Abstract
Anti-angiogenesis has been recognized as a crucial strategy in anti-tumor therapy, and the early assessment of its efficacy is equally significant. In this study, we developed a magnetic resonance (MR) probe specifically targeting angiogenesis to facilitate targeted imaging for the early evaluation of anti-angiogenic effects. We synthesized DOTA-G3CNGRC, conjugated it with gadolinium(III), and subsequently evaluated the labeled probe in vitro. The tumor-bearing mouse models of HT-29 (negative for CD13 expression) and HT-1080 (positive for CD13 expression) were successfully established. Magnetic resonance imaging was conducted via intraperitoneal injection of labeled probes and Gd-DOTA, both before and after treatment with ubenimex at a dose of 0.5 mg/kg/day for seven consecutive days. The average signal intensity ratio of the transplanted tumor (target tissue, T) to the left hind leg (non-target tissue, NT) was determined using the region of interest technique (ROI), while changes in tumor size were meticulously recorded. Additionally, APN/CD13 expression levels in transplanted tumors were assessed both prior to and following treatment. The labeling rate of probes was 88.99%. The IC50 of the probes was 7.03 µM. The T/NT ratio of HT-1080 was significantly higher than that of HT-29 (P < 0.001, n = 5). Following treatment, the T/NT ratio of the HT-1080 transplanted tumors was significantly reduced (P < 0.001, n = 5), accompanied by a notable decrease in CD13 expression and negligible changes in the sum of the long and short diameters (P = 0.39, n = 5). The research findings revealed that Gd-DOTA-G3CNGRC can serve as a highly specific gadolinium-based magnetic resonance imaging probe for monitoring the efficacy of anti-angiogenic therapy.
Similar content being viewed by others
Introduction
The healing process of almost all chronic diseases and acute injuries is accompanied by endothelial cell activation and angiogenesis, and the process of angiogenesis has an important impact on the final prognosis of different diseases. For some diseases, providing conditions to promote angiogenesis is considered to be one of the measures to promote the improvement of these diseases, such as fractures and wound healing disorders. However, for other diseases, especially malignant tumors, anti-angiogenesis is considered one of the therapeutic means to achieve better outcomes for these diseases. Therefore, the search for and identification of new receptors selectively expressed by activated endothelial cells and the discovery of vascular function regulators or new ligands that can interact with them has become a hot spot in vascular target imaging and therapy research1,2,3,4,5.
Aminopeptidase N (APN/CD13) (EC 3.4.11.2) is a type II exonuclide of approximately 150–240 kDa that is involved in the degradation of neutral or alkaline N-terminal residues of biologically active peptides. This protein is a member of the zinc metallopeptidase M1 family and consists of an enzyme-promoting extracellular domain, a transmembrane region and a short cytoplasmic domain involved in signal transduction6. It was found that the expression of this enzyme was up-regulated in angiogenic vessels caused by various etiologies, while it was little or no expression in normal vessels7,8. Studies have shown that APN/CD13 plays an important role in cancer development, progression and even treatment, especially the importance of APN expression and activities related to cancer characteristics, especially tumor angiogenesis and metastasis9,10.
The Asn-Gly-Arg (NGR) peptide was originally discovered by selecting peptide-phage libraries in tumor mice11. A large number of previous studies have shown that the NGR peptide can bind specifically to aminopeptidase N (APN/CD13) in vivo and in vitro. Because of these properties, the NGR peptide has been used by many researchers as a ligand-directed vector for the delivery of various therapeutics and imaging agents to angiogenic vessels12,13,14,15. Most of the previous research reports focused on targeted therapeutic drugs or imaging probes based on NGR carriers, while relatively few reports have evaluated the efficacy of anti-angiogenic drugs based on NGR carrier probes10,11,12. Therefore, in this study, Gd-DOTA-G3CNGRC was prepared, and imaging was performed on tumor-bearing mouse models to provide a magnetic resonance molecular imaging method for the early efficacy evaluation of antiangiogenic drugs.
