Abstract
Pyroptosis, a programmed form of cell death, typically increases during the atherosclerosis development. Cyclodextrins are oligosaccharides with anti-atherosclerotic properties. Therefore, the present study aims to explore the role of methyl-ß-cyclodextrin (Mß-CD), a representative of cyclodextrin, in suppressing the development of atherosclerosis. Atherosclerosis animal and cell models were established by feeding ApoE−/− mice a high-fat diet for 12 weeks and by inducing oxidized low-density lipoprotein (ox-LDL) (75 µg/mL for 24 h) in rat vascular smooth muscle cells (VSMCs), respectively. Meanwhile, Mß-CD (2.0 g/kg, twice a week in vivo and 5mM in vitro) was used to manipulate GSDMD-mediated pyroptosis.The present study revealed a reduction in atherosclerotic plaques in the aorta (19.46 ± 2.38% vs. 8.10 ± 1.28%,P < 0.01), accompanied by a decrease in the number of CD68+ cells in the aortic sinus of atherosclerotic mice following Mß-CD intervention. Additionally, there were reduced levels of lipids and cholesterol, as well as lower levels of IL-1ß and IL-18 cytokines, alongside decreased activation of the TLR4/NF-κB/NLRP3 pathway. This resulted in decreased pyroptosis proteins in both in vivo (GSDMD-NT: 1.06 ± 0.24 vs. 0.26 ± 0.03, P < 0.01) and in vitro (GSDMD-NT: 1.37 ± 0.15 vs. 0.62 ± 0.14, P < 0.01) models after Mß-CD treatment. Moreover, the number of PI-positive cells was reduced in the atherosclerotic cell model after treatment with Mß-CD. This study provides evidence that Mß-CD may reduce atherosclerosis by inhibiting the TLR4/NF-κB/NLRP3 pathway and GSDMD-mediated pyroptosis, highlighting the need for further investigation of Mß-CD as a potential treatment option for atherosclerosis.
Similar content being viewed by others
Introduction
Atherosclerosis is a progressive inflammatory disease characterized by the accumulation of lipids in the arterial vessel wall, which begins in early life. The disease develops with the formation of atherosclerotic plaques, leading to narrowing of the arterial lumen. Atherosclerotic plaques often remain stable for years but can rapidly become unstable, rupture, and trigger thrombus formation1. Risk factors such as family history, hypercholesterolemia, hypertension, obesity, and cigarette smoking promote the development and progression of the disease. Accordingly, in addition to the restriction of the vessel lumen, the presence of atherosclerotic plaques is associated with an increased risk of acute cardiovascular events, such as myocardial infarction (MI) and stroke. Current therapies reduce the risk of cardiovascular disorders through the treatment of hyperlipidemia, hypertension, and surgical interventions. However, atherosclerotic cardiovascular disease (ASCVD) remains one of the leading causes of death. Additional therapeutic approaches are needed to further reduce the risk of cardiovascular events.
Cells in atherosclerotic plaques undergo subtype transitions and participate in the progression of regulated necrosis, which plays a significant role in atherosclerosis2. One of the best-defined forms of regulated necrosis is pyroptosis, a pro-inflammatory form of regulated cell death, characterized by the formation of plasma membrane pores via members of the gasdermin (GSDM) protein family3,4. The subsequent activation step leads to the assembly of the NOD-like receptor protein 3 (NLRP3) inflammasome, which facilitates self-cleavage and activation of caspase-1, accelerates the maturation of interleukin-1ß (IL-1ß) and interleukin-18 (IL-18) precursors, and cleaves GSDMD into its active form, the amino-terminal GSDMD (GSDMD-N). Once activated, GSDMD-N promotes the formation of membrane pores, releases IL-1ß and IL-18, induces DNA degradation, and causes cell swelling5,6.
Recent studies reveal that up to 30–70% of the damaged cells in atherosclerotic lesions originate from vascular smooth muscle cells (VSMCs)7,8. Cholesterol accumulation in vascular smooth muscle cells (VSMCs) leads to the formation of lipid-loaded cells, subsequently triggering key events in atherogenesis. Moreover, VSMCs are major mediators of the propagation and perpetuation of inflammation throughout the vessel wall. During the development of atherosclerosis, the accumulation of oxidized low-density lipoprotein (ox-LDL) in the arterial wall leads to the transformation of VSMCs into foam cells. Additionally, ox-LDL accumulation in the plaque promotes VSMC dysfunction, followed by the activation of a pro-inflammatory response. Therefore, targeting cholesterol metabolism and the inflammatory response in VSMCs presents promising strategies for the prevention and treatment of atherosclerosis9.
Cyclodextrins are ring-shaped oligosaccharides formed in nature through the digestion of cellulose by bacteria. They consist of various numbers of α-(1,4)-linked glucopyranose subunits, such as α-cyclodextrin (α-CD; six subunits), ß-cyclodextrin (ß-CD; seven subunits), and γ-cyclodextrin (γ-CD; eight subunits). Due to their barrel-shaped structure, which features a hydrophobic inner cavity and a hydrophilic outer surface, cyclodextrins are considered efficient carriers for food and medicine, enhancing water solubility. Various cyclodextrin derivatives, such as methyl-ß-cyclodextrin, have been developed to further improve water solubility10. Studies have reported that cyclodextrins have anti-atherosclerotic efficacy through various mechanisms, such as promoting cholesterol efflux from atherosclerotic plaques/macrophages, inhibiting the oxidation of plasma LDL, increasing plasma HDL levels, reducing cholesterol crystal-induced complement activation, and modifying gut flora11,12. However, it remains unclear whether cyclodextrin attenuates atherosclerosis by inhibiting pyroptosis. Therefore, this study aims to investigate whether methyl-ß-cyclodextrin (Mß-CD), a representative cyclodextrin, attenuates GSDMD-mediated pyroptosis in atherosclerotic animal models and ox-LDL-induced VSMCs.
