for qPCR assays measuring telomere and β-globin sequences. The telomere primer pair targets the repetitive sequence TTAGGG, while the β-globin primer pair targets a stable region in the β-globin gene, ensuring accurate relative quantification through the T/S ratio. In the telomere analysis, the data obtained by quantitative PCR were expressed as the T/S ratio of the number of gene copies. This T/S ratio is proportional to the average telomere length.
Sample size
We tested the means Wilcoxon sign rank test (match pairs) to test means for power calculations concerning maternal and neonatal telomere lengths. With a total sample size of 47 patients, an α-level is 0.05 and a power (1-β err prob) of 95%, the analyze was conducted.
Statistical analyses
Participants with missing data for a specific outcome were excluded from the corresponding analyses. Statistical analyses were conducted using SPSS statistical package, version 26 from IBM, Armonk, NY, USA. Categorical data were represented using absolute and relative frequencies. Group differences between categorical variables were tested using the Pearson Chi-square test. Numerical data in case of a normal distribution, and in other instances, by the median and interquartile range limits. Student’s t-test was used to assess group differences for normally distributed continuous variables, while differences for non-normally distributed continuous variables were evaluated using the Mann–Whitney U test. Data that was not normally distributed underwent log transformation (natural logarithm) prior to analysis. To assess the relationship between maternal and neonatal telomere lengths, Pearson’s correlation coefficient was used. Regression with the Spearman correlation coefficient (rrho) was performed on non-normally distributed data. The Wilcoxon signed-rank test was to measure continuous data between maternal and neonatal telomere. Finally, multiple linear regression analyses were applied to identify maternal and neonatal LTL associated with gestational weight gain (GWG), TSH, and BDNF. All P-values are two-sided and P-values p < 0.05.
Results
Based on TSH values, participants were categorized into the subclinical hypothyroid group (SHG, n = 35) and euthyroid group (ETG, n = 35). All pregnancies were terminated by caesarean section. The euthyroid group (ETG) had significantly lower TSH concentration than the subclinical hypothyroid group (p < 0.001). The two study groups had no notable differences in age, duration and onset of T1DM, BMI, gestational weight gain, HbA1c, fT3, fT4, BDNF or maternal glucose. Maternal telomere lengths significantly differ between the two groups. Further details are presented in Table 1.
The neonates to subclinical hypothyroid mothers exhibited greater birth weights and lengths compared to neonates born to euthyroid mothers (P = 0.040, p = 0.030, respectively). Neonatal telomere lengths were also longer in the euthyroid maternal group than in the group of maternal subclinical hypothyreoidism (P = 0.010, Wilcoxon sign-rank test). Additionally, the prevalence of Large for Gestational Age (LGA) and fetal macrosomia were significantly higher in the subclinical hypothyroid mothers (P = 0.034 and P = 0.029). There is no difference between TSH, fT3, fT4, C-peptide, glucose, insulin resistance, and leptin in umbilical vein in two study groups.
Significant differences were observed between maternal telomere lengths and neonatal telomere length (1.7 ± 1.1:2.3 ± 1.2), p < 0.001; Wilcoxon sign test). Further details are presented in Table 2.
Figure 1 illustrates the linear correlation between maternal and neonatal telomere lengths.
Figure 1 depicts the linear correlation between maternal and neonatal telomere lengths, demonstrating a strong positive relationship (r = 0.750, 95% Confidence interval 0.548- 0.837) with a highly significant P-value (P < 0.001), indicating a robust association between the telomere lengths of mothers and their neonates.
Furthermore, a significant nonparametric correlation was identified between maternal telomere lengths and BDNF levels in maternal vein serum (rrho = 0.370, p = 0.003). Neonatal telomere length exhibited a negative correlation with maternal glucose concentration (rrho = -0.411, P = 0.006) and C-peptide levels in umbilical vein serum (rrho = -0.275; p = 0.045). BDNF levels in maternal vein serum during the first trimester also correlated with neonatal telomere length (rrho = 0.510; P < 0.001). Conversely, an inverse nonparametric correlation was observed between neonatal telomere lengths and maternal TSH concentration (rrho = -0.296, P = 0.018) (Table 3).
Table 4 illustrates the adjusted relationships between maternal telomere lengths and various factors. Specifically, it shows a negative association with gestational weight gain (β = -0.259, 95% CI -0.122 to -0.010), maternal thyroid-stimulating hormone (TSH) levels (β = -0.231, 95% CI –0.422 to -0.015), and a positive relationship with maternal brain-derived neurotrophic factor (BDNF) in the first trimester (β = 0.523, 95% CI 0.001 to 0.002). Furthermore, the table also depicts the adjusted relationship between neonatal telomere lengths and these same factors, highlighting a negative correlation with gestation weight gain (β = -0.266, 95% CI -0.138 to – 0.009), and maternal TSH levels (β = -0.237, 95% CI -0.493 to -0.010), alongside a positive association with maternal BDNF levels in the first trimester (β = 0.395, 95% CI 0.000 to 0.002).
