Abstract
The Nuclear Factor of Activated T-cells cytoplasmic 1 (NFATc1) is identified to be an oncogene in several human cancers. However, its specific role in HCC progression and the relevant mechanisms remain unclear. To investigate this, we analyzed NFATc1 expression in clinical HCC samples (n = 289) using RT-qPCR, Western blotting, and immunohistochemistry (IHC). In vitro assays assessed HCC cell proliferation, migration, invasion, apoptosis, and cell cycle, while in vivo studies using BALB/c nude mice evaluated the effects of NFATc1 on HCC growth and metastasis. Mechanistic studies were conducted to explore NFATc1’s downstream signaling pathways. Results revealed that NFATc1 is significantly upregulated in HCC tissues compared to adjacent non-cancerous tissues, correlating with poor prognosis. Overexpression of NFATc1 enhanced HCC cell proliferation, migration, and invasion both in vitro and in vivo, while silencing NFATc1 reduced these malignant traits. Mechanistic analysis indicated that NFATc1 activation correlates with NF-κB/TMP21 signaling and senescence-associated secretory phenotype (SASP) amplification, independent of growth arrest. These findings suggest that NFATc1 plays a pivotal role in HCC progression, potentially through the modulation of SASP activation. Targeting NFATc1 and its signaling pathways could be a promising therapeutic approach for HCC.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) takes up approximately 90% of primary liver cancers and ranks fourth among the factors causing cancer-related deaths worldwide1. Early-stage HCC patients are ideal candidates for liver resection and other surgical treatments, which yield a five-year survival rate of 75–80%2. Unfortunately, due to the subtle onset and high invasiveness of HCC, more than 50% of cases are diagnosed at advanced stages1. Despite advancements in systemic therapy, the persistently high mortality rates indicate that more effective treatment options are required for advanced-stage HCC3. Understanding the mechanisms driving HCC progression is key to developing effective targeted therapy.
Nuclear factor of activated T cells (NFAT) proteins comprising the calcium-responsive transcription factor (NFATc1-NFATc4) family, were originally identified as critical regulators for T cell activation and differentiation4. Recent studies indicates that NFAT proteins also exert significant effects on tumor occurrence and development5. Generally, these proteins reside in the cytosol and are hyperphosphorylated in quiescent cells. Upon activation, NFAT proteins undergo dephosphorylation and translocate to the nucleus, where they facilitate gene transcription5. NFATc1, as a member of the NFAT family, has been shown to be activated in various solid tumors, including those in the colon, pancreas, glioblastoma, breast, and lung6,7,8,9,10. This dysregulation can drive tumor proliferation, invasion, metastasis, and recurrence by increasing self-renewal capacity8, inducing stem cell-like properties11, or facilitating epithelial-mesenchymal transition (EMT)12. However, the involvement of the NFATc1 pathway in HCC and its specific role in its underlying mechanisms remain insufficiently understood.
The activation of NFATc1 signaling has been shown to upregulate various cytokines and establish an inflammatory microenvironment13. Chronic inflammation is widely recognized as a hallmark of cancr, including HCC. Emerging evidence suggests that the secretome derived from senescent cells constitutes a major source of chronic inflammation in cancer14. Senescent cells exhibit a secretome composed mainly of growth factors, inflammatory factors, matrix remodeling factors, known as the senescence-associated secretory phenotype (SASP), which has been confirmed to enhance HCC malignant progression by promoting cell growth, EMT, angiogenesis, invasion, or establishing an immunosuppressive microenvironment15,16,17,18,19. Previous studies have reported intracellular calcium accumulation and calcium-dependent signaling pathway activation are crucial in regulating cellular senescence20,21. However, the role of NFATc1 involved in hepatocyte senescence and the secretion of the SASP in HCC remains unexplored.
In the present study, we investigated the effect and underlying mechanisms of NFATc1 on HCC progression, with a particular focus on hepatocyte senescence and SASP.
Materials and methods
Patients and tissue samples
Formalin-fixed, paraffin-embedded tissue samples were acquired from patients with HCC (n = 289) who underwent surgery without prior radiotherapy or chemotherapy at the Affiliated Hospital of Jining Medical University. Table 1 presents the clinical and pathological data of these patient. Tissue microarrays (TMAs) with a core diameter of 2.0 mm were constructed from the collected samples for subsequent analysis. Immunohistochemistry (IHC) examinations were performed on all the samples, and an additional 12 paired fresh HCC and matched non-cancerous tissue samples were further analyzed using quantitative reverse transcription polymerase chain reaction (RT-qPCR) and Western blotting. All procedures followed were approved by the Research Ethics Committee of the Affiliated Hospital of Jining Medical University (approval number: 2020C046) and complied with the principles of the 1975 Helsinki Declaration, revised in 2008. Informed consent was obtained from all participants or their legal guardians.
Cells and cell culture
Both LM3 and Huh-7 human HCC cells were obtained from American Type Culture Collection (ATCC). LM3 cells were cultivated in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (ExCell, USA) and 1% penicillin-streptomycin (Gibco, USA), while Huh-7 cells were grown in DMEM added with 1.5 g/L sodium bicarbonate (iCell, China), 10% FBS, and 1% penicillin-streptomycin. The cells were subject to incubation at 37 °C in 5% CO2.
Cell transfection for overexpression and knockdown
LM3 and Huh-7 cells (5 × 104/well) were inoculated in six-well plates prior to overnight incubation. To generate NFATc1-overexpressing or NFATc1-silenced cell lines, we used the following vectors (all from Genechem Co., Ltd.): LV-NFATc1 (23019), LVCON335 (overexpression control), LV-NFATc1-RNAi (129449, 129450, and 129451), and LVCON313 (RNAi control). The specific sequence can be found in the supplementary materials (Table S1). Opti-MEM (Gibco, USA) supplemented with HitransG P (Genechem, China) was adopted for all transfections. Transfection efficiency was assessed 48 h after transfection using a fluorescence microscope (RVL-100-G, ECHO, San Diego, CA, USA). Then, stable NFATc1-overexpressing or NFATc1-silenced cells were generated by selecting cells with 2 µg/mL puromycin (GeneChem, China) for about two weeks.
Cell counting kit-8 (CCK-8) assay
A CCK-8 (TargetMol, China) assay was carried out to assess cell viability following a specific protocol. After transfection, the cells were inoculated in 96-well plates (1,000 cells/well). The CCK-8 reagent (10 µL) was supplemented to each well at 0, 24, 48, and 72 h after inoculation, followed by 1 h of incubation at 37 °C. Subsequently, the absorbance was measured through a Synergy H1 Hybrid Reader (BioTek, VT, USA) at 450 nm.
Colony formation assay
After transfection, the cells (500 cells/well) were inoculated in six-well plates, followed by culture in 2 mL of medium containing 10% FBS, with the medium changed at two-day intervals. After 14 days, 4% paraformaldehyde was added to fix the colonies, and then, crystal violet (Biosharp, China) was added for cell staining. Images were taken for colony quantification.
Scratch assay
Transfected cells were inoculated into six-well plates for forming the confluent monolayer. A scratch was then made at the center of each well with a 200 µL pipette tip. Serum-free medium was added to incubate cells for 48 h, and images were obtained with the phase-contrast microscope.
Transwell assays
Transwell assays were performed in polycarbonate filter chambers (pore size, 8.0 μm; Corning, USA), and Matrigel (Corning, USA)was coated for invasion assays. Briefly, 5 × 105 transfected LM3 cells and 1 × 105 transfected Huh7 cells were suspended in a serum-free medium before inoculation in the upper Transwell chambers. The lower chamber was filled with a medium which contained 20% FBS as a chemoattractant. Following incubation for 48 h for the migration of Huh7 cells, 72 h for migration of LM3 cells and the invasion of Huh7 cells, and 96 h for the invasion of LM3 cells, we eliminated non-migrated or non-invasive cells from the upper membrane surface, fixed migrated or invasive cells on the lower membrane surface, stained them with crystal violet, and captured them with a microscope (RVL-100-G, ECHO, San Diego, CA, USA). The cells were quantified using ImageJ software.
Cell cycle and apoptotic analysis
We conducted a flow cytometry assay to evaluate transfected cell apoptosis and the cell cycle. After transfection, the cells were harvested and rinsed with ice-cold PBS. Next, the apoptosis rate was examined using the APC Annexin-V Apoptosis Detection Kit with 7-AAD (BioLegend, USA) according to specific protocols. For cell cycle analysis, after transfection, the cells were fixed for 24 h in 75% ice-cold ethanol at 4 °C, followed by staining with propidium iodide (PI) using a Cell Cycle Analysis Kit (Biosharp, China). Analysis was performed on the DxFLEX flow cytometer (Beckman Coulter, USA).
In vivo tumor xenograft and lung metastasis models
A total of 36 4-week-old male BALB/c nude mice were purchased in Jinan PengYue Laboratory Animal Breeding Co., Ltd. (Jinan, China) and raised under specific pathogen-free conditions. For the xenograft model, each mouse was given subcutaneous injection of 2 × 106 transfected Huh7 cells (6 mice/group, 4 groups). We determined tumor size at 3-day intervals, and computed tumor volume below: volume = 0.5 × length × width² (mm³). For the lung metastatic model, each mouse was given injection of 1.5 × 106 transfected Huh7 cells through tail vein (3 mice/group, 4 groups). The mice were anesthetized with intraperitoneal injections of sodium pentobarbital (60 mg/kg body weight) and subsequently euthanized by an overdose of sodium pentobarbital (150 mg/kg body weight) at the end of the experiment. Tumors and lung metastases were assessed using hematoxylin and eosin (H&E) staining, IHC, or fluorescence microscopy. All animal experiments were conducted in compliance with the National Institutes of Health (NIH) guidelines and the ARRIVE guidelines. Furthermore, these protocols were sanctioned by the Jining Medical University Ethics Committee for Animal Experiments (approval number: 2020B007).
RT-qPCR
Total liver and cellular RNA was isolated through an RNA-Quick Purification Kit (Feijie, China). Next, mRNA was reverse-transcribed into cDNA following the protocol offered with the reverse transcription kit (ABM, China). Quantitative PCR was performed with UltraSYBR Mixture (Kangwei Century, China) on a Bio-Rad fluorescence quantitative PCR system. Gene expression was calculated based on the expression of GAPDH or β-actin using the 2−ΔΔCT approach. The primers can be found in the supplementary materials (Table S2).
Protein extraction and Western blotting
Liver or cellular proteins were isolated with RIPA lysis buffer which contained 1% protease inhibitor and 1% PMSF (Beyotime, China). A cytosolic and nuclear extraction kit (INVENT, USA) was used to isolate nuclear proteins following specific guidelines. The concentrations of the extracted proteins were determined through a BCA protein concentration assay kit (Beyotime, China). Next, proteins were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Epizyme, China) before the proteins were transferred onto PVDF membranes (Millipore, USA). When the membranes were blocked with defatted milk powder, they were subject to incubation overnight at 4 °C with primary antibodies. Subsequently, secondary antibodies were added and incubated for 1 h at ambient temperature. Antibody binding was visualized using an enhanced chemiluminescence (ECL) Western blotting substrate (Millipore, USA). Full-length blots images were analyzed in ImageJ, and Target/GAPDH ratios were statistically evaluated in GraphPad Prism v9.0. All experiments were conducted with biological replicates, and within-blot normalization to GAPDH ensured loading control.
IHC
After xylene dewaxing, the paraffin-embedded tissue samples underwent gradient ethanol rehydration. Citrate buffer (pH 6; Biosharp, China) was adopted for antigen retrieval, followed by quenching of endogenous peroxidase with 3% (vol/vol) hydrogen peroxide (H2O2; Boster, USA). Following permeabilization with 0.3% Triton X-100 (Yuanye, China), the sections were blocked with goat serum (Boster, USA) and incubated overnight with primary antibodies at 4 °C. The next day, the sections were treated with HRP-conjugated secondary antibodies before diaminobenzidine (DAB; Maixi_Bio, China) staining and hematoxylin counterstaining (Solarbio, China) were performed. The IHC staining index was calculated as the product of staining intensity score and the positive area score. Staining intensities were graded into 0 (none), 1 (weak), 2 (moderate), or 3 (strong). Positive cell frequencies were categorized into 0 (< 5%), 1 (6–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%). Scores of 0–8 and 9–12 stood for low and high expression separately.
Senescence-associated β-galactosidase (SA-β-gal) staining
We conducted SA-β-gal staining with a senescent cell histochemical staining kit (Beyotime, China). Sections were prepared by washing, fixing, and incubating with the kit’s working solution overnight under 37 °C. Senescent cells were later monitored with an optical microscope.
Statistical analysis
Data are expressed as mean ± standard deviation. Statistical analyses were conducted using Student’s t-test for two-group comparisons, Chi-square test for categorical variables, and one-way ANOVA for comparisons among multiple groups. Spearman correlation and logistic regression analyses were performed for correlation analysis. Survival analyses employed Kaplan-Meier and multivariable Cox regression controlling for age, gender, BCLC staging, AFP levels, vascular invasion, necrosis, and hyaline globules. Benjamini-Hochberg FDR correction (q < 0.1) was applied to survival/correlation p-values. All results were of statistical significance at p < 0.05. GraphPad Prism v9.0 and R v4.2.1 (Cox models) was adopted for the statistical analyses.
Results
Elevated expression of nuclear NFATc1 predicts poor prognostic outcomes of HCC patients
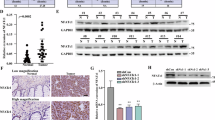
For investigating the effect of NFATc1 on HCC development, we assessed its relative abundance in fresh tissues in HCC patients (n = 12) using RT-qPCR and Western blotting. Our results showed a significant increase in both NFATc1 mRNA and protein levels in HCC tissues compared to matched non-cancerous controls (Fig. 1a and b). Further, nuclear/cytoplasmic fractionation followed by Western blotting revealed a notable NFATc1 overexpression in the nuclei of HCC cells (Fig. 1c).
We next performed IHC analysis on a HCC tissue microarray with 289 paired HCC and adjacent non-cancerous samples. Consistent with Western blot data, IHC analysis confirmed a pronounced nuclear accumulation of NFATc1 in tumor tissue (Fig. 1d and e). Additionally, HCC tissues had markedly higher NFATc1 levels compared to adjacent non-cancerous tissues (Fig. 1d). Then, tumors were divided in NFATc1-high or NFATc1-low group according to staining intensity scores. An overview of NFATc1 levels and clinicopathological factors are presented in Table 1. Notably, elevated NFATc1 protein levels in HCC were associated with more aggressive tumor traits, including higher alpha-fetoprotein (AFP) levels (p = 0.049), frequent vascular invasion (p = 0.034), severe necrosis (p = 0.003), and hyaline globules (p = 0.032). As suggested by Kaplan-Meier survival analyses, those with higher NFATc1 expression had significantly higher recurrence rates and poorer overall survival (p = 0.0116 and p = 0.0025, respectively). To minimize false positives from multiple comparisons, the Benjamini-Hochberg procedure was used to adjust the p-values. After adjustment, high NFATc1 expression remained significantly associated with severe necrosis (FDR-adjusted p = 0.045) (Table 1), shorter disease progression (FDR-adjusted p = 0.0116), and poorer overall survival (FDR-adjusted p = 0.005) (Fig. 1f). A multivariable Cox regression analysis, accounting for potential confounders such as age, gender, BCLC staging, AFP levels, vascular invasion, necrosis, and hyaline globules, confirmed that high NFATc1 expression remained independently associated with shorter disease progression (HR = 1.59, 95%CI 1.069–2.363, p = 0.022) and poorer overall survival (HR = 3.428, 95%CI 1.679–6.999, p = 0.001). These findings suggest that NFATc1 may promote HCC progression and could serve as a biomarker for predicting HCC prognosis.
NFATc1 is upregulated in HCC patients and correlates with poor prognosis. (a) RT-qPCR analysis of NFATc1 expression in paired tumor (T) and adjacent non-tumor (A) tissues (n = 12). (b) Representative Western blot images and quantification of NFATc1 protein levels in HCC samples (n = 12). (c) NFATc1 localization assessed by nuclear and cytoplasmic protein fractionation (n = 4). (d) Representative IHC images and quantitative IHC analysis of NFATc1 expression in HCC tissue microarrays (n = 289). (e) Representative IHC images illustrating varying staining intensities: none, weak, moderate, strong. (f) Kaplan-Meier survival analysis of time to progression and overall survival in HCC patients, stratified by NFATc1 expression (n = 289). Statistical analysis: Student’s t-test for two-group comparisons, Chi-square for categorical variables, Kaplan-Meier for survival, with Benjamini-Hochberg FDR correction. *p < 0.05, **p < 0.01, ****p < 0.0001.
NFATc1 activation promotes HCC cell proliferation, migration and invasion in vitro
This study analyzed NFATc1’s effect on HCC cell growth, migration and invasion through various in vitro assays. We utilized an overexpression and three short hairpin RNA (shRNA) lentiviruses that targeted backsplicing site of NFATc1 (designated as shNFATc1#1, shNFATc1#2, and shNFATc1#3) to stably overexpress or knock down NFATc1 in LM3 and Huh7 cells. NFATc1 expression was validated through RT-qPCR and Western blotting (Fig. 2a). Among the shRNA constructs, shNFATc1#3 showed the most efficient knockdown and was chosen for further studies (Fig. 2a). CCK-8 assays revealed that NFATc1-overexpressing LM3 and Huh7 cells had greater viability at 24, 48, and 72 h, whereas NFATc1-knockdown cells had reduced viability (Fig. 2b). Colony formation assays showed larger and more numerous colonies in NFATc1-overexpressing cells, with the opposite effect in NFATc1-knockdown cells (Fig. 2c). Flow cytometry indicated a reduced cell proportion at G1 phase within NFATc1-overexpressing cells, while an increase was observed in NFATc1-knockdown cells (Fig. 2d). LM3 cells presented higher apoptosis rates in the NFATc1-knockdown group and lower rates in the NFATc1-overexpressing group than in the control group, but no significant differences in apoptosis rates were detected across groups in Huh7 cells (Fig. 2f). Wound healing assays showed enhanced migration in NFATc1-overexpressing cells and reduced migration in NFATc1-knockdown cells (Fig. 2e). Furthermore, transwell assays demonstrated that NFATc1 overexpression significantly increased both HCC cell migration and invasion, while NFATc1 silencing exerted opposite effects (Fig. 2g and h). Overall, our findings suggest that NFATc1 exerts the crucial effect on promoting HCC cell growth, migration and invasion.
NFATc1 exerts oncogenic roles in HCC in vitro. (a) LM3 and Huh7 cells were transfected with lentiviruses expressing either NFATc1, shNFATc1(short hairpin RNA targeting NFATc1), or corresponding controls: NC (overexpression Negative Control) and shNC (short hairpin RNA Negative Control), and NFATc1 expression was confirmed by RT-qPCR and Western blotting. (b) and (c) Cell proliferation was assessed using CCK-8 and colony-forming assays in transfected LM3 and Huh7 cells. (d) Flow cytometry analyzed cell cycle distribution following NFATc1 overexpression or knockdown. (e) Wound healing assays were conducted to evaluate the migratory capacity of transfected LM3 and Huh7 cells. (f) Flow cytometry analyzed apoptosis of transfected LM3 and Huh7 cells. (g) and (h) Transwell assays assessed the impact of NFATc1 modulation on the migration and invasion capabilities of transfected LM3 and Huh7 cells (200× magnification). Statistical analysis: Student’s t-test for two-group comparisons, one-way ANOVA for comparisons among multiple groups. n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: no significance.
NFATc1 expression increases during hepatocyte senescence and enhances SASP production and secretion
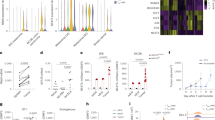
To investigate whether NFATc1 is involved in hepatocyte senescence and the secretion of the SASP in HCC, we established a hydrogen peroxide (H₂O₂)-induced cellular senescence model (Fig. 3a and c), as previously described22. RT-qPCR and Western blot analyses revealed significant upregulation of NFATc1, p16, nuclear factor kappa B (NF-κB) and main SASP components (IL-1β, IL-6, IL-8) in H₂O₂-treated senescent cells in comparison with control cells (Fig. 3b and c). When NFATc1 was knocked down in these senescent HCC cells, a significant suppression of NF-κB, IL-1β, IL-6, and IL-8 expression, as well as the expression of the intracellular membrane transport protein TMP21 was observed, without altering the levels of cell cycle maker p16 (Fig. 3d and e). Conversely, overexpression of NFATc1 in HCC cells resulted in an increase in NF-κB, TMP21 and SASP levels, while cell cycle maker levels remained unchanged (Fig. 3f and g). These findings suggest that NFATc1 activation occurs in senescent hepatocytes and is associated with NF-κB/TMP21 signaling and the amplification of the SASP.
NFATc1 correlates with SASP activation in HCC cells. (a) SA-β-gal staining of H2O2-induced senescent LM3 and Huh7 cells. (b) Representative Western blot images displaying the expression of NFATc1, NF-κB, and the cell cycle marker p16 in H2O2-induced senescent LM3 and Huh7 cells. (c) RT-qPCR analysis of NFATc1, p16 and SASP (NF-κB, IL-1β, IL-6, IL-8) in H2O2-induced senescent LM3 and Huh7 cells. (d) and (e) Western blot and RT-qPCR analysis of the effects of NFATc1 knockdown on NFATc1, TMP21, SASP and p16. (f) and (g) Western blot and RT-qPCR analysis of the effects of NFATc1 overexpression on NFATc1, TMP21, SASP and p16. Statistical analysis: Student’s t-test for two-group comparisons. n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: no significance.
NFATc1 promotes tumor growth and lung metastasis in vivo
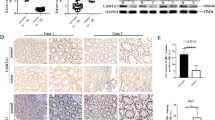
For investigating NFATc1’s impact on tumor growth in vivo, this study employed the xenograft model by subcutaneously injecting NFATc1-overexpressing or NFATc1-knockdown Huh7 cells into nude mice (Fig. 4a). As demonstrated in Fig. 4c, tumors derived from NFATc1-overexpressing cells were significantly larger than the control tumors, while those from NFATc1-knockdown cells were smaller than control group. IHC staining revealed an increased proportion of NF-κB -positive cells in NFATc1-overexpressing tumors, while a decrease was observed in NFATc1-knockdown tumors (Fig. 4d). RT-qPCR analyses confirmed that the expression levels of the SASP factors IL-1β, IL-6, and IL-8 were significantly elevated in NFATc1-overexpressing tumors, whereas a reduction in their expression was observed in NFATc1-knockdown tumors (Fig. 4e). To assess metastasis, we established a lung metastatic model by injecting NFATc1-overexpressing or NFATc1-knockdown Huh7 cells into the tail vein of nude mice (Fig. 4b). Whole-slide scans of H&E-stained sections and fluorescence images revealed that NFATc1 overexpression greatly increased lung metastatic lesions, while NFATc1-knockdown substantially reduced lung metastasis (Fig. 4f). Collectively, the above results highlight NFATc1’s critical effect on enhancing both tumor growth and metastasis in vivo.
NFATc1 enhances HCC growth and metastasis in vivo. (a) Schematic of the subcutaneous tumorigenesis model using nude mice (n = 6 mice per group, 4 groups). (b) Schematic of the lung metastasis model using nude mice (n = 3 mice per group, 4 groups). (c) Tumor size and volume measurements in mice after subcutaneous injection of Huh7 cells transfected with either NC, NFATc1, shNC, or shNFATc1. (d) Representative images of H&E and NF-κB IHC staining in tumor tissue derived from subcutaneously injected mice. (e) RT-qPCR analysis of SASP factors IL-1β, IL-6, IL-8 in tumors derived from subcutaneously injected mice. (f) Representative H&E-stained sections and fluorescence images of lung tissue from mice injected via the tail vein with Huh7 cells transfected with either NC, NFATc1, shNC, or shNFATc1. Statistical analysis: Student’s t-test for two-group comparisons, Chi-square test for categorical variables. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: no significance.
Clinical relevance of NFATc1 and SASP expression within HCC
To validate the relation of NFATc1 with SASP expression during HCC progression, we analyzed senescence markers and main SASP components in HCC tissues obtained from 12 freshly collected clinical samples. Our results demonstrated significantly higher levels of p53, p21, p16, and NF-κB in HCC tissues compared to matched non-cancerous counterparts (Fig. 5a and b). RT-qPCR analysis further confirmed the upregulation of mRNA expression for various SASP components (IL-1β, IL-6, IL-8, TNF-α) in HCC tissues (Fig. 5c). Spearman correlation analysis, adjusted using the Benjamini-Hochberg method, revealed that elevated expression levels of senescence markers (p53, p21, p16) and key SASP components (NF-κB, IL-1β, IL-6, IL-8, and TNF-α) were positively correlated with NFATc1 expression in HCC tissues (Fig. 5d).
Expression of senescence markers and SASP components in HCC patients. (a) Representative Western blot images, and (b) quantitative analysis of NF-κB, p53, p21, and p16 expression in tumor (T) and adjacent non-tumor (A) tissues. (c) RT-qPCR analysis of p53, p21, p16, and SASP component (IL-1β, IL-6, IL-8, TNF-α) levels in tumor (T) and adjacent non-tumor (A) tissues. (d) Correlation analysis of NFATc1 levels with cell cycle markers (p53, p21, p16) and SASP components (NF-κB, IL-1β, IL-6, IL-8, TNF-α) in tumor samples from HCC patients. Statistical analysis: Student’s t-test for two-group comparisons, Spearman correlation for the associations between NFATc1 and cell cycle markers or SASP components. Benjamini-Hochberg FDR correction (q < 0.1) was applied to the correlation p-values. n = 12, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
In recent years, NFATc1 has been shown to be an oncogene in several human cancers6,7,8,9,10,11,12,23,24. Nonetheless, its impact on HCC is still elusive, with current studies yielding inconsistent findings25,26,27. In our study, we observed a significant upregulation of NFATc1 in HCC tissues, and this increased NFATc1 expression was related to poor survival outcomes. Moreover, overexpression of NFATc1 enhanced HCC cell viability, growth, invasion, and migration in vitro and in vivo. On the contrary, silencing NFATc1 reduced malignant phenotype of HCC cells. These findings provide robust evidence that NFATc1 functions as a potent oncogene, significantly contributing to the initiation and progression of HCC. The discrepancies between our results and some previous studies may be due to differences in the cellular models used. For instance, the HepG2 cells, which harbor a wild-type p53 gene, may exhibit a distinct response to NFATc1 signaling compared to the LM3 and Huh7 HCC cells with p53 mutations. Evidence indicates that the p53 status of cells could significantly influence the effects of NFATc1 activation28,29. This raises the possibility that NFATc1’s oncogenic potential may be context-dependent, with p53 mutations potentially altering its impact on tumorigenesis. Future studies will investigate the interaction between NFATc1 and p53 to further elucidate how they cooperate or antagonize each other in HCC cells.
Cellular senescence, an irreversible growth arrest triggered by various stressors or DNA damage, plays a context-dependent role in cancer initiation and progression, acting as both a tumor suppressor and promoter30,31. Central to this duality is the SASP, a dynamic mediator that influences tumor microenvironment (TME) remodeling, immune modulation, and therapy resistance32. In HCC, Eggert and colleagues demonstrