Abstract
The hippocampus is one of the first regions affected in various neurodegenerative diseases. In this study, we investigated the vascular factors contributing to its susceptibility, aiming to elucidate the underlying vascular mechanisms. Utilizing publicly available single-cell datasets, we analyzed the differential expression of genes in the endothelial cells and other blood-brain barrier (BBB)-associated cells within the hippocampus and compared them to those in the cortex. The identified hub genes were further validated using naïve mouse and ischemia rat models. We identified differentially expressed genes (DEGs) in endothelial cells, pericytes, and astrocytes in the BBB. Subsequent gene ontology (GO) enrichment analysis and protein-protein interaction (PPI) network analysis identified key hub genes: Kdr, Fn1, Pecam1, Cd34, Cd93, and Emcn that related to angiogenesis. Their differential expression was then experimentally verified using microvessels from mouse and rat brains. In the naïve mouse, compared to the hippocampus, the expression of Fn1 and Pecam1 was significantly higher in cortical microvessels, while Kdr, Cd34, and Cd93 exhibited a clear trend of increased expression. In the rat model of ischemia, we observed an upregulation of angiogenesis-related genes—Kdr, Cd34, and Cd93—within the microvasculature of both the hippocampus and cortex. However, their expression levels were relatively lower in the hippocampus compared to the cortex. These findings suggest that the hippocampus has a reduced angiogenic capacity compared to the cortex, which may contribute to its increased vulnerability to neurological disorders.
Similar content being viewed by others
Introduction
In mammals, significant differences exist between the cerebral cortex and hippocampus in terms of anatomy, cellular structure, and function1. The cortex is a later-evolved brain structure composed of six layers of neurons and encompasses widespread functional areas2whereas the hippocampus has a relatively simpler structure, consisting of three main regions that are critically involved in learning, memory, and emotional regulation3. As one of the most extensively studied regions of the brain, the hippocampus is highly sensitive to hypoxia and shows early functional impairments in conditions such as Alzheimer’s disease (AD), epilepsy, vascular dementia, and aging4,5. The level of reactive oxygen species-scavenging tetrahydrobiopterin (BH4) in the hippocampus is lower than that in the whole brain, suggesting that the hippocampus may be more vulnerable to oxidative stress6. In addition, the interaction between the vascular and nervous systems offers a novel perspective for investigating hippocampal vulnerability7,8: The normal function of neurons is maintained by the blood-brain barrier (BBB), which is primarily composed of brain microvascular endothelial cells, astrocytes, and pericytes. It was reported that in neurodegenerative and vascular diseases, the hippocampal BBB is more vulnerable than in the cerebral cortex. Previous studies in our group also confirmed the vulnerability of BBB in the hippocampus: One year after stroke, the permeability of BBB in the hippocampus of rhesus monkeys was significantly higher than that in the cortex, suggesting that the damage to the BBB in the hippocampus was more severe compared to the frontal cortex9. In addition, in the brain-gut axis-related studies of our group, continuous increase in BBB permeability in the hippocampus of rhesus monkeys with intestinal flora disorder was reported10. Furthermore, in the mouse model with intestinal flora disorder, we found that the permeability of BBB in the hippocampus was significantly higher than that in the cortex. Lastly, studies in other groups also demonstrated the vulnerability of the hippocampus: It was reported that elevated extracellular histones in the blood serve as a biomarker of vascular dysfunction in severe trauma or sepsis, with histone-induced BBB leakage occurring more frequently in the hippocampus than in the cortex11. In addition, Shaw. K et al. in 2021 found that, due to the difference in vascular network, pericyte, and endothelial cell function, the blood flow, blood oxygen, and neurovascular coupling in the hippocampus were less than those in the neocortex, and these characteristics may limit the supply of oxygen. This also explains its sensitivity to injury in neurological diseases8. Visualization of cerebral vasculature revealed that, compared to the cortex, the hippocampus exhibits lower vascular diameter, length density, and volume fraction, indicating its higher vulnerability to cerebrovascular dysfunction12,13. Furthermore, single-cell level sequencing and proteomics are essential tools for investigating the underlying mechanisms. These techniques also help to explain the vascular heterogeneity of the BBB in the hippocampus and cortex2,14.
In light of the evidence, we believe that investigating the mechanisms of BBB injury in the hippocampus as well as its vulnerability, are essential for preserving its normal structure and function. However, current studies on the heterogeneity of hippocampal and cortical damage mainly focus on the neurons15,16,17,18,19. Most reports focused on the phenotypes associated with specific biological processes6,11while some have investigated the overall structure and molecular basis of cerebral vessels8. However, comparative analyses of cortical and hippocampal brain microvessels in the BBB remain limited. In this study, we investigated why the BBB in the hippocampus was more vulnerable compared to the cerebral cortex. Identifying the factors underlying this increased vulnerability may offer important insights into neurovascular dysfunction and its effects on hippocampal health, which is crucial for advancing our understanding of cerebral circulation and cerebrovascular diseases.
