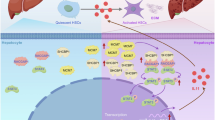
Abstract
Hepatic stellate cells (HSCs) serve as pivotal mediators of liver fibrogenesis. This study investigates the pro-inflammatory effects of melanoma differentiation-associated gene-7/interleukin-24 (MDA-7/IL-24) within both murine and cellular models. By employing the hydrodynamic injection of a plasmid expressing IL-24/mda7 (PMDA-7) in mice, significant inflammatory histopathological alterations were observed in the liver tissue. In human LX-2 HSCs, treatment with recombinant IL-24 (rIL-24), as well as transfection with PMDA-7 or RGD-modified PMDA-7-RGD plasmids, resulted in an increased expression of pro-inflammatory cytokines (IL-1β, IL-6, IL-17, IL-18, IL-23) and regulatory molecules (SOCS1, SOCS3). Importantly, the RGD-modified variant exhibited the most pronounced effects, with PMDA-7-RGD inducing the highest levels of IL-1β, IL-6, IL-17, and IL-18 expression, whereas rIL-24 was noted to most effectively upregulate IL-23 (P < 0.05). These findings substantiate that MDA-7/IL-24 exerts pro-inflammatory effects on HSCs, which are further amplified by RGD conjugation, thus suggesting its potential involvement in the pathogenesis of liver inflammation and fibrosis.
Similar content being viewed by others
Introduction
Liver diseases such as liver fibrosis, a wound-healing response, follow chronic liver damage and ultimately result in liver cirrhosis1,2. HSCs are significant players in liver fibrosis; understanding their behavior during inflammation is valuable for future treatments. When liver inflammation resolves, activated HSCs decrease or become inactive, thus reducing liver fibrosis and promoting its regression3. HSCs are resident mesenchymal cells located in the perisinusoidal area between the endothelial cells and hepatocytes, known as the “space of disuse.” HSCs are primarily considered in developing fibrosis in response to liver injury, as they are the main contributors to liver extracellular matrix (ECM) deposition in all etiologies of liver injury. The central dogma for stellate cells is that they are resting in the normal liver and are “activated” in response to liver damage4. Liver disorders, including liver fibrosis, are associated with inflammation5. Liver fibrosis is initially linked to chronic inflammation, leading to further tissue deterioration. This process is mainly attributed to the activation of HSCs6.
Cytokines of the IL-10 family play crucial roles in fibrosis-related inflammation associated with fibroproliferative diseases such as liver fibrosis7. IL-24 is a member of the IL-10 cytokine superfamily. Melanoma differentiation-associated gene-7 (MDA-7) is one of the transcripts whose expression was induced in terminally differentiated cells8. MDA-7/IL-24 possesses several activities at physiological and supra-physiological levels. It’s most impressive functions include a pro-inflammatory role, suppression of keratinocyte proliferation during wound healing, anti-microbial defense, autoimmune induction, and the elimination of cancer cells9.
In a study, IL-24 was identified as a protective cytokine during liver inflammation, which may significantly influence the healing of fibrogenic wounds in the liver10. Another study demonstrated the beneficial role of IL-24 in antagonizing IL-20-promoted liver fibrogenesis, suggesting that IL-24 could have translational potential for the clinical treatment of liver fibrosis5. Therefore, considering reports regarding IL-24’s inflammatory role, its contribution to liver fibrosis should be explored as a novel target for managing the disease.
On the other side, the fusion of cytokines with tumor homing peptides (THPs), such as arginine-glycine-aspartate (RGD) peptide, facilitates specific binding to integrins αVβ3 and αVβ5, which are highly expressed on the surface of certain cancerous and transformed cells11. The over-expression of αvβ3 integrin has also been well documented in hepatic stellate cells (HSCs)12; however, the impact of RGD-receptors on the immune-inflammatory response in HSCs has yet to be investigated.
This study aims to investigate the pro-inflammatory activity of endogenous MDA-7/IL-24 (with or without RGD peptide) and recombinant IL-24 in the mouse liver and human HSC line, respectively.
Materials and methods
Animal and material
In this experiment, an in-vivo evaluation was conducted utilizing 15 male BALB/c mice, aged between 6 and 8 weeks and weighing between 20 and 30 g. The mice were sourced from the Animal Breeding Laboratory Center at Shiraz University of Medical Sciences, located in Shiraz, Iran. Before their utilization in the laboratory, the mice were housed for a minimum of one week under controlled conditions, which included a temperature range of 19–23 °C, humidity levels of 40–50%, and a light cycle of 12 h (from 6 am to 6 pm). They were also provided with daily access to food and water. All experimental protocols were approved by the Ethics Committee of Shiraz University of Medical Sciences (License IR.SUMS.REC.1395.S1126) and conducted in accordance with institutional guidelines for the care and use of laboratory animals, adhering to the ARRIVE guidelines.