Discussion
As expected, our study confirmed that maternal telomeres are generally shorter than neonatal telomeres, consistent with previous research findings17. We also found a strong positive correlation between maternal and neonatal telomere lengths, indicating that mothers with longer telomeres gave birth to offspring with longer TL, as reported by Daneels and colleagues18. This suggests a hereditary component influencing neonatal telomere length. Several other studies have also reported longer neonatal than maternal telomeres and demonstrated an association between maternal and neonatal telomere lengths19,20.
It is important to note that telomere shortening is influenced not only by ageing but also by various genetic and environmental factors and diseases21. While our study focused on mothers with T1DM, we did not directly compare maternal and neonatal telomere lengths between diabetic and non-diabetic pregnant women. Research, such as the meta-analysis conducted by Wang et al.12, has shown that diabetes can affect telomere length, with individuals with diabetes having shorter telomeres compared to healthy individuals. However, the exact impact of diabetes on telomere length concerning age remains unclear. Wang and colleagues suggested that diabetes might lead to senescence or apoptosis of islet β-cells, causing gradual telomere shortening, which could contribute to diabetes complications. Understanding how diabetes affects telomere length is crucial for diabetes prevention and treatment22. It is worth noting that other studies, like the one by Cross et al.23, found no significant difference in cord blood telomere length between pregnancies of women with diabetes control subjects. Similarly, Gilfillan et al.24 did not find a difference in neonatal TL when comparing healthy pregnant women with gestational and pregestational diabetes. However, their sample sizes were relatively small.
In our study, we observed that neonates born to mothers with subclinical hypothyroidism had shorter telomeres compared to neonates born to euthyroid mothers.
The diagnosis of subclinical hypothyroidism is based on TSH values (TSH < 2.5–5.0 mIU/L) during the first trimester of pregnancy. We evaluated thyroid hormones, including TSH, fT3, and fT4, in pregnant women during their first trimester, specifically between the 10th and 11th weeks. Before the 12th week of pregnancy, the fetus depends on the mother’s thyroxine for hormone production. Even after this milestone, the fetus is still partially reliant on the mother’s pregnancy hormones. Thyroid hormones are crucial in the regulation of glucose, lipid and calcium metabolism in pregnant women and fetuses. In addition, they are crucial for the proper growth and neurodevelopment of the fetus. Maternal subclinical hypothyroidism during pregnancy is associated with an increased risk of adverse neonatal outcomes, including delayed intellectual and motor development, low birth weight, preterm birth, fetal distress, and fetal growth restriction25. The findings of a meta-analysis conducted by Liu et al. are convincing25. This highlights the importance of monitoring thyroid hormone levels during pregnancy to ensure optimal cognitive and physical development of the offspring. Authors Ohadi et al.26 conducted a study comparing fetal TSH and newborn telomere length. They found an inverse relationship between TSH levels and relative telomere length in cord blood of newborns, which is consistent with our study. Their finding suggests that lower fetal thyroid hormones may influence “biological” age at birth and, consequently, potential longevity. Stier et al. conducted a study that found that high thyroid hormone levels during pregnancy in birds can lead to longer telomeres6. This effect was observed both at birth and at the end of the growth period. Their research is consistent with our results.
It is noteworthy that in our study, approximately 50.8% of the participants were diagnosed with subclinical hypothyroidism during the first trimester of pregnancy, with 12 (18.2%) pregnant women having Hashimoto’s thyroiditis before pregnancy. In our study, pregnant women received levothyroxine therapy after diagnosis of subclinical hypothyroidism. Based on our findings, we emphasize the urgent need to regulate thyroid hormone levels before pregnancy. This is essential not only to protect the health of the mother, but also to ensure optimal fetal development and overall well-being.
We also observed a correlation between BDNF levels in maternal blood and maternal and neonatal telomere size. Neonates with telomeres shorter than 2.2 T/S had significantly (P = 0.012) lower BDNF levels [median 691.4 (IQR 543.9; 953.2)] compared to those with longer telomeres longer than 2.2 T/S [median 874.8 (IQR 662.7; 1152.0)]. Additionally, higher maternal BDNF levels were associated with increased neonatal telomere length. This correlation aligns with numerous psychiatric studies that have reported a connection between BDNF levels and telomere length27,28. This finding suggests a potential link between neurodevelopment factors and telomere biology. Mothers who have hypothyroidism during the first trimester of pregnancy were found to have lower levels of BDNF compared to those without the condition. However, the difference was not statistically significant. A study conducted on rats by Liu D et al. showed that subclinical hypothyroidism caused a decrease in the expression of BDNF in both genetic and protein levels29. The connection between BDNF and telomere biology influences various cellular processes, such as cell proliferation, neuronal differentiation, and neuronal survival, as reported by Greenberg and colleagues30.