To address this, we employed the single-cell sequencing method, which allows us to analyze the different cellular components of the BBB and their heterogeneity as our starting point. By mining publicly available cerebral vascular-related single-cell sequencing datasets, we were able to identify and analyze the differentially expressed genes (DEGs) in vascular endothelial cells from the hippocampus and cortex of the brain. After analysis, key hub genes: kinase insert domain receptor (Kdr), fibronectin 1 (Fn1), platelet and endothelial cell adhesion molecule 1 (Pecam1), CD34 antigen (Cd34), CD93 antigen (Cd93), and endomucin (Emcn) that related to the angiogenesis were identified and validated by experiments with naïve mouse and rat models.
Materials and methods
Single-cell RNA sequencing (scRNA-seq) datasets
In this study, we employed relevant datasets obtained from the GEO datasets (http://www.ncbi.nlm.nih.gov), including the mouse datasets GSE185862 (> 1.3 million cells)20SRP135960 (509,876 cells)21GSE60361 (3,005 cells)22and the human dataset GSE163577 (143,793 single-nucleus)23. These datasets encompass brain regions such as the hippocampus and cortex, and provide sequencing data for vascular-associated cell types. Quality control (QC) was conducted to assess mitochondrial gene content, gene count, and other indicators to identify and exclude potential multi-cell or doublet contamination. After QC, the data were imported into Seurat objects. Subsequently, we applied the Seurat 4.0 package for data normalization, dimensionality reduction, and clustering analysis to remove batch effects and obtain reliable data. Using the FindClusters function, we clustered the cells into distinct groups based on the reduced dimensions. Cell type annotation was carried out by referencing known cell markers from the literature, as well as markers commonly used in similar studies. Additionally, the machine learning tool scType was employed to automate the annotation of cell types for each cluster.
Identification of differentially expressed genes (DEGs)
Based on the marker gene information provided by the datasets, we independently identified vascular-associated cell type—including endothelial cells, pericytes, and astrocytes—within the hippocampal and cortical regions of the brain. Differential gene expression analysis was performed using the FindMarkers function implemented in the Seurat (version 4.0) package. In this analysis, the cortex was used as the reference group, while the hippocampus served as the comparison group, allowing for the identification of genes that exhibit region-specific expression patterns. To enhance interpretability and facilitate visual exploration of the results, volcano plots were generated using the ggplot2 package in R. These plots provide a graphical representation of the distribution of DEGs based on their statistical significance and magnitude of change. Genes were considered significantly differentially expressed if they met the criteria of an absolute log2 fold change (log2FC) greater than 0.25 and a P-value less than 0.05, thereby ensuring both biological relevance and statistical rigor.
Functional and pathway enrichment analysis
To investigate the potential biological roles of the DEGs, we performed functional enrichment analysis using Gene Ontology (GO) with a specific focus on the Biological Process (BP) category. Enrichment analyses were conducted using the clusterProfiler package (version 4.4.4) in R, based on annotation datasets derived from the Ensembl genome assemblies GRCh38.p13 (human) and GRCm39 (mouse), as well as curated GO terms. To facilitate accurate mapping and annotation of gene identifiers across datasets, we employed the biomaRt package (version 2.52.0), which serves as an interface to a range of biologically relevant datasets.
PPI network construction and hub genes identification
We employed an online tool (http://www.string-db.org/) for identifying and predicting interactions between genes or proteins and constructed the PPI network of intersecting DEGs. Next, we used the Cytoscape software to visualize the PPI network of the DEGs. The key nodes in the network were investigated using the Cytoscape plug-in Cytohubba to explore the central genes contained in the PPI network.
Animal
C57BL/6J mice (male, 6-8weeks old, #219, Beijing Vital River Laboratory Animal Technology Co., Ltd. China) and Wistar rats (male, 9–12 weeks old, #102, Beijing Vital River Laboratory Animal Technology Co., Ltd. China) were used in this study. Animals were housed under controlled specific pathogen-free (SPF) conditions (12-hour light/dark cycle, 22 ± 2℃, 50 ± 10% humidity) with food and water provided ad libitum.
At the end of the experiment, animals were euthanized using carbon dioxide (CO2) inhalation, in accordance with institutional and national ethical guidelines. CO2 was introduced gradually into a chamber at a rate of 20–30% of the chamber volume per minute. Death was confirmed by the absence of heartbeat and respiration, followed by cervical dislocation to ensure death. Cerebral microvessel specimens were then immediately collected from mice and rats after euthanasia.
All experimental protocols involving animals were reviewed and approved by the Ethical Committee of West China Hospital, Sichuan University, (Approval No. 20230515008). All procedures were performed in strict accordance with the relevant institutional guidelines and national regulations for the care and use of laboratory animals.
Furthermore, the reporting of animal experiments in this study adheres to the ARRIVE guidelines (https://arriveguidelines.org) to ensure transparency and reproducibility.
Two-vessel occlusion (2VO) modelling
We established a 2VO rat model via bilateral common carotid artery occlusion to induce cerebral ischemia and hypoxia. Rats designated for modelling were anesthetized with halothane inhalation, the bilateral common carotid arteries (CCA) of the rats were exposed through a midline cervical incision and permanently ligated. After carefully incising the carotid artery sheath and separating the vagus nerve from the CCAs, only the CCAs were ligated. During the procedure, the body temperature of the rats was maintained using a heating pad. The sham operation group only underwent neck skin incision and CCAs separation (SHAM, n = 6). Brain microvessels from the hippocampus and cortex were isolated and extracted from rats at day 1 (2VO D1, n = 6) and 3 days (2VO D3, n = 6) after the establishment of the 2VO model. The mortality rate of 2VO modeling was about 20%.