Professor Scott Friedman generously provided the human hepatic stellate cell (HSC) line, LX-2, from Mount Sinai School of Medicine, New York. Comprehensive details regarding the generation of this unique cell line have been documented previously13. Two plasmids, specifically PMDA-7 and pmda-RGD, were prepared in our laboratory. These plasmids incorporate an RGD peptide (the standard cyclic RGD, designated as the RGD4C sequence) fused to the MDA-7/IL-24 sequence. The empty pcDNA3.1 vector was utilized as a negative control, referred to as pcDNA3.114. Leptin and recombinant interleukin-24 (r-IL-24) were acquired from Sigma-Aldrich, USA, as well as R&D Systems. The transfection reagent Lipofectamine 2000 (Catalog Number: 11668019) and all agents for cell culture were purchased from Invitrogen Company. The RevertAid™ First Strand cDNA Synthesis Kit (Catalog Number: K1622) was supplied by Fermentas Inc. The RealQ Plus 2 × Master Mix (Catalog Number: A323402) Green was sourced from Ampliqon Inc. Additionally, the human IL-6 ELISA Kit (Plate Number: EK0410) was obtained from Boster Biological Technology Co., Ltd., located in Pleasanton, California, USA. All other materials were purchased from Sigma Company, USA. All kits and reagents were employed per the manufacturers’ protocols.
Hydrodynamics injection and histology
The animals were group-caged and maintained in a humidity-controlled room under standard conditions. The present study was performed according to the recommendations in the Shiraz University of Medical Sciences Guideline for the Care and Use of Laboratory Animals.
The hydrodynamic injection method was used as described previously15. A 1.2 µg plasmid per gram body weight (BW) dissolved in phosphate-buffered saline (PBS) was injected into the mouse tail vein at approximately 7 s. Animal groups included a group that did not receive the plasmid, a group that received the empty pcDNA3.1 vector, which was also enrolled as the negative control, and a group that received the plasmid expressing PMDA-7. This was performed twice within 7 days.
The livers of the mice were harvested 3 days after a hydrodynamic injection. Briefly, after animals were anesthetized (pentobarbital, 50 mg/kg, IP), their livers were removed and washed in cold 0.9% (w/v) sodium chloride. Paraffin-embedded liver Sects. (5-μm-thick sections) fixed in paraformaldehyde (4%, pH 7.4) were prepared, and pathological liver changes were examined with hematoxylin and eosin (H&E) staining before being microscopically examined.
LX-2 cell culture, treatments, and cell transfection
The LX-2 cells in the negative control and experimental groups were grown in Dulbecco’s modified Eagle medium (DMEM, high glucose) supplemented with 5% fetal bovine serum (Invitrogen), 100 IU/ml penicillin, and 100 mg/ml streptomycin. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. Culture media were replaced every three days.
The activation procedure, serum starvation, and leptin addition, concomitant in the positive control group, were applied to LX-2 cells for 24 h. The purified Leptin was utilized at a concentration of 100 ng/ml (Sigma-Aldrich, USA). For serum starvation conditions, 1% DMEM was employed instead of 5% DMEM.
In this study, different groups included LX-2 cells (Normal LX-2, as negative control), which were transfected with empty pcDNA 3.1 plasmid (pcDNA 3.1. negative control of plasmid), LX-2 cells that were transfected with a pcDNA 3.1 expressing MDA-7 (PMDA-7), the LX-2 cells which were transfected with a vector expressing RGD-modified MDA-7 (PMDA-7-RGD), LX-2 cells that received recombinant IL-24 protein (100 ng/mL, R&D Systems, Minneapolis, MN, USA), and LX-2 cells that underwent a stress condition by Leptin and serum starvation (Leptin group).
Cells with 70–80% confluency and between 6 and 8 passages were used for the experiments; they were transfected with 2.5 μg DNA plasmid using Lipofectamine 2000 reagent in a serum-free DMEM medium, according to the manufacturer’s instructions. After transfection (about seven hours), the medium was substituted with fresh complete medium (DMEM with 5% FBS, 100 U/ml streptomycin, and 100 U/ml penicillin) and maintained at 37 °C in a humidified atmosphere with 5% CO2 for up to 48 h.
The cells treated with IL-24 protein were harvested after 24 h for real-time quantitative PCR analysis. Also, after 48 h, culture supernatants were collected to detect protein by ELISA.
RNA isolation and real-time quantitative PCR analysis
RNA isolation was performed on the cell samples using a commercial RNA extraction kit (Cinnagen Inc., Iran) using the manufacturer’s protocol. The RNA’s purity, integrity, and concentration were assessed by measuring the 260/280 optical density ratio (Spectrophotometer NanoDrop™ Lite, Thermo Scientific™, USA) and performing 1% agarose gel electrophoresis. Reverse mRNA transcription was carried out using 1 μg of RNA and the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas Life Sciences, USA). Real-time PCR was performed using the RealQ Plus 2 × Master Mix Green (Ampliqon, Odense, Denmark) on an Applied Biosystems StepOne™ Instrument (ABI, Step One, USA). Primer pairs for IL-1β, IL-6, IL-17, IL-18, IL-23, SOCS1, SOCS3, TGF-β, and PGK were designed, as listed in Table 1. The mRNA expression levels were normalized to the housekeeping gene Phosphoglycerate Kinase (PGK). The PCR conditions included an initial denaturation step at 94 °C for 10 min, followed by 40 cycles of denaturation at 94 °C for 15 s and annealing/extension at 60 °C for 60 s.