The strength of our study. This study is the first to investigate the dynamics of telomere length in pregnant women with subclinical hypothyroidism and T1DM, as well as in their newborns. To ensure the accuracy of the results, blood samples were collected from the mothers and umbilical veins of the newborns during labor, reducing the impact of the postpartum period. Additionally, the study was designed to eliminate any potential influence from the mode of delivery or the nutritional status of the women by ensuring that they had been fasting for at least 8 h before the cesarean section. While the study offers valuable insights, it is important to acknowledge its limitations. The relatively small sample size and the lack of healthy control group may impact the study’s findings. However, the study’s limitations are its small sample size and the absence of a healthy control group. The limitation of this research is the failure to measure the percentage of immature blast cells using flow cytometry. This emphasizes the need for employing flow cytometry in future studies to ensure accurate assessments.
In conclusion, a study reveals that hereditary factors and the intrauterine environment in mothers with T1DM can influence neonatal telomere lengths. We also found that subclinical hypothyroidism during pregnancy may contribute to shorter neonatal telomeres. Brain-derived neurotrophic factor plays a pivotal in mediating the relationship between subclinical hypothyroidism and telomere length. Understanding these relationships is essential for gaining insights into the factors that influence neonatal telomere length, which is considered a marker of biological ageing and could have implications for future health outcomes.
References
Lu, W., Zhang, Y., Liu, D., Songyang, Z. & Wan, M. Telomeres structure, function, and regulation. Exp. Cell Res. 319, 133–134. https://doi.org/10.1016/j.yexer.2012.09.005 (2013).
Notterman, D. A. & Schneper, L. Telomere time-why we should treat biological age cautiously. JAMA Netw. Open 3(5), e204352. https://doi.org/10.1001/jamanetworkopen.2020.4352 (2020).
Holt, R. I. G. et al. The management of type 1 diabetes in adults. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 44, 2589–2625. https://doi.org/10.2337/dci21-0043 (2021).
Garey, C. et al. Preeclampsia and other pregnancy outcomes in nulliparous women with type 1 diabetes: A retrospective survey. Gynecol. Endocrinol. 36, 982–985. https://doi.org/10.1080/09513590.2020.1749998 (2020).
Simeoni, U. & Barker, D. J. Offspring of diabetic pregnancy: Long-term outcomes. Semin. Fetal Neonatal Med. 14, 119–124. https://doi.org/10.1016/j.siny.2009.01.002 (2009).
Stier, A. et al. Born to be young? Prenatal thyroid hormones increase early-life telomere length in wild collared flycatchers. Biol. Lett. 16, 20200364. https://doi.org/10.1098/rsbl.2020.0364 (2020).
Shields, B. M. et al. Fetal thyroid hormone level at birth is associated with fetal growth. J. Clin. Endocrinol. Metab. 96, E934–E938. https://doi.org/10.1210/jc.2010-2814 (2011).
Eom, Y. S., Wilson, J. R. & Bernet, V. J. Links between thyroid disorders and glucose homeostasis. Diabetes Metab. J. 46, 239–256. https://doi.org/10.4093/dmj.2022.0013 (2022).
Haddow, J. E. et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 341, 549–555. https://doi.org/10.1056/NEJM199908193410801 (1999).
de Escobar, G. M., Obregón, M. J. & del Rey, F. E. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract. Res. Clin. Endocrinol. Metab. 18, 225–248. https://doi.org/10.1016/j.beem.2004.03.012 (2004).
Binder, D. K. & Scharfman, H. E. Brain-derived neurotrophic factor. Growth Factors 22, 123–131. https://doi.org/10.1080/08977190410001723308 (2020).
Briana, D. D. & Malamitsi-Puchner, A. Developmental origins of adult health and disease: The metabolic role of BDNF from early life to adulthood. Metabolism 81, 45–51. https://doi.org/10.1016/j.metabol.2017.11.019 (2018).
Guzzardi, M. A. et al. Elevated glycemia and brain glucose utilization predict BDNF lowering since early life. J. Cereb. Blood Flow Metab. 38, 447–455. https://doi.org/10.1177/0271678X17697338 (2018).
Miller, S. A., Dykes, D. D. & Polesky, H. F. A simple salting-out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215. https://doi.org/10.1093/nar/16.3.1215 (1988).