Cerebral microvessels sample Preparation
We isolated the brain microvessels from mice and rats separately. 14 naïve C57BL/6J mice were used for the extraction of cerebral microvessels from mice. For the extraction of cerebral microvessels from rats, we used 6 rats in the sham group, 6 rats in the 2VO modeling group for one day, and 6 rats in the 2VO modeling group for 3 days, respectively. The microvessels of the hippocampus and cortex were extracted by density gradient centrifugation following the reported protocol24.
After sacrificing the animals, their brains were extracted, and the cortex and hippocampus were isolated immediately. The brain tissue was homogenized with a loose-fit Dounce grinder of KIMBLE® Dounce tissue grinder set (Sigma, #D9063, German), centrifuged (4℃) for 5 min at 2,000g, and the supernatant was then removed. The pellet was resuspended in 15% Dextran 70 (Sangon, #9004-54-0, China) and centrifuged (4℃) for 15 min at 10,000g to separate the upper and lower layers, with the microvessels pellet remaining in the lower-most layer. The microvessels pellet was resuspended in DPBS and transferred onto the 40 μm cell strainer. After washing with the cold DPBS, the microvessels was obtained by inverting the strainer and washing with DMEM medium containing 0.5% (wt/vol) bovine serum albumin (Beyotime, #ST2254, China). The suspension was then centrifuged (4℃) for 10 min at 5,000g to isolate pure microvessels pellet.
RT-qPCR
Total RNA was extracted from the tissue samples using the TRIzol™ Reagent (Invitrogen, #15596026, USA) according to the manufacturer’s instructions. Reverse transcription was performed using the HiScript III RT SuperMix for qPCR (Vazyme, #R323-01, China). The synthesized cDNA was stored at -80℃ until further use. RT-qPCR was conducted using the ChamQ Universal SYBR qPCR Master Mix (Vazyme, #Q711-02, China). The relative expression levels of the target genes were calculated using the 2^(-ΔΔCt) method, with β-actin (Actb) as the internal reference gene. The PCR primers used in this study were listed in Table 1 below.
Western blot (WB) analysis
Proteins were extracted from microvessels samples from the hippocampus and the cortex using RIPA buffer (Beyotime, #P0013B, China) containing protease inhibitors (Beyotime, #P1005, China). The protein concentration was determined by BCA assay. Equal amounts of protein were separated by SDS-PAGE on 10% polyacrylamide gels and transferred to PVDF membranes. After blocking with 5% non-fat milk in TBST for 1 h at room temperature, the membranes were incubated with primary antibodies against vascular endothelial growth factor receptor 2, KDR (Abcam, #ab315238, UK), complement component C1q receptor, CD93 (Abcam, #ab134079, UK), and β-actin (Bioss, #bs-0061R, China) overnight at 4 ℃, followed by incubation with HRP-conjugated secondary antibodies for 1 h at room temperature. Protein bands were visualized using an ECL detection kit (Millipore, #WBKlS0100) and analyzed with ImageJ software. β-actin was used as a loading control. The blot was sectioned prior to antibody incubation and reassembled during imaging using TOUCH IMAGER™ (eBlot, China) to ensure accurate detection and visualization.
Statistical analysis
Graphs and statistical analyses were performed using GraphPad Prism (GraphPad 8.0 Software, USA). Comparisons among multiple groups were conducted using two-way ANOVA, while a two-tailed unpaired Student’s t-test was applied for comparisons between two groups. Data were presented as mean ± standard error of the mean (SEM), with a P < 0.05 considered statistically significant.
Results
DEGs between the cortical and hippocampal BBB were identified and validated
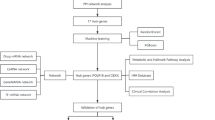
To explore the differences in BBB between the cerebral cortex and hippocampal regions in both the mouse and human brain, we investigated the DEGs in three cellular components of the BBB: endothelial cells, pericytes, and astrocytes. We then compared the DEGs of these three types of cells in the cerebral cortex and the hippocampal region, respectively. The DEGs were presented with volcano plots. The general workflow of this study is shown in Fig. 1 below.
We selected four datasets for our analysis: the mouse datasets GSE185862, SRP135960, GSE60361, and the human dataset GSE163577. From these datasets, we extracted the data related to the endothelial cells. We then compared the DEGs of these cell types between the cerebral cortex and hippocampus of the brain, followed by functional enrichment analysis of the identified DEGs. In the mouse datasets, differentially co-expressed genes shared among the three key cellular components of the BBB—endothelial cells, pericytes, and astrocytes—were identified. These genes were subjected to functional enrichment analysis, and hub genes were subsequently identified and validated through animal experiments.
