Abstract
Plants inhabiting saline areas develop specific morpho-anatomical and physiological features to survive. Sporobolus ioclados is among the few grass species that dominate highly saline habitats. This is a salt excretory species and can potentially be important for phytoremediation of salt-affected lands. Three ecotypes of Sporobolus ioclados (Trin.) Nees (DF-Derawar Fort (LSE), BD-Bailahwala Dahar (MSE), LS-Ladam Sir) from the Cholistan Desert were evaluated to investigate structural and functional modifications for salt tolerance under controlled conditions in hydroponic growth medium using half-strength Hoagland’s nutrient solution. Three salinity (NaCl) treatments were provided, namely 0 (control), 150, and 300 mM. All three ecotypes showed different structural and physiological modifications under salinity stress. Structural and functional traits were more developed in the HSE. Modifications. Structural features include intensity of sclerification and thicker leaves. Functional features were high concentration of toxic ions excretion, organic osmolytes accumulation, and maintenance of leaf turgor, photosynthesis and water use efficiency. All these confer it an excellent material for the phytoremediation as well as revegetation of highly saline lands.
Similar content being viewed by others
Introduction
Salinity stress is a major threat to growth and biomass production of plants, particularly those colonizing arid and semiarid regions. Plants develop specific structural and functional features that are critical for survival therein1. Salt tolerance is a complex mechanism2, therefore, salt tolerant (or halophytic) species are the model plants to explore adaptive traits. These traits can be incorporated in glycophytes for the enhancement of salt tolerance traits3
Halophytes develop very specific features to handle high salinity. Structural-based mechanisms include well-developed rooting system4, salt-excreting microhairs5, increased succulence6, intensive sclerification7, salt excretory glands8, and stomatal size, density and orientation9. Functional features are restricted or selective ion uptake10 and toxic ion excretion or compartmentalization11,12. More importantly, turgor maintenance by accumulating organic osmolytes13. High concentration of Na+ and Cl– in saline soils causes ionic imbalance in plants. This affects the uptake by root cells of several other metabolically active ions, which hampers several metabolic activities in different parts of plants i.e. leaf, stem and root14.
There are about 250 species in the genus Sporobolus. In Pakistan, ten species are reported15. The genus Sporobolus has many species that are known for high degree of salinity tolerance. The examples are Sporobolus airoides16, S. spicatus (Vahl) Kunth17, S. arabicus18, and S. virginicus19. Sporobolus ioclados (pan dropseed) dominates salt-affected inter-dune flats in the Cholistan Desert20.
Halophytic C4 grasses, including Sporobolus species, have developed specific mechanisms to cope salinity stress. Among these, “salt glands” which are bicellular leaf epidermal structures eliminating excess saline ions from shoots by excretion under salinity stress21. It is a perennial, stoloniferous grass. This species is widely distributed in coastal areas and desert habitats22. It is a highly palatable grass that is often over-grazed and can form a major component of plant communities23. Plant features, specifically anatomical characteristics, are strongly influenced by environmental heterogeneity24. However, genetically fixed characteristics during the evolutionary history of a plant express themselves under controlled environments25. Since hydroponic system provides a uniform and homogeneous growth medium, so it is possible to study evolutionary fixed characteristics of S. ioclados ecotypes. This led us to hypothesize that ecotypes of S. ioclados might respond independently to salt stress. Mechanisms for tolerating high levels of salts may be different in these ecotypes. The present study was conducted to explore tolerance mechanism at structural and functional levels, to evaluate phytoremediation potential, and to correlate structural and functional features under different salinities. This species was previously evaluated for structural and functional features along salinity gradient from its natural habitats20. For the present study, S, ioclados was examined under controlled conditions to evaluate genetically fixed traits in differently adapted ecotypes. The present study is a part of same project in which two grasses, Lasiurus scindicus26 and Aeluropus lagopoides27 were evaluated under controlled conditions.
Materials and methods
Collection sites
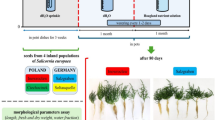
Three ecotypes of Sporobolus ioclados (Trin.) Nees from the Cholistan Desert (Fig. 1) were evaluated to investigate structural and functional modifications. The least saline ecotype (LSE) was collected from Derawar Fort. Moderately saline ecotype (MSE) was from Bailahwala Dahar. The highest saline ecotype (HSE) was from Ladam Sir.
Experimentation
Vegetative buds from naturally growing plants of each ecotype of S. ioclados were collected and grown in Faisalabad condition for six months to acclimatize under local conditions. A total of 160 ramets (vegetative buds), each with three tillers of equal size, were detached. The ramets were planted in a hydroponic medium using half-strength Hoagland’s solution following Hoagland and Arnon28. Plastic containers (capacity 25 L) were used for experimentation. The salt treatments were 0 (control), 150, and 300 mM of NaCl. Salinity levels were maintained gradually by adding 50 mM solution daily to prevent sudden salinity shock. The experiment was conducted for 8 weeks.
Structural and functional traits
Detailed structural and functional methodology has already been presented in Naz et al.26 and Naz et al.27.
Morphological traits
Morphological characters were measured at the end of the experiment. The number of leaves per plant was manually counted. Leaf area was calculated by the formula devised by Lopes et al.29.
Plant fresh weight was recorded by a portable digital balance immediately after uprooting the plants from growth medium.
Anatomical traits
Formalin acetic alcohol solution was used for the preservation of plant material for anatomical studies. The material was kept for 24 h and then transferred to acetic alcohol following Ruzin30. Sections were cut by a sharp-edge razor blade. Ethyl alcohol grades (30, 50, 70, 90 and 100% in distilled water) were used for dehydration of the sections. Biological stains safranin was used for staining lignified tissue (sclerenchyma and xylem vessels). Fast green will stain primary walls (parenchyma, phloem). Ocular micrometer was calibrated with stage micrometer. Measurements of different tissues and cells were taken with an ocular micrometer. Photographs were taken with a compound microscope (Meiji Techno Japan).
Abaxial stomatal area (µm2).
Leaf sheath anatomy
-
HTh − Leaf sheath thickness (µm)
-
HDE − Adaxial epidermal cell area (µm2)
-
HBE − Abaxial epidermal cell area (µm2)
-
HCC − Parenchymatous cell area (µm2)
-
HST − Sclerenchyma thickness (µm)
-
HVB − Vascular bundle area (µm2)
-
HMX − Metaxylem area (µm2)
-
HPA − Phloem area (µm2)
Leaf blade anatomy
-
LTh − Leaf thickness (µm)
-
LST − Sclerenchymatous thickness (µm)
-
LDE − Adaxial epidermal cell area (µm2)
-
LBE − Abaxial epidermal cell area (µm2)
-
LBC − Bulliform cell area (µm2)
-
LVB − Vascular bundle area (µm2)
-
LMX − Metaxylem area (µm2)
-
LPA − Phloem area (µm2)
Epidermal appendages
-
LDM − Adaxial microhair density
-
LBM − Abaxial microhair density
-
LTD − Trichome density
-
LTL − Trichome length (µm)
-
LDS − Adaxial stomatal density
-
LBS − Abaxial stomatal density
-
LDA − Adaxial stomatal area (µm2)
-
LBA − Abaxial stomatal area (µm2)
Gas exchange traits
Gas-exchange parameters were recorded by an infrared gas analyzer (LCA-4 ADC, Analytical Development Company, Hoddesdon, England). Specifications are presented in (Table 1). Leaf water potential was attained by a M-615 Scholander chamber (MMM—Mosler Tech Support, UK) from 8:00–10:00 a.m. Osmotic potential was recorded by a Wescor 5500 Vapor Pressure Osmometer (Artisan Technology Group, USA) (Table 2).
Gas exchange parameters
-
NAR-Net assimilation rate (µmol m−2 s−1)
-
TrR-Transpiration rate (mmol m−2 s−1)
-
StC-Stomatal conductance (mmol m−2 s−1)
-
SCC-Substomatal CO2 concentration (µmol mol−1)
-
WUE-Water use efficiency
Organic osmolytes
Total amino acids were recorded at optical density 570 nm by a UV–Visible spectrophotometer (Hitachi 220, Japan) following Moore and Stein31. Total soluble proteins were estimated at optical density 620 nm by a spectrophotometer (Hitachi, 220, Japan) following Lowry et al.32. Total soluble sugars were estimated at optical density 620 nm by a spectrophotometer (Hitachi, 220, Japan) in accordance with Yemm and Willis33. Proline was recorded according to Bates et al.34 and absorbance was recorded at 520 nm.