Following each real-time PCR run, gel electrophoresis and melting curve analysis were conducted to verify the specificity of the amplified products. The amplification signals from different samples were normalized to the cycle threshold (Ct) values of PGK. The relative mRNA expression levels between the test and control groups were compared using the delta-delta CT (2^-ΔΔCT) method, and the results were expressed as fold changes in the data analysis.
Enzyme-linked immunosorbent assay (ELISA)
Nearly 48 h after LX-2 cell transfection and treatment with plasmids and IL-24 protein, respectively, the culture supernatants were collected for measurement of the released IL-6 by a human IL-6 ELISA Kit (Boster Biological Technology Co., Ltd., Pleasanton, CA, USA) according to the manufacturer’s instructions. The final absorbance was read at 450 nm by the ELISA reader using a FLUO star OMEGA microplate reader and software (BMG Labtech Ltd., Aylesbury, UK), and the concentration of IL-6 protein was assessed compared with a standard curve. Total samples were analyzed in triplicate.
Statistical analysis
The results were analyzed by the Independent Samples T-test or one-way analysis of variance (ANOVA) using Prism version 6.0 software (GraphPad Software Inc., San Diego, CA, USA). Statistically, p-values less than 0.05 and 0.01 (*P < 0.05, **P < 0.01) were considered significant.
Results
Hydrodynamic injection of MDA-7/IL-24 developed a pro-inflammatory pattern in the liver
Our histopathological analysis revealed compelling evidence of IL-24-mediated hepatic inflammation (Fig. 1). Real-time qPCR analysis of liver tissues demonstrated successful IL-24 gene expression following PMDA-7 transfection, showing a 2.8-fold increase compared to the controls (p < 0.01, Fig. 1A). Histological examination showed striking differences between the groups; while liver sections from pcDNA3.1-transfected control mice maintained normal hepatic architecture with intact lobules (Fig. 1B), PMDA-7-transfected animals exhibited marked inflammatory cell infiltration (particularly in portal areas) and significant disruption of liver parenchyma structure (Fig. 1C, yellow arrows). These findings were consistent across all examined sections (n = 5 animals per group), confirming that hydrodynamic delivery of PMDA-7 induces robust inflammatory responses in the liver tissue. The inflammatory pattern observed—characterized primarily by mononuclear cell aggregates—suggests IL-24 may preferentially activate specific immune pathways that warrant further investigation.
(A) IL-24 gene expression in the liver of hydrodynamics transfection by quantitative real-time PCR. Expression data relative to those of the reference gene from at least three independent assays are given as mean ± SEM. Statistical significance was tested using one-way ANOVA. * P value < 0.05; **, P value < 0.01. (B) and (C) Hematoxylin and eosin (H&E) staining of mouse liver (magnification, × 20) tissue sections from mice transfected with pcDNA 3.1 and PMDA-7, respectively. Histopathological changes that indicate inflammation were observed in animal tissues transfected with pada-7, and this finding was absent from all examined sections transfected with pcDNA 3.1. For each experimental condition (transfected or not non-transfected groups), six tissue sections from five biological replicates (five animals) were examined. Livers were transfected with pada-7; massive inflammatory cell aggregation was detected in this group (yellow arrow). Scale bars (100 µm) are indicated for each picture.
Both endogenous and recombinant MDA-7/IL-24 showed an inflammatory impact
To determine whether MDA-7/IL-24 can stimulate pro-inflammatory cytokine production in LX-2 cells, qPCR and ELISA assays were employed. The expression levels of inflammatory genes, including IL-1β, IL-6, IL-17, IL-18, and IL-23, were measured.
Overall, these findings emphasized degrees of pro-inflammatory role for IL-24/mda7 either in recombinant or endogenous forms and, more significantly, in fusion with RGD. However, PMDA-7-RGD and rIL-24 significantly up-regulated the expression of pro-inflammatory molecules compared to the cells alone or empty plasmid, respectively. The results indicated a two-fold enhancement of the IL-1β (2.25-fold, P = 0.0181) expression following pmda7-RGD transfection, compared to the empty plasmid. Moreover, the rIL-24 protein significantly up-regulated IL-1β (1.44 fold) expression compared to the control group, normal LX-2 cells (P = 0.0050). The level of IL-6 protein increased significantly after all MDA-7/IL-24 treatments, as determined by ELISA assay. The IL-6 protein concentrations were determined to be near 54.12 pg/ml, 55.29 pg/ml, and 56.00 pg/ml for PMDA-7, PMDA-7-RGD, and rIL-24 protein treatments, respectively, while it was 28.78 pg/ml for the cells transfected with pcDNA 3.1 and 18.76 pg/ml for normal cells (Fig. 2).