Angiogenesis-related genes exhibited higher expression levels in cortical endothelial cells than in those of the hippocampus
To investigate the disparities in BBB characteristics between the hippocampus and the cortex, as well as to elucidate the susceptibility of the hippocampus, we conducted a comparative analysis of differential gene expression in the endothelial cells, which are the major components of the BBB, between the hippocampus and cortex, using the mouse brain datasets: GSE185862, SRP135960, GSE60361, and human brain dataset GSE163577, respectively. The volcano plots of the highly or lowly expressed genes were shown in Fig. 2A-D. We identified that the expressions of genes basigin (Bsg), inhibitor of DNA binding 1 (Id1), C-X-C motif chemokine ligand 12 (Cxcl12) (Fig. 2A), transmembrane 4 L six family member 1 (Tm4sf1), calmodulin 2 (Calm2) (Fig. 2B), glutathione peroxidase 4 (Gpx4,) cystatin C (Cst3), semaphorin 3E (Sema3e) (Fig. 2C), EGF like domain multiple 7 (EGFL7), nuclear paraspeckle assembly transcript 1 (NEAT1), and parathyroid hormone 1 receptor (PTH1R) (Fig. 2D), which were related to angiogenesis, were higher in cortical endothelial cells than in the hippocampus. Moreover, the expression levels of inflammation-related genes Jun proto-oncogene (Jun), JunB proto-oncogene (Junb) (Fig. 2B), and interferon induced transmembrane protein 3 (IFITM3) (Fig. 2D) in cortical endothelial cells were lower than those in the hippocampus. Such inflammatory signaling may suppress neurogenesis in the hippocampus, which may partially explain the vulnerability of the hippocampus4,23. After the GO enrichment, we discovered that genes highly expressed in cortical endothelial cells, as compared to those in the hippocampus, were enriched in pathways directly or indirectly related to angiogenesis, such as the cell-cell adhesion, positive regulation of angiogenesis, angiogenesis, negative regulation of extracellular matrix disassembly, response to oxidative stress, and vascular endothelial growth factor receptor signaling pathway (Fig. 2E-H, related DEGs were listed in Table 2). The pathways enriched with genes that were lowly expressed in the cortex endothelial cells compared to the hippocampus were shown in Fig. 2I-L: only the pathway “negative regulation of angiogenesis” was identified to be associated with angiogenesis. Collectively, our findings suggest that, compared to the cortex, the hippocampus may have a limited angiogenic capacity, making it less equipped to cope with damage caused by various diseases.
Identification of DEGs and functional enrichment in the endothelial cells (cortex VS hippocampus). (A–D) Volcano plots of DEGs (cortex VS hippocampus) for GSE185862, SRP135960, GSE60361, and GSE163577 datasets, respectively. The following items were presented in the same order. Data points in red represent highly expressed genes while the blue represent lowly expressed genes. The differences were set as |log FC|>0.25, P < 0.05. (E–H) GO enrichment analysis of highly expressed genes. (I–L) GO enrichment analysis of lowly expressed genes. Pathways identified to be associated with angiogenesis were highlighted in red color. The counts represented the number of genes enriched in the corresponding pathway, with larger dots indicating a higher number of enriched genes. The different colors corresponded to varying -log10(p-value), with values closer to red indicating smaller p-values, and those closer to green representing larger p-values.
Angiogenesis-related genes exhibited higher expression levels in cortical BBB cells than in those of the hippocampus in mouse
To broaden the scope of analysis and obtain more comprehensive information—and considering that the extracted brain microvessels contain three cellular components of the BBB: endothelial cells, astrocytes, and pericytes24—we further integrated these three cells into a single cell population termed as the “BBB cells” in three mouse datasets (GSE185862, SRP135960, GSE60361) and examined their differential gene expression between the cortex and hippocampal regions. Considering species differences, the human dataset GSE163577 was not included in this analysis. The results showed that, compared to the hippocampus, 94 genes were commonly lowly expressed in the cortex (Fig. 3A), with their GO analysis presented in Fig. 3C. Conversely, 62 genes were commonly highly expressed (Fig. 3B), with their GO analysis shown in Fig. 3D. Specifically, compared to the hippocampus, although none of the pathway was found associated with angiogenesis in the lowly expressed genes (Fig. 3C), pathways related to angiogenesis were enriched in the highly expressed genes in the cortex (Fig. 3D, highlighted in red). These results further confirmed that the hippocampus has a reduced capacity for angiogenesis compared to the cortex. The protein-protein interaction (PPI) network was then constructed to visualize the interactions between proteins encoded by angiogenesis-related genes (Fig. 3E). From the results, several hub genes such as Kdr, Fn1, and Pecam1, were identified and are known to play essential roles in angiogenesis which is essential for the repair of vascular and neural injuries25,26,27. Interestingly, among these angiogenesis-related genes in BBB cells, Cd34, Cd93, and Cxcl12 were also highly expressed in cortical endothelial cells (cortex vs. hippocampus, shown in Table 2). This observation underscored the reliability and robustness of our predicted hub gene results.