Organic osmolytes
-
TFP-Total free amino acids (µg g−1)
-
TSP-Total soluble proteins (µg g−1)
-
TSS-Total soluble sugars (mg g−1)
-
TPr-Total proline (µg g−1)
Chlorophyll pigments
Chlorophylls a and b were attained in accordance with Arnon35. Carotenoids were estimated by the Wellburn36 method. Absorbance recorded at 645, 663 and 480 nm using a spectrophotometer (Hitachi-220 Japan).
Photosynthetic pigments
-
Cha-Chlorophyll a (mg g−1)
-
Chb-Chlorophyll b (mg g−1)
-
Car-Carotenoids (mg g−1)
Ionic content
Tissue ionic content was measured in accordance with Wolf37. Cations (Na+, K+ and Ca2+) were estimated with a flame photometer (Model 410, Sherwood Scientific Ltd., Cambridge, UK). Chloride content was attained by a 926-chloride meter (Sherwood Scientific Ltd., Cambridge, UK).
Excreted ions
Ten fresh leaves were incised from the plant and washed with 100 ml of deionized H2O. Excreted ions were then estimated from the washed off water. The Na+ and K+ were attained with a 410-flame photometer (Sherwood Scientific Ltd., Cambridge, UK). The Ca2+ and Mg2+ were measured with an Analyst 3000-atomic absorption spectrophotometer (Perkin Elmer, Norwalk, USA). The Cl─ was attained with a 926-chloride meter (Sherwood Scientific Ltd., Cambridge, UK).
Leaf water content and succulence
Leaf water content and leaf succulence were calculated by the formulae:
Succulence was calculated in accordance with Tiku38.
Phytoremediation traits
Phytoremediation traits were recorded in accordance with of Diwan et al.39.
Dilution factor was calculated in accordance with Abbas et al.39.
where DW is the dry weight.
Statistical analysis
A two factorial completely randomized design with 4 replications was used for experimentation. The data were analyzed by a two-way analysis of variance using Minitab statistical software (v. 17). Means were compared by a Tukey test. A principal component analysis (PCA) was run to investigate relationship among growth, anatomical, physiological and excreted ions using R Studios (V 1.1.463). Heatmaps were constructed to evaluate the relationship of excreted ions with morpho-anatomical and physiological traits using a customized R code. Estimated response was calculated for excreted ions and different morho-anatomical and physiological traits. Transpiration rate and sub-stomatal CO2 concentration exhibited a significant variation (p < 0.05) of interaction, while interaction in chlorophyll b varied significantly at p < 0.01. In ionic content, root, shoot and leaf Na+, K+, Ca2+ and Cl– showed significant variation of ecotypes and treatment, while interaction varied significantly in root Cl– and root K+ (p < 0.05). In excreted ions, interaction varied significantly in excreted Na+ (p < 0.01), excreted K+ (p < 0.05), excreted Ca2+ (p < 0.01) and excreted Cl– (p < 0.05). Root water content depicted a significant variation of interaction (p < 0.01), while stem succulence varied significantly at p < 0.01. In Bioconcentration factor, ecotypes, treatments and their interaction varied significantly in all traits. In leaf translocation factor, interaction varied significantly at p < 0.001. All traits of dilution factor showed significant variation regarding ecotypes, treatments and their interaction.
Results
Leaf area showed significant variation of ecotypes (p < 0.001), treatments (p < 0.05) and interaction of ecotypes and treatments (p < 0.05) as presented in (Table 3). Ecotypes varied significantly in root fresh weight (p < 0.01), root dry weight (p < 0.001) and shoot dry weight (p < 0.05). In all leaf sheath anatomical traits, ecotypes, treatments and their interaction varied significantly. In leaf blade anatomical traits, interaction showed varied significantly in bulliform cell area (p < 0.001), vascular bundle area (p < 0.05) and phloem area (p < 0.001). In epidermal appendages, interaction revealed significant variation in adaxial and abaxial microhair density (p < 0.001), trichome density (p < 0.05), trichome length (p < 0.01) and abaxial stomatal density (p < 0.001).
Morphology
Morphological traits like leaf number and area, and root fresh weight decreased with salinity in the Derawar Fort (LSE), whereas leaf dry weight increased (Table 3). Traits such as stem fresh weight, dry weight and root dry weight increased only at 150 mM. Leaf fresh weight decreased significantly at 150 mM but thereafter increased at 300 mM. The Bailahwala Dahar (MSE) showed a decrease in leaf number and leaf area with salinity levels. Traits such as root and leaf fresh increased with salinity, while stem fresh weight increased only at 300 mM. Stem dry weight decreased only at 300 mM, while an increase was observed in leaf dry weight of Bailahwala Dahar (MSE). The Ladam Sir (HSE) responded differently with increasing salinity levels. Traits such as leaf number, leaf area, root fresh weight, leaf fresh weight and leaf dry weight increased with salinity. Stem fresh weight and root dry weight increased only at 300 mM, while no change was recorded in stem dry weight.
Among habitats, the morphological number of leaves, total leaf area, root fresh weight and stem fresh weight showed a significant decrease in the least saline ecotype (LSE) at highest salinity level (300 mM), while number of leaves and root fresh weight were highest in highly saline ecotype at 300 mM and 150 mM respectively. Stem fresh weight, leaf fresh weight, root dry weight, stem dry weight and leaf dry weight were the maximum in highly saline ecotype (HSE) at 300 mM salinity level.
Leaf sheath anatomy
Leaf sheath anatomical traits such as thicknesses of leaf sheath, adaxial and abaxial epidermis and sclerenchyma, and areas of vascular bundle and metaxylem increased with salinity in the Derawar Fort (LSE), (Table 3, Fig. 2), Phloem area decreased with salinity, while parenchymatous cell area decreased only at 300 mM in this ecotype. In the Bailahwala Dahar (MSE), a consistent increase with salinity was recorded in leaf sheath anatomical traits such as adaxial and abaxial epidermal thickness, parenchymatous cell area, sclerenchymatous thickness and metaxylem area. Leaf sheath thickness, vascular bundle area and phloem area decreased with salinity in the Bailahwala Dahar (MSE). In the Ladam Sir (HSE), leaf sheath anatomical traits such as leaf sheath thickness, adaxial epidermal thickness and metaxylem area decreased with salinity, while all other leaf sheath anatomical traits increased.
Among habitats, leaf sheath thickness and adaxial epidermal cell area were the maximum in highly saline ecotype (HSE) at control level while minimum in moderately saline ecotype (MSE) at 300 mM and 0 mM respectively. Abaxial epidermal cell area and parenchymatous cell area were the highest in least saline ecotype at 150 mM salinity, while the minimum values for these parameters were recorded in moderately saline ecotype at 0 mM salinity level. Sclerenchyma thickness was maximum in least saline ecotype at 300 mM salinity level, while the minimum in moderately saline ecotype at 0 mM salinity level. Vascular bundle area was the highest in highly saline ecotype at 300 mM level, while lowest in moderate saline ecotype at 150 mM level. Metaxylem area was recorded highest in highly saline ecotype at 0 mM level, the lowest in least saline ecotype at 0 mM level. Phloem area was observed the maximum in highly saline ecotype at 300 Mm, while the minimum in moderately saline ecotype at 300 Mm.
Leaf blade anatomy
Leaf blade anatomical traits such as sclerenchymatous thickness, adaxial and abaxial epidermal thicknesses, and metaxylem area increased with salinity in all ecotypes, while leaf thickness and bulliform cell area decreased (Table 3, Fig. 3 and Fig. 4). Vascular bundle area increased only at 150 mM in the Derawar Fort (LSE), decreased with salinity in the Bailahwala Dahar (MSE) and increased with salinity in the Ladam Sir (HSE). The phloem area increased only at 150 mM level, while decreased at 300 mM in the Derawar Fort (LSE). Thuss trait decreased with salinity in the Bailahwala Dahar (MSE) and Ladam Sir (HSE).
Among habitats, leaf thickness, bulliform cell area, vascular bundle area and phloem area were the maximum in moderately saline ecotypes at 0 mM salinity level, while were recorded as the minimum in moderately saline (300 mM), highly saline ecotype (300 mM), least saline ecotype (0 mM) and highly saline ecotype (300 mM) respectively. Sclerenchymatous thickness, adaxial epidermal cell area, abaxial epidermal cell area and metaxylem area were recorded highest in highly saline ecotype at 300 mM salinity level, while the lowest values for the Sclerenchymatous thickness was observed in least saline ecotype at 0 mM, adaxial epidermal cell area, abaxial epidermal cell area and metaxylem area in highly saline ecotype 0 mM salinity level.
Epidermal appendages
Traits like microhair density in abaxial and adaxial leaf surfaces, trichome density and trichome length increased invariably with salinity in all ecotypes, however all these were significantly higher in the Ladam Sir (HSE) (Table 3). Stomatal density on abaxial surface increased with salinity in the Derawar Fort (LSE), while decreased in the Bailahwala Dahar (MSE) and HSE. Adaxial stomatal density and abaxial stomatal area decreased with salinity in the S. ioclados ecotypes. The Stomata area decreased with salinity in the Derawar Fort (LSE) and Bailahwala Dahar (MSE), while not altered in the Ladam Sir (HSE).
Among habitats, the maximum adaxial microhair density, abaxial microhair density, trichome density and trichome length were recorded in highly saline ecotype at 300 mM level, while the minimum values for these traits were observed in highly saline ecotype (0 mM), least saline ecotype (0 mM), moderately saline ecotype (0 mM) and moderately saline at (0 mM) respectively. The highest adaxial stomatal density, adaxial stomatal area and abaxial stomatal area were observed in moderately saline ecotype at 0 mM level. The maximum abaxial stomatal density was recorded in least saline ecotype at 300 mM, while minimum in least saline ecotype at 0 mM salinity level.
Physiological parameters
Leaf water relations
The leaf osmotic potential and water potential invariably became more negative with salinity. Leaf turgor potential increased significantly with salinity in the Ladam Sir (HSE). It decreased in the Bailahwala Dahar (MSE) as salinity level increased. In the Bailahwala Dahar (MSE), turgor potential was not affected at lower salt levels, but it decreased at 300 mM (Table 4).
Among habitats, the highest osmotic potential was observed in highly saline ecotype at 300 mM salinity, while the minimum osmotic potential was recorded in least and highly saline ecotypes at 0 mM level. Water potential was observed maximum in least saline ecotype at 300 mM salinity level, while was the minimum in highly saline ecotype at 0 mM. The maximum turgor potential was observed in moderately saline ecotype at 0 and 150 mM salinity levels.
Organic osmolytes
Organic osmolytes increased with salinity (Table 4). The HSE accumulated significantly higher concentration of free amino acids, soluble proteins, soluble than Derawar Fort (LSE) or Bailahwala Dahar (MSE). Concentration of all these were significantly lower in the Derawar Fort (LSE). Among habitats, the maximum total free amino acids, total soluble proteins, total soluble sugars and proline were recorded in highly saline ecotype at 300 mM salt level, while the minimum in all these parameters in least saline ecotype at 0 mM level.
Gas exchange traits
Water use efficiency (WUE) increased invariably under stress conditions (Table 4). Transpiration rate (E), stomatal conductance (g) and sub-stomatal CO2 concentration (Ci) consistently decreased with salinity. In particular, g and Ci were significantly higher in the Ladam Sir (HSE) under 300 mM. Net assimilation rate (Pn) decreased significantly in the Derawar Fort (LSE) with salinity. This trait increased only at 150 mM in the Bailahwala Dahar (MSE), while increased significantly with salinity in the Ladam Sir (HSE).
Among habitats, the highest transpiration rate, stomatal conductance and substomatal CO2 concentration were recorded in least saline ecotype at 0 mM, while the lowest value for these traits were observed in least saline ecotype at 300 mM salinity level.
Photosynthetic pigments
Chlorophyll a and b invariably decrease with salinity (Table 4). Carotenoids increased in the Derawar Fort (LSE) at 150 mM, while not change in the Bailahwala Dahar (MSE). In the Ladam Sir (HSE), carotenoids increased significantly with salinity. Among habitats, the maximum chlorophyll a and chlorophyll b were recorded in highly saline ecotype at 0 mM and, carotenoids in least saline at 1500 mM level, while all these traits were the minimum in least saline ecotypes at 300 mM salinity level.
Tissue ionic content
The root, stem and leaf Na+ and Cl– significantly increased in all cases (Table 5). The Derawar Fort (LSE) accumulated significantly higher concentration of Cl– in roots and Na+ in the leaves than its counterparts. The Bailahwala Dahar (MSE) accumulated more Cl– in stem. The stem Ca2+ increased up to 300 mM in all three ecotypes, but the Ladam Sir (HSE) accumulated more Ca2+ than its counterparts. Leaf Ca2+, however, increased invariably in response to increasing salt stress. Concentration of K+ in root, stem and leaf of Derawar Fort (LSE) and Bailahwala Dahar (MSE) decreased with salinity. In the Ladam Sir (HSE), stem K+ decreased with salinity, whereas root and leaf K+ increased (Table 5).
Leaf excreted ions
In the HSE, concentration of excreted ions (Na+ and Cl−) than its counterparts (Table 5). The Derawar Fort (LSE) and Bailahwala Dahar (MSE) excreted mire K+ and Ca2+ than Ladam Sir (HSE). A significant decrease in excreted K+ and Ca2+ was noted at the highest salt level in this ecotype.
Water content
Root water content decreased consistently in the Derawar Fort (LSE) with salinity, while it increased in the Bailahwala Dahar (MSE). In the highest saline ecotype Ladam Sir (HSE), root water content increased at 150 mM salinity level, while decreased significantly at the highest level (Table 6). Stem water content increased only at 300 mM in the Bailahwala Dahar (MSE) and Ladam Sir (HSE), while remained unaffected in the Derawar Fort (LSE) under salinity stress. Leaf water content was the highest at 300 mM level in the Derawar Fort (LSE), while it was maximum at 300 mM salinity in the Bailahwala Dahar (MSE) and at 150 mM in the Ladam Sir (HSE).
Root succulence was the highest at 0 mM in the Derawar Fort (LSE), while it was maximum at 300 mM level in the MSE and at 150 mM level in the Ladam Sir (HSE) (Table 6). Stem succulence was the highest at 300 mM NaCl in all ecotypes, the maximum was recorded in the Bailahwala Dahar (MSE). Leaf succulence was the greatest at 0 mM NaCl in the Derawar Fort (LSE) and Ladam Sir (HSE), while it remained unchanged in the Bailahwala Dahar (MSE).
Phytoremediation
Root, stem and leaf Na+ and Cl− bioconcentration factor decreased consistently with increasing salinity levels in all ecotypes of S. ioclados (Table 6). Excreted Na+ bioconcentration factor was the maximum at 150 mM salinity in the Bailahwala Dahar (MSE) and Ladam Sir (HSE), while it was the highest in the Derawar Fort (LSE) at 0 mM NaCl. The excreted Cl− bioconcentration factor, however, decreased with salinity in all three ecotypes.
Stem Na+ (STN) and Stem Cl− (STC) translocation factor was the greatest in all three ecotypes at 0 mM NaCl (Table 6). Leaf Na+ (LTN) translocation factor increased significantly as salinity levels increased in all three ecotypes. Leaf Cl− (LTC) translocation factor was the maximum at 300 mM NaCl in the Bailahwala Dahar (MSE) and Ladam Sir (HSE), while at 150 mM in the Derawar Fort (LSE). Root, stem and leaf Na+ and Cl− dilution factor decreased consistently with salinity increasing levels in all three ecotypes.
