Abstract
Previous studies suggest that butyrylcholinesterase (BChE), an enzyme involved in the cholinergic anti-inflammatory pathway, may be linked to inflammation, disease severity, and risk of death in COVID-19 patients. Extending earlier work on BChE and COVID-19 severity, this study investigates additional factors such as age, sex, vaccination status, and symptom profiles. We analyzed 462 patients with polymerase chain reaction (PCR)-confirmed COVID-19 from the first epidemic wave in Spain, examining the association between BChE activity, clinical outcomes, demographic factors, and symptoms. The cohort consisted of 78 asymptomatic patients, 200 patients with mild symptoms, 122 patients with severe symptoms, and 62 critically ill patients. Of the patients in the severe symptoms group and critically ill patients, 26 died within 30 days of diagnosis. Our results showed that BChE activity was not affected by sex but decreased significantly with age (P < 0.0001). Patients with severe COVID-19 symptoms and critically ill patients exhibited lower BChE activity than asymptomatic or mildly symptomatic individuals (P < 0.0001). Furthermore, lower BChE activity was observed in patients with respiratory symptoms, such as pneumonia (P = 0.0027) and dyspnea (P = 0.0120), while higher BChE activity was seen in patients with neurological symptoms, such as anosmia (P < 0.0001), ageusia (P = 0.0012), and headache (P = 0.0005). No significant association was found between BChE activity and gastrointestinal, algesic, musculoskeletal, or systemic inflammatory symptoms. Additionally, vaccinated patients, particularly those who received two doses, had lower BChE activity compared to unvaccinated individuals (P = 0.0465). In conclusion, serum BChE activity is significantly associated with the severity, mortality, and specific symptoms of COVID-19, and is influenced by age and vaccination status. These findings imply that BChE may be a potential biomarker to support prognosis and risk stratification in COVID-19 patients. However, further research is needed to understand the underlying mechanisms and to validate the role of BChE in clinical practice.
Similar content being viewed by others
Introduction
The accurate prediction of COVID-19 severity and mortality remains a significant challenge due to the complex interplay of host and viral factors. Identifying reliable biomarkers could aid in risk stratification and improve clinical decision-making. Recent studies, including our own, have shown that serum butyrylcholinesterase (BChE) activity may be an important predictor of COVID-19 mortality1,2,3,4. BChE, also known as serum cholinesterase or pseudocholinesterase, is a cholinesterase primarily produced in the liver. It is widely distributed in various tissues, with particularly high activity in the liver and plasma5. Because BChE is produced in the liver, its plasma levels can reflect both hepatic dysfunction and systemic inflammation, which are commonly observed in severe cases of COVID-196,7. Although it does not have a well-defined physiological function, BChE may provide protective benefits, such as scavenging nerve agents and organophosphate pesticide poisoning, aid in the treatment of drug addiction, and potentially contribute to metabolic management8,9,10. Alteration of BChE activity has been shown to be associated with dysfunctions of the cholinergic system, which may be related to several neurological and metabolic disorders11,12,13,14. The cholinergic system, which plays an essential role in modulating an appropriate immune response during systemic inflammation, is dysregulated in immunosenescence, contributing to low-grade inflammation (inflammaging) and potentially worsening COVID-19 outcomes15,16,17,18,19.
COVID-19, caused by the SARS-CoV-2 virus, displays a wide range of clinical characteristics, from asymptomatic to critical status or fatal outcome20. The disease can manifest with different types of symptoms, varying in severity, including respiratory (e.g., cough, pneumonia, dyspnea), gastrointestinal (e.g., vomitus, diarrhea), neurological (e.g., anosmia, ageusia, headache), algesic (e.g., myalgia, arthralgia, odynophagia), and inflammatory (e.g., fever, chills)21. The most common symptoms are fever, fatigue, and dry cough, while less common symptoms include headache, sore throat, myalgia, diarrhea, vomiting, chills, and loss of smell and taste, which can vary with different variants of the virus22,23.
From a clinical perspective, the majority of COVID-19 patients exhibit mild to moderate symptoms (approximately 81%), while approximately 14% progress to severe pneumonia, necessitating hospitalization, and in some cases, ventilation in an intensive care unit (ICU)20,21. Approximately 5% of cases develop critical conditions, including acute respiratory distress syndrome (ARDS), septic shock, and multiple organ dysfunction or failure20. However, there have also been numerous reports of patients without any symptoms who unexpectedly deteriorated into severe conditions or died, despite lacking early symptoms24,25,26,27. These asymptomatic cases pose significant challenges in the diagnosis of the disease and contribute to the heightened risk of viral transmission25,27. Moreover, the condition of COVID-19 could quickly progress from asymptomatic or mildly symptomatic to severe, involving multi-organ dysfunction or even death27,28,29. Several conventional inflammatory and anti-inflammatory markers have been explored, but none fully capture the severity of COVID-19’s cytokine storm. Novel biomarkers such as monocyte distribution width, electrolyte imbalances, pentameric C-reactive protein, pentraxin-3, chemerin, adiponectin, leptin, apelin, visfatin, resistin, and galectin-3, the complexity of cytokine storm have shown promise, but clinical applicability remains limited4,30,31.
Therefore, given the mounting evidence that cholinergic activity, particularly BChE activity, may play an important role in the inflammation associated with COVID-19, we tested the hypothesis that BChE activity could be associated with COVID-19 mortality1,2,3,4,16and could serve as a predictor of COVID-19 severity, it is crucial to consider the implication of these findings. Previous studies have mainly focused on the relationship between COVID-19 mortality rates and BChE activity1,2,3,4,16with less emphasis on the severity of the disease. In contrast, the present study explores a more comprehensive range of outcomes, including mortality rates, as well as the relationship between BChE activity and disease severity and symptoms. Building upon our prior investigation1we further explore the impact of age, sex, and vaccine doses on COVID-19 outcomes and their association with BChE activity in a more extensive cohort. Given the global impact of COVID-1932 and the possibility of future pandemics, robust biomarkers remain essentials for patient risk stratification. Therefore, assessing serum BChE activity as an indicator of COVID-19 status remains important.