Experimental materials and instruments
Experimental materials
2-Chloro-triphenylmethyl chloride resin, Fmoc-AA-OH (N-[fluorent-methoxycarbonyl]-l-alanyl-L-alanine), 5 g of indanhydrone-100 mL of ethanol, pyridine, N-(9-fluorent-methoxycarbonyl)-S-triphenylmethyl-L-cysteine (Fmoc-CYS (TRT)-Oh), Fmoc-ARG (PBF)-OH (N- (9-fluorene methoxycarbonyl)-S-triphenylmethyl-L-cysteine), N-fluorenyl methoxyl-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine (FMOC-Arg(Pbf) OH), and DOTA-NHS (1,4,7,10-tetraazecyclododecane-1,4,7,10-tetraacetic acid) were of analytical grade and purchased from Hefei Bairdi Chemical Technology Co., LTD., China. Anhui). Analytical grade DCM (dichloromethane), DIPEA (N, N-diisopropyl ethylamine), methanol, DMF (N, N-dimethylformamide), HOBT (1-hydroxybenzotriazole), and DIC (N, N’-diisopropyl carbodiimide) were purchased from Tianjin Concord Technology Co., LTD. (Tianjin, China). Bestatin (Ubenimex), GdCl3.6H2O, Gd-DOTA, and xylenol orange were purchased from Sigma‒Aldrich. The cell culture and immunohistochemical staining related reagents used in this study were purchased from Gibco, a brand owned by Thermo Fisher Scientific Inc.
Main experimental instruments
Vacuum freeze-dryer/freeze-dryer (LGJ-30FD, Beijing Songyuan Huaxing Technology Development Co., LTD.), preparation-grade HPLC (P3000I, Beijing Innovation Tongheng Chromatography Technology Co., LTD.), Shimadzu Liquid chromatocl-Mass Spectrometry Instrument (LCMS2020) and Shimadzu HPLC analysis instrument (LC-10AT 2010 A) are the products of Jinna Instrument Equipment (Shanghai) Co., LTD., continuous electric deionization ultra-pure water machine (YL-EDI60, Shanghai Yilan Environmental Protection Technology Co., LTD.) magnetic resonance imaging instrument (SIEMENS, MAGNETOM, skyra3.0T).
Methods
Synthesis of DOTA-G3CNGRC
Firstly, the 2-chloro-triphenylmethyl chloride resin (500 mg) was immersed in DCM for 30 min, followed by three washes with DMF and one wash with DCM. Then, a mixture containing 0.15 mM of Fmoc-CYS (TRT) OH, 8 mL of anhydrous DCM, and 800 µL of DIPEA was added and reacted under a nitrogen bubble condition for 1.5 h. After washing with DMF three times, a mixture containing 0.5 mL of DIPEA, 0.5 mL of methanol, and 2 mL of DCM was added, and the reaction continued for 20 min. Then, the liquid in the reactor was drained using a circulating water vacuum pump, and DMF, approximately three times the volume of the resin, was added to the reactor. The mixture was washed for 30 s, the liquid was drained, and this operation was repeated four times. Secondly, a 20% piperidine/DMF solution (with a volume three times that of the resin) was added to the reactor and bubbled with nitrogen for 20 min. The reactor was then washed four times using the aforementioned washing method. Finally, take the aforementioned 10–20 pieces of resin, add two drops of detection reagents A and B, and heat in a 100℃ incubator for 2 min to observe the color change of the resin. If the resin changes color, it indicates that the Fmoc has been successfully removed; if no color change occurs, the procedure must be repeated.Coupling: Dissolve 0.5 mM of Fmoc-Arg(Pbf)-OH and 0.5 mM of HOBT (75 mg) in 1 mL of DMF, followed by the addition of 100 µL of DIC. Mix for 1 min, then add the mixture to the drained resin for a nitrogen bubble reaction lasting 1 h.The resin was subsequently tested and washed multiple times. Following peptide synthesis, the Fmoc group was removed and washed off, after which 2 mL of DMF containing 0.3 nM DOTA-NHS and 100 µL of DIPEA were sequentially added and allowed to react for 20 min to modify the N-terminal of the polypeptide with DOTA. Finally, following the method described above, the resin is washed three times with methanol, after which the liquid is removed and the resin is vacuum-dried until it appears as a granular substance.Cut the polypeptide from the resin and purify the polypeptide: add the cleavage solution (100 mL of which contains 95 mL TFA, 1 mL water, 2 mL EDT, and 2 mL Tis) to the dried resin. Then, add methyl tert-butyl ether (pre-cooled to -20℃) in a volume ten times that of the cleavage solution to precipitate the cleaved polypeptide. Finally, the peptide was purified using an 8-micron 30 × 250 mm diagesol column. The mobile phase consisted of A: 0.1% TFA aqueous solution and B: 0.1% TFA acetonitrile solution; the flow rate was set at 12 mL/min. The sample was loaded through pump A, and subsequently, a 10% acetonitrile water solution was used to balance the gradient for 5 min. The sample peak was prepared for collection and detection. When the purity of the analysis exceeded 80%, the sample was deemed suitable for freezing and subsequent use.