Materials and methods
Antibodies and reagents
The following reagents were used for the Oil Red O staining: Oil Red O saturated solution (Servicebio, G1015). For immunofluorescence analysis, primary and secondary antibodies were used as follows: anti-alpha smooth muscle actin antibody (Abcam, ab7817), anti-CD68 rabbit pAb (Servicebio, GB113150-100), Alexa Fluor® 488 (ZSGB-BIO, ZF-0512), and Alexa Fluor® 594 (ZSGB-BIO, ZF-0516). Pre-coated plates for ELISA from Cloud-Clone Corp. were used for IL-1ß (L230904388) and IL-18 (L230904397) detection. For western blotting, the following reagents, primary, and secondary antibodies were used: human ox-LDL (Yiyuan Biotechnologies, YB-002), methyl-ß-cyclodextrin (MedChemExpress, M. Wt: 1310, 128446-36-6), anti-TLR4 rabbit pAb (Servicebio, GB11519-100), NF-κB p65 (D14E12) XP® rabbit mAb (Cell Signaling Technology, 8242), phospho-NF-κB p65 (Ser536) (93H1) rabbit mAb (Cell Signaling Technology, 3033), anti-NLRP3 antibody (Abcam, ab263899), anti-GSDMD antibody (Abcam, ab219800), goat anti-mouse IgG (Servicebio, GB23301), and goat anti-rabbit IgG (Servicebio, GB23303). The Hoechst33342/PI double-staining kit was purchased from Solarbio (Beijing, China).
Mice model
Male apolipoprotein E knockout (ApoE−/−) mice (GemPharmatech Co., Ltd., T00145B/APOE) and C57BL/6J (wt/wt) mice (GemPharmatech Co., Ltd., N000013/C57BL/6JGPt), aged 6–8 weeks, were purchased. All ApoE−/− mice were randomly divided into two groups (n = 18 per group): ApoE−/− mice with the high-fat diet (ApoE−/−), and ApoE−/− mice with the high-fat diet plus Mß-CD (ApoE−/−+Mß-CD). Similarly, all C57BL/6J (wt/wt) mice were randomly divided into two groups (n = 18 per group): C57BL/6J (wt/wt) mice with the regular diet (wild-type), and C57BL/6J (wt/wt) mice with the regular diet plus Mß-CD (wild-type + Mß-CD). ApoE−/− mice were fed a high-fat diet (Jiangsu Synergy Pharmaceutical Biological Engineering Co., Ltd.; Cat no: XT108C; HFD 40%, 1.25% cholesterol) for 12 weeks. The ApoE−/−+Mß-CD and wild-type + Mß-CD groups were injected subcutaneously with Mß-CD (2.0 g/kg) twice a week, while the other two groups received subcutaneous injections of NaCl (2.0 g/kg). During the treatment, the body weight of the mice was monitored. All mice were housed in a climate-controlled room (24 °C, 55% humidity) in individually ventilated cages, exposed to a 12-hour light/dark cycle. Throughout the experiment, the mice had ad libitum access to food and water. The animal experiments were approved by the Laboratory Animal Welfare and Ethics Committee of Fujian Medical University (Approval No. 2017-068) and were conducted in accordance with the ARRIVE guidelines.
Histological analyses
The aorta, including the iliac bifurcation, was dissected and longitudinally cut open. The aortic roots were isolated, embedded in an optimum cutting temperature (OCT) compound, and sectioned at 8–10 μm thickness. The tissue sections were stained with oil-saturated Oil Red O, following the manufacturer’s protocols. Images were captured using a 40x objective and oil immersion lenses (NIKON DS-U3, Nikon, Japan) mounted on a Nikon 80i microscope (Nikon, Japan). The plaque area was quantified using ImageJ software.
Immunofluorescence analysis
The protocol for Immunofluorescence analysis was described in our previous studies13,14,−15. Frozen slides were left at room temperature for 10–15 min to equilibrate and prevent moisture buildup. Rat VSMCs were washed once with PBS before fixation. Both the tissue and rat VSMCs were fixed with 4% paraformaldehyde for 10 min. After three washes with PBS, slides were treated with 0.3-0.5% Triton X-100 for 20 min, followed by blocking with 5% BSA for 1–2 h. After incubation with primary antibodies (anti-alpha smooth muscle actin and anti-CD68 rabbit pAb) and secondary antibodies (Alexa Fluor® 488 and Alexa Fluor® 594), slides were washed and incubated with DAPI solution. Imaging was performed using a Nikon 80i microscope, and analysis was conducted with ImageJ software.
Blood lipid test analysis
The sample was incubated at room temperature for 2 h, then centrifuged at 3,000 rpm for 15 min at 2–8 °C. The serum was separated and stored at -80 °C. The concentrations of serum triglycerides (TG), total cholesterol (TCHO), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and blood glucose were measured using biochemical reagent kits supplied by Shenzhen Rayto Life Science Co., Ltd., following the manufacturer’s protocols. The following kits were used: TG Kit (S03027), TCHO Kit (S03042), HDL-c Kit (S03025), LDL-c Kit (S03029), and Glucose Kit (S03039). The experiment was conducted in the Department of Clinical Laboratory, The First Affiliated Hospital of Fujian Medical University, Fuzhou, People’s Republic of China. The corresponding parameters were set on the automatic biochemical analyzer (Cobas® 8000, Cobas ISE). After testing, the results were exported directly from the analyzer and sent in Excel format for further analysis.
Enzyme-linked immunosorbent assay (ELISA)
The protocol was described in our previous studies13,14. Blood samples were centrifuged at 3,000 × g for 20 min in a refrigerated centrifuge. The serum was then separated and stored at -80 °C. The concentrations of IL-1ß and IL-18 in the serum were measured using high-sensitivity ELISA kits, according to the supplier’s protocol. Data were analyzed using Excel. Duplicate wells were run in each experiment.
Primary VSMC isolation, culture, and treatment
The animal experiments were approved by the Laboratory Animal Welfare and Ethics Committee of Fujian Medical University (Approval No. 2017-068) and were conducted in accordance with the ARRIVE guidelines.
Rat VSMCs were isolated from male Sprague-Dawley rats (120–180 g) thoracic aorta and cultured using methods previously described in our individual studies of rat VSMCs16,17,18. Cells were cultured in DMEM containing high glucose (BasalMedia), supplemented with 20% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin (100 mg/mL) in a humidified chamber at 37 °C with 5% CO2. Rat VSMCs from 4 to 5 passages were seeded in 6-well plates. Once the cells reached 75-85% confluency, they were starved for 24 h in high-glucose DMEM medium containing 0% FBS. The VSMCs were then treated with varying concentrations of ox-LDL (50, 75, and 100 µg/mL) for 6, 12, 18, and 24 h. Additionally, the cells were treated with different concentrations of Mß-CD (1, 3, 5, and 10mM).
Extracting proteins and Western blotting
The methods for protein extraction from tissues and cultured cells, as well as Western blotting, were based on our previous studies13,19. Briefly, the following procedure was used: The mice’s thoracic aorta was snap-frozen in liquid nitrogen and stored at -80 °C. To extract proteins from the tissue, a 1:1 ratio of tissue to RIPA buffer (Sigma, USA) was maintained. For cultured cells, the plate was rinsed twice with PBS. Then, 200µL of ice-cold lysis buffer (Beyotime, Shanghai, China) containing 1mM PMSF (Beyotime, Shanghai, China) and 1% protease inhibitor cocktail (Roche, Switzerland) was added to each well, and the plate was incubated on ice for 15 min to allow protein extraction. The protein lysates were heated at 100 °C for 10 min, then stored at -80 °C for long-term storage or at -20 °C for short-term use. For Western blotting analysis, proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (7.5%, 10%) and electroblotted onto PVDF membranes. The PVDF membranes were blocked with 5% skimmed milk and incubated overnight at 4 °C with primary antibodies (anti-TLR4, NF-κB p65, phospho-NF-κB p65, anti-NLRP3, anti-GSDMD; dilution: 1:1000). After primary antibody incubation, the membranes were washed with 1× tris-buffered saline with Tween (TBST) and incubated with appropriate secondary antibodies. The membranes were detected using an ECL kit (Meilunbio, MA0186-1). The relative intensities of the protein bands were analyzed using ImageJ software.
Hoechst/propidium iodide (PI) staining
Rat VSMCs were cultured and differentiated into mature cells in 6-well or 12-well cell culture plates. The medium was replaced with serum-free DMEM high-glucose medium, and the cells were starved for 24 h. The cells were then treated with 75 µg/mL ox-LDL and 5mM Mß-CD. Once the cells reached the designated time point, they were stained with a solution consisting of cell staining buffer, Hoechst33342 staining solution, and PI staining solution, in the following ratio: 1mL cell staining buffer, 5µL Hoechst33342, and 5µL PI staining solution. The cells were incubated in the dark at 4 °C for 30 min. After incubation, the cells were washed twice with 1× PBS and then observed and imaged using a Nikon 80i Microscope (Nikon, Japan). PI-positive cells were quantified using ImageJ software. To calculate the percentage of PI-positive cells, divide the number of PI-positive cells by the number of Hoechst-positive cells. This protocol was carried out according to our previous study, “Hoechst33342/propidium iodide (PI) staining”13.
Statistical analyses
Statistical analyses of in vivo experiments were performed with at least eight mice per group. In vitro data were obtained from four to six independent experiments. The data are presented as the mean ± SEM. GraphPad Prism 10 software (GraphPad Software, Inc., San Diego, CA, USA) was used for data analysis. An unpaired Student’s t-test was used to compare two groups, while one-way and two-way ANOVA were applied for comparisons among multiple groups, followed by the least significant difference (LSD) test. Differences with P < 0.05 were considered statistically significant.
Results
Mß-CD reduced atherosclerotic plaque and decreased CD68+ cell expression in ApoE −/− mice
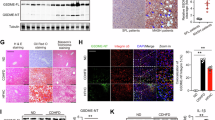
Oil Red O staining was performed to compare the size of atherosclerotic plaques in the entire aortic root and sinus among the wild-type, ApoE−/−, and ApoE−/−+Mß-CD groups. The results showed that ApoE−/− mice developed significantly larger atherosclerotic plaque areas in both the aortic root (19.46 ± 2.38% vs. 2.7 ± 0.82%,P < 0.01) and the aortic sinus (38.60 ± 3.48 vs. 5.03 ± 1.33 × 103 µm2, P < 0.01) compared to wild-type mice, confirming the successful establishment of the atherosclerosis model in vivo. However, the ApoE−/− mice treated with Mß-CD showed a significant reduction in the atherosclerotic plaque area in the entire aortic root (19.46 ± 2.38% vs. 8.10 ± 1.28%,P < 0.01) and in the aortic sinus (38.60 ± 3.48 vs. 12.49 ± 2.01 × 103 µm2, P < 0.01) (Fig. 1A–D).
Additionally, immunofluorescence analysis was conducted to assess the presence of CD68+ cells across the three groups. ApoE−/− mice exhibited a marked increase in CD68+ cell infiltration compared to wild-type controls (52.16 ± 4.69% vs. 15.20 ± 3.78%,P < 0.01), indicating enhanced macrophage recruitment. Treatment with Mß-CD significantly reduced CD68+ cell levels in ApoE−/− mice (52.16 ± 4.69% vs. 30.37 ± 3.17%,P < 0.05) (Fig. 1E, F). These findings suggest that Mß-CD attenuates atherosclerotic plaque formation and macrophage accumulation in ApoE−/− mice.
Mß-CD reduced atherosclerotic plaque and decreased CD68+ cell expression in ApoE−/− mice. (A,C) Oil Red O staining showed atherosclerotic lesions in the entire aortic root and aortic sinus. Scale bars = 4 mm (A) and 250 μm (C), respectively. (B,D) Quantitative analysis revealed significantly larger plaques in the aortic root and aortic sinus in the ApoE−/− group compared to the wild-type group. Mß-CD treatment reduced plaque size in the ApoE−/−+Mß-CD group (n = 8, **P < 0.01). (E) Representative immunofluorescence images showing macrophage deposition (CD68+ cells) in the aortic sinus of the ApoE−/−, wild-type, and ApoE−/−+Mß-CD groups. Immunostaining for CD68 (red), α-SMA (green), and DAPI (blue) is shown. Scale bar = 100 μm. (F) Quantification of CD68+ cell area in the aortic sinus. Statistical analysis revealed a significant reduction in CD68+ cells in the ApoE−/−+Mß-CD group compared to the ApoE−/− group (n = 8, *P < 0.05 and **P < 0.01).
Mß-CD inhibited lipids and cholesterol substances levels and lowered plasma IL-1ß and IL-18 in ApoE−/− mice.