Methods
Study design
This monocentric, cross-sectional, observational study was conducted between April 2020 and September 2021, after approval by the Ethics Committees of the Hospital Universitari de Sant Joan. The study was conducted in accordance with the World Medical Association’s Code of Ethics (Declaration of Helsinki). During the study period, the variants of the SARS-CoV-2 virus in Spain were B.1.177 (April 2020 to January 2021), Alpha B.1.1.7 (until May 2021), and Delta B.1.617.2 (until the end of September 2021)33. We enrolled consecutive PCR-confirmed COVID-19 patients who met the inclusion criteria during the study period at Hospital Universitari de Sant Joan, after obtaining informed consent. Individuals of both sexes, over 18 years of age, with confirmed SARS-CoV-2 virus by polymerase chain reaction (PCR) testing were included in the study. PCR testing was performed using either the VIASURE SARS-CoV-2 Real Time PCR Detection Kit (CerTest Biotec, Zaragoza, Spain) or the Procleix® method with a Panther automated extractor and amplifier (Grifols Laboratories, Barcelona, Spain). The date of infection onset was defined as either the date of first symptoms or the date of the first positive PCR test. Patients were classified according to the recommendations of the Health Department of the regional Government of Catalonia as follows: asymptomatic infected patients had a positive PCR test but no symptoms of COVID-19; mildly symptomatic patients had a positive PCR test and COVID-19-related symptoms but did not require hospitalization (oxygen saturation, SpO2, > 94%, respiratory rate < 22 breaths/min, and no dyspnea); severely symptomatic patients had a positive test with evidence of lower respiratory tract disease on clinical assessment or imaging, with an SpO2 < 94% on room air at sea level and a respiratory rate > 22 breaths/min; critically ill patients had a positive test and required high-flow oxygen therapy, mechanical ventilation (invasive or non-invasive), or extracorporeal membrane oxygenation. Symptom classification was confirmed through clinical evaluation, review of medical records, and follow-up interviews when applicable. The clinical characteristics of the patients are presented in Table 1.
Data collection
Demographic data and clinical assessments were obtained in pseudonymized form from the hospital electronic medical record system. Information on age, sex, and clinical outcomes for each patient was obtained from hospital discharge or death summaries. Current comorbidities were assessed using the Charlson Comorbidity Index (CCI)34 and categorized according to the relevant organ system group. The clinical parameters and serum BChE activity of the patients were evaluated throughout the hospitalization.
Butyrylcholinesterase activity assessment
Whole blood samples were collected in 7.5 ml S-Monovette Serum-Gel tubes (SARSTEDT, Product No. 01.1602.001) and centrifuged at 2500×g for 10 min at room temperature. The resulting supernatants, representing sera, were then stored at − 80 °C until subsequent analysis in Slovakia.
An Ellman’s method optimized for biological samples was used to detect BChE activity in sera35. Each sample was assayed in duplicate. Ellman’s reaction is based on the enzymatic hydrolysis of butyrylthiocholine (BTC) to produce thiocholine, which then reacts with Ellman’s reagent, 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), to produce a yellow color. A 2.5 µl serum sample was preincubated with 0.5 mM DTNB in HEPES buffer, pH 7.5, for 20 min to allow interaction with free sulfhydryl groups. Subsequently, 1 mM BTC iodide (Sigma-Aldrich, Product No. B3253) was added, and the absorbance was immediately measured at 405 nm using a BioTek ELx800 spectrophotometer for 20 min at 46-second intervals to assess BChE activity. Activity is expressed as ΔmO.D. (405 nm), calculated from the slope of the linear phase of the saturation curve.
Data analysis
Data were processed and analyzed using GraphPad PRISM (version 9.3.1, 2021) and JMP Statistical Software (version 16.1) with a significance level of P < 0.05. Demographic data and clinical parameters, including age, sex, vaccination status, and BChE activity, were presented as mean with interquartile range (IQR) or median with IQR. Patients were divided into subgroups based on sex (male/female), age (< 65 years/ ≥65 years), vaccine dose, and symptoms. One-way analysis of variance (ANOVA) followed by post hoc tests and unpaired t-tests for parametric continuous data, or Wilcoxon/Kruskal-Wallis tests for non-normally distributed data, were used to compare groups. The Spearman rank correlation test was used to assess the relationship between BChE activity and relevant clinical parameters. Confounding factors were controlled through appropriate stratification into study subgroups, representing a direct, robust, and mathematically straightforward approach to confounder control. These factors were also internally validated using multivariate regression analysis (MVRA), which confirmed the statistical significance of the factor and confounder under investigation. A detailed MVRA analysis leading to a prediction model will be the subject of further work.
Result
Demographics and clinical characteristics
The study included a total of 462 patients (Table 1). Out of these patients, 78 (16.8%) were asymptomatic, 200 (43.2%) were mildly symptomatic, 122 (26.4%) were severely symptomatic, and 62 (13.4%) were critically ill patients.
The asymptomatic group did not display any clinical symptoms throughout the study. In the mildly symptomatic group, the predominant symptoms were cough and fever, whereas in severely symptomatic group and critically ill patients, cough, dyspnea and fever were the most common symptoms (Table 2).
The patient population was comprised of 265 females (57.3%) and 197 males (42.6%) (Table 1). Females exhibited a higher prevalence compared to males in the asymptomatic (62.8% vs. 37.2%) and mildly symptomatic (71.0% vs. 29.0%) groups, while male patients demonstrated a higher frequency compared to females in the severely symptomatic (41.8% vs. 58.2%) and critically ill (37.1% vs. 62.9%) groups.
The mean age of the patients was 57.48 ± 20.44 years (Table 1). Patients < 65 years were more prevalent in the asymptomatic (61.5% vs. 38.5%) and mildly symptomatic (83.5% vs. 16.5%) groups, whereas patients ≥ 65 years were more frequent in the severely symptomatic (41% vs. 59%) and critically ill (41.9% vs. 58.1%) groups.
Within 30 days of analysis, fatalities occurred in 2.6% of severely symptomatic patients (0.8% female vs. 1.6% male) and 37.1% of critically ill patients (11.3% female vs. 25.8% male). Additionally, 3.8% of the deceased patients were < 65 years and 96.2% were ≥ 65 years (Table 1).
Information regarding vaccination status was provided for 457 patients, and unvaccinated patients predominated in all symptom groups. Within the asymptomatic group, 47 patients were unvaccinated, 10 had received a single dose, and 19 had received two doses of vaccine. Within the mildly symptomatic group, 157 patients had not received vaccination, 10 had received a single dose and 33 had received two doses. Among the severely symptomatic patients, 75 were unvaccinated, 15 had received one dose of the vaccine, and 29 had been vaccinated twice. Among the critically ill patients, 45 were unvaccinated, while nine and eight had received one or two vaccine doses, respectively.
Serum BChE activity
Subgroup analysis based on sex revealed that there were no significant differences in BChE activity between female and male patients (P = 0.3644, Fig. 1A). In contrast, the analysis based on age revealed a significant decline in BChE activity in patients ≥ 65 years compared to patients < 65 years (P < 0.0001, Fig. 1B). Furthermore, BChE activity was related to mortality, with a significant decline in BChE activity observed in deceased patients compared to discharged patients (P < 0.0001, Fig. 1C).
Serum BChE activity according to sex (A), age (B) and mortality (C). There was no significant difference in BChE activity between female (n = 265) and male (n = 197) patients (P = 0.3644), while there was a significant decline in BChE activity in patients ≥ 65 years (n = 171) compared to those < 65 years (P < 0.0001, n = 291). The results showed a significant reduction in BChE activity in deceased patients (n = 26) compared to discharged (n = 436) patients (P < 0.0001). BChE - Butyrylcholinesterase; ≥65 years - of age 65 of older; <65 years - younger than 65 years.
A statistically significant difference in the mean BChE activity was identified among the study outcome categories (P < 0.0001), with a notable decline in BChE activity corresponding to the severity of COVID-19 (Fig. 2A). The asymptomatic and mildly symptomatic groups showed higher BChE activity, followed by the severely symptomatic group, with the critically ill group displaying the lowest serum BChE activity. A post hoc test revealed significant differences between the critically ill group and the asymptomatic group (P = 0.0046), the mildly symptomatic group (P < 0.0001), and the severely symptomatic group (P = 0.0381). Additionally, a significant difference was observed between the severely symptomatic group and the mildly symptomatic group (P = 0.0060). However, the comparison of the asymptomatic and mildly symptomatic groups (P = 0.3434) and the severely symptomatic (P = 0.7086) groups did not show significant differences.
A more detailed analysis of BChE activity in the symptom groups revealed significant differences between symptom groups for patients < 65 years (P = 0.0033, Fig. 2B) but not for patients ≥ 65 years (P = 0.1333, Fig. 2C). The post hoc test in patients < 65 years indicated significant differences between mildly symptomatic vs. severely symptomatic (P = 0.0170) and critically ill patients (P = 0.0058) but not between asymptomatic vs. mildly symptomatic (P = 0.6253), severely symptomatic (P = 0.2021), and critically ill (P = 0.0535), or critically ill vs. severely symptomatic (P = 0.3380) groups.
Serum BChE activity in the symptom groups: (A) All patients, (B) patients of age younger than 65 years, and (C) patients of age 65 years or older. Significant differences were observed among the symptom groups (P < 0.0001, A), particularly between critically ill (n = 62) vs. asymptomatic (P = 0.0046, n = 78), mildly symptomatic (P < 0.0001, n = 200), and severely symptomatic (P = 0.0381, n = 122) groups. Differences were also significant between severely symptomatic vs. mildly symptomatic (P = 0.0060), while comparisons involving asymptomatic vs. mildly symptomatic and asymptomatic vs. severely symptomatic groups were not significant (P = 0.3434 and P = 0.7086, respectively). For patients of age younger than 65 years, symptoms group differences were significant (P = 0.0033, B), but not for patients of age 65 years or older (P = 0.1333, C). These significances were between mildly symptomatic (n = 167) vs. severely symptomatic (P = 0.0170, n = 50) and mildly symptomatic vs. critically ill (P = 0.0058, n = 26) groups. BChE Butyrylcholinesterase.
Serum BChE activity with regards to COVID-19 symptoms
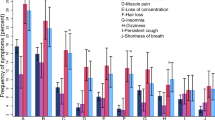
An analysis of BChE activity, informed by the presence of various symptoms associated with COVID-19 symptoms, including respiratory, gastrointestinal, neurological, musculoskeletal and systemic symptoms yielded inconsistent results. No significant differences were observed in BChE activity in patients with cough (P = 0.1089, Fig. 3.A1) or pulmonary failure (P = 0.5811, Fig. 3.A4), while lower BChE activity was observed in patients with pneumonia (P = 0.0027, Fig. 3.A2) and dyspnea (P = 0.0120, Fig. 3.A3). Furthermore, BChE activity exhibited no significant differences among patients with vomiting (P = 0.5775, Fig. 3.B1), diarrhea (P = 0.2773, Fig. 3.B2), or anorexia (P = 0.5341, Fig. 3.B3). Conversely, higher BChE activity was observed in patients with anosmia (P < 0.0001, Fig. 3.C1), ageusia (P = 0.0012, Fig. 3.C2), and headache (P = 0.0005, Fig. 3.C3). Notably, there were no significant differences in BChE activity in patients with myalgia (P = 0.6085, Fig. 3.D1), arthralgia (P = 0.7449, Fig. 3.D2), and odynophagia (P = 0.5113, Fig. 3.D3). Similarly, no significant alterations in BChE activity were observed in patients experiencing fever (P = 0.0571, Fig. 3.E1) or chills (P = 0.9169, Fig. 3.E2).