Labeling DOTA-G3CNGRC with Gd(III)
Firstly, 1 mg (0.9µM) of DOTA-NGR was dissolved in 9 mL of acetic acid buffer with a pH of 5.4, followed by 10 mL of GdCl3 solution with a concentration of 10 µM/L, the molar ratio of Gd(III) to NGR was about 11:1, and the reaction was oscillated in a 38℃ water bath for 30 min. Secondly, the mixture was transferred into a 10 mL dialysis tube (Spectra/Por® Flot-A-Lyzer ®, MWCO: 500 − 100), then the tube was placed in a beaker containing 500 mL of deionized water and dialyzed with a magnetic stirrer for 1 h. Finally, the marking rate was determined: 6 mL of the prepared xylenol orange solution (concentration 2 g/L) was added to a 12-well cell culture plate at 1 mL per well, GdCl3 solution at different concentrations (0.1 mL) was added, and the enzyme marker (Varioskan LUX, Thermo Fisher Scientific Inc.) was used. The OD value of each well was determined at 575 nm, and the standard curve and equation of Gd(III) content were established. After dialysis, the liquid in the beaker was added to the xylenol-containing orange hole at a rate of 0.1 mL per hole, the OD value of each hole was determined, the concentration of free Gd(III) was calculated, and the loading rate of Gd(III) was calculated according to the concentration. To verify the accuracy of this method, we conducted simultaneous HPLC and mass spectrometry analyses on the labeled probes, as well as performed magnetic resonance imaging and relaxivity measurement on the probes. The sequence used for the imaging was T1-weighted fast low-angle shot 2D (t1_fl2d), with the following parameters: TR: 200 ms, TE: 2.46 ms, flip angle: 70 degrees, DFOV: 40.4 × 20.0 cm, Matrix: 320 × 272, and a slice thickness of 3 mm. Ammonium acetate buffer (0.2 µM/mL) was set as the negative control, gadolinium(III) labeled DOTA-G3CNGRC (0.9µM/mL) as the labeled probe concentration, and Gd-DOTA (2.5 µM/mL) as the positive control.The measurement of relaxation rates was performed with reference to previous literature24,25, under the conditions of 128 MHz and 298 K.
Cell binding experiment
Cell binding experiments were carried out according to the methods reported in the references16 and were summarized as follows: HT-1080 cells (1 × 106 cells/well) (purchased from the Cell Bank of the Chinese Academy of Sciences) were plated at uniform cell density and incubated overnight. The cells were washed 3 times with cold buffer (25 mM HEPES and 1% BSA) for 2 min. The cells were then mixed with 5 µL of 99mTc-labelled G3CNGRC [the preparation conditions were as follows: freshly washed 99mTcO4− 5 mCi (185 MBq) and 5 µg of stannous chloride (SnCl2) were added to 2 mg of DOTA G3CNGRC dissolved in deionized water, mixed evenly, and oscillated in a water bath at 38 °C for 30 min. The reaction solution was transferred into a dialysis tube and oscillated at room temperature for 30 min (the dialysate was 500 mL of deionized water)], and different concentrations of 99mTc-DOTA-G3CNGRC were incubated for 1 h. After washing with cold binding buffer 3 times, the cells were lysed with 200 µL lysis buffer. A gamma counter was then used to measure the radioactivity counts in each well. SPSS 29.0 software was used to perform nonlinear regression fitting on the data, and the 50% inhibition concentration (half-inhibition concentration, IC50) value of the best fit was calculated.
Establishment of tumor-bearing animal models and magnetic resonance imaging
Culture of cell lines and establishment of the tumor model
HT-1080 (human fibrosarcoma) (CD13 positive expression) and HT-29 (human colorectal adenocarcinoma) (CD13 negative expression) cells (purchased from Cell Bank of the Chinese Academy of Sciences) were cultured in a 75 mL flask with 10 mL complete DMEM (HT-1080)/RM1570 (HT-29)/medium with 1% penicillin and 1% streptomycin. The culture conditions were 37 °C, 10% FBS, and 5% CO2. BALB/c nude mice were 8 weeks old (purchased from the Experimental Animal Center of Zunyi Medical University), with a total of 14 males and females randomLy weighing approximately 25 g per mouse. The mice were kept in an environment free of special pathogens (SPF). HT-29 and HT-1080 cells were collected and inoculated into the right axilla of mice at 5 × 106 cells per mouse and continued to be fed, and magnetic resonance imaging was performed when the tumor grew up to approximately 1 cm.