Blood lipid and cytokine levels were assessed in wild-type, ApoE−/−, and ApoE−/−+Mß-CD mice to determine the impact of Mß-CD treatment on lipids, cholesterol, and inflammatory mediators. After 12 weeks on the high-fat diet, ApoE−/− mice exhibited significantly higher levels of TG, TCHO, LDL-c, and blood glucose, alongside lower levels of HDL-c, compared to wild-type mice on a regular diet (LDL-c: 8.11 ± 1.16 vs. 0.2 ± 0.01 mmol/L,P < 0.01). These findings indicate that elevated serum lipids and cholesterol crystals are associated with the progression of atherosclerosis in this model. However, ApoE−/− mice treated with Mß-CD showed a significant reduction in LDL-c (8.11 ± 1.16 vs. 3.47 ± 0.83 mmol/L,P < 0.01), blood glucose, and an increase in HDL-c, suggesting that Mß-CD can mitigate atherosclerosis-related lipid and glucose abnormalities (Fig. 2A-E).
Furthermore, to evaluate the inflammatory response, serum levels of IL-1ß and IL-18 were measured using enzyme-linked immunosorbent assay (ELISA). ApoE−/− mice exhibited significantly higher levels of IL-1ß (17.28 ± 0.31 vs. 15.87 ± 0.42 pg/ml,P < 0.05) and IL-18 (12.77 ± 0.34 vs. 10.51 ± 0.38 pg/ml,P < 0.05) compared to wild-type mice, correlating with atherosclerosis development. Treatment with Mß-CD significantly reduced IL-1ß (17.28 ± 0.31 vs. 14.85 ± 0.21 pg/ml,P < 0.05) and IL-18 (12.77 ± 0.34 vs. 10.82 ± 0.22 pg/ml,P < 0.05) levels in ApoE−/− mice, further supporting Mß-CD’s potential anti-atherosclerotic and anti-inflammatory effects (Fig. 2F, G).
Mß-CD inhibited lipids and cholesterol substances levels and lowered plasma IL-1ß and IL-18 in ApoE−/− mice. (A–D) The graphs show that ApoE−/− mice on the high-fat diet had significantly higher plasma levels of TG, TCHO, LDL-c, and blood glucose compared to both the wild-type and ApoE−/− +Mß-CD groups. (E) The wild-type and ApoE−/−+Mß-CD groups exhibited significantly higher HDL-c levels. (F–G) Similarly, plasma levels of the inflammatory mediators IL-1ß and IL-18 were significantly reduced in both the wild-type and ApoE−/−+Mß-CD groups. Statistical analysis revealed significant differences between the ApoE−/−, wild-type, and ApoE−/− +Mß-CD groups (n = 8, *P < 0.05, **P < 0.01).
Mß-CD inhibited GSDMD-mediated pyroptosis in atherosclerotic ApoE −/− mice, along with the Inhibition of TLR4/NF-κB/NLRP3 pathway
To investigate the role of GSDMD-mediated pyroptosis and the TLR4/NF-κB/NLRP3 pathway in the development of atherosclerosis, and to assess the effectiveness of Mß-CD in reducing atherosclerosis through this pathway, we analyzed the sequential expression of TLR4, NF-κB, and p-NF-κB in ApoE−/− mice using Western blotting. Our data revealed a significant increase in the expression of NLRP3 (0.96 ± 0.03 vs. 0.29 ± 0.05,P < 0.01), GSDMD, and GSDMD-NT (1.06 ± 0.25 vs. 0.39 ± 0.03,P < 0.01) in ApoE−/− mice compared to wild-type mice, confirming that atherosclerosis progression is associated with GSDMD-mediated pyroptosis. Treatment with Mß-CD significantly reduced the expression of TLR4, NF-κB, p-NF-κB, NLRP3 (0.96 ± 0.03 vs. 0.34 ± 0.06,P < 0.01), GSDMD, and GSDMD-NT (1.06 ± 0.25 vs. 0.26 ± 0.03,P < 0.01) in ApoE−/− mice, indicating that Mß-CD inhibits the activation of this pathway and prevents GSDMD cleavage, thereby blocking the formation of cell membrane pores (Fig. 3).
Mß-CD inhibited GSDMD-mediated pyroptosis in atherosclerotic ApoE−/− mice, along with the inhibition of TLR4/NF-κB/NLRP3 pathway. (A–G) Representative blottings and quantitative analysis showed higher protein expression of TLR4, NF-κB, p-NF-κB, NLRP3, GSDMD, and GSDMD-NT in the ApoE−/− group compared to the wild-type group. However, treated with Mß-CD, the expression of these proteins was reduced in the ApoE−/−+Mß-CD group (n = 8, *P < 0.05, **P < 0.01). Full blot images for (3 A) are provided in the supplemental data (Supplementary Fig. 3 A).
Identification of rat VSMCs and ox-LDL-induced pyroptosis via GSDMD involving the TLR4/NF-κB/NLRP3 pathway
Rat VSMCs were isolated from the thoracic aorta of rats, and their purity was confirmed by immunofluorescence staining of alpha-smooth muscle actin (α-SMA) (Fig. 4A, B). It is important to note that α-SMA is an intracellular filamentous actin and a vital marker molecule of VSMCs, making it essential for the accurate verification of the cells.
Additionally, to confirm the role of GSDMD-mediated pyroptosis and the TLR4/NF-κB/NLRP3 pathway in the development of atherosclerosis in the in vitro model, rat VSMCs were treated with 50, 75, and 100 µg/mL of ox-LDL for 6, 12, 18, and 24 h, respectively. The expression of pyroptosis-related proteins was then analyzed using Western blotting. Our findings showed that after 24 h, 75 µg/mL and 100 µg/mL of ox-LDL significantly increased the expression levels of TLR4, NF-κB, p-NF-κB, NLRP3, GSDMD, and GSDMD-NT proteins in rat VSMCs compared to the control (ctrl) group. Moreover, the ox-LDL 75 µg/mL group had a more significant impact on activating pyroptosis in rat VSMCs than the 100 µg/mL group after 24 h. Consequently, we conducted subsequent experiments using 75 µg/mL of ox-LDL (Fig. 4C-I). The results indicated that GSDMD-mediated pyroptosis facilitated the development of atherosclerosis.
Identification of Rat VSMCs and ox-LDL-induced Pyroptosis via GSDMD involving the TLR4/NF-κB/NLRP3 pathway. (A) Representative image of rat primary VSMCs (4th generation). (B) Immunofluorescence of α-SMA used to identify VSMCs in primary cultures of rat thoracic aortas. Anti-α-SMA (green fluorescence) was used to stain actin in VSMCs, and DAPI (blue fluorescence) was used to stain nuclei. (C–I) Representative Western blotting and quantitative analysis showing that ox-LDL triggered pyroptosis in rat VSMCs through GSDMD. (C) Protein levels of TLR4, NF-κB, p-NF-κB, NLRP3, GSDMD, and GSDMD-NT were higher in the ox-LDL 75 µg/mL group compared to the ctrl group after 24 h. (D–I) Significant differences between groups were observed (n = 4/5), with P-values ranging from *P < 0.05 to **P < 0.01. Full blot images for (4 C) are provided in the supplemental data (Supplementary Fig. 4 C).
Mß-CD suppressed ox-LDL-induced GSDMD-mediated pyroptosis in rat VSMCs
To investigate whether Mß-CD can alleviate GSDMD-mediated pyroptosis in rat VSMCs induced by ox-LDL by inhibiting the GSDMD signaling pathway, we analyzed the expression levels of GSDMD pathway-related proteins in different treatment groups. Representative Western blotting and quantitative analysis demonstrated that induction with ox-LDL (75 µg/mL, 24 h) significantly increased the protein expression levels of TLR4, NF-κB, p-NF-κB, and NLRP3 (1.53 ± 0.17 vs. 1.00 ± 0.00,P < 0.05), as well as GSDMD and GSDMD-NT (1.37 ± 0.15 vs. 1.00 ± 0.00, P < 0.01) in the ox-LDL 75 µg/mL group compared to the ctrl group. This indicates that the GSDMD signaling pathway is involved in the activation of pyroptosis in ox-LDL-induced rat VSMCs.
To further explore the potential protective effect of Mß-CD, rat VSMCs were pretreated with various concentrations of Mß-CD (ranging from 1 to 10mmol/L) two hours before the addition of 75 µg/mL ox-LDL. Our findings showed that treatment with 5mM Mß-CD significantly decreased the protein expression levels of TLR4, NF-κB, p-NF-κB, and NLRP3 (1.53 ± 0.17 vs. 0.62 ± 0.21,P < 0.01), as well as GSDMD and GSDMD-NT (1.37 ± 0.15 vs. 0.62 ± 0.14,P < 0.01), in the ox-LDL 75 µg/mL + 5mM Mß-CD group compared to the ox-LDL 75 µg/mL group (Fig. 5) .
These results strongly suggest that Mß-CD inhibits ox-LDL-induced GSDMD-mediated pyroptosis by downregulating the GSDMD signaling pathway.
Mß-CD suppressed ox-LDL-induced GSDMD-mediated pyroptosis in rat VSMCs. (A–G) Representative Western blotting and quantitative analysis revealed that Mß-CD inhibited the ox-LDL-induced expression of pyroptosis-related molecules in rat VSMCs. (A) TLR4, NF-κB, p-NF-κB, NLRP3, GSDMD, and GSDMD-NT protein levels were significantly lower in the treatment ox-LDL 75 µg/mL + 5mM Mß-CD group compared to the ox-LDL 75 µg/mL group. Mß-CD was added two hours before ox-LDL. (B–G) Quantitative analysis revealed significant differences between the groups (n = 5,*P < 0.05,**P < 0.01,P = no significant). Full blot images for (5 A) are provided in the supplemental data (Supplementary Fig. 5 A).
Mß-CD improved the cell membrane integrity of rat VSMCs damaged by ox-LDL
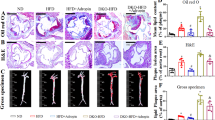
To confirm the effect of Mß-CD on cell damage caused by ox-LDL-induced pyroptosis in rat VSMCs, Hoechst 33,342/propidium iodide (PI) double immunofluorescence staining was used to analyze cell membrane permeability. Experimental results revealed that the positive rate of PI staining in the ox-LDL 75 µg/mL group was significantly higher (60.68 ± 1.78% vs. 26.03 ± 4.49%,P < 0.01) than that in the ctrl group. These results indicate that cell membrane integrity was compromised in the presence of ox-LDL.
However, after treatment with 5mM Mß-CD, the positive rate of PI staining was reduced (60.68 ± 1.78% vs. 29.74 ± 3.84%,P < 0.01) in rat VSMCs treated with ox-LDL 75 µg/mL compared to the ox-LDL 75 µg/mL + 5mM Mß-CD group (Fig. 6). Thus, the data suggest that Mß-CD alleviated the inflammatory response and improved cellular viability following GSDMD-mediated pyroptosis in rat VSMCs.
Mß-CD improved the cell membrane integrity of rat VSMCs damaged by ox-LDL. (A) Immunofluorescence images of Hoechst 33,342/PI double staining. (B) Quantitative analysis revealed that the positive rate of PI staining in the ox-LDL 75 µg/mL group was significantly higher than that in the ctrl group (**P < 0.01). However, after treatment with 5mM Mß-CD, the positive rate of PI staining was reduced (**P < 0.01) in the ox-LDL 75 µg/mL + 5mM Mß-CD group. The experiments were performed independently (n = 6). Scale bar = 100 μm.
Discussion
In this study, we demonstrated that both a high-fat diet and ox-LDL induced the development of atherosclerosis in ApoE−/− mice and rat VSMCs via the TLR4/NF-κB/NLRP3 signaling pathway, primarily by triggering GSDMD-mediated pyroptosis. Our research primarily focused on evaluating the effects of Mß-CD on this signaling cascade and its role in modulating pyroptosis in atherosclerosis models.
Cyclodextrins, including Mß-CD, are well recognized for their cholesterol-depleting properties. Therefore, we acknowledge that Mß-CD may influence lipid levels. Notably, previous research has shown that membrane cholesterol-rich lipid rafts function as key platforms for inflammatory signaling, including NOX–ROS–NLRP3 inflammasome activation20. Disruption of lipid rafts by agents such as Mß-CD can inhibit the recruitment and activation of upstream regulators of pyroptosis pathways, even without causing extensive lipid depletion. Our findings revealed that Mß-CD effectively inhibited GSDMD-mediated pyroptosis, thereby preventing the progression of atherosclerosis induced by both a high-fat diet and ox-LDL exposure. This study is the first to provide evidence of Mß-CD’s potential to reduce atherosclerosis by inhibiting GSDMD-mediated pyroptosis via the TLR4/NF-κB/NLRP3 pathway. Our findings also demonstrate that Mß-CD treatment leads to reduced atherosclerotic plaque areas in the aorta, lower levels of CD68+ cells in aortic plaques, and decreased plasma levels of lipids, cholesterol, and pro-inflammatory cytokines IL-1ß and IL-18. Additionally, Mß-CD treatment enhances VSMCs membrane integrity.
Atherosclerosis is a condition in which cholesterol accumulates beneath artery walls, leading to inflammation and plaque formation. Various forms of cell death, including apoptosis, necrosis, autophagy, and pyroptosis, play critical roles in its progression. While apoptosis and autophagy have been well-documented in atherosclerosis, the mechanisms underlying pyroptosis in the disease remain incompletely understood. Pyroptosis was first discovered in 1986 as a form of programmed cell death triggered by various factors. It is characterized by the formation of inflammasomes, membrane pores, and the release of inflammatory cytokines such as IL-1ß and IL-18. In 2001, Cookson and colleagues formally named this process “pyroptosis.” Certain risk factors associated with atherosclerosis, such as ox-LDL and cholesterol crystals, have been found to promote pyroptosis21. In 2015, researchers discovered that GSDMD is the key executor of pyroptosis and is essential for the maturation and secretion of the inflammatory mediators IL-1ß and IL-1822,23,24. On the other hand, intracellular and extracellular risk factors, such as ox-LDL and cholesterol crystals, can trigger inflammasome assembly. Inflammasomes are immune complexes that drive inflammation and include proteins from the NLR and PYHIN families, as well as Pyrin proteins. Among them, NLRP3 is the most extensively studied inflammasome and is known for its role in initiating the innate immune response. Its close association with atherosclerosis has been widely documented25. The assembly process of NLRP3 consists of two steps: priming and activation. The priming stage involves the recognition of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) by pattern recognition receptors (PRRs), primarily toll-like receptors (TLRs). This recognition leads to the nuclear translocation of NF-κB and the transcription of pro-IL-1ß and IL-18. Following priming, the activation stage is initiated, leading to NLRP3 inflammasome assembly and the subsequent induction of pyroptosis21,26.
Human atherosclerosis differs greatly from the mice model. However, in the present study, the ApoE−/− mice model was selected because it is the most widely used and well-characterized model for atherosclerosis research. ApoE is a multifunctional protein expressed by hepatocytes, macrophages, and vascular cells, regulating lipoprotein metabolism, inflammation, and monocyte/macrophage function—all of which are central to the development of atherosclerosis. ApoE deficiency leads to spontaneous hypercholesterolemia and the formation of atherosclerotic lesions, which are accelerated under high-fat diet feeding. The lesions closely mirror human disease progression, evolving from fatty streaks to complex plaques containing lipid cores, fibrous caps, inflammatory infiltrates, and calcifications. Comparative studies using human ApoE isoform knock-in mice (e.g., ε2, ε3, ε4) have further reinforced the translational relevance of findings obtained in ApoE−/− models. Critically, ApoE−/− mice display heightened inflammatory responses, including activation of pathways such as NLRP3 inflammasome signaling and enhanced IL-1ß secretion—both of which are central to GSDMD-mediated pyroptosis. This inflammatory environment makes the model particularly suitable for studying the role of GSDMD in atherosclerosis progression. Experimentally, ApoE−/− mice offer several advantages: they develop all stages of atherosclerosis, including foam cell formation, necrotic core development, fibrous cap formation, and late-stage calcification, closely resembling human plaque morphology. The model also faithfully reproduces key pathological features such as macrophage infiltration, smooth muscle cell migration, and lipid accumulation. Additionally, it allows the dissection of specific genetic contributions (e.g., GSDMD) to atherogenesis, providing a platform to study gene-environment interactions. Importantly, ApoE deficiency sensitizes vascular cells to pyroptosis-relevant stimuli, making it ideal for investigating inflammatory cell death mechanisms. Moreover, therapeutic responses in ApoE−/− mice often mirror those observed in human clinical settings. Overall, while no animal model can completely replicate human atherosclerosis, the ApoE−/− mice remains the gold standard, particularly for investigating inflammatory and pyroptotic mechanisms within plaques27,28,29,30.
Consistent with previous studies on pyroptosis and atherosclerosis, our data provide evidence that the abnormal metabolism induced by a high-fat diet in ApoE−/− mice (in vivo) and ox-LDL in vitro cultured cells leads to increased expression levels of TLR4, NF-κB, p-NF-κB, NLRP3, GSDMD, and GSDMD-NT proteins. This upregulation indicates the successful development of atherosclerosis driven by GSDMD-mediated pyroptosis.
According to a recent study, Mß-CD was found to exert an anti-atherosclerotic effect in a mice model of hyperhomocysteinemia (HHcy)-induced atherosclerosis. In our vivo experiments, a dose of 2.0 g/kg was selected with reference to the study “HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in ApoE−/− Mice.” Furthermore, the study reported a significant reduction in the levels of IL-1ß and IL-1820. In our preliminary in vitro experiments, multiple concentrations of Mß-CD (1, 3, 5, and 10mM) were tested to assess cytotoxicity and efficacy. Based on these results, 5mM was selected as an effective and non-toxic concentration for VSMCs exposed to ox-LDL. This concentration was further supported by the findings of the study “Effects of MßCD on Lipoxygenase-Induced LDL Oxidation”, in which Mß-CD effectively prevented LDL oxidation by extracting cholesterol from membranes at concentrations ranging from 1 to 10mM”31. Consistent with these findings, our experimental results demonstrated that Mß-CD treatment mitigated the abnormal metabolism induced by a high-fat diet in ApoE−/− atherosclerotic mice (in vivo), as well as ox-LDL-induced atherosclerotic changes in in vitro cultured cells. This effect was associated with decreased protein expression levels of TLR4, NF-κB, p-NF-κB, NLRP3, GSDMD, and GSDMD-NT. Similarly, our findings confirmed that ApoE−/− mice fed a high-fat diet exhibited elevated plasma levels of the pro-inflammatory cytokines IL-1ß and IL-18. However, treatment with Mß-CD resulted in a significant reduction in IL-1ß and IL-18 levels (Fig. 7). Our data, in agreement with previous studies, further support the conclusion that Mß-CD exerts an anti-atherosclerotic effect.
Elevated levels of LDL-c and cholesterol crystals are hallmark features that promote atherosclerosis. Cholesterol accumulates in cells and transforms into cholesterol crystals through the enzymatic actions of acetyl-CoA acetyltransferase and neutral cholesterol ester hydrolase. These crystals activate inflammasomes, triggering an inflammatory response. Additionally, LDL undergoes oxidative modification, transforming into ox-LDL, which further amplifies inflammation through a series of ligand interactions. Ox-LDL serves as a critical link between lipid metabolism disorders and the inflammatory response. These mechanisms illustrate how ox-LDL triggers pyroptosis32. Therefore, after feeding ApoE−/− mice a high-fat diet, we observed increased levels of TG, TCHO, LDL-c, and blood glucose, along with a decrease in HDL-c levels in their plasma. Meanwhile, a separate study investigated the role of pyroptosis in the development of chronic liver injury and the therapeutic mechanism of DHM. The study found that treatment with Hydroxypropyl-ß-cyclodextrin (HP-ß-CD) led to a decrease in serum TG, TC, and LDL levels33. Notably, we observed reduced serum TG, TCHO, LDL-c, and blood glucose levels, along with an increase in HDL-c levels following Mß-CD treatment.
The stability of an atherosclerotic plaque depends on the thickness of its fibrous cap and the level of inflammation. If the cap thin due to VSMC death and ECM breakdown, the risk of complications, such as stroke, increases. Additionally, VSMC-derived macrophage-like cells can exacerbate inflammation. However, recent research suggests that VSMCs play a crucial role in preventing fibrous cap rupture in advanced plaques. The abnormal death and proliferation of VSMCs accelerate plaque formation, ultimately contributing to atherosclerosis34. Furthermore, atherosclerosis develops due to interactions between blood monocytes and the activated endothelium, leading to arterial dysfunction. Monocytes differentiate into macrophages, which accumulate lipoproteins and form fatty deposits. In the early stages, arterial endothelial cells express adhesion molecules, attracting monocytes and T-lymphocytes, which subsequently transform into lipid-engulfing macrophages. Over time, fat accumulation causes cellular damage, while macrophages secrete enzymes that weaken the plaque structure, thereby increasing the risk of rupture35,36,37. The findings of our study strongly indicate that ApoE−/− mice fed a high-fat diet developed atherosclerotic plaques at a rapid pace. As the study progressed, we confirmed the presence of VSMC-derived macrophages within the plaques by using CD68 antibodies as a macrophage marker and α-SMA as a molecular marker to identify VSMCs. This approach enabled us to evaluate the inflammatory response in the atherosclerotic plaques. Our study supports previous research demonstrating that VSMC-derived macrophages are recruited to atherosclerotic plaques, emphasizing their critical role in atherosclerosis development. Additionally, a recent study investigated the effects of HDL and hydroxypropyl-ß-cyclodextrin (HP-ß-CD) on cholesterol extraction from aggregated LDL, given LDL’s essential role in the early stages of atherosclerosis. Their results demonstrated that HP-ß-CD treatment reduced macrophage cholesterol uptake and foam cell formation, highlighting its potential therapeutic effect38. Meanwhile, our data provided clear imaging evidence confirming that Mß-CD reduced plaque area and decreased the population of CD68+ cells. Based on these findings, we observed a strong anti-atherosclerotic effect of Mß-CD. According to a recent study, macrophage pyroptosis induced by oxidative stress from ox-LDL led to reduced cell permeability39. We further examined the correlation between these factors. Our data provided strong evidence that ox-LDL induction in VSMCs led to an increase in PI-positive cells. Notably, after Mß-CD treatment, the number of PI-positive VSMCs decreased. Our study has several limitations. Firstly, Mß-CD exhibited unexpected behavior in Western blot analysis within the control group, and further investigation is needed to clarify this finding. Secondly, additional analysis is required to confirm the presence of cytokines such as IL-1ß and IL-18 in vitro. Thirdly, while this study focused specifically on GSDMD-mediated pyroptosis in atherosclerosis, future studies could include direct comparisons with other regulated cell death pathways, such as apoptosis, ferroptosis, and necrosis, to better elucidate their relative contributions to disease progression. Finally, although key markers of inflammasome activation—such as caspase-1 activation, ASC speck formation, and LDH release—are currently being investigated in a separate study, these important aspects remain part of our group’s ongoing work. We are actively addressing these points in future experiments to further strengthen and validate our findings. In conclusion, our study unveils a novel mechanism by which Mß-CD attenuates atherosclerosis by reducing GSDMD-mediated pyroptosis, a key pathogenic factor in atherosclerosis development through the TLR4/NF-κB/NLRP3 pathway. Understanding the molecular mechanisms of pyroptosis and the anti-atherosclerotic effects of Mß-CD provides deeper insight into the pathogenesis of atherosclerosis and offers a potential therapeutic strategy for its treatment.
Data availability
All data generated and analyzed during this study are included in this article and its Supplementary Information files. All data are available from the corresponding author on reasonable request.
References
Emini Veseli, B. et al. Animal models of atherosclerosis. Eur. J. Pharmacol. 816, 3–13 (2017).
Depuydt, M. A. C. et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. 127(11), 1437–1455 (2020).
Galluzzi, L. et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535(7610), 153–158 (2016).
Tong, Y. et al. NLRP3 inflammasome and its central role in the cardiovascular diseases. Oxid. Med. Cell Longev. 429, 3206 (2020).
Wu, D. et al. Gasdermin family: A promising therapeutic target for cancers and inflammation-driven diseases. J. Cell Commun. Signal. 14(3), 293–301 (2020).
Shankman, L. S. et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21(6), 628–637 (2015).
Jacobsen, K. et al. Diverse cellular architecture of atherosclerotic plaque derives from clonal expansion of a few medial SMCs. JCI Insight. 2(19), e95890 (2017).
Chistiakov, D. A., Melnichenko, A. A., Myasoedova, V. A., Grechko, A. V. & Orekhov, A. N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. (Berl). 95(11), 1153–1165 (2017).
Duchêne, D. & Bochot, A. Thirty years with cyclodextrins. Int. J. Pharm. 514(1), 58–72 (2016).
Zimmer, S. et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 8(333), 333ra50 (2016).
Chen, G. et al. Methyl-β-cyclodextrin suppresses the monocyte-endothelial adhesion triggered by lipopolysaccharide (LPS) or oxidized low-density lipoprotein (oxLDL). Pharm Biol. 59(1), 1036–1044 (2021).
Wu, J. et al. TNF-α contributes to sarcopenia through caspase-8/caspase-3/GSDME-mediated pyroptosis. Cell Death Discov. 9(1), 76 (2023).
Zhuang, W. et al. Pulmonary arterial hypertension induced by a novel method: Twice-intraperitoneal injection of monocrotaline. Exp. Biol. Med. (Maywood). 243(12), 995–1003 (2018).
Chen, A. et al. Zinc promotes cell proliferation via regulating metal-regulatory transcription factor 1 expression and transcriptional activity in pulmonary arterial hypertension. Cell Cycle 22(10), 1284–1301 (2023).
Chen, H. F., Xie, L. D. & Xu, C. S. The signal transduction pathways of heat shock protein 27phosphorylation in vascular smooth muscle cells. Mol. Cell Biochem. 333(1–2), 49–56 (2010).
Huang, J. et al. Silencing heat shock protein 27 (HSP27) inhibits the proliferation and migration of vascular smooth muscle cells in vitro. Mol. Cell Biochem. 390(1–2), 115–121 (2014).
Chen, H. F., Xie, L. D. & Xu, C. S. Role of heat shock protein 27 phosphorylation in migration of vascular smooth muscle cells. Mol. Cell Biochem. 327(1–2), 1–6 (2009).
Gao, G. et al. Comprehensive analyses of m6A RNA methylation patterns and related immune microenvironment in idiopathic pulmonary arterial hypertension. Front Genet. 14, 1222368 (2023).
Liu, S., Tao, J., Duan, F., Li, H. & Tan, H. HHcy induces pyroptosis and atherosclerosis via the lipid raft-mediated NOX-ROS-NLRP3 inflammasome pathway in ApoE-/- mice. Cells 11(15), 2438 (2022).
Wang, Q. et al. Pyroptosis: A pro-inflammatory type of cell death in cardiovascular disease. Clin. Chim. Acta 510, 62–72 (2020).
He, W. T. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25(12), 1285–1298 (2015).
Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic. Cell Death Nature 526(7575), 660–665 (2015).
Ding, J. et al. Poreforming activity and structural autoinhibition of the gasdermin family. Nature 535(7610), 111–116 (2016).
Wang, L., Negro, R. & Wu, H. TRPM2, linking oxidative stress and Ca2+ permeation to NLRP3 inflammasome activation. Curr. Opin. Immunol. 62, 131–135 (2020).
He, X., Bai, Q., Zhang, X. & Zhang, L. MgCl2 attenuates ox-LDL-induced vascular smooth muscle-derived foam cells pyroptosis by downregulating the TLR4/NF-κB signaling pathway. Biol. Trace Elem. Res. 201(11), 5242–5256 (2023).
Getz, G. S. & Reardon, C. A. ApoE knockout and knockin mice: The history of their contribution to the understanding of atherogenesis. J. Lipid Res. 57(5), 758–766(2016).
Linton, M. F. et al. Apolipoprotein E and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 35(5), e10–e25 (2015).
Nakashima, Y. et al. ApoE-deficient mice develop lesions. Arterioscler. Thromb. 14(1), 133–140 (1994).
Wang, N. et al. Plaque macrophages in atherosclerosis. J. Mol. Cell. Cardiol. 89(Pt B), 179–185 (2015).
Ao, M. & Chen, Y. Effects of MβCD on lipoxygenase-induced LDL oxidation. Chem. Pharm. Bull. (Tokyo). 65(2), 200–203 (2017).
Song, Y. et al. TLR4/NF-κB/ Ceramide signaling contributes to Ox-LDL-induced calcification of human vascular smooth muscle cells. Eur. J. Pharmacol. 794, 45–51 (2017).
Cheng, Q. C. et al. Dihydromyricetin ameliorates chronic liver injury by reducing pyroptosis. World J. Gastroenterol. 26(41), 6346–6360 (2020).
Durham, A. L., Speer, M. Y., Scatena, M., Giachelli, C. M. & Shanahan, C. M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 114(4), 590–600 (2018).
Braunersreuther, V., Mach, F. & Steffens, S. The specific role of chemokines in atherosclerosis. Thromb. Haemost. 97(5), 714–721 (2007).
Schulz, C. et al. Chemokine fractalkine mediates leukocyte recruitment to inflammatory endothelial cells in flowing whole blood: A critical role for P-selectin expressed on activated platelets. Circulation 116(7), 764–773 (2007).
McLaren, J. E., Michael, D. R., Ashlin, T. G. & Ramji, D. P. Cytokines,macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog. Lipid Res. 50(4), 331–347 (2011).
Singh, R. K., Lund, F. W., Haka, A. S. & Maxfield, F. R. High-density lipoprotein or cyclodextrin extraction of cholesterol from aggregated LDL reduces foam cell formation. J. Cell Sci. 132(23), 1 (2019).
Qiu, Y. et al. Exogenous spermine inhibits high glucose/oxidized LDL-induced oxidative stress and macrophage pyroptosis by activating the Nrf2 pathway. Exp. Ther. Med. 23(4), 310 (2022).
Acknowledgements
The authors would like to express their sincere gratitude to Mr. Li Yongsheng and Mr. Zhou Chao for initiating and recommending this program.
Funding
This work was supported by the grants from National Natural Science Foundation of China (82370351 and 82170355) and the Joint Funds for the Innovation of Science and Technology, Fujian province (2019Y9124) .
Author information
Authors and Affiliations
Contributions
Mohammad Ismail Hajary Sagor, Xie Liangdi, and Lian Guili conceptualized and designed the research, while Mohammad Ismail Hajary Sagor conducted the experiments, collected the data, performed the analysis, and wrote the manuscript. Lian Guili , Wang Qiuran, Wang Junshu , Yan Yan, Tang Huibin , Gao Gufeng , and Lin Huakan contributed to data visualization and formal analysis. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of ethical approval and compliance with ARRIVE guidelines
This study was performed strictly according to recommendations. The animal experiments were approved by the Laboratory Animal Welfare and Ethics Committee of Fujian Medical University (Approval No. 2017-068) and were conducted in accordance with the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sagor, M.I.H., Wang, Q., Wang, J. et al. Cyclodextrin attenuates atherosclerosis by diminishing gasdermin D (GSDMD)-mediated pyroptosis. Sci Rep 15, 21605 (2025). https://doi.org/10.1038/s41598-025-04889-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04889-2
Keywords
This article is cited by
-
Targeting pyroptosis in atherosclerosis: emerging pharmacologic strategies and natural compound-based therapeutics—a narrative review
International Journal of Clinical Pharmacy (2025)