Serum BChE activity in patients with various symptoms. (A) Respiratory symptoms: No significant differences in BChE levels were observed with cough (P = 0.1089, n = 179, A1), pulmonary failure P = 0.5811, n = 18, A4), or odynophagia (P = 0.5113, n = 23, D3), but levels were significantly lower in patients with pneumonia (P = 0.0027, n = 50, A2) and dyspnea (P = 0.0120, n = 120, A3) compared to patients without these symptoms (n = 283, n = 444, n = 412, n = 317, n = 439 respectively). (B) Gastrointestinal symptoms: No significant differences were seen in patients with vomitus (P = 0.5775, n = 24, B1), diarrhea (P = 0.2773, n = 52, B2), or anorexia (P = 0.5341, n = 13, B3) compared to those without these symptoms (n = 438, n = 410, and n = 449, respectively). (C) Neurological symptoms: BChE activity was significantly higher in patients with anosmia (P < 0.0001, n = 44, C1), ageusia (P = 0.0012, n = 40, C2), or headache (P = 0.0005, n = 73, C3) compared to those without these symptoms (n = 418, n = 422, n = 389, respectively). (D) Musculoskeletal symptoms: No significant difference in BChE activity were observed in patients with myalgia (P = 0.6085, n = 45, D1), or arthralgia (P = 0.7449, n = 30, D2) compared to those without these symptoms (n = 417, n = 432, respectively). (E) Systemic symptoms: No significant difference in BChE activity were found in patients with fever (P = 0.0571, n = 187, E1) or chills (P = 0.9169, n = 11, E2) compared to those without these symptoms (n = 275, n = 451, respectively). BChE Butyrylcholinesterase.
Subsequent analysis of BChE activity revealed a dose-dependent effect of vaccination (P = 0.0465, Fig. 4), with a reduction in activity in patients vaccinated with two doses compared to unvaccinated patients (P = 0.0451). However, there were no significant differences in BChE activity between unvaccinated patients and those who had received a single dose (P = 0.9410), nor between patients who had received a single dose and those who had received two doses (P = 0.1606).
Serum BChE activity in patients according to vaccine dose. There was a significant change between groups (P = 0.0465). Post hoc tests revealed that patients vaccinated with two doses (n = 89) had significantly lower BChE activity compared to unvaccinated patients (n = 324, P = 0.0451), while no significant differences were observed between the other groups. BChE - Butyrylcholinesterase.
Discussion
In this study, we tested our hypothesis that serum BChE activity could serve as a predictor of clinical status progression and outcome in COVID-19 patients. To investigate this, we analyzed BChE activity in the sera of 462 patients with PCR-confirmed COVID-19 from the first epidemic wave in Spain. The cohort consisted of 78 asymptomatic, 200 mildly symptomatic, 122 severely symptomatic, and 62 critically ill patients. Twenty-six patients from the two most severe groups died within 30 days of diagnosis. The most common symptoms reported were cough and fever, observed in approximately half of the cohort. While most symptoms were reported in mildly symptomatic patients, pneumonia and pulmonary failure were more prevalent in severely symptomatic and critically ill patients, respectively.
Although both males and females were present in comparable numbers, women predominated in the asymptomatic and mildly symptomatic groups, whereas males were more common in the two more severe groups. Deaths were more frequent in males, accounting for 69% of the total mortality. Several COVID-19 studies have reported that males were more severely affected than females36,37,38,39consistent with previous findings from epidemiological data of SARS-CoV and MERS-CoV that also highlighted sex differences in disease manifestation39,40,41. There is a lack of understanding of how sex influences COVID-19 outcomes. However, observed genetic and behavioral differences, which are multifactorial in nature, may play a significant role in susceptibility to viral infections36. For example, the effects of the X chromosome and sex hormones on immune responses have been reported, potentially providing females with greater plasticity and adaptability to infections42. Toll-like receptor 7 (TLR7), encoded on the X chromosome, plays a critical role in the immune response43. Several studies have shown that plasmacytoid dendritic cells (pDCs) from females produce more IFNα/β than males following TLR7 activation by viral RNA36,43,44potentially giving females an advantage in combating COVID-19. Additionally, males are more likely to engage in risky behaviors such as smoking and alcohol consumption and tend to have a wider range of underlying health conditions, which may increase their risk for severe COVID-19 outcomes36,37.
Seventy-five percent of the patients in the study were unvaccinated, with half of whom were in the mildly symptomatic group. For vaccinated patients, 38% of the asymptomatic group, 21% of the mildly symptomatic group, and 37% of the severely symptomatic and critically ill groups had received either one or two doses of the vaccine. Among those who had received two doses of the vaccine, only 29% developed severe symptoms and 9% became critically ill. Additionally, 11% of the severely symptomatic and 34% of the critically ill vaccinated patients died, and all these individuals were aged ≥ 65 years. Hence, our results are consistent with other studies demonstrating the protective effect of COVID-19 vaccination, although factors such as age may influence the efficacy of the vaccine45.
It is well established that advanced age is a recognized risk factor for severe COVID-19 outcomes and increased mortality27,39. Consistent with previous findings by Markuskova et al. (2023), we confirmed this association in our study1. The chronological age of 65 years and older is commonly used to define elderly individuals in clinical practice46and was also used in this study. In the studied cohort, both age groups, < 65 years and ≥ 65 years, were well represented in all symptom groups. However, consistent with the warnings and recommendations regarding the risks associated with older age and COVID-19 issued by various health organizations, governments, and researchers27,33,47our results revealed that younger patients (< 65 years) were more represented in the two less severe groups, whereas older patients (≥ 65 years) predominated in the two groups with more severe clinical status. Furthermore, mortality was almost exclusively observed in the elderly patients (96%).
Our previous analysis of 148 patients with severe COVID-19 revealed significantly lower serum BChE activity in patients who succumbed to the disease1consistent with observations by other research groups2,3,48,49,50. Furthermore, we showed dynamic changes in serum BChE activity corresponding to the progression of clinical status1reflecting the severity of the disease. Here, we confirmed lower BChE activity in deceased patients. Moreover, we observed an alignment between serum BChE activity and COVID-19 severity and symptoms.
Serum BChE activity differed among the symptom groups, showing higher activities in the asymptomatic and mildly symptomatic patients as compared to the two more severe groups. The lowest serum BChE activities were observed in the critically ill patients. Despite the distribution of patients across the symptom groups, BChE activity did not appear to be influenced by sex, as no significant difference was observed between females and males. Nevertheless, the lower BChE activity observed in the sera of elderly patients, as reported both here and previously by us1 and others2,3,4,15,16,51,52,53prompted us to perform a follow-up age-sensitive analysis. While results from patients < 65 years were preserved and BChE activity was a function of severity, decreasing in severely symptomatic and even more so in critically ill groups, age ≥ 65 years flattened the effect with only a tendency for lower BChE in the critically ill group. The lack of significant variation in patients ≥ 65 years may be attributed to the overall lower BChE activity in elderly patients.
Patients who received two doses of the vaccine demonstrated reduced BChE activity. This unanticipated discovery is counterintuitive and not yet fully understood. One potential explanation for this phenomenon is that it may reflect immune system activation triggered by vaccination. Confounding factors, such as age, comorbidities, or the timing of sample collection relative to vaccination, may also influence the results. Given the complexity of immune and cholinergic interactions, further research is needed to clarify the underlying mechanisms and potential clinical implications.
To the best of our knowledge, this is the first study to investigate the relationship between BChE activity and the symptoms in COVID-19 patients. Our subgroup analysis revealed that BChE activity was significantly associated with specific COVID-19 symptoms.
Lower BChE activity was associated with respiratory symptoms such as pneumonia and dyspnea, but not with cough or pulmonary failure. Conversely, higher BChE activity was associated with neurological symptoms such as anosmia, ageusia, and headache, potentially reflecting lower systemic inflammation in patients with isolated neurological manifestations. However, this observational finding does not imply causality. BChE activity was not associated with gastrointestinal, algesic, musculoskeletal, or fever and chills.
Regarding respiratory symptoms, previous studies have reported a reduction in BChE activity in patients with chronic obstructive pulmonary disease (COPD), suggesting a possible mechanistic link, although the exact mechanism remains unclear54,55,56. In contrast, increased BChE activity has been reported in the early stages of some neurological diseases associated with inflammation13,57although the exact mechanism remains unclear due to the complexity of inflammatory responses involving multiple organs58,59,60. In addition, factors such as the grade or severity of the disease and age may induce different inflammatory responses. Nevertheless, it is important to mention that the assessment of certain symptoms, particularly neurological and musculoskeletal symptoms, may not have been fully accurate in critically ill patients who required high-flow oxygen therapy, mechanical ventilation, or extracorporeal membrane oxygenation. Given that these patients were often unable to communicate effectively with medical staff, and considering the challenging conditions during the COVID-19 pandemic, some symptoms may have been underreported or overlooked.
Our findings support the potential role of BChE as a prognostic marker in patients with COVID-19, though causal mechanisms remain to be confirmed. The relationship between BChE activity and clinical status remains, however, unclear. Our previous study revealed a correlation between serum BChE activity and inflammatory markers, including C-reactive protein and interleukin-61. This is further supported by the work of others suggesting the prognostic potential of BChE in inflammation and sepsis54,61,62,63. In recent years, a growing body of evidence has highlighted the role of acetylcholine in inflammatory processes13,54,64. Several immune cells, including T cells, B cells, and NK cells, have been shown to produce acetylcholine, with its release being triggered by infection65. Acetylcholine modulates the immune response by activating α7 nicotinic receptors on macrophages, inhibiting nuclear factor (NF-κβ) activation, preserving high mobility group box 1 protein (HMGB1) in its nuclear form, and reducing pro-inflammatory cytokine production13,17,66. Immune cell-derived acetylcholine plays an important role in the response to sepsis, viral infections, and autoimmune diseases67. BChE, by mediating the degradation of acetylcholine, may be indirectly involved in the regulation of pro-inflammatory factors as acetylcholine plays a crucial role in the cholinergic anti-inflammatory pathway13,54. However, the activity of cholinesterases, specifically BChE, in metabolic homeostasis and inflammation is complex and appears to be even more so in various pathological situations. Hence, BChE activity likely follows different mechanisms in conditions caused by chronic or acute inflammation5,50,54. In pathological conditions caused by low-grade systemic inflammation, such as type 1 or type 2 diabetes57hypertension, hyperlipidemia57Alzheimer’s disease58Parkinson’s disease59and multiple sclerosis, an increase in BChE has been observed in the early stages of these diseases13,57,59. However, as the diseases progress to more advanced stages, a decrease in BChE activity has been reported, which has been associated with an increased risk of death in these diseases15,57,61. Therefore, the reduction of BChE in severe and deceased COVID-19 patients, as similarly observed in diseases such as cancer68,69chronic obstructive pulmonary disease70cardiovascular disease71,72HIV73hemodialysis74and stroke75could be considered an indicator of disease progression and poor prognosis, further supporting its potential role as a biomarker for severe outcomes in various pathological conditions.
The association of BChE activity with COVID-19 severity, mortality, age, and specific symptoms underscores its potential as a multifaceted biomarker for COVID-19. Given the complexity of the cytokine storm and the intricate interplay of various factors, integrating BChE activity into a composite index with other biomarkers may improve diagnostic accuracy and prognostic assessment. Additionally, considering the upstream role of the cholinergic system in inflammation, BChE activity may provide insight into therapeutic targets aimed at modulating the inflammatory response in COVID-19 patients.
Our study has several limitations. First, its observational nature limits the ability to establish causality between BChE activity and COVID-19 outcomes. Second, as a monocentric study, the generalizability of the findings may be limited; however, it offers detailed patient phenotyping from a single institution. Third, the demographics of the study cohort may affect the broader applicability of the findings. Fourth, although we recognize that BChE activity can be influenced by medications and comorbidities such as diabetes, chronic obstructive pulmonary disease (COPD), and liver disease, and we were unable to comprehensively assess these potential confounding factors due to the emergency conditions of the COVID-19 pandemic, which limited the availability of detailed clinical records. This may have affected the observed associations between BChE and disease severity. Future studies should aim to incorporate detailed comorbidity profiles to better control for these variables. Fifth, due to the specific circumstances of the pandemic, it was not possible to collect blood samples from all patients at consistent time points, potentially introducing variability. Finally, in some cases, the assessment of patient symptoms and characteristics may have been hindered by the patients’ unconscious state and their inability to communicate, compounded by the constraints imposed by the emergency situation.
In summary, in this study, we confirmed our previous findings of significantly lower serum BChE activity in patients with severe COVID-19 and in critically ill COVID-19 patients. In addition, we demonstrated that BChE activity was associated with COVID-19 severity, mortality, specific symptoms, age, and vaccination status. While BChE activity was not influenced by sex, it decreased with age and disease severity. Respiratory symptoms, such as pneumonia and dyspnea, and neurological symptoms, such as anosmia, ageusia and headache, as well as vaccination status were associated with variation in BChE activity. Our findings suggest that BChE may serve as a valuable tool for assessing the severity of COVID-19, predicting outcomes, and informing therapeutic strategies.
Data availability
All data contained in this study are available from the authors upon reasonable request.
References
Markuskova, L. et al. Serum butyrylcholinesterase as a marker of COVID-19 mortality: results of the monocentric prospective observational study. Chem. Biol. Interact. 381, 110557 (2023).
Sipahioglu, H. et al. Does serum butyrylcholinesterase level determine the severity and mortality of COVID-19 pneumonia? Prospective study. Front. Med. (Lausanne). 9, 940533 (2022).
Espeter, F. et al. Critically ill COVID-19 patients show reduced point of Care-Measured butyrylcholinesterase Activity-A prospective, monocentric observational study. Diagnostics (Basel). 12, 2150 (2022).
Goncharov, N. V. et al. Searching for new biomarkers to assess COVID-19 patients: A pilot study. Metabolites 13, 1194 (2023).
Sun, T. et al. New insights into butyrylcholinesterase: pharmaceutical applications, selective inhibitors and multitarget-directed ligands. Eur. J. Med. Chem. 275, 116569 (2024).
Melo, A. K. G. et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis. PLoS One. 16, e0253894 (2021).
Mao, R. et al. Manifestations and prognosis of Gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 5, 667–678 (2020).
Santarpia, L., Grandone, I., Contaldo, F. & Pasanisi, F. Butyrylcholinesterase as a prognostic marker: a review of the literature. J. Cachexia Sarcopenia Muscle. 4, 31–39 (2013).
Jasiecki, J., Targońska, M. & Wasąg, B. The role of butyrylcholinesterase and Iron in the regulation of cholinergic network and cognitive dysfunction in alzheimer’s disease pathogenesis. Int. J. Mol. Sci. 22, 2033 (2021).
Przybyłowska, M., Dzierzbicka, K., Kowalski, S. & Chmielewska, K. Inkielewicz-Stepniak, I. Therapeutic potential of multifunctional derivatives of cholinesterase inhibitors. Curr. Neuropharmacol. 19, 1323–1344 (2021).
Chen, V. P., Gao, Y., Geng, L. & Brimijoin, S. Butyrylcholinesterase regulates central Ghrelin signaling and has an impact on food intake and glucose homeostasis. Int. J. Obes. (Lond). 41, 1413–1419 (2017).
Inácio Lunkes, G. et al. Serum cholinesterase activity in diabetes and associated pathologies. Diabetes Res. Clin. Pract. 72, 28–32 (2006).
Das, U. N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med. Sci. Monit. 13, RA214–221 (2007).
Xu, L. et al. Exploring butyrylcholinesterase expression in diseases using a promising fluorescent imaging tool. Sens. Actuators B. 394, 134432 (2023).
Pomara, N. & Imbimbo, B. P. Impairment of the cholinergic anti-inflammatory pathway in older subjects with severe COVID-19. Med. Hypotheses. 144, 110274 (2020).
Belinskaia, D. A. et al. Albumin is a component of the esterase status of human blood plasma. Int. J. Mol. Sci. 24, 10383 (2023).
Pavlov, V. A., Wang, H., Czura, C. J., Friedman, S. G. & Tracey, K. J. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 9, 125–134 (2003).
Mehranfard, D. & Speth, R. C. Cholinergic anti-inflammatory pathway and COVID-19. Bioimpacts 12, 171–174 (2022).
Qin, Z., Xiang, K., Su, D. F., Sun, Y. & Liu, X. Activation of the cholinergic Anti-Inflammatory pathway as a novel therapeutic strategy for COVID-19. Front. Immunol. 11, 595342 (2021).
Li, M. et al. COVID-19 vaccine development: milestones, lessons and prospects. Signal. Transduct. Target. Ther. 7, 146 (2022).
Safiabadi Tali, S. H. et al. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin. Microbiol. Rev. 34, e00228–e00220 (2021).
Li, L. Q. et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 92, 577–583 (2020).
Zhu, J. et al. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis. J. Med. Virol. 92, 1902–1914 (2020).
Zaher, A. & Ali, S. Why asymptomatic COVID19 patient could face a sudden death. Int. J. Innovative Res. Med. Sci. 6, 178–183 (2021).
Nahshon, C., Bitterman, A., Haddad, R., Hazzan, D. & Lavie, O. Hazardous postoperative outcomes of unexpected COVID-19 infected patients: A call for global consideration of sampling all asymptomatic patients before surgical treatment. World J. Surg. 44, 2477–2481 (2020).
Yadav, R., Bansal, R., Budakoty, S. & Barwad, P. COVID-19 and sudden cardiac death: A new potential risk. Indian Heart J. 72, 333–336 (2020).
Ewing, A. G. et al. Review of organ damage from COVID and Long COVID: A disease with a spectrum of pathology. Med. Rev. 5, 66–75 (2021).
Yu, L., Liu, Y. & Feng, Y. Cardiac arrhythmia in COVID-19 patients. Ann. Noninvasive Electrocardiol. 29, e13105 (2024).
Hojyo, S. et al. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 40, 37 (2020).
Capra, A. P. et al. The prognostic value of Pentraxin-3 in COVID-19 patients: A systematic review and Meta-Analysis of mortality incidence. Int. J. Mol. Sci. 24, 3537 (2023).
Grewal, T. & Buechler, C. Adipokines as diagnostic and prognostic markers for the severity of COVID-19. Biomedicines 11, 1302 (2023).
Du, J. et al. Global trends in COVID-19 incidence and case fatality rates (2019–2023): a retrospective analysis. Front. Public. Health. 12, 1355097 (2024).
Troyano-Hernáez, P., Reinosa, R. & Holguín, Á. Evolution of SARS-CoV-2 in Spain during the first two years of the pandemic: Circulating variants, amino acid conservation, and genetic variability in structural, Non-Structural, and accessory proteins. Int. J. Mol. Sci. 23, 6394 (2022).
Charlson, M. E., Carrozzino, D., Guidi, J. & Patierno, C. Charlson comorbidity index: A critical review of clinimetric properties. Psychother. Psychosom. 91, 8–35 (2022).
Dingova, D. et al. Optimal detection of cholinesterase activity in biological samples: modifications to the standard ellman’s assay. Anal. Biochem. 462, 67–75 (2014).
Alwani, M. et al. Sex-based differences in severity and mortality in COVID-19. Rev. Med. Virol. 31, e2223 (2021).
Gebhard, C., Regitz-Zagrosek, V., Neuhauser, H. K., Morgan, R. & Klein, S. L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex. Differ. 11, 29 (2020).
Ueyama, H. et al. Gender difference is associated with severity of coronavirus disease 2019 infection: an insight from a Meta-Analysis. Crit. Care Explor. 2, e0148 (2020).
Sarri, G. et al. Prognostic factors of COVID-19: an umbrella review endorsed by the international society for pharmacoepidemiology. Clin. Pharmacol. Ther. 114, 604–613 (2023).
Jansen, A. et al. Sex matters - a preliminary analysis of middle East respiratory syndrome in the Republic of korea, 2015. Western Pac. Surveill Response J. 6, 68–71 (2015).
Karlberg, J., Chong, D. S. Y. & Lai, W. Y. Y. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 159, 229–231 (2004).
Wu, H. et al. Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron 81, 103–119 (2014).
Meier, A. et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 15, 955–959 (2009).
Berghöfer, B. et al. TLR7 ligands induce higher IFN-alpha production in females. J. Immunol. 177, 2088–2096 (2006).
Watanabe, A., Iwagami, M., Yasuhara, J., Takagi, H. & Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 41, 1783–1790 (2023).
Singh, S. & Bajorek, B. Defining ‘elderly’ in clinical practice guidelines for pharmacotherapy. Pharm. Pract. (Granada). 12, 489 (2014).
Stróż, S., Kosiorek, P. & Stasiak-Barmuta, A. The COVID-19 inflammation and high mortality mechanism trigger. Immunogenetics 76, 15–25 (2024).
Nakajima, K. et al. Serum cholinesterase associated with COVID-19 pneumonia severity and mortality. J. Infect. https://doi.org/10.1016/j.jinf.2020.08.021 (2020). https://pubmed.ncbi.nlm.nih.gov/32822684
Bahloul, M. et al. Clinical characteristics and outcomes of critically ill COVID-19 patients in sfax, Tunisia. Acute Crit. Care. 37, 84–93 (2022).
Bahloul, M. et al. Prognostic value of serum cholinesterase activity in severe SARS-CoV-2-Infected patients requiring intensive care unit admission. Am. J. Trop. Med. Hyg. 107, 534–539 (2022).
Karasova, J. Z. et al. Activity of cholinesterases in a young and healthy middle-European population: relevance for toxicology, Pharmacology and clinical praxis. Toxicol. Lett. 277, 24–31 (2017).
Abou-Hatab, K., O’Mahony, M. S., Patel, S. & Woodhouse, K. Relationship between age and plasma esterases. Age Ageing. 30, 41–45 (2001).
Maul, J. D. & Farris, J. L. The effect of sex on avian plasma cholinesterase enzyme activity: a potential source of variation in an avian biomarker endpoint. Arch. Environ. Contam. Toxicol. 47, 253–258 (2004).
Peng, Z. L. et al. Association between early serum cholinesterase activity and 30-day mortality in sepsis-3 patients: A retrospective cohort study. PLoS One. 13, e0203128 (2018).
Sicinska, P. et al. Decreased activity of butyrylcholinesterase in blood plasma of patients with chronic obstructive pulmonary disease. Arch. Med. Sci. 13, 645–651 (2017).
Boudinot, E. et al. Effects of acetylcholinesterase and butyrylcholinesterase Inhibition on breathing in mice adapted or not to reduced acetylcholinesterase. Pharmacol. Biochem. Behav. 80, 53–61 (2005).
Rao, A. A., Sridhar, G. R. & Das, U. N. Elevated butyrylcholinesterase and acetylcholinesterase May predict the development of type 2 diabetes mellitus and alzheimer’s disease. Med. Hypotheses. 69, 1272–1276 (2007).
Jasiecki, J. & Wasąg, B. Butyrylcholinesterase protein ends in the pathogenesis of alzheimer’s disease-could BCHE genotyping be helpful in alzheimer’s therapy? Biomolecules 9, 592 (2019).
Dong, M. X. et al. Serum Butyrylcholinesterase Activity: A Biomarker for Parkinson’s Disease and Related Dementia. Biomed Res Int 1524107 (2017). (2017).
Hughes, C. G. et al. Association between cholinesterase activity and critical illness brain dysfunction. Crit. Care. 26, 377 (2022).
Zivkovic, A. R. et al. Reduced serum butyrylcholinesterase activity indicates severe systemic inflammation in critically ill patients. Mediat. Inflamm. 274607 (2015).
Zivkovic, A. R. et al. A sustained reduction in serum cholinesterase enzyme activity predicts patient outcome following sepsis. Mediat. Inflamm. 1942193 (2018).
Asad, Z. U. A. et al. The role of vagus nerve stimulation in Sepsis. Bioelectron. Med. 3, 51–62 (2020).
Sridhar, G. R. & Gumpeny, L. Emerging significance of butyrylcholinesterase. World J. Exp. Med. 14, 87202 (2024).
Cox, M. A. et al. Choline acetyltransferase-expressing T cells are required to control chronic viral infection. Science 363, 639–644 (2019).
Kimura, H. et al. Neural anti-inflammatory action mediated by two types of acetylcholine receptors in the small intestine. Sci. Rep. 9, 5887 (2019).
Cox, M. A. et al. Beyond neurotransmission: acetylcholine in immunity and inflammation. J. Intern. Med. 287, 120–133 (2020).
Koie, T. et al. Significance of preoperative butyrylcholinesterase as an independent predictor of survival in patients with muscle-invasive bladder cancer treated with radical cystectomy. Urol. Oncol. 32, 820–825 (2014).
Klocker, E. V. et al. Decreased activity of Circulating butyrylcholinesterase in blood is an independent prognostic marker in pancreatic Cancer patients. Cancers (Basel). 12, 1154 (2020).
Chen, Z. et al. Prognostic value of serum cholinesterase levels for In-Hospital mortality among patients with acute exacerbation of chronic obstructive pulmonary disease. COPD 20, 178–185 (2023).
Goliasch, G. et al. Butyrylcholinesterase activity predicts long-term survival in patients with coronary artery disease. Clin. Chem. 58, 1055–1058 (2012).
Shiba, M. et al. Serum cholinesterase as a prognostic biomarker for acute heart failure. Eur. Heart J. Acute Cardiovasc. Care. 10, 335–342 (2021).
Xu, L. et al. Butyrylcholinesterase levels on admission predict severity and 12-month mortality in hospitalized AIDS patients. Mediat. Inflamm. 5201652 (2018).
Stojanov, M. D. et al. Butyrylcholinesterase activity and mortality risk in Hemodialysis patients: comparison to HsCRP and albumin. Clin. Biochem. 42, 22–26 (2009).
Ben Assayag, E. et al. Serum cholinesterase activities distinguish between stroke patients and controls and predict 12-month mortality. Mol. Med. 16, 278–286 (2010).
Acknowledgements
We would like to acknowledge the use of AI tools for assistance in proofreading and language editing. Specifically, ChatGPT (developed by OpenAI) and DeepL were used to improve the clarity and coherence of the manuscript. While these tools provided linguistic support, the responsibility for the content of this work remains entirely with the authors.
Funding
This work was supported by The Ministry of Education, Research, Development and Youth of the Slovak Republic [grant numbers VEGA 1/0283/22 and VEGA 1/0293/25 to A.H.], The Slovak Research and Development Agency [grant numbers APVV-22–0541 to A.H], and the Fundació La Marató de TV3, Barcelona, Spain [grant number 201807-10 to J.C.].
Author information
Authors and Affiliations
Contributions
Tomáš Fazekaš conducted data validation and statistical analyses, interpreted the results, reviewed and revised the manuscript for important intellectual content, and prepared the figures. Lucia Kováčik collected and managed patient data and performed laboratory analyses. Morteza Motahari Rad provided technical support, assisted in data validation, drafted the manuscript and prepared the figures. Xavier Gabaldó Barrios collected and managed patient data. Simona Mihaela Iftimie collected and managed patient data. Jordi Camps collected and managed patient data, and secured funding. Anna Hrabovska conceptualized the study, designed the methodology, supervised the project, secured funding, and provided overall project oversight, also participated on data validation, and critically reviewed and revised the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fazekaš, T., Kováčik, L., Rad, M.M. et al. Serum butyrylcholinesterase activity as a predictor of severity and mortality in COVID-19 patients. Sci Rep 15, 23437 (2025). https://doi.org/10.1038/s41598-025-07017-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07017-2