Magnetic resonance imaging of the tumor model
Gd-DOTA-G3CNGRC (200 µg per mouse) was injected intraperitoneally, mice were fixed on a flat board, and a 3.0T MRI T1WI scan was performed with a special coil for small animals. The sequence used was T1-weighted fast spin-echo 2D (t1_fl2d) with the following imaging parameters: TR: 200 ms, TE: 2.46 ms, flip angle: 70 degrees, DFOV: 40.4 × 20.0 cm, Matrix: 320 × 272, slice thickness: 3 mm. Image processing: the region of interest technique (ROI) was adopted, the tumor was selected as target tissue (T), and the muscle tissue of the left hind leg was selected as nontarget tissue (NT). The average signal intensitys of the ROI region were counted and the T/NT ratio was calculated. After the first imaging, HT-1080 tumor-bearing mice were placed in SPF environment and given 0.5 mg/kg/ day intraperitoneal administration of ubenimex for 7 days, and magnetic resonance imaging was performed again on the 8th day to detect the changes in tumor T/NT values. To confirm the specificity of the probe, Gd-DOTA imaging of the transplanted tumor was conducted using the same sequence as Gd-DOTA-G3CNGRC, following an intraperitoneal injection of 1 mg/kg. The scanning time points were set at 0.5 h, 1 h, 2 h, and 4 h post-injection, respectively.
Pathological examination
Following the initial MRI scan, two mice bearing HT-1080 and HT-29 tumors were humanely euthanized via cervical dislocation, and their tumor tissues were harvested. The specimens were subsequently fixed in a formaldehyde solution for 48 h before being embedded in paraffin blocks to prepare sections with a thickness of 5 μm. Immunohistochemistry was then conducted to assess the expression of APN/CD13 in each sample. At the conclusion of the second MRI scan, all mice were humanely euthanized to detect the expression of APN/CD13 in the transplanted tumors using the same method.
Statistical analysis
The statistical software utilized for all data analysis was SPSS version 29.0. A one-way analysis of variance was employed to compare the means of multiple groups. A statistically significant difference was set at P < 0.05, and measurement data were expressed as the mean ± standard deviation (mean ± std.).
Results
Synthesis of DOTA-G3CNGRC and Gd(III) labeling
HPLC results showed that the peak retention time of DOTA-G3CNGRC was 15.136 min, and the purity was up to 96.39% (Supplementary Fig. 1). Mass spectrometry results showed that the actual molecular weight of DOTA-G3CNGRC was 1107.20, which was very close to the theoretical molecular weight of 1107.18 (Supplementary Material 1). Based on the established GdCl3 standard curve (Fig. 1), the labeling rate of Gd-DOTA-G3CNGRC was determined to be 88.99%. This outcome is in close agreement with the HPLC results (90.93%), and the actual molecular weight of the labeled probe is 1264.10 (Supplementary Material 2). The labeled probe (Fig. 2C) and Gd-DOTA (Fig. 2A) both exhibited high signal intensity on T1-weighted images, while the ammonium acetate solution showed low signal intensity (Fig. 2B). The proton relaxivities of Gd-DOTA-G3CNGRC—R1 and R2 —are 7.2 mM− 1S− 1 and 7.4 mM− 1S− 1 respectively, while those of Gd-DOTA are 4.0 mM− 1S− 1 and 4.8 mM− 1S− 1 respectively. The chemical structure diagram of the labeled probe was shown in Supplementary Material 3.
The standard curve illustrated the relationship between the concentration of Gd(III) and the optical density (OD) value of the xylenol orange mixture. The absorbance (x) at a wavelength of 570 nm, after combining xylenol orange with varying concentrations of gadolinium(III), shows a strong correlation with the concentration of gadolinium(III) (y).
Results of the cell binding experiment
The CD13 receptor-binding affinity of DOTA − G3CNGRC was measured by a competitive cell binding assay using HT-1080 cells, and the 99mTc-labelled DOTA − G3CNGRC peptide was used for competitive substitution as a CD13-specific radioligand. The IC50 of DOTA − G3CNGRC was 7.03 µM (95% CI: 6.47 µM to7.62 µM, n = 5) (Fig. 3).
Cell binding experiment of DOTA-G3CNGRC. In HT-1080 cells, the binding of 99mTc-DOTA-G3CNGRC to the CD13 receptor was inhibited by DOTA-G3CNGRC in a dose-dependent manner.The concentration of the DOTA-G3CNGRC peptide ranged from 0.01 to 60.00 µM. The IC50 value of Gd-DOTA-G3CNGRC was determined to be 7.03 µM (95% CI: 6.47 µM to 7.62 µM, n = 5).
Results of magnetic resonance imaging in animal models
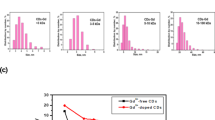
Upon conducting T1-weighted magnetic resonance imaging following the intraperitoneal injection of Gd-DOTA, we noted that the transplanted tumors of HT-1080 (Fig. 4A, Mouse 1) and HT-29 (Fig. 4A, Mouse 2) demonstrated comparable enhancement effects. However, subsequent to the injection of the labeled probe, the HT-1080 transplanted tumor (Fig. 4A, Mouse 3) exhibited a high signal intensity, whereas the HT-29 transplanted tumor presented an isointense signal (Fig. 4A, Mouse 4). The signal intensity of the HT-29 xenografts remained constant before (Fig. 4B, mouse 5) and after treatment (Fig. 4B, mouse 5’), despite a notable increase in size. Conversely, the signal intensity of the HT-1080 xenografts was greater before treatment (Fig. 4B, mouse 6) than after treatment (Fig. 4B, mouse 6’). Statistical analysis indicated that at 2 h post-injection of the labeled probe, the T/NT ratios of HT-1080 xenografts were significantly higher than those of HT-29 xenografts, irrespective of pre-treatment or post-treatment status (Table 1). Nevertheless, no significant difference in T/NT values was detected between HT-1080 xenografts (treated or untreated) and HT-29 xenografts at 1 h post Gd-DOTA injection (Table 1). The T/NT value-time curve indicated that for HT-1080 and HT-29 xenografts, the peak value appeared at 1 h post-injection of Gd-DOTA, whereas the peak value emerged at 2 h post-injection of Gd-DOTA-G3CNGRC (Fig. 5).
Magnetic resonance imaging of tumor-bearing mouse model. Following intraperitoneal injection of Gd-DOTA or Gd-DOTA-G3CNGRC (A), high signal intensity was observed in HT-1080 xenograft tumors (Mouse 1 and Mouse 3), with peak times occurring at 1 h and 2 h post-injection, respectively. In contrast, HT-29 xenograft tumors exhibited high signal intensity only after Gd-DOTA injection (Mouse 2), with peak time consistent with that of HT-1080 xenografts. However, no significant high signal was observed after Gd-DOTA-G3CNGRC injection (Mouse 4). Before and after treatment (B), the signal intensity of the post-treatment HT-29 xenografts (mouse 5’) exhibited no significant change compared to pre-treatment (mouse 5), despite a marked increase in tumor size. In contrast, the signal intensity of the post-treatment HT-1080 xenografts (mouse 6’) significantly decreased compared to pre-treatment (mouse 6), while the tumor size showed no notable variation.The solid and dashed green circles represent the ROI sampling points.
T/NT-time curves of HT-1080 and HT-29 xenografts after injection with different contrast agents. In the HT-1080 and HT-29 xenograft tumor models, the peak T/NTratio occurred at 1 h post Gd-DOTA injection, with no significant difference in T/NT ratios observed between the two xenografts before or after treatment. In contrast, Gd-DOTA-G3CNGRC exhibited a T/NT peak at 2 h post-injection in the HT-1080 model, accompanied by significant differences before and after treatment, whereas no distinct peak was observed in the HT-29 model either before or after treatment.
To assess changes in the size of transplanted tumors before and after treatment, we measured the sum of the long and short diameters of the tumor’s largest cross-section on transverse MRI images.The results indicated that the sum of the long and short diameters of the HT-1080 transplanted tumors did not significantly change before and after treatment (3.1 ± 0.1 cm vs. 2.9 ± 0.1 cm, n = 5, P = 0.39). In contrast, the diameters of the HT-29 transplanted tumors were significantly larger after one week (3.5 ± 0.2 cm vs. 2.9 ± 0.1 cm, P = 0.001).
Pathology
Immunohistochemical staining showed that APN/CD13 was not expressed in HT-29 transplanted tumors (Fig. 6A, B), but was highly expressed in HT-1080 transplanted tumors (Fig. 6C). The expression of APN/CD13 in HT-1080 grafts decreased significantly one week after treatment with ubenimex (Fig. 6D).
Immunohistochemical staining of ANP/CD13 expression in transplanted tumors. HT-29 grafts (A, B) exhibited no significant expression of ANP/CD13, whereas HT-1080 grafts demonstrated high expression of ANP/CD13 prior to treatment with ubenimex (indicated by arrows). Expression of ANP/CD13 was markedly reduced at 7 days post-treatment with ubenimex (indicated by arrows) (20×).
Discussion
In addition to pathological examination methods, various imaging techniques are employed to evaluate the status of tumor angiogenesis, each with its own distinct advantages and applications. For example, nuclear medicine offers high sensitivity but suffers from low spatial resolution16,17. Magnetic resonance imaging (MRI) can provide a clearer anatomical structure; however, MRI technology often cannot differentiate between normal (or mature) and new blood vessels18. Optical devices exhibit high sensitivity and a straightforward imaging process. However, the clinical application of such imaging technology is currently constrained by the weak penetration of fluorescence photons and the phenomenon of fluorescence quenching19,20. MR angiography and MR perfusion imaging are prevalent clinical techniques for evaluating tumor vasculature18,21, yet they commonly suffer from a lack of specificity for neovascularization. In contrast, magnetic resonance imaging utilizing paramagnetic materials labeled with neovasculature-targeting peptides holds promise for addressing the resolution inadequacies in nuclear medicine imaging, potentially yielding images with superior tissue and spatial resolution while avoiding the risks associated with ionizing radiation22,23,24.The intensity of the T1-weighted image signal from the magnetic resonance probe correlates with the Gd(III) loading rate of the probe and the concentration of the Gd(III) chelate25. The Gd-DOTA-G3CNGRC probes prepared in this study had a loading rate comparable to those reported in previous studies22,23,24. Nevertheless, there has been limited research on the application of magnetic resonance imaging targeting probes to assess the effectiveness of antiangiogenic or antitumor therapeutic drugs. Our results indicated that the T/NT ratio in the group of tumor-bearing mice decreased significantly after 7 days of ubenimex treatment, despite the sum of the long and short diameters of the tumor not changing significantly. This suggested that the CD13 activity expressed by the tumor was inhibited post-treatment. Additionally, on Gd-DOTA imaging, both pre- and post-treatment HT-1080 transplanted tumors, as well as HT-29 transplanted tumors, exhibited significant enhancement. Furthermore, the enhancement time differed from that of Gd-DOTA-G3CNGRC, and there was no variation in T/NT values. This indicated that the enhancement effect of gadopentetate dimeglumine on tumors is non-specific, and verifies the high specificity of Gd-DOTA-G3CNGRC for CD13-positive tumors. In the mouse tumor model tested in this experiment, HT-29 tumors with negative expression of APN/CD13 exhibited little uptake of Gd-DOTA-G3CNGRC, whereas HT-1080 tumors with positive expression of CD13 clearly took up Gd-DOTA-G3CNGRC. This suggested that during the synthesis and labeling of DOTA-G3CNGRC, the activity of G3CNGRC was not significantly compromised. Compared with previous reports, our imaging results basically reached the same conclusion15,22,23,24. However, in contrast to these reports, our study mainly focused on the feasibility of early evaluation of the efficacy of antiangiogenic drugs, so DOTA-G3CNGRC was not used for blocking experiments in our study.
Studies have shown that APN/CD13 is upregulated in the blood vessels of many solid tumors, and the expression of cancer cells, stroma and/or vasculature can be observed depending on the tumor type12,13. The formation of neovascularization is closely related to tumor growth, metastasis and invasion, so reducing the expression of APN/CD13 in tumor cells has a significant promoting effect on improving the therapeutic effect of antitumour drugs26,27,28. At present, a variety of antagonists targeting APN/CD13 have entered the clinical application or clinical trial stage, such as bestatin (ubenimex), amastatin, and probestin13,29,30, among which ubenimex is the most commonly used in the clinic. Its mechanism of action may be through targeting the CD13/EMP3/FAK/NF-κB pathway, affecting autophagy and apoptosis, improving chemotherapy drug sensitivity, and thus reversing drug resistance30. Anti-tumour angiogenesis is currently a commonly used method, including many types of drugs, which have also been clinically applied31,32. Most patients may experience significant side effects for a period of time after usage, and resistance may develop subsequently33,34. Therefore, in addition to detecting serum tumor markers, imaging of a target should also be performed for direct evaluation, especially early assessment. Our findings introduced a new aspect to these approaches, but further research should be needed on various types of tumor models.
Molecular probes targeting APN/CD13 using NGR peptides as carriers have been reported in previous studies, such as 64Cu-labeled NGR1 and NGR216, and 68Ga-labeled NOTA-G3-NGR35, which achieved encouraging results in micro-positron emission tomography (PET) imaging. However, this type of probe carries potential radiation damage risks and exhibits relatively low tissue resolution in images, making it difficult to accurately delineate the boundary between tumors and surrounding tissues. Although the dual-modal magnetic resonance probe RGD10–NGR9–USPIO demonstrates good contrast for tumor diagnosis15, its preparation process is relatively complex. Additionally, T2-weighted imaging (T2WI) provides lower tissue resolution compared to T1-weighted imaging (T1WI). Moreover, there may be nonspecific enhancement of the reticuloendothelial system in organs such as the liver, lymph nodes, and bone marrow. While magnetic resonance contrast agents can assess tumor vascularity to differentiate benign from malignant lesions, they lack specificity and may be more suitable for evaluating tumor response after multiple treatment cycles.
Conclusion
Our research findings indicated that the labeled probe exhibits outstanding targeting capabilities in both in vivo and in vitro experiments. Consequently, we are confident that this labeled probe can function as a highly specific gadolinium-based MRI tracer, suitable for additional clinical studies to evaluate the efficacy of anti-angiogenic therapy.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Kuai, J., Han, C. & Wei, W. Potential regulatory roles of GRK2 in endothelial cell activity and pathological angiogenesis. Front. Immunol. 12, 698424 (2021).
Crippa, L. et al. A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood 121, 392–402 (2013).
Liu Chao, Castillo Alesha, B. Targeting Osteogenesis-Angiogenesis coupling for bone repair. J. Am. Acad. Orthop. Surg. 26 (7), e153–e155 (2018).
Griffioen, A.W. & Dudley, A.C. The rising impact of angiogenesis research. Angiogenesis 25, 435–437 (2022).
Lidonnici Jacopo, S., Massimo, M. & Oberkersch Roxana, E. Cancer-Induced metabolic rewiring of tumor endothelial cells. Cancers 14 (11), 2735 (2022).
Santos, A. N. et al. Aminopeptidase N/CD13 is directly linked to signal transduction pathways in monocytes. Cell. Immunol. 201, 22–32 (2000).
Malin, W. et al. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 102, 501-08 (2011).
Di Matteo, P. et al. Enhanced expression of CD13 in vessels of inflammatory and neoplastic tissues. J. Histochem. Cytochem. 59, 47–59 (2011).
Mina-Osorio, P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol. Med. 14, 361–371 (2008).
Pang, L. et al. Serum APN/CD13 as a novel diagnostic and prognostic biomarker of pancreatic cancer. Oncotarget 7, 77854–77864 (2016).
Arap, W., Pasqualini, R. & Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279, 377–380 (1998).
Angelo Corti, M., Fiocchi, F. & Curnis Targeting CD13 with Asn-Gly-Arg (NGR) peptide-drug Conjugates. 101 – 22 (Next-Generation Therapies and Technologies for Immune-Mediated Inflammatory Diseases, 2017).
Barnieh, F. M., Loadman, P. M. & Falconer, R. A. Is tumour-expressed aminopeptidase N (APN/CD13) structurally and functionally unique? Biochimica et biophysica acta. Reviews cancer. 1876 (2), 188641 (2021).
Gopal Pathuri, J. E. et al. Solid phase synthesis and biological evaluation of probestin as an angiogenesis inhibitor. Bioorg. Med. Chem. Lett. 23, 3561–3564 (2013).
Wu, T. et al. Magnetic resonance imaging of tumor angiogenesis using dual-targeting RGD10-NGR9 ultrasmall superparamagnetic iron oxide nanoparticles. Clin. Transl Oncol. 20, 599–606 (2018).
Chen, K. et al. Synthesis and evaluation of 64Cu-Labelled monomeric and dimeric NGR peptides for micropet imaging of CD13 receptor expression. Mol. Pharm. 10, 417–427 (2013).
João, D. G. et al. Radiometallated peptides for molecular imaging and targeted therapy. Dalton Trans. https://doi.org/10.1039/C0DT01599G (2011).
Qian, J. et al. Recent advances in explainable artificial intelligence for magnetic resonance imaging. Diagnostics 13, 1571 (2023).
Huang, R. et al. In vivo tumor angiogenesis imaging using Peptide-Based Near-Infrared fluorescent probes. Methods Mol. Biol. 1444, 73–84 (2016).
Pretze, M. et al. αvβ3-Specific gold nanoparticles for fluorescence imaging of tumor angiogenesis. Nanomaterials 11, 138 (2021).
Stippich, C. et al. Task-based presurgical functional MRI in patients with brain tumors. Clin. Funct. MRI. 121 – 95 (2021).
Koudrina, A. et al. Exploring the unique contrast properties of Aptamer – Gadolinium conjugates in magnetic resonance imaging for targeted imaging of thrombi. ACS Appl. Mater. Interfaces. 13, 9412–9424 (2021).
Ren, B. X. et al. Magnetic resonance tumor targeting imaging using gadolinium labelled human telomerase reverse transcriptase antisense probes. Cancer Sci. 103 (8), 1434–1439 (2012).
Park, J.-A. et al. Improved tumor-targeting MRI contrast agents: Gd (DOTA) conjugates of a cycloalkane-based RGD peptide. Biochem. Biophys. Res. Commun. 455(3–4), 246 – 50. (2014).
Divya Rajendran, J. et al. Synthesis, characterization and relaxivity validations of Gd(III) complex of DOTA Tetrahydrazide as MRI contrast agent. J. Mol. Struct. 1255, 132474 (2022).
Pereira Flavia, E. et al. CD13 is essential for inflammatory trafficking and infarct healing following permanent coronary artery occlusion in mice. Cardiovascular. Res. 100 (1), 74–83 (2013).
Juho, J. et al. Aminopeptidase expression in multiple myeloma associates with disease progression and sensitivity to Melflufen. Cancers 13 (7), 1527 (2021).
Xiu, T. et al. CD13 downregulation mediated by Ubenimex inhibits autophagy to overcome 5-FU resistance by disturbing the EMP3/FAK/NF-κB pathway in gastric cancer cells. Transl Cancer Res. 11 (8), 2487–2500 (2022).
Yangyang Liu, D. et al. Development of hydroxamate derivatives containing a pyrazoline moiety as APN inhibitors to overcome angiogenesis. Molecules 27, 8339 (2022).
Jiangying Cao, W. et al. Development of a Bestatin-SAHA hybrid with dual inhibitory activity against APN and HDAC. Molecules 25, 4991 (2020).
Samman Munir, A. A. et al. Anti-angiogenesis potential of phytochemicals for the therapeutic management of tumors. Curr. Pharm. Design. 26 (2), 265–278 (2020).
Teleanu, R. I. et al. Tumor angiogenesis and Anti-Angiogenic strategies for Cancer treatment. J. Clin. Med. 9 (1), 84 (2019).
Nakamichi, S. et al. A Phase II study of Ubenimex combined with pembrolizumab, nab-paclitaxel, and carboplatin for previously untreated advanced squamous non-small-cell lung cancer: TORG2241 (UBE-Q) 2023. Clin. Lung Cancer 25 (1), 85–90 (2024).
Yunyan Shi, Q. et al. Ubenimex suppresses Glycolysis mediated by CD13/Hedgehog signaling to enhance the effect of cisplatin in liver cancer. Transl Cancer Res. 12 (10), 2823–2836 (2023).
Adrienn Kis, N. et al. In vivo assessment of aminopeptidase N (APN/CD13) specificity of different 68Ga-labelled NGR derivatives using PET/MRI imaging. Int. J. Pharm. 589, 119881 (2020).
Acknowledgements
Thanks to all the staff of the central laboratory of the Third Affiliated Hospital of Zunyi Medical University (Zunyi First People’s Hospital) for their selfless help in this study. Thanks for the funding of this study from Guizhou Science and Technology Department project: [2019] 1325.
Author information
Authors and Affiliations
Contributions
Authors in order: The author, Songsong Liu, participated in the research process, data collection, analysis and interpretation of data, drafting papers, statistical analysis; Sheng Han participated in the research process, data collection, analysis and interpretation of data, drafting papers, statistical analysis; Gongwei Jing participated in research process, data collection, analysis and interpretation of data, statistical analysis; Pinqin Wang participated in the research process, analysis and interpretation of MRI data, and supporting work; Yanteng Zhang participated in the research process, MRI data collection, statistical analysis, technical and material support; Ling Xiong participated in the research process, data collection, administration, technical and material support; Yingfang Zhang , participated in the research process, IC50 data collection, literature review and material support; Huasheng Qu participated in the research process, 99mTc-DOTA-G3CNGRCdata collection, literature review and material support; Bingxiu Ren participated in the design, implementation research, data collection, analysis and interpretation of data, drafting papers, critical review of the intellectual content of the manuscript, access to research funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, S., Liu, SS., Jing, GW. et al. Early evaluation of anti-angiogenic effects with gadolinium(III) labeled APN/CD13 specific binding peptides magnetic resonance imaging. Sci Rep 15, 21269 (2025). https://doi.org/10.1038/s41598-025-05905-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05905-1