Effect of MDA-7/IL-24 on the expression of the IL-1β and the IL-6. Expression levels were estimated by real-time quantitative PCR (IL-1β and IL-6 genes) and ELISA (IL-6) in normal LX-2 cells, 48 h (plasmids) or 24 h (protein) after the transfection with pcDNA 3.1, PMDA-7, PMDA-7-RGD, and treatment with IL-24 protein (100 ng/ml). (A) IL-1βmRNA expression levels in LX-2 cells. (B) The effect of MDA-7/IL-24 on the IL-6 mRNA expression levels. (C) An increase in the amount of IL-6 protein by ELISA at 48 h following administration of MDA-7/IL-24 was observed. Data (mean ± standard error of the mean) are shown. The analysis of ELISA and reverse transcription-quantitative polymerase chain reaction demonstrated that MDA-7/IL-24 increased IL-1β and IL-6 expression. The cell group treated with leptin significantly differed from the control group in all the genes studied. *P < 0.05, **P < 0.01 vs. control.
The levels of other pro-inflammatory genes, including IL-17, IL-18, and IL-23, showed uneven results. As regards qPCR assessment for IL-17 expression, while all groups showed a similar pattern of increment, PMDA-7 and PMDA-7-RGD revealed a significant inductive effect in LX-2 cells (1.56 and 1.52 folds, respectively) (P = 0.0247 and P = 0.0386, respectively). However, all groups’ induction mean was lower than that of other pro-inflammatory genes. According to the results, IL-18 and IL-23 gene expression analysis revealed a similar upward trend in the test groups compared to the controls. Accordingly, the highest levels of IL-18 gene expression were detectable following PMDA-7-RGD transfection (1.98 fold), compared to the regular LX-2 group (P = 0.0242). Besides, the rIL-24 protein significantly increased the expression of IL-18 in the LX-2 cells, compared to the normal LX-2 cells (1.55 fold) (P = 0.0117). Regarding the influence of protein/plasmid on IL-23 expression, a significant up-regulation of the gene was achieved similarly for rIL-24 and PMDA-7-RGD (P = 0.0486 and P = 0.0176, respectively) (Fig. 3). This suggests that IL-24 may synergize with the IL-23/IL-17 axis to amplify pro-inflammatory responses in HSCs because IL-23 is a known inducer of IL-17 production in Th17 cells, and our data show concurrent upregulation of IL-17 (Fig. 3A).
MDA-7/IL-24 stimulates the gene expression of pro-inflammatory genes. Lx-2 cells were transfected and treated with plasmids and IL-24 protein. The mRNA levels of IL-17, IL-18, and IL-23 were determined in normal Lx-2 by quantitative real-time PCR. (A) The mRNA expression analysis for IL-17 gene expression level. (B) MDA-7/IL-24 effect on IL-18 expression in LX-2 cells. (C) IL-23 mRNA expression levels in LX-2 cells. The cell group treated with leptin significantly differed from the control group in all the genes studied. Expression data relative to those of the reference gene from at least three independent assays are given as mean ± SEM. Statistical significance was tested using one-way ANOVA. * P value < 0.05; **, P value < 0.01.
Different types of IL-24 /mda7 triggered the SOCS1, SOCS3 and TGF-β genes expression
The adverse regulatory effects of MDA-7/IL-24 were assessed by analysis of the level of SOCS1 and SOCS3 suppressor molecules. The SOCS1 and SOCS3 gene expression patterns showed a similar trend for all test groups, albeit in varying degrees. These results indicated that gene expression of both regulatory genes elevated significantly following PMDA-7-RGD, PMDA-7, and rIL-24 (2.31-fold, 2.76-fold, and 1.52-fold, respectively). With a glance, it was clear that the mean enhanced level of SOCS1 expression in the test groups was detected to be more than that in SOCS3 (P < 0.05). The results showed that the levels of SOCS3 in Lx-2 cells transfected with PMDA-7 and PMDA-7-RGD were higher than the control group (1.67-fold and 1.76-fold, respectively) (P = 0.0001 and P = 0.0046, respectively). Also, SOCS3 mRNA levels were significantly higher in the cells treated with rIL-24 protein (P = 0.0099) (Fig. 4).
MDA-7/IL-24 effects on TGF-β and SOCS regulatory genes. Lx-2 cells were transfected and treated with plasmids and IL-24 protein. The TGF-β, SOCS1, and SOCS3 mRNA levels were determined in normal Lx-2 by quantitative real-time PCR. (A) Validation of the expression of TGF-β gene by real-time RT-PCR. (B) Quantitative real-time PCR gene expression of SOCS1. (C) Quantitative real-time PCR gene expression for SOCS3. The cell group treated with leptin significantly differed from the control group in all the genes studied. Expression data relative to those of the reference gene from at least three independent assays are given as mean ± SEM. Statistical significance was tested using one-way ANOVA. * P value < 0.05; **, P value < 0.01.
Discussion
Liver disorders, including liver fibrosis, are associated with inflammation5. As HSCs are significant players in liver fibrosis, unraveling their behavior during inflammation is valuable for upcoming treatment. When liver inflammation ends, activated HSCs decrease or become inactive, causing liver fibrosis to decrease and fibrosis to regress3.