Relationship between structural and functional traits in Sporobolus ioclados
Principle component analysis between growth and leaf anatomical traits showed three distinct clusters (Fig. 5a). Leaf metaxylem area (LMX) and trichome length (LTL) showed close association with growth attributes leaf Na+ (LfN) and Cl− (LfL) in the Derawar Fort (LSE) at 150 mM. The second cluster showed strong association between adaxial epidermal thickness with excretory Na+ (ExN) in the Ladam Sir (HSE) at 300 mM, while adaxial microhairs density (LDM), abaxial microhairs density (LBM), trichome density (LTD) showed strong association with growth attributes like excretory Cl− (ExL), and leaf Ca+ (ExC) in the Ladam Sir (HSE) at 150 mM. The third cluster showed a strong relationship of excretory K+ (ExK) with shoot fresh weight (MSF) and sclerenchymatous thickness (LST) in the Bailahwala Dahar (MSE) at 300 mM. The anatomical attributes like abaxial stomatal area (LBS), phloem area (LPA), leaf thickness (LTh), bulliform cell area (LBC), adaxial stomatal density (LDS) and vascular bundle (LVB) were strongly associated with morphological traits, leaf area (MLA) and leaves per plant (Number of leaves) in the Bailahwala Dahar (MSE) at 150 mM (Fig. 5a).
Principal component analysis showing relationship among structural and functional traits in Sporobolus ioclados from the Cholistan Desert grown hydroponically under different levels of salt. (a) Relationship between growth, leaf anatomy, ionic content and excretory ions, (b) Relationship between growth, leaf sheath anatomy, ionic content and excretory ions. Ecotypes: DF-Derawar Fort (LSE), BD-Bailahwala Dahar (MSE), LS-Ladam Sir (HSE); Morphological characteristics: MLN − Number of leaves (plant−1). MLA − Total leaf area (cm2), MRF − Root fresh weight (g plant−1), MSF − Stem fresh weight (g plant−1), MLF − Leaf fresh weight (g plant−1), MRD − Root dry weight (g plant−1), MSD − Stem dry weight (g plant−1), MLD − Leaf dry weight (g plant−1). Leaf blade anatomy: LTh − Leaf thickness (µm), LST − Sclerenchymatous thickness (µm), LDE − Adaxial epidermal cell area (µm2), LBE − Abaxial epidermal cell area (µm2), LBC − Bulliform cell area (µm2), LVB − Vascular bundle area (µm2), LMX − Metaxylem area (µm2), LPA − Phloem area (µm2). Epidermal appendages: LDM − Adaxial microhair density, LBM − Abaxial microhair density, LTD − Trichome density, LTL − Trichome length (µm), LDS − Adaxial stomatal density, LBS − Abaxial stomatal density, LDA − Adaxial stomatal area (µm2), LBA − Abaxial stomatal area (µm2). Leaf sheath anatomy: HTh − Leaf sheath thickness (µm), HDE − Adaxial epidermal cell area (µm2), HBE − Abaxial epidermal cell area (µm2), HCC − Parenchymatous cell area (µm2), HST − Sclerenchyma thickness (µm), HVB − Vascular bundle area (µm2), HMX − Metaxylem area (µm2), HPA − Phloem area (µm2).
Growth and leaf sheath anatomical traits presented three isolated groups (Fig. 5b). Morphological attributes like plant leaf area (MLA), leaves per plant (MLN), shoot fresh weight (MSF) showed strong relationship with microhair density (HMD) in the Bailahwala Dahar (MSE) at 300 mM. The second cluster showed strong association among morphological characteristics and leaf sheath metaxylem area (HMX) with excretory K+ (ExK), excretory Na+ (ExN) and leaf Ca2+ (LfC) in the Ladam Sir (HSE) at 150 mM. Leaf Na+ (LfN), leaf Cl− (LfL) and excretory Cl− (ExL) showed strong association with leaf sheath thickness (HTh), sclerenchymatous thickness (HST), phloem area (HPA) and abaxial epidermal thickness (HBE) in the Ladam Sir (HSE) at both 150- and 300-mM levels. The third cluster showed a strong association between parenchymatous cell area (HCC) and adaxial epidermal thickness (HDE).
Chlorophyll pigments (chlorophyll a (Cha) and chlorophyll b (Chb) closely grouped with o mM levels of all three ecotypes (Fig. 6a), as were the gas exchange traits (transpiration rate (TrR), stomatal conductance (StC) and sub-stomatal CO2 concentration (SCC). Water use efficiency (WUE) was strongly associated with the Bailahwala Dahar (MSE) at the highest salt level (300 mM). Among leaf water (Fig. 6b), Leaf water potential (WtP) and osmotic potential (OsP) were linked to 300 mM salt level, while turgor potential (TuP) was associated with the Ladam Sir (HSE) and Bailahwala Dahar (MSE) at 150 mM. Organic osmolytes such as total soluble proteins (TSP), total soluble sugars (TSS) and proline content (TPr) were clustered with the Ladam Sir (HSE) at 0 and 150 mM, and MSD at 150 mM.
Principal component analysis showing relationship among structural and functional traits in Sporobolus ioclados from the Cholistan Desert grown hydroponically under different levels of salt. (a) Relationship between growth, physiology, ionic content and excretory ions, (b) Relationship between growth, leaf water relations, ionic content and excretory ions. Ecotypes: DF-Derawar Fort (LSE), BD-Bailahwala Dahar (MSE), LS-Ladam Sir (HSE). Morphological characteristics: MLN − Number of leaves (plant−1). MLA − Total leaf area (cm2), MRF − Root fresh weight (g plant−1), MSF − Stem fresh weight (g plant−1), MLF − Leaf fresh weight (g plant−1), MRD − Root dry weight (g plant−1), MSD − Stem dry weight (g plant−1), MLD − Leaf dry weight (g plant−1). Leaf water relations: OsP − Osmotic potential (− MPa), WtP − Water potential (− MPa), TuP − Turgor potential (MPa). Organic osmolytes: TFA − Total free amino acids (µg g−1), TSP − Total soluble proteins (µg g−1), TSS − Total soluble sugars (mg g−1), TPr − Total proline (µg g−1). Gas exchange parameters: NAR − Net assimilation rate (µmol m−2 s−1), TrR − Transpiration rate (mmol m−2 s−1), StC − Stomatal conductance (mmol m−2 s−1), SCC − Substomatal CO2 concentration (µmol mol−1), WUE − Water use efficiency. Photosynthetic pigments: Cha − Chlorophyll a (mg g−1), Chb − Chlorophyll b (mg g−1), Car − Carotenoids (mg g−1). Root ionic content: RtN − Root Na+ (mg g−1), RtK − Root K + (mg g−1), RtC − Root Ca2+ (mg g−1), RtL − Root Cl− (mg g−1). Stem ionic content: StN − Stem Na+ (mg g−1), StK − Stem K+ (mg g−1), StV − Stem Ca2+ (mg g−1), StL − Stem Cl− (mg g−1). Leaf ionic content: LfN − Leaf Na+ (mg g−1), LfK − Leaf K+ (mg g−1), LfC − Leaf Ca2+ (mg g−1), LfL − Leaf Cl− (mg g−1). Excreted ions: ExN − Na+ (mg L−1), ExK − K+ (mg L−1), ExC − Ca2+ (mg L−1), ExL − Cl− (mg L−1).
Heatmaps showing relationship of structural and functional traits
Among leaf sheath anatomical traits, leaf area was negatively correlated with leaf sheath metaxylem area and excretory K in the Derawar Fort (LSE) at 0 mM, (Fig. 7a). A negative correlation of leaf number and leaf area was observed with microhair density and metaxylem area in the Derawar Fort (LSE) at 300 mM. Leaf Na+ negatively influenced leaf sheath anatomical traits such as abaxial epidermal thickness, leaf sheath thickness, vascular bundle area and phloem area. A positive correlation of leaf Na+ was recorded with parenchymatous cell area, abaxial epidermal thickness, sclerenchymatous thickness and leaf sheath thickness in the Derawar Fort (LSE)at 150 mM. Leaf blade anatomical traits like leaf thickness, adaxial stomatal density and vascular bundle area showed positive correlation with leaf area in the Bailahwala Dahar (MSE) at 0 mM. A closely associated cluster was noticed among excretory ions (Na+, Cl− and K+) with leaf anatomical traits such as adaxial epidermal thickness, adaxial microhair density, sclerenchymatous thickness, trichome length and trichome density, and morphological traits such as leaf number and stem fresh weight (Fig. 7b).
Heatmaps showing relationship among structural and functional traits in Sporobolus ioclados from the Cholistan Desert grown hydroponically under different levels of salt. (a) Relationship between growth, leaf sheath anatomy and excretory ions, b. Relationship between growth, leaf anatomy and excretory ions, c. Relationship between growth, physiology and excretory ions, d. Relationship between growth, leaf water relations and excretory ions. Ecotypes: DF-Derawar Fort (LSE), BD-Bailahwala Dahar (MSE), LS-Ladam Sir (HSE). Morphological characteristics: MLN − Number of leaves (plant−1). MLA − Total leaf area (cm2), MSF − Stem fresh weight (g plant−1). Leaf sheath anatomy: HTh − Leaf sheath thickness (µm), HDE − Adaxial epidermal cell area (µm2), HBE − Abaxial epidermal cell area (µm2), HCC − Parenchymatous cell area (µm2), HST − Sclerenchyma thickness (µm), HVB − Vascular bundle area (µm2), HMX − Metaxylem area (µm2), HPA − Phloem area (µm2). Leaf blade anatomy: LTh − Leaf thickness (µm), LST − Sclerenchymatous thickness (µm), LDE − Adaxial epidermal cell area (µm2), LBE − Abaxial epidermal cell area (µm2), LBC − Bulliform cell area (µm2), LVB − Vascular bundle area (µm2), LMX − Metaxylem area (µm2), LPA − Phloem area (µm2). Epidermal appendages: LDM − Adaxial microhair density, LBM − Abaxial microhair density, LTD − Trichome density, LTL − Trichome length (µm), LDS − Adaxial stomatal density, LBS − Abaxial stomatal density, LDA − Adaxial stomatal area (µm2), LBA − Abaxial stomatal area (µm2). Leaf water relations: OsP − Osmotic potential (− MPa), WtP − Water potential (− MPa), TuP − Turgor potential (MPa). Organic osmolytes: TFA − Total free amino acids (µg g−1), TSP − Total soluble proteins (µg g−1), TSS − Total soluble sugars (mg g−1), TPr − Total proline (µg g−1). Gas exchange parameters: NAR − Net assimilation rate (µmol m−2 s−1), TrR − Transpiration rate (mmol m−2 s−1), StC − Stomatal conductance (mmol m−2 s−1), SCC − Substomatal CO2 concentration (µmol mol−1), WUE − Water use efficiency. Photosynthetic pigments: Cha − Chlorophyll a (mg g−1), Chb − Chlorophyll b (mg g−1), Car − Carotenoids (mg g−1). Root ionic content: RtN − Root Na+ (mg g−1), RtK − Root K + (mg g−1), RtC − Root Ca2+ (mg g−1), RtL − Root Cl− (mg g−1). Stem ionic content: StN − Stem Na+ (mg g−1), StK − Stem K+ (mg g−1), StV − Stem Ca2+ (mg g−1), StL − Stem Cl− (mg g−1). Leaf ionic content: LfN − Leaf Na+ (mg g−1), LfK − Leaf K+ (mg g−1), LfC − Leaf Ca2+ (mg g−1), LfL − Leaf Cl− (mg g−1). Excreted ions: ExN − Na+ (mg L−1), ExK − K+ (mg L−1), ExC − Ca2+ (mg L−1), ExL − Cl− (mg L−1).
Excretory ions such as Na+, Cl− and Ca2+ were positively correlated with leaf Cl−, leaf Ca2+ and water use efficiency in Ladam Sir (HSE) and Bailahwala Dahar (MSE) at 300 mM (Fig. 7c). Net CO2 assimilation rate showed positive correlation with leaf number, leaf area and stem fresh weight in the HSE at 300 mM, while negatively correlated with the Derawar Fort (LSE) at 300 mM. Organic osmolytes such as soluble protein, soluble sugars and proline positively correlated with excretory K+ in the HSE at 150 and 300 mM, whereas negatively in the Derawar Fort (LSE) at 0 mM (Fig. 7d). Free amino acids and osmotic potential positively correlated with excretory Na+ and Cl− and leaf Ca2+ in the Ladam Sir (HSE) at 300 mM.
Estimated response curves
Response curves among excretory ions and morphological traits showed high variability, particularly in the Derawar Fort (LSE) (Fig. 8a). The influence of excreted ions in leaf sheath anatomical traits was relatively linear, however slight variation was recorded in the Derawar Fort (LSE) (Fig. 8b). Response of excreted ions on leaf blade anatomical traits showed variability, especially in the Ladam Sir (HSE). Variability was relatively low in the Derawar Fort (LSE) and Bailahwala Dahar (MSE) (Fig. 8c). Variability was the maximum regarding influence of excretory ions on epidermal appendages, the Derawar Fort (LSE) showed greater variability (Fig. 8d). Response of excreted ions to leaf water relations and organic osmolytes was linear (Fig. 8e), while high deviation was seen in gas exchange traits and chlorophyll pigments in response to excretory ions (Fig. 8f). Leaf ionic content deviated strongly in response to excreted ions, the Derawar Fort (LSE) was relatively more deviated (Fig. 8g).
Estimated response of structural and functional traits in Sporobolus ioclados from the Cholistan Desert grown hydroponically under different levels of salt. (a) Response of excretory ions to growth parameters, (b) Response of excretory ions to leaf sheath anatomical parameters, (c) Response of excretory ions to leaf anatomical parameters, (d) Response of excretory ions to leaf epidermal appendages, (e) Response of excretory ions to leaf water relations and osmoprotectants, (f) Response of excretory ions to gas exchange parameters and chlorophyll pigments, (g) Response of excretory ions to leaf ionic content. Ecotypes: DF-Derawar Fort (LSE), BD-Bailahwala Dahar (MSE), LS-Ladam Sir (HSE). Morphological characteristics: MLN − Number of leaves (plant−1). MLA − Total leaf area (cm2), MSF − Stem fresh weight (g plant−1). Leaf sheath anatomy: HTh − Leaf sheath thickness (µm), HDE − Adaxial epidermal cell area (µm2), HBE − Abaxial epidermal cell area (µm2), HCC − Parenchymatous cell area (µm2), HST − Sclerenchyma thickness (µm), HVB − Vascular bundle area (µm2), HMX − Metaxylem area (µm2), HPA − Phloem area (µm2). Leaf blade anatomy: LTh − Leaf thickness (µm), LST − Sclerenchymatous thickness (µm), LDE − Adaxial epidermal cell area (µm2), LBE − Abaxial epidermal cell area (µm2), LBC − Bulliform cell area (µm2), LVB − Vascular bundle area (µm2), LMX − Metaxylem area (µm2), LPA − Phloem area (µm2). Epidermal appendages: LDM − Adaxial microhair density, LBM − Abaxial microhair density, LTD − Trichome density, LTL − Trichome length (µm), LDS − Adaxial stomatal density, LBS − Abaxial stomatal density, LDA − Adaxial stomatal area (µm2), LBA − Abaxial stomatal area (µm2). Leaf water relations: OsP − Osmotic potential (− MPa), WtP − Water potential (− MPa), TuP − Turgor potential (MPa). Organic osmolytes: TFA − Total free amino acids (µg g−1), TSP − Total soluble proteins (µg g−1), TSS − Total soluble sugars (mg g−1), TPr − Total proline (µg g−1). Gas exchange parameters: NAR − Net assimilation rate (µmol m−2 s−1), TrR − Transpiration rate (mmol m−2 s−1), StC − Stomatal conductance (mmol m−2 s−1), SCC − Substomatal CO2 concentration (µmol mol−1), WUE − Water use efficiency. Photosynthetic pigments: Cha − Chlorophyll a (mg g−1), Chb − Chlorophyll b (mg g−1), Car − Carotenoids (mg g−1). Root ionic content: RtN − Root Na+ (mg g−1), RtK − Root K + (mg g−1), RtC − Root Ca2+ (mg g−1), RtL − Root Cl− (mg g−1). Stem ionic content: StN − Stem Na+ (mg g−1), StK − Stem K+ (mg g−1), StV − Stem Ca2+ (mg g−1), StL − Stem Cl− (mg g−1). Leaf ionic content: LfN − Leaf Na+ (mg g−1), LfK − Leaf K+ (mg g−1), LfC − Leaf Ca2+ (mg g−1), LfL − Leaf Cl− (mg g−1). Excreted ions: ExN − Na+ (mg L−1), ExK − K+ (mg L−1), ExC − Ca2+ (mg L−1), ExL − Cl− (mg L−1).
Discussion
Plant adaptations develop in species of even in ecotypes when exposed to a specific set of environments for longer periods40. Several researchers reported structural and functional variations in ecotypic of various plant species of the family Poaceae. The examples are Qian et al.41 in Kentucky bluegrass (Poa pratensis), Horie et al.42 in rice (Oryza sativa), Hameed et al.18 in Sporobolus arabicus, Hameed et al.43 in Imperata cylindrica, Rahat et al.44 in Diplanchne fusca, Naz et al.45 in Suaeda vera and Fatima et al.46 in Aristida adscensionis. Ecotypes of Sporobolus ioclados were collected at a distance at least 50 km from each other. Structural and functional mechanisms were different in these ecotypes suggesting the independent evolution within the Cholistan Desert.
Leaf number and area significantly reduced by salinity in the Derawar Fort (LSE) and Bailahwala Dahar (MSE). A decrease in leaf number and area is an important modification that significantly reduces transpiration rate. Leaf fresh and dry weights increased in Derawar Fort (LSE) under stress, whereas root, stem and leaf fresh weight increased in Bailahwala Dahar (MSE) and Ladam Sir (HSE). Dry weights of underground and above ground organs generally increased in Ladam Sir (HSE), which is a clear sign of high salinity tolerance47,48. The HSE maintained growth and development under high salinities, as was reported in halophytic species17,49. The Derawar Fort (LSE) showed better growth at 150 mM salt level, but growth parameters reduced at higher salinities.
Leaf anatomical traits responded similarly in all three ecotypes with few exceptions. Leaf traits like sclerenchymatous thickness, epidermal cell area on both leaf surfaces, and metaxylem area increased with salinity levels. Sclerification is linked to mechanical strength of soft tissue45. It also plays a role in prevention of water loss especially when in and outside vascular tissue50. Intensity of sclerification, however, was significantly higher in Ladam Sir (HSE) than its counterparts. Thick epidermis on leaf surface is a characteristic of desert species, a critical modification for water conservation in harsh arid, saline and hot conditions23. Epidermis at both leaf surfaces was significantly thicker in HSE than Derawar Fort (LSE) or Bailahwala Dahar (MSE), which indicates a higher degree of salt tolerance in this ecotype. Metaxylem vessel diameter increased significantly under salinity. Broader vessels are associated with better conduction of water and nutrients7, and under high salinities this modification contributes towards nutrient translocation for various metabolic processes51. Leaf vascular bundle area increased with salinity stress only on the Ladam Sir (HSE). This ecotype maintained normal development of vascular tissue under high salinities, hence more tolerant than other ecotypes. Leaf thickness significantly decreased under salinity stress in all ecotypes, as was the thickness of bulliform cells. Grooved bulliform cells in the Ladam Sir (HSE) and Derawar Fort (LSE), in addition to thinner leaves is a critical modification for easier leaf rolling44. Such condition controls transpiration rate by protecting stomata and adaxial leaf surface from direct contact with external environment52. Large parenchymatous bulliform cells on entire adaxial side of midrib in the Bailahwala Dahar (MSE) may not involve in leaf rolling but immensely important in storing additional water53.
Epidermal traits like microhairs density on both leaf surfaces, trichome length and density increased invariably in all ecotypes under salinity. Microhairs are small appendages specifically associated with salt excretion by bursting and releasing salts outside plant body45. Trichomes, in contrast, is a characteristic of desert species. Trichomes lower the leaf temperature54 break wind intensity and reflect solar radiation, that results in lowering of transpiration rate and critically important to colonize plants in saline desert environments55. Density of microhairs and trichomes and trichome length were significantly larger in the Ladam Sir (HSE) than in the other two ecotypes, indicating its better adaptation for high salinities. Size of bundle sheath cells increased only in the Derawar Fort (LSE) under salinity, which is associated with C4 photosynthesis56.
Thicker leaf sheaths were observed in the Derawar Fort (LSE) under salinity stress, which was primarily due to storage parenchyma proportion. This feature is helpful under longer periods of drought (or physiological drought) by storing more water in parenchymatous tissue9. Abaxial epidermal thickness increased under salinity in all ecotypes, a characteristic of desiccation tolerant desert species44. Sclerification on outer surface of leaf sheath, especially outside vascular bundles also increased significantly under salinity. This is not only associated with mechanical strength to metabolically active tissues54, but also prevents water loss57. Other leaf sheath anatomical traits like adaxial epidermal thickness, and areas of parenchymatous cell, vascular bundle, metaxylem and phloem responded invariably to salinity stress, i.e., increased in some ecotypes while decreased in others under salinity stress.
The Ladam Sir (HSE) showed distinct behaviour in terms of gas exchange parameter (especially water use efficiency), accumulation of organic osmolytes and concentration of chlorophyll pigments. All these were significantly higher in this ecotype at the highest salt level. This is a clear-cut indication of a higher degree of tolerance than its counterparts53. The Ladam Sir (HSE) accumulated all osmolytes in greater amounts, which confers its better adaptation58. Another unique feature of Ladam Sir (HSE) was the increased net assimilation rate under salinity stress, which is directly linked to water use efficiency. This is important for survival under high salinities59.
Ionic content like Na+, Ca2+ and Cl− increased under salinity in all ecotypes, while K+ content decreased in all ecotypes. In S. ioclados, K+ may not be involved in neutralizing Na+ toxicity, instead Ca2+ uptake is more important. Uptake of Ca2+ among with Na+ has earlier been reported by Ievinsh et al.60 and Kapadia et al.61 in tomato; Naz et al.26 in sewan grass (Lasiurus scindicus Henr.), Tadayyon et al.62 in castor oil plant (Ricinus communis L.) plant and Hassan et al.63 in in barley (Hordeum vulgare L.). Effective excretion of toxic salts out of the plant body is a major adaptation of many halophytic species64,65. Increased density of salt-excretory microhairs under salinity was noticed in all ecotypes. In the Ladam Sir (HSE), density of microhairs on both leaf surfaces was exceedingly high, particularly at higher salt levels. This may justify the higher rate of Na+ and Cl– excretion in this ecotype. Single-celled trichomes were also seen in the Ladam Sir (HSE), which were absent in other ecotypes. This may provide additional protection in minimizing water loss from leaf surface44.
Tissue water content and succulence generally increased in the Bailahwala Dahar (MSE) and Ladam Sir (HSE) under salinity, which is a critical adaptation in these ecotypes. This will increase their capability of surviving in prolonged periods of drought49. The bioconcentration factor and dilution factor of Na+ and Cl− invariably decreased with salinity levels in all ecotypes. The major salt tolerance mechanism might not the salt compartmentalization, rather this species relied on salt excretion. This was confirmed by leaf translocation factor of Na+ and Cl−, which increased in the Bailahwala Dahar (MSE) and HSE under salinity66.
Response to salinity stress of all three ecotypes was different under controlled conditions, which is an indication of their independent evolution while growing in differently salt-affected habitats54. The Derawar Fort (LSE) showed better growth at a lower salt level. Specific features for salinity tolerance were thick leaves, larger vascular bundles and phloem area in the leaf blade. Additionally, thick epidermis equipped with dense microhairs is associated with salinity tolerance. The major physiological traits that caused reduction in biomass production were poorly maintained turgor and photosynthesis rate. The Bailahwala Dahar (MSE) depended on proportion of parenchymatous cells and increased photosynthesis rate, for salinity tolerance. Furthermore, high concentration of Ca2+ and K+ in leaves can neutralize the Na+ or Cl− toxicity.
Salinity tolerance in the Ladam Sir (HSE) relied on several structural and functional features for high degree of salinity tolerance. Structural features were high proportion of sclerenchyma, thick epidermis, high density of salt-excretory microhairs and trichomes, thicker leaves, large vascular bundles and greater proportion of phloem. Functional features were better maintenance of turgor and high concentration of osmolytes. In addition, better maintenance of photosynthesis (and water use efficiency) and better excretion of toxic ions indicated high salinity tolerance of the Ladam Sir (HSE).
Conclusion
All three ecotypes showed different structural and functional modifications to cope with saline stress of the growth medium. Overall, the mechanism based on morpho-anatomical and physiological features is very much developed in the Ladam Sir (HSE). This ecotype can safely be concluded as an excellent genotype for phytoremediation and revegetation of saline lands. More importantly, features such as proportion of sclerenchyma, density of trichomes and microhairs, salt excretion, leaf succulence, photosynthesis rate and accumulation of organic osmolytes are the yardstick to judge degree of salinity tolerance in S. ioclados ecotypes.
Data availability
Raw data can be accessed by Corresponding author Syed Mohsan Raza Shah, Assistant Professor Department of Botany, University of Education Dera Ghazi Khan Campus, Pakistan). The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Hashem, H. A. & Mohamed, A. H. Strategies for drought tolerance in xerophytes. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses (ed. Hasanuzzaman, M.) (Springer, 2020).
Wani, S. H. et al. Engineering salinity tolerance in plants: progress and prospects. Planta 251, 1–29 (2020).
Yeduguri Himabindu, Y. H. et al. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Botany 124, 39–63 (2016).
Ortiqova, L. S. Fodder halophytes for saline lands of Kyzylkum desert. Am. J. Plant Sci. 10, 1517–1526 (2019).
Céccoli, G. et al. Salt glands in the Poaceae family and their relationship to salinity tolerance. Bot. Rev. 81, 162–178 (2015).
Rozema, J. & Schat, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 92, 83–95 (2013).
Mumtaz, S. et al. Structural and functional determinants of physiological pliability in kyllinga brevifolia rottb. for survival in hyper-saline saltmarshes. Water Air Soil Pollut. 232, 1–21 (2021).
Nesterov, V., Bogdanova, E., Makurina, O., Rozina, S. & Rozentsvet, O. Effect of NaCl, copper and cadmium ions on halophytes with different types of salt resistance: accumulation, physiological and biochemical reactions. Funct. Plant Biol. 48, 1053–1061 (2021).
Iqbal, U. et al. Contribution of structural and functional modifications to wide distribution of Bermuda grass Cynodon dactylon (L) Pers. Flora 286, 151973 (2022).
Kumar, A., Mann, A., Kumar, A., Kumar, N. & Meena, B. L. Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. Int. J. Phytoremed. 23, 1041–1051 (2021).
Garrote-Moreno, A. et al. Short-term impacts of salinity pulses on ionic ratios of the seagrasses Thalassia testudinum and Halodule wrightii. Aquat. Bot. 120, 315–321 (2015).
Shaygan, M., Mulligan, D. & Baumgartl, T. The potential of three halophytes (Tecticornia pergranulata, Sclerolaena longicuspis, and Frankenia serpyllifolia) for the rehabilitation of brine-affected soils. Land Degrad Dev. 29, 2002–2014 (2018).
Sharma, A. et al. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9, 285 (2019).
Flowers, T. J. Improving crop salt tolerance. J. Exp. Bot. 55, 307–319 (2004).
Peterson, P. M., Romaschenko, K., Arrieta, Y. H. & Saarela, J. M. A molecular phylogeny and new subgeneric classification of Sporobolus (Poaceae: Chloridoideae: Sporobolinae). Taxon 63, 1212–1243 (2014).
Mata-González, R., Hovland, M., Abdallah, M. A., Martin, D. W. & Noller, J. S. Nutrient uptake and gas exchange of Great Basin plants provide insight into drought adaptations and coexistence. J. Plant Ecol. 14, 854–869 (2021).
Zamin, M. et al. Performance of Aeluropus lagopoides (mangrove grass) ecotypes, a potential turfgrass, under high saline conditions. Environ. Sci. Poll. Res. 26, 13410–13421 (2019).
Hameed, M. et al. Physioanatomical adaptations in response to salt stress in Sporobolus arabicus (Poaceae) from the Salt Range, Pakistan. Tur. J. Bot. 37, 715–724 (2013).
Tada, Y. et al. Functional screening of salt tolerance genes from a halophyte Sporobolus virginicus and transcriptomic and metabolomic analysis of salt tolerant plants expressing glycine-rich RNA-binding protein. Plant Sci. 278, 54–63 (2019).
Naz, N. et al. Adaptations for salinity tolerance in Sporobolus ioclados (Nees ex Trin.) Nees from saline desert. Flora 223, 46–55 (2016).
Weragodavidana, P. S. Salt gland excretion efficiency and salinity tolerance of Sporobolus species. (2016).
Tassadduq, S. S. et al. Ecological distribution patterns of wild grasses and abiotic factors. Sustainability 14, 11117 (2022).
Wasim, M. A., Naz, N. & Zehra, S. S. Anatomical characteristic, ionic contents and nutritional potential of Buffel grass (Cenchrus ciliaris L.) under high salinity. South Afr. J. Bot. 144, 471–479 (2022).
Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014, 208747 (2014).
Bakhtiari, M., Formenti, L., Caggìa, V., Glauser, G. & Rasmann, S. Variable effects on growth and defense traits for plant ecotypic differentiation and phenotypic plasticity along elevation gradients. Ecol. Evol. 9, 3740–3755 (2019).
Naz, N. et al. Morpho-anatomical and physiological attributes for salt tolerance in sewan grass (Lasiurus scindicus Henr.) from Cholistan Desert, Pakistan. Acta Physiol. Plant. 36, 2959–2974 (2014).
Nargis Naz, N. N. et al. Structural and functional aspects of salt tolerance in differently adapted ecotypes of Aeluropus lagopoides from saline desert habitats. Int. J. Agric. Biol. 20, 41–51 (2018).
Hoagland, D. R. & Arnon, D. I. The water-culture method for growing plants without soil. 347, 32 (1938).
Lopes, D. M., Walford, N., Viana, H. & Sette, C. R. A proposed methodology for the correction of the leaf area index measured with a ceptometer for Pinus and Eucalyptus forests. Rev. Arv. 40, 845–854 (2016).
Ruzin, S. E. Plant Microtechnique and Microscopy (Oxford University Press, 1999).
Moore, S. & Stein, W. H. Photometric nin-hydrin method for use in the ehromatography of amino acids. J. Biol. Chem. 176, 367–388 (1948).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Yemm, E. W. & Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57, 508 (1954).
Bates, L. S., Waldren, R. P. A. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973).
Arnon, D. I. Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1 (1949).
Wellburn, A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144, 307–313 (1994).
Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 13, 1035–1059 (1982).
Tiku, B. L. Ecophysiological aspects of halophyte zonation in saline sloughs. Plant Soil 43, 355–369 (1975).
Abbas, A., Ahmad, M. S. A., Ashraf, M., Ali, Q. & Alvi, A. K. Role of antioxidative defense system in amelioration of cadmium-induced phytotoxic effects in germinating seeds of maize (Zea mays). Crop Past. Sci. 73, 599–613 (2021).
Bhuiyan, M., Raman, A., Hodgkins, D., Mitchell, D. & Nicol, H. Physiological response and ion accumulation in two grasses, one legume, and one saltbush under soil water and salinity stress. Ecohydrology 8, 1547–1559 (2015).
Qian, Y., Follett, R. F. & Kimble, J. M. Soil organic carbon input from urban turfgrasses. Soil Sci. Soc. Am. J. 74, 366–371 (2010).
Horie, T., Karahara, I. & Katsuhara, M. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice 5, 1–18 (2012).
Hameed, M. et al. Physiological adaptative characteristics of Imperata cylindrica for salinity tolerance. Biologia 69, 1148–1156 (2014).
Rahat, Q. U. A. et al. Structural determinants of phytoremediation capacity in saltmarsh halophyte Diplachne fusca (L.) P. Beauv. ex Roem. & Schult. Subsp. fusca. Int. J. Phytoremed. 25, 630–645 (2022).
Naz, N. et al. Contribution of structural and functional adaptations of hyper-accumulator suaeda vera forssk. ex JF gmel. for adaptability across salinity gradients in hot desert. Environ. Sci. Poll. Res. 29, 64077–64095 (2022).