Cawthon, R. M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, e47. https://doi.org/10.1093/nar/30.10.e47.) (2002).
Joglekar, M. V. et al. An optimised step-by-step protocol for measuring relative telomere length. Methods Protoc. 3, 27. https://doi.org/10.3390/mps3020027 (2020).
Panelli, D. M. & Bianco, K. Cellular aging and telomere dynamics in pregnancy. Curr. Opin. Obstet. Gynecol. 34, 57–61. https://doi.org/10.1097/GCO.0000000000000765 (2022).
Daneels, L. et al. Maternal vitamin D and newborn telomere length. Nutrients 13, 2012. https://doi.org/10.3390/nu13062012 (2021).
Martens, D. S. et al. Newborn telomere length predicts later life telomere length: Tracking telomere length from birth to child- and adulthood. EBioMedicine 63, 103164. https://doi.org/10.1016/j.ebiom.2020.103164 (2021).
Send, T. S. et al. Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology 42, 2407–2413. https://doi.org/10.1038/npp.2017.73 (2017).
Schneider, C. V. et al. Association of telomere length with risk of disease and mortality. JAMA Intern. Med. 182, 291–300. https://doi.org/10.1001/jamainternmed.2021.7804 (2022).
Wang, J. et al. Association between telomere length and diabetes mellitus: A meta-analysis. J. Int. Med. Res. 44, 1156–1173. https://doi.org/10.1177/0300060516667132 (2016).
Cross, J. A. et al. Cord blood telomere length, telomerase activity and inflammatory markers in pregnancies in women with diabetes or gestational diabetes. Diabet. Med. 27, 1264–1270. https://doi.org/10.1111/j.1464-5491.2010.03099.x (2010).
Gilfillan, C. et al. Leukocyte telomere length in the neonatal offspring of mothers with gestational and pre- gestational diabetes. PLoS ONE 11, e0163824. https://doi.org/10.1371/journal.pone.0163824 (2016).
Liu, Y., Chen, H., Jing, C. & Li, F. The association between maternal subclinical hypothyroidism and growth, development, and childhood intelligence: A meta-analysis. J. Clin. Res. Pediatr. Endocrinol. 10, 153–161. https://doi.org/10.4274/jcrpe.4931 (2018).
Ohadi, H. et al. Umbilical cord blood thyroid hormones are inversely related to telomere length and mitochondrial DNA copy number. Sci. Rep. 14, 3164. https://doi.org/10.1038/s41598-024-53628-6 (2024).
Vasconcelos-Moreno, M. P. et al. Telomere length, oxidative stress, inflammation and BDNF levels in siblings of patients with bipolar disorder: Implications for accelerated cellular aging. Int. J. Neuropsychopharmacol. 20, 445–454. https://doi.org/10.1093/ijnp/pyx001 (2017).
Delgado-Losada, M. L. et al. Loneliness, depression, and genetics in the elderly: Prognostic factors of a worse health condition?. Int. J. Environ. Res. Public Health 19, 15456. https://doi.org/10.3390/ijerph192315456 (2022).
Liu, D. et al. The effect of maternal subclinical hypothyroidism during pregnancy on brain development in rat offspring. Thyroid 20, 909–915. https://doi.org/10.1089/thy.2009.0036 (2010).
Greenberg, M. E. et al. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 29, 12764–12767. https://doi.org/10.1523/JNEUROSCI.3566-09.2009 (2009).
Acknowledgements
The authors would like to express their sincere gratitude to Prof. Nino Sinčić, Ph.D., the esteemed head of the epigenetic biomarker research group (epiMark), for his invaluable guidance. We also extend our thanks to Dr. Dora Raos for her expertise in determining telomere length in the laboratory, and to Dr. Marina Horvatiček for her dedication to collecting blood samples from mothers and umbilical veins, which was crucial to our research.
Funding
This research was supported by the Croatian Science Foundation under grant PRE-HYPO No. IP2018-01-1284.
Author information
Authors and Affiliations
Contributions
J.D. conceived the experiment, J.D. and M.I. conducted the experiment, J.D. and M.I. analysed the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statements
The Ethics Committee at the School of Medicine, the University of Zagreb, approved the study (No. 380-59-10106-19-111/26). All women in the study provided informed consent for themselves and their newborns. All the methods were performed following the Declaration of Helsinki—Ethical principles for medical research involving human subjects.
Data availability
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Djelmis, J., Ivanisevic, M. Influence of subclinical hypothyroidism and brain-derived neurotropic factor on telomere length dynamics in type 1 diabetic pregnancies and their newborns. Sci Rep 15, 194 (2025). https://doi.org/10.1038/s41598-024-84430-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84430-z