It is essential to study cytokines and their roles in diseases to develop therapeutics for various diseases, including liver fibrosis16. Besides the regular physiological role that MDA-7/IL-24 plays in the immune system, its role in cancer has been extensively studied. In this regard, the known functions of MDA-7/IL-24 are Apoptosis, Autophagy, Anti-angiogenesis, Inhibition of Invasion, and Metastasis17. In recent years, there have been several studies on the potential therapeutic applications of IL-24, most of which focused on the protective role of IL-24 in cancer. IL-24 has recently attracted much attention due to its antiproliferative and anticancer activities5,18.
IL-24 and its functions in liver fibrosis have been poorly understood. Nevertheless, there are studies regarding liver injury. IL-24 effectively inhibits the growth and metastasis of hepatoma cells19,20, plays an essential protective role in the development of liver injury, and has the potential to repair liver damage in mice21.
A study published by Wang et al. demonstrated that hepatic fibrosis, associated with cirrhosis and NASH, correlated with the liver’s imbalanced expression of IL-20 and IL-24. They revealed the significant protection of IL-24 in the liver injury process, and demonstrated a novel function of IL-24, and identified a potential therapeutic candidate for treating liver fibrosis10.
Our recent findings show that both endogenous and soluble forms of MDA-7/IL-24 induce apoptosis and senescence in activated LX-2 cells and, more importantly, the fusion of the RGD peptide with this cytokine improves these activities. Therefore, RGD-modified MDA-7/IL-24 may be a suitable candidate for adjunctive fibrosis treatment22.
IL-24 also exhibits pro-inflammatory and anti-inflammatory properties, depending on the type of disease23. Our recent study suggests a pro-inflammatory role for this cytokine in fibrosis24. Given the abundant expression of IL-24 in regular mouse or human liver25, the present study was designed to answer some questions about the role of IL-24 in the inflammatory state of HSCs. The points, including the effect of IL-24 on the mouse liver tissue histology, the impact of IL-24 on the HSCs’ inflammatory response, and its role in the expression of regulatory molecules such as SOCS1 and SOCS3, were addressed herein.
The results showed that MDA-7/IL-24 hydrodynamics transfection could develop an inflammatory state in vivo. As its receptor is present in the liver tissue, it is predictable that IL-24 could stimulate inflammation in the liver7. However, an in vivo study showed that the administration of IL-24, with its antioxidant effects, could reduce inflammation in injured mouse livers. They showed that the reduction in hepatic inflammation was mediated by inhibiting activated stellate cells21. Their study in patients with severe liver fibrosis also showed that IL-24 expression was higher in the non-fibrotic liver and decreased with increasing disease severity21.
While one study showed that administering IL-24 to mice with CCL4-induced liver injury did not improve liver inflammation, another study showed that IL-24 expression was reduced in patients with acute liver failure. This was associated with disease progression25. Our recent study also revealed that IL-24 expression increased with the activation of HSCs and that IL-24 and its cognate receptors might play a role in fibrosis progression24.
Interestingly, the in-vitro results corresponded to the in-vivo findings. In brief, in this study, the expression pattern of the inflammatory response of HSC revealed a significant pro-inflammatory gene, as determined by qPCR and ELISA. The levels of the inflammatory cytokines, including IL-1β, IL-6, IL-17, IL-18, and IL-23, increased significantly following physiologic and ectopic forms of IL-24. These results confirmed the innate inflammatory behavior of the MDA-7/IL-24 during LX-2 cell activation. A recent study has shown that knockdown of MDA-7/IL-24 has decreased the expression of IL-1β, IL-6, and IL-1726. Thus, all these results further support the idea of inflammatory properties for IL-2427.
In addition, a study by Hosseini et al. revealed that IL-24 with RGD could enhance its inflammatory potential28. The possible mechanisms for this biological activity have been explained by activating the cascade of the JAK/STAT pathway signaling29. This effect has been seen in the liver and reported in other cells, such as monocytes, through receptor binding and proinflammatory responses during wound healing30.
IL-1β also inhibits liver regeneration31. Finally, the mice treated with IL-1 receptor antagonists showed better hepatocyte regeneration and more significant improvement in liver damage caused by chronic ethanol consumption compared to untreated mice32.
Liver regeneration seems to be dependent on inflammatory cytokines like IL-1β33 and IL-1834, and their results further support this, which comes from a study that has shown the role of the mentioned cytokines in fibrosis35. Interestingly, our results were in line with these observations. This study showed that MDA-7/IL-24 could increase IL-1β, IL-6, and IL-18 gene expression in the normal Lx-2 cells. The possible explanation for this result is that LX2 produces these cytokines to participate in liver regeneration and fibrosis36,37. This biological activity is precisely the one that has been reported about MDA-7/IL-24 in the keratinocyte, which plays a pivotal role in the early stages of wound healing38. MDA-7/IL-24 can induce the expression of other cytokines, such as IL-17 and IL-23, by MDA-7/IL-24 -IL-24-containing vectors and IL-24 protein, suggesting that IL-24 may be a member of complex pathways of cytokines involved in inflammation39.
A study has shown that HSC treatments with exosomes derived from CCl4-treated hepatocytes significantly elevated the expression of IL-1β, IL-17A, and IL-23 in HSCs. Exosome-mediated activation of TLR3 (toll-like receptor 3) in HSCs increases the expression of these cytokines40. The results of this study was compared with those of previous studies. Accordingly, we suggest that IL-24 may be a member of complex cytokine pathways involved in liver inflammation.
In the third part of this project, we evaluated the harmful regulator genes of fibrosis, which include TGF-β and SOCSs. The earned results showed that the expression of these genes only significantly increased when the IL-24 protein was added to the LX-2 cell line. Such biological functions of the TGF-β and SOCSs, which are negative regulators of fibrosis, can be expected in these cells after transfection by MDA-7/IL-24. These findings may partly explain why the IL-24 protein binds to its receptors, which may induce immunomodulatory effects. These results are consistent with other studies, which have also shown that the MDA-7/IL-24 can increase the expression of SOCS family members and TGF-β26. Therefore, these two members can be essential in regulating inflammation in Lx-2 cells.
Other studies on IBD patients further support the idea that the MDA-7/IL-24 can play a protective role in the disease based on inflammation. This effect is mediated by activating the JAK1/STAT3/SOCS3 cascade and is considered a potential target in treating and regulating inflammation in IBD patients41,42,43. Moreover, it has been shown in various contexts that the SOCS proteins contribute to the role of apoptotic and antitumor activity of the MDA-7/IL-24. For example, the expression of SOCS3 protein in apoptotic HepG2 cells increases after exposure to the MDA-7/IL-2444,45,46.
Several studies have proposed that HSCs contribute considerably to liver immunity47. In this case, the HSCs have been primarily characterized as the primary effector cells in liver immunology. Several immune cells interact directly or indirectly (by soluble mediators) with HSCs. For example, these cells can induce T gamma delta to produce some inflammatory cytokines such as IL-17. In addition, our results and those of other studies reveal that these cells in a specific environment can produce some cytokines. Herein, we suggest that although this cell does not belong to the specified immune cells, it can exhibit an immunomodulatory property to drive the liver into an inflammatory phenotype. Consequently, all this evidence leads us to investigate the role of HSCs as principal regulators of liver immunology and evaluation of HSCs inflammatory function48. Therefore, it seems that manipulating these cells could open new therapeutic strategies for chronic liver diseases.
The results showed that the PMDA-7-RGD vector was more effective than the PMDA-7 vector. It has been confirmed that binding RGD peptide or other tumor-homing peptides to proteins can increase the efficiency of MDA-7/IL-24 in tumor cells. However, this achievement has yet to be reported on HSCs. In a study, it was demonstrated that similar to tumor cells, integrin αvβ3, the receptor of RGD, is expressed profoundly on HSCs49. Our last findings showed that both endogenous and soluble forms of MDA-7/IL-24 induced apoptosis and senescence in activated LX-2 cells. More importantly, a fusion of RGD peptide to this cytokine enhanced these activities. Thus, RGD-modified MDA-7/IL-24 could be a suitable candidate for further molecular therapy of fibrosis22.
Another study found that an antifibrotic drug carrier based on RGD peptide modified with a polymersome significantly inhibited activated HSCs. They showed that the drug carrier modified with RGD peptide had a higher antifibrotic activity than the carrier without50. As a result, targeting MDA-7/IL-24 by RGD peptide can improve the specificity and potency of secreted cytokine.
At the molecular level, it has been demonstrated that vitamin A stored in normal HSCs suppresses pro-inflammatory cytokines (IL-1β, TNF-α) and the production of nitric oxide (NO)51. Our results showed that MDA-7/IL-24 could increase the pro-inflammatory cytokines. Consequently, MDA-7/IL-24 takes the normal HSCs to the activated cell in the early stages, but we are convinced that this cytokine can prevent the activation of HSCs in the final stages. Such properties have also been observed in keratinocytes and wound healing52.
In conclusion, it was shown that MDA-7/IL-24 had a pro-inflammatory effect in normal Lx-2 cells. Both high endogenous and physiological soluble IL-24 /mda7 levels exhibited a pro-inflammatory pattern on HSCs. Hence, tethering an RGD peptide to its endogenous form could also enhance this response. Selective targeting of activated HSCs (which overexpress αvβ3/αvβ5 integrins) via RGD-modified IL-24 could offer a novel strategy for modulating liver inflammation in fibrosis, and reduced off-target effects compared to systemic cytokine therapy.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Unagolla, J. M., Das, S., Flanagan, R., Oehler, M. & Menon, J. U. Targeting chronic liver diseases: Molecular markers, drug delivery strategies and future perspectives. Int. J. Pharm. 660, 124381. https://doi.org/10.1016/j.ijpharm.2024.124381 (2024).
Zuñiga-Aguilar, E. & Ramírez-Fernández, O. Fibrosis and hepatic regeneration mechanism. Transl. Gastroenterol. Hepatol. 7, 9. https://doi.org/10.21037/tgh.2020.02.21 (2022).
Higashi, T., Friedman, S. L. & Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 121, 27–42. https://doi.org/10.1016/j.addr.2017.05.007 (2017).
Kamm, D. R. & McCommis, K. S. Hepatic stellate cells in physiology and pathology. J. Physiol. 600, 1825–1837. https://doi.org/10.1113/jp281061 (2022).