Common DEGs analysis and functional enrichment in BBB cells (cortex VS hippocampus). Venn diagram of common DEGs in combined set of endothelial cells, astrocytes, and pericytes across three mouse datasets (GSE185862, SRP135960 and GSE60361). (A) 94 common DEGs were identified to be lowly expressed in the three datasets. (B) 62 common DEGs were identified to be highly expressed in the three datasets. (C) GO enrichment analysis of commonly lowly expressed DEGs. (D) GO enrichment analysis of commonly highly expressed DEGs. The counts represented the number of genes enriched in the corresponding pathway, with larger dots indicating a higher number of enriched genes. Different colors corresponded to varying -log10(p-value), with values closer to red indicating smaller p-values, and those closer to green representing larger p-values. The pathways highlighted in red color are associated with angiogenesis. (E) PPI network of highly expressed genes associated with angiogenesis. The size and color of the nodes represented their relative significance or connectivity within the network.
Angiogenic capacity is lower in the hippocampus than in the cortex of the mouse brain
To further validate our observation that the hippocampus has a lower angiogenic capacity than the cortex, we isolated the brain microvessels from both the cortex and hippocampus of the naïve mice, respectively, to investigate the expression changes of the top 6 hub genes that were identified to be associated with angiogenesis (shown in Fig. 3E). Our results demonstrated that, compared to the hippocampus, the genes expression levels of Fn1 and Pecam1 were significantly higher in the cortical microvessels (P < 0.05), while the genes Kdr, Cd34, and Cd93 showed a notable trend towards higher expression levels (Fig. 4).
The expression of hub genes in the microvessels of mouse brain. The relative mRNA expression levels of target genes were quantified using RT-qPCR and normalized to the expression level of housekeeping gene β-actin (Actb). Data were presented as fold changes relative to the hippocampus. HC: hippocampus, CTX: cortex. (A) Kdr, (B) Fn1, (C) Pecam1, (D) Cd34, (E) Cd93, (F) Emcn. Data were presented as mean ± SEM, (n = 3), *P < 0.05.
Angiogenic capacity is lower in the hippocampus compared to the cortex in rat 2VO model
It has been reported that, in the brain, genes associated with angiogenesis were up-regulated under ischemic and hypoxic conditions, potentially leading to the formation of new blood vessels to provide increased oxygen and nutrient support to the ischemic area28,29. To explore differences in angiogenic capacity between the hippocampus and cortex, we established a rat 2VO model by ligating the bilateral common carotid arteries. This procedure reduces cerebral blood flow and mimics the pathological changes of chronic cerebral ischemia, making it a widely used model in related research30. We then isolate brain microvessels from the cortex and hippocampus in both sham-operated and 2VO rats. The subsequent RT-qPCR analysis with above mentioned hub genes (Kdr, Fn1, Pecam1, Cd34, Cd93, Emcn, shown in Fig. 3E) revealed that Kdr, Cd34, and Cd93, were up-regulated in the microvessels of both the hippocampus and cortex, but their expression levels were significantly lower in the hippocampus compared to the cortex (Fig. 5A, D, E). Fn1 and Pecam1 showed differences between the hippocampus and cortex only for three days and one day after modelling, respectively (Fig. 5B, C), which may be explained as the temporal dynamics of hypoxia-regulated angiogenesis31. Interestingly, three days after modelling, the expression of the Emcn gene in hippocampal microvessels was significantly higher than that in the cortex (Fig. 5F).
Furthermore, we validated the protein expression of angiogenesis-related genes Kdr and Cd93, which represent different aspects of angiogenesis - initiation of neovascularization and maintaining endothelial barrier function, respectively32,33. The WB analysis result was shown in Fig. 5G. Although there was a trend toward higher KDR protein expression in cortical microvessels compared to the hippocampus in the sham-operated group, the difference was not statistically significant. Moreover, following 2VO induction for one and three days, KDR expression levels in hippocampal microvessels was lower than in the cortex (Fig. 5H). In contrast, CD93 expression was lower in hippocampal microvessels compared to cortical samples in the sham group. Compared to the sham-operated group, CD93 expression was elevated in the microvessels of both the hippocampus (SHAM vs. 2VO D1, P < 0.01; SHAM vs. 2VO D3, P < 0.05) and cortex (SHAM vs. 2VO D3, P < 0.01) following 2VO modeling. Notably, by day three post-2VO, CD93 expression in cortical microvessels was higher than in hippocampal microvessels (Fig. 5I). These findings collectively demonstrated that these hub genes were involved in the angiogenesis in the brain and played different role in hippocampus and cortex.
Validation of hub genes using microvessels in the brain of 2VO rat. The relative mRNA expression levels of target genes (A) Kdr, (B) Fn1, (C) Pecam1, (D) Cd34, (E) Cd93, (F) Emcn were analyzed in sham-operation, day 1 and day 3 after 2VO modelling. Results of the RT-qPCR analysis were normalized to the housekeeping gene β-actin (Actb). (G) WB analysis of KDR and CD93 in microvessels from groups of sham-operation, day 1 and day 3 after 2VO modelling. Quantification of the WB analysis were shown in (H) and (I). The relative expression level was normalized to that of β-actin. Data were presented as fold changes relative to sham-operated rats and shown as mean ± SD (n = 3).*P < 0.05, **P < 0.01. HC: hippocampus, CTX: cortex.
Discussion
Numerous studies have demonstrated that the hippocampus is more susceptible to various harmful factors compared to other brain regions, exhibiting a high degree of vulnerability6,8,9,11,34,35,36,37. The introduction of the neurovascular unit (NVU) further underscores the critical role of vascular factors in hippocampal vulnerability38. A compromised BBB may be a common element in various types of insults34. We therefore investigated the intrinsic transcriptomic differences between hippocampal and cortical BBB-related cells. This study utilized bioinformatics analysis to show that, in both mouse and human, higher expressed genes in the cortex, compared to the hippocampus, were enriched in angiogenesis-related pathways. Angiogenesis is essential for repairing neurovascular damage by supplying oxygen and nutrients to ischemic areas, supporting neuronal survival, and clearing metabolic waste and inflammation28,29,39. It also works closely with neurogenesis, and together they promote tissue regeneration and functional recovery, partly through the VEGF signaling pathway40,41,42.
Furthermore, we extracted the microvessels tissue samples from mouse brain and experimentally verified the hub genes identified from the bioinformatics analysis of mouse datasets. The results showed that the expression levels of hub genes Kdr, Fn1, Pecam1, Cd34, and Cd93 were higher in the cortex compared to the hippocampus, aligning with the findings from our bioinformatics analysis. In both rats and their 2VO models, these hub genes also exhibited higher expression in the cortex compared to the hippocampus, consistent with the patterns observed in mice. This concordance may be attributed to the genetic homology between the two species.
Additionally, we established a rat 2VO model to induce cerebral ischemia and hypoxia. In other studies, to induce cerebral hypoperfusion in mouse, a bilateral common carotid artery stenosis (BCAS) model was established by partially restricting blood flow with micro-coils placed around the bilateral common carotid arteries, resulting in chronic hypoperfusion43. In rats, permanent ligation of the bilateral common carotid arteries rapidly induces cerebral hypoperfusion. Compared to the establishment of mouse BCAS model, the procedure we used to build the 2VO rat model is straightforward, and produced a more severe and rapid ischemic condition that led to significant global brain damage44. Interestingly, in the 2VO ischemic rat model, genes associated with angiogenesis, such as Kdr, Cd34, and Cd93, showed compensatory increases. In addition, the levels of these genes in the hippocampus remained lower than in the cortex, further supporting our hypothesis that the hippocampus has a reduced capacity for angiogenesis relative to the cortex. However, the expression of Emcn did not exhibit significant differences between the hippocampus and cortex in naïve mice or sham-operated rats, but showed higher expression in the hippocampus three days after 2VO in rats. This suggests the complexity of the molecular regulatory network governing angiogenesis under hypoxic conditions and highlights the need for extended investigations into macroscopic angiogenic phenotypes in response to hypoxia and other pathological insults. In addition, hypoxia altered Kdr and Cd93 mRNA levels significantly, yet protein expression didn’t follow the same pattern. This discrepancy may result from post-transcriptional regulation, including differences in translation efficiency and protein degradation, reflecting a decoupling between transcription and translation under hypoxic stress45,46. Despite this, hippocampal brain microvessels showed weaker hypoxia tolerance than the cortex in terms of angiogenesis at the protein level, a phenomenon that warrants deeper investigation in future studies.
Despite the significant insights gained from this study, several limitations should be discussed. Firstly, datasets GSE185862 and SRP135960 provide extensive single-cell transcriptomic coverage of both the cortex and hippocampus, whereas GSE60361 is restricted to the primary somatosensory cortex and the CA1 subregion of the hippocampus. Similarly, due to the scarcity of samples, we were unable to include more human datasets. Additionally, the GSE163577 dataset utilized single - nucleus RNA sequencing rather than fresh cells and only obtained partial samples from the cortical and hippocampal regions. Although the overlap of DEGs at the individual gene level is limited, many of the identified genes converge on similar biological processes or pathways, suggesting functional convergence across datasets. In the future, the transcriptome bioinformation of the BBB in different brain regions under disease conditions could be studied. Secondly, our study did not include diverse disease models. The lack of validation using mouse 2VO model and other disease models, such as those for neurodegenerative diseases and PTSD, is a notable gap. These models are crucial for exploring specific pathological mechanisms and assessing potential treatments. Future research should incorporate a broader range of disease models to allow for a more comprehensive assessment of hippocampal vulnerability and BBB dysfunction. Due to the challenges in obtaining human tissue samples, it’s also important to validate the angiogenesis-related genes identified from human datasets in suitable models, such as in vitro human brain organoid disease models. Thirdly, future research should incorporate targeted interventions to better understand their impact. For instance, the development of therapeutic approaches that promote hippocampal angiogenesis could be explored. Also, it would be beneficial to investigate the effects of various therapeutic approaches on hippocampal vulnerability and cognitive outcomes. This could provide a foundation for creating more effective treatment strategies for conditions linked to hippocampal dysfunction, thereby enhancing both the translational potential and clinical relevance of the research.