Fatima, S. et al. Structural and functional responses in widespread distribution of some dominant grasses along climatic elevation gradients. Flora 289, 152034 (2022).
Hammami, H., Saadatian, B. & Hosseini, S. A. H. Geographical variation in seed germination and biochemical response of milk thistle (Silybum marianum) ecotypes exposed to osmotic and salinity stresses. Ind. crops Prod. 152, 112507 (2020).
Pirasteh-Anosheh, H. & Hedayati-Firoozabadi, A. Sorghum [Soghum bicolor (L.) Moench.] growth, and soil moisture and salt content as affected by irrigation water salinity. Int. J. Appl. Exp. Biol. 1, 33–37 (2022).
Kaleem, M., Hameed, M., Ahmad, F., Ashraf, M. & Ahmad, M. S. A. Anatomical and physiological features modulate ion homeostasis and osmoregulation in aquatic halophyte Fimbristylis complanata (Retz.) link. Acta Physiol. Plant. 44, 59 (2022).
Shah, S. M. R., Hameed, M., Ahmad, M. S. A. & Wahid, M. A. Invasive success of Ipomoea carnea Jacq. through plasticity in physio-anatomical and phytochemical traits across diversified habitats. Biol. Inv. 25, 197–219 (2023).
Asghar, N., Hameed, M. & Ahmad, M. S. A. Ion homeostasis in differently adapted populations of Suaeda vera Forssk. ex JF Gmel. for phytoremediation of hypersaline soils. Int. J. Phytoremed. 25, 47–65 (2023).
Wall, S. et al. Stomata on the abaxial and adaxial leaf surfaces contribute differently to leaf gas exchange and photosynthesis in wheat. New Phytol. 235, 1743–1756 (2022).
Ali, Z. et al. Wetting mechanism and morphological adaptation; leaf rolling enhancing atmospheric water acquisition in wheat crop—A review. Environ. Sci. Poll. Res. 29, 30967–30985 (2022).
Waseem, M. et al. Dew absorption by leaf trichomes in Caragana korshinskii: an alternative water acquisition strategy for withstanding drought in arid environments. Physiol. Plant. 172, 528–539 (2021).
Fatima, S. et al. Survival strategies in khavi grass [Cymbopogon jwarancusa (Jones) Schult.] colonizing hot hypersaline and arid environments. Water, Air, Soil Poll. 232, 1–17 (2021).
Zahra, N. et al. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 178, 55–69 (2022).
Karabourniotis, G., Liakopoulos, G., Bresta, P. & Nikolopoulos, D. The optical properties of leaf structural elements and their contribution to photosynthetic performance and photoprotection. Plants 10, 1455 (2021).
Grigore, M. N., Boscaiu Neagu, M. T. & Vicente Meana, Ó. Assessment of the relevance of osmolyte biosynthesis for salt tolerance of halophytes under natural conditions. Eur. J. Plant Sci. Biotechnol. 5, 12–19 (2011).
Akhter, M. S. et al. Influence of salinity stress on PSII in barley (Hordeum vulgare L.) genotypes, probed by chlorophyll-a fluorescence. J. King Saud Uni. Sci. 33, 101239 (2021).
Ievinsh, G., Ieviņa, S., Andersone-Ozola, U. & Samsone, I. Leaf sodium, potassium and electrolyte accumulation capacity of plant species from salt-affected coastal habitats of the Baltic Sea: Towards a definition of Na hyperaccumulation. Flora 274, 151748 (2021).
Kapadia, C. et al. Halotolerant microbial consortia for sustainable mitigation of salinity stress, growth promotion, and mineral uptake in tomato plants and soil nutrient enrichment. Sustainability 13, 8369 (2021).
Tadayyon, A., Nikneshan, P. & Pessarakli, M. Effects of drought stress on concentration of macro-and micro-nutrients in Castor (Ricinus communis L.) plant. J. Plant Nutr. 41, 304–310 (2018).
Hassan, A. et al. Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 28, 4276–4290 (2021).
Grigore, M. N., & Toma, C. Morphological and anatomical adaptations of halophytes: A review. Handbook of halophytes: from molecules to ecosystems towards biosaline agriculture, 1079–1221 (2021).
Ibraheem, F., Al-Zahrani, A. & Mosa, A. Physiological adaptation of three wild halophytic Suaeda species: Salt tolerance strategies and metal accumulation capacity. Plants 11, 537 (2022).
Guarino, F., Ruiz, K. B., Castiglione, S., Cicatelli, A. & Biondi, S. The combined effect of Cr (III) and NaCl determines changes in metal uptake, nutrient content, and gene expression in quinoa (Chenopodium quinoa Willd.). Ecotoxicol. Environ. Saf. 193, 110345 (2020).
Acknowledgements
The authors would like to extend their sincere appreciation to the Ongoing Research Funding program (ORF-2025-134), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
N.N: Planned and carried out the experimental work; M.H: Supervisor who planned the research work; F.A.: Supervised research planning, collection and data analysis; M.S.A.A., K.S.A.: Biostatistician; data visualization, modeling and interpretation; S.M.R.S., S.B., S.F., A.A., Z.A.: Research execution, biochemical analysis, anatomical photography and data collection; K.F.A., G.D.A.Q., A.H. and E.F.A.: writing, reviewing and editing; A.H. and E.F.A: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study does not include any animal or human subjects and no specific ethical approval is needed. Other necessary guidelines set by University of Agriculture, Faisalabad for handling of plant material during conduction of laboratory work were followed. All samplings were done with the least possible disturbances to plant communities and environment. After completion of study, all experimental materials were properly discarded/incinerated in a controlled environment to avoid bio-contamination.
Plant ethics statement
The voucher specimens used for plant identification are deposited to the herbarium facility of the Department of Botany, University of Agriculture, Faisalabad, and are available for verification on request. Voucher number were 19-1-2018 (Moderately saline Bailahwala Dahar), 19-2-2018 (least saline Derawar Fort), and 19-3-2018 (highly saline Ladam Sir). The specimens were verified from Dr. Mansoor Hameed, Professor and Plant Taxonomist, Department of Botany, University of Agriculture Faisalabad, Pakistan and Dr. Farooq Ahmad, Associate Professor and expert in Grass Systematics, Department of Botany, University of Agriculture Faisalabad, Pakistan. Specimens can be accessed by Corresponding author Syed Mohsan Raza Shah, Assistant Professor Department of Botany, University of Education Dera Ghazi Khan Campus, Pakistan). Anatomical slides, photographs and raw data calculated from these photographs are available can be requested if needed can be accessed by Syed Mohsan Raza Shah, Assistant Professor Department of Botany, University of Education Dera Ghazi Khan Campus, Pakistan.
Publication ethics statements
It is certified that the manuscript is the product of an original study and is submitted solely to this Journal for consideration. It is not submitted to any other Journal, in part or full, for simultaneous consideration nor has been previously published in any form or language (other than as a thesis of the first author, which is properly acknowledged). There is no plagiarism/self-plagiarism, salami-slicing/publishing, secondary publication nor near verbatim. All data presented in this manuscript is product of our own study and the manuscript does not contain any copyrighted material (data tables or figures). All results and data are presented clearly, honestly, and without fabrication, falsification or inappropriate data manipulation (including image-based manipulation).
Consent to participate
The contribution of all participants/parties involved in this study have been appreciated either in authorship OR acknowledged in the acknowledgement section. All contributors listed in this manuscript have substantially participated in this study and preparation of the manuscript.
Consent for publication
All authors agree to publish and there is no conflict for publication of this manuscript for publication in this Journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Naz, N., Shah, S.M.R., Basharat, S. et al. Phytoremediation of hypersaline soils by salt excretory C4 halophytic pan dropseed grass (Sporobolus ioclados Nees ex Trin.) through alteration in foliar architecture. Sci Rep 15, 24882 (2025). https://doi.org/10.1038/s41598-025-01291-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01291-w
Keywords
This article is cited by
-
Taraxacum officinale as sentinel for heavy metal bioaccumulation and phytostabilization in urban soils
International Journal of Environmental Science and Technology (2026)