Petrescu, A. D. & DeMorrow, S. Interleukin-24 therapy- a potential new strategy against liver fibrosis. EBioMedicine 65, 103245. https://doi.org/10.1016/j.ebiom.2021.103245 (2021).
Tanwar, S., Rhodes, F., Srivastava, A., Trembling, P. M. & Rosenberg, W. M. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J. Gastroenterol. 26, 109–133. https://doi.org/10.3748/wjg.v26.i2.109 (2020).
Sziksz, E. et al. Fibrosis related inflammatory mediators: Role of the IL-10 cytokine family. Mediat. Inflamm. 2015, 764641. https://doi.org/10.1155/2015/764641 (2015).
Jiang, H., Lin, J. J., Su, Z. Z., Goldstein, N. I. & Fisher, P. B. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene 11, 2477–2486 (1995).
Menezes, M. E. et al. Role of MDA-7/IL-24 a multifunction protein in human diseases. Adv. Cancer Res. 138, 143–182. https://doi.org/10.1016/bs.acr.2018.02.005 (2018).
Wang, H. H. et al. Interleukin-24 protects against liver injury in mouse models. EBioMedicine 64, 103213. https://doi.org/10.1016/j.ebiom.2021.103213 (2021).
Bogdanović, B., Fagret, D., Ghezzi, C. & Montemagno, C. Integrin targeting and beyond: Enhancing cancer treatment with dual-targeting RGD (Arginine–Glycine–Aspartate) strategies. Pharmaceuticals 17, 1556 (2024).
Umemoto, T. et al. Integrin αvβ3 enhances the suppressive effect of interferon-γ on hematopoietic stem cells. EMBO J. 36, 2390–2403. https://doi.org/10.15252/embj.201796771 (2017).
Yang, C. et al. Liver fibrosis: Insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology 124(1), 147–159 (2003).
Hosseini, E., Hosseini, S. Y., Hashempour, T., Fattahi, M. R. & Sadeghizadeh, M. Effect of RGD coupled MDA-7/IL-24 on apoptosis induction in a hepatocellular carcinoma cell line. Mol. Med. Rep. 15, 495–501 (2017).
Liu, F., Song, Y. & Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6, 1258–1266 (1999).
He, Y. et al. Immunopathobiology and therapeutic targets related to cytokines in liver diseases. Cell Mol. Immunol. 18, 18–37. https://doi.org/10.1038/s41423-020-00580-w (2021).
Emdad, L. et al. Recent insights into apoptosis and toxic autophagy: The roles of MDA-7/IL-24, a multidimensional anti-cancer therapeutic. Semin. Cancer Biol. 66, 140–154. https://doi.org/10.1016/j.semcancer.2019.07.013 (2020).
Wang, K. et al. The role of interleukin-20 in liver disease: Functions, mechanisms and clinical applications. Heliyon 10, e29853. https://doi.org/10.1016/j.heliyon.2024.e29853 (2024).
Caparrós, E. & Francés, R. The interleukin-20 cytokine family in liver disease. Front. Immunol. 9, 1155. https://doi.org/10.3389/fimmu.2018.01155 (2018).
Chen, W. Y. et al. IL-24 inhibits the growth of hepatoma cells in vivo. Genes Immun. 6, 493–499. https://doi.org/10.1038/sj.gene.6364233 (2005).
Wang, H.-H. et al. Interleukin-24 protects against liver injury in mouse models. EBioMedicine 64, 103213 (2021).
Jamhiri, I. et al. Enhancing the apoptotic effect of IL-24/mda-7 on the human hepatic stellate cell through RGD peptide modification. Immunol. Invest. 47, 335–350. https://doi.org/10.1080/08820139.2018.1433202 (2018).
Zhong, Y., Zhang, X. & Chong, W. Interleukin-24 immunobiology and its roles in inflammatory diseases. Int. J. Mol. Sci. 23, 627. https://doi.org/10.3390/ijms23020627 (2022).
Jamhiri, I. et al. The pattern of IL-24/mda-7 and its cognate receptors expression following activation of human hepatic stellate cells. Biomed. Rep. 7, 173–178. https://doi.org/10.3892/br.2017.931 (2017).
Wang, J. et al. Intracellular XBP1-IL-24 axis dismantles cytotoxic unfolded protein response in the liver. Cell Death Dis. 11, 17. https://doi.org/10.1038/s41419-019-2209-6 (2020).
Ross, B. X. et al. IL-24 promotes Pseudomonas aeruginosa keratitis in C57BL/6 mouse corneas. J. Immunol. 198, 3536–3547 (2017).
Mitamura, Y., Nunomura, S., Furue, M. & Izuhara, K. IL-24: A new player in the pathogenesis of pro-inflammatory and allergic skin diseases. Allergol. Int. 69, 405–411. https://doi.org/10.1016/j.alit.2019.12.003 (2020).
Hosseini, E., Hosseini, S. Y., Hashempour, T., Fattahi, M. R. & Sadeghizadeh, M. Effect of RGD coupled MDA-7/IL-24 on apoptosis induction in a hepatocellular carcinoma cell line. Mol. Med. Rep. 15, 495–501. https://doi.org/10.3892/mmr.2016.6009 (2017).
Smith, S. et al. Interleukin 24: Signal transduction pathways. Cancers (Basel) 15, 3365. https://doi.org/10.3390/cancers15133365 (2023).
Kolumam, G. et al. IL-22R Ligands IL-20, IL-22, and IL-24 Promote Wound Healing in Diabetic db/db Mice. PLoS ONE 12, e0170639. https://doi.org/10.1371/journal.pone.0170639 (2017).
Mathews, S. et al. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell. Mol. Immunol. 13, 206–216 (2016).
Iracheta-Vellve, A. et al. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int. 37, 968–973 (2017).
Ma, H., Shi, X., Yuan, X. & Ding, Y. IL-1β siRNA adenovirus benefits liver regeneration by improving mesenchymal stem cells survival after acute liver failure. Ann. Hepatol. 15, 260–270. https://doi.org/10.5604/16652681.1193723 (2016).
Zhang, J. et al. Interleukin 18 accelerates the hepatic cell proliferation in rat liver regeneration after partial hepatectomy. Gene 537, 230–237. https://doi.org/10.1016/j.gene.2013.12.062 (2014).
Moschen, A. R. et al. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 17, 840 (2011).
Robinson, M. W., Harmon, C. & O’farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 13, 267 (2016).
DeSantis, D. A., Ko, C. W., Wang, L., Lee, P. & Croniger, C. M. Constitutive activation of the Nlrc4 inflammasome prevents hepatic fibrosis and promotes hepatic regeneration after partial hepatectomy. Mediat. Inflamm. 2015, 909827. https://doi.org/10.1155/2015/909827 (2015).
Feng, K., Cen, J., Zou, X. & Zhang, T. Novel insight into MDA-7/IL-24: A potent therapeutic target for autoimmune and inflammatory diseases. Clin. Immunol. 266, 110322. https://doi.org/10.1016/j.clim.2024.110322 (2024).
Halwani, R. et al. Th-17 regulatory cytokines IL-21, IL-23, and IL-6 enhance neutrophil production of IL-17 cytokines during asthma. J. Asthma 54(9), 893–904 (2017).
Seo, W. et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology (Baltimore, MD) 64, 616–631. https://doi.org/10.1002/hep.28644 (2016).
Andoh, A. et al. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J. Immunol. 183, 687–695 (2009).
Rawlings, J. S., Rosler, K. M. & Harrison, D. A. The JAK/STAT signaling pathway. J. Cell Sci. 117, 1281–1283. https://doi.org/10.1242/jcs.00963 (2004).
Hu, X., Li, J., Fu, M., Zhao, X. & Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduction Target. Therapy 6, 402. https://doi.org/10.1038/s41392-021-00791-1 (2021).
Shen, M. & Shi, H. Estradiol and estrogen receptor agonists oppose oncogenic actions of leptin in HepG2 cells. PLoS ONE 11, e0151455 (2016).
Trindade-da-Silva, C. A. et al. 15-Deoxy-Δ12, 14-prostaglandin J2 induces apoptosis and upregulates SOCS3 in human thyroid cancer cells. PPAR Res. 2016, 4106297 (2016).
Bina, S. et al. Impact of RGD peptide tethering to IL24/mda-7 (melanoma differentiation associated gene-7) on apoptosis induction in hepatocellular carcinoma cells. Asian Pac. J. Cancer Prev. 16, 6073–6080 (2015).
Weiskirchen, R. & Tacke, F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg. Nutr. 3, 344 (2014).
Troeger, J. S. et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 143, 1073-1083.e1022. https://doi.org/10.1053/j.gastro.2012.06.036 (2012).
Reetz, J. et al. Development of adenoviral delivery systems to target hepatic stellate cells in vivo. PLoS ONE 8, e67091 (2013).
Chen, Z., Jain, A., Liu, H., Zhao, Z. & Cheng, K. Targeted drug delivery to hepatic stellate cells for the treatment of liver fibrosis. J. Pharmacol. Exp. Ther. 370, 695–702. https://doi.org/10.1124/jpet.118.256156 (2019).
Weiskirchen, R. & Tacke, F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg. Nutr. 3, 344–363. https://doi.org/10.3978/j.issn.2304-3881.2014.11.03 (2014).
Poindexter, N. J. et al. IL-24 is expressed during wound repair and inhibits TGFα-induced migration and proliferation of keratinocytes. Exp. Dermatol. 19, 714–722 (2010).
Author information
Authors and Affiliations
Contributions
All authors have contributed equally.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethics approval and consent to participate
The Institutional Animal Care and Ethics Committee at Shiraz University of Medical Sciences approved all experimental protocols. Additionally, the study was conducted following the relevant guidelines and regulations, as well as the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Daneshparvar, A., Hosseini, S.Y., Hamidizadeh, N. et al. Pro-inflammatory effects of endogenous and recombinant MDA-7/IL-24 with RGD peptide on human hepatic stellate cells. Sci Rep 15, 24127 (2025). https://doi.org/10.1038/s41598-025-06850-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06850-9