Conclusion
In this study, our bioinformatics analysis initially revealed that angiogenesis-related genes were expressed at lower levels in hippocampal endothelial cells compared to those in the cortex. Subsequently, we identified the key hub genes that were downregulated in mouse hippocampal BBB-associated cells and enriched in angiogenesis-related pathways. In addition, we validated angiogenesis-related hub genes—Kdr, Fn1, Pecam1, Cd34, Cd93, and Emcn—using brain microvessels samples from naïve mice and 2VO rat models. Our findings provided important insights into the intrinsic vulnerability of the hippocampus, suggesting that it possesses a reduced angiogenic capacity relative to the cortex. This diminished capacity may impair the hippocampus’s ability to deliver oxygen and nutrients to injured tissue, clear harmful mediators, and support neuronal survival and neurogenesis—functions essential for effective brain repair and recovery.
Data availability
The datasets analyzed in the present study are publicly available in the NCBI Gene Expression Omnibus (GEO) and the Sequence Read Archive (SRA) under the following accession numbers: GSE185862, GSE60361, GSE163577 (GEO), and SRP135960 (SRA).
References
Pessoa, L., Medina, L., Hof, P. R. & Desfilis, E. Neural architecture of the vertebrate brain: implications for the interaction between emotion and cognition. Neurosci. Biobehav Rev. 107, 296–312 (2019).
Macdonald, J. A., Murugesan, N. & Pachter, J. S. Endothelial cell heterogeneity of blood-brain barrier gene expression along the cerebral microvasculature. J. Neurosci. Res. 88, 1457–1474 (2010).
Bienkowski, M. S. et al. Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat. Neurosci. 21, 1628–1643 (2018).
Montagne, A. et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Kuroiwa, T., Bonnekoh, P. & Hossmann, K. A. Laser doppler flowmetry in CA1 sector of hippocampus and cortex after transient forebrain ischemia in gerbils. Stroke 23, 1349–1354 (1992).
Austin, S. A., Santhanam, A. V., d’Uscio, L. V. & Katusic, Z. S. Regional heterogeneity of cerebral microvessels and brain susceptibility to oxidative stress. PLoS One. 10, e0144062. https://doi.org/10.1371/journal.pone.0144062 (2015).
Segarra, M., Aburto, M. R., Hefendehl, J. & Acker-Palmer, A. Neurovascular interactions in the nervous system. Annu. Rev. Cell. Dev. Biol. 35, 615–635 (2019).
Shaw, K. et al. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat. Commun. 12 https://doi.org/10.1038/s41467-021-23508-y (2021).
Zhang, Y. et al. Chronic cerebral hypoperfusion and blood-brain barrier disruption in uninjured brain areas of rhesus monkeys subjected to transient ischemic stroke. J. Cereb. Blood Flow. Metab. 42 (7), 1335–1346 (2022).
Wu, Q. et al. Potential effects of antibiotic-induced gut Microbiome alteration on blood-brain barrier permeability compromise in rhesus monkeys. Ann. N Y Acad. Sci. 1470, 14–24 (2020).
Villalba, N., Baby, S., Cha, B. J. & Yuan, S. Y. Site-specific opening of the blood-brain barrier by extracellular histones. J. Neuroinflamm. 17 https://doi.org/10.1186/s12974-020-01950-x (2020).
Miyawaki, T. et al. Visualization and molecular characterization of whole-brain vascular networks with capillary resolution. Nat. Commun. 11 https://doi.org/10.1038/s41467-020-14786-z (2020).
Zhang, X. et al. High-resolution mapping of brain vasculature and its impairment in the hippocampus of alzheimer’s disease mice. Natl. Sci. Rev. 6, 1223–1238 (2019).
Saubaméa, B., Cochois-Guégan, V., Cisternino, S. & Scherrmann, J. M. Heterogeneity in the rat brain vasculature revealed by quantitative confocal analysis of endothelial barrier antigen and P-glycoprotein expression. J. Cereb. Blood Flow. Metab. 32, 81–92 (2012).
Zhu, H. et al. Why are hippocampal CA1 neurons vulnerable but motor cortex neurons resistant to transient ischemia? J. Neurochem. 120, 574–585 (2012).
Schmidt-Kastner, R. Genomic approach to selective vulnerability of the hippocampus in brain ischemia-hypoxia. Neuroscience 309, 259–279 (2015).
Perez-Rodriguez, D., Anuncibay-Soto, B., Llorente, I. L., Perez-Garcia, C. C. & Fernandez-Lopez, A. Hippocampus and cerebral cortex present a different autophagic response after oxygen and glucose deprivation in an ex vivo rat brain slice model. Neuropathol. Appl. Neurobiol. 41, e68–79 (2015).
Nair, P. K., Buerk, D. G. & Halsey, J. H. Jr. Comparisons of oxygen metabolism and tissue PO2 in cortex and hippocampus of gerbil brain. Stroke 18, 616–622 (1987).
Liu, X., Guo, Z., Liu, W., Sun, W. & Ma, C. Differential proteome analysis of hippocampus and Temporal cortex using label-free based 2D-LC-MS/MS. J. Proteom. 165, 26–34 (2017).
Yao, Z. et al. A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell 184, 3222–3241e3226 (2021).
Amit, Z. et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014e1022 (2018).
Zeisel, A. et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Yang, A. C. et al. A human brain vascular atlas reveals diverse mediators of alzheimer’s risk. Nature 603(7903), 885–892 (2022).
Lee, Y. K., Uchida, H., Smith, H., Ito, A. & Sanchez, T. The isolation and molecular characterization of cerebral microvessels. Nat. Protoc. 14, 3059–3081 (2019).
Guo, H. et al. Vascular endothelial growth factor: an attractive target in the treatment of hypoxic/ischemic brain injury. Neural Regen Res. 11, 174–179 (2016).
Mastorakos, P., Russo, M. V., Zhou, T., Johnson, K. & McGavern, D. B. Antimicrobial immunity impedes CNS vascular repair following brain injury. Antimicrobial immunity impedes CNS vascular repair following brain injury. Nat. Immunol. 22, 1280–1293 (2021).
Du, Y. T., Pan, Z. G., Chen, B. C. & Sun, F. Y. Carotid artery transplantation of brain endothelial cells enhances neuroprotection and neurorepair in ischaemic stroke rats. Acta Pharmacol. Sin. 45, 2487–2496 (2024).
Beck, H. & Plate, K. H. Angiogenesis after cerebral ischemia. Acta Neuropathol. 117, 481–496 (2009).
Fang, J., Wang, Z. & Miao, C. Y. Angiogenesis after ischemic stroke. Acta Pharmacol. Sin. 44, 1305–1321 (2023).
Washida, K., Hattori, Y. & Ihara, M. Animal models of chronic cerebral hypoperfusion: from mouse to primate. Int. J. Mol. Sci. 20(24), 6176. https://doi.org/10.3390/ijms20246176 (2019).
Hayashi, T., Noshita, N., Sugawara, T. & Chan, P. H. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J. Cereb. Blood Flow. Metab. 23, 166–180 (2003).
Ferrara, N. Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. 25, 581–611 (2004).
Lugano, R. et al. CD93 maintains endothelial barrier function by limiting the phosphorylation and turnover of VE-cadherin. FASEB J. 37, e22894. https://doi.org/10.1096/fj.202201623RR (2023).
Davidson, T. L. & Stevenson, R. J. Vulnerability of the Hippocampus to Insults: Links to Blood–Brain Barrier Dysfunction. Int J Mol Sci. https://doi.org/10.3390/ijms25041991 (2024).
Boekhoorn, K., Joels, M. & Lucassen, P. J. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile alzheimer hippocampus. Neurobiol. Dis. 24, 1–14 (2006).
Burke, M. J. et al. Morphometry of the hippocampal microvasculature in post-stroke and age-related dementias. Neuropathol. Appl. Neurobiol. 40, 284–295 (2014).
Du, A. T. et al. Effects of subcortical ischemic vascular dementia and AD on entorhinal cortex and hippocampus. Neurology 58, 1635–1641 (2002).
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R. & Zlokovic, B. V. Blood-Brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78 (2019).
Manoonkitiwongsa, P. S., Jackson-Friedman, C., McMillan, P. J., Schultz, R. L. & Lyden, P. D. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J. Cereb. Blood Flow. Metab. 21, 1223–1231 (2001).
Fujioka, T., Kaneko, N. & Sawamoto, K. Blood vessels as a scaffold for neuronal migration. Neurochem Int. 126, 69–73 (2019).
Robin, A. M. et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 26, 125–134 (2006).
Sun, Y. et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 111, 1843–1851 (2003).
Zhao, Y. et al. Vascular endothelium deploys caveolin-1 to regulate oligodendrogenesis after chronic cerebral ischemia in mice. Nat. Commun. 13 https://doi.org/10.1038/s41467-022-34293-7 (2022).
Farkas, E., Luiten, P. G. & Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 54, 162–180 (2007).
Buccitelli, C. & Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 21, 630–644 (2020).
Liu, L. & Simon, M. C. Regulation of transcription and translation by hypoxia. Cancer Biol. Ther. 3, 492–497 (2004).
Acknowledgements
The authors would like to thank Sichuan Junhui Biotechnology Co., Ltd. and Singapore HumanSim Pte. Ltd. for providing the experimental platforms for this study.
Funding
This study was supported by: (1) National Natural Science Foundation of China (82071349); (2) Sichuan Science and Technology Program (2025ZNSFSC0703); (3) National Key Research and Development Program of China (2021YFF0702000).
Author information
Authors and Affiliations
Contributions
The project was conceived and supervised by Z.H. Zhong and Q.X. Zhong. Bioinformatics analysis was conducted by X.J. Li, N.J. Li and J.Y. Zu; X.J. Li and Y. He carried out the biological experiments and drafted the manuscript. The manuscript was revised by Z.H. Zhong and Q.X. Zhong. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experimental protocols involving animals were reviewed and approved by the Ethical Committee of West China Hospital, Sichuan University (Approval No. 20230515008).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Li, N., He, Y. et al. Diminished angiogenic capacity in the hippocampus compared to the cortex indicates regional vulnerability. Sci Rep 15, 21768 (2025). https://doi.org/10.1038/s41598-025-06201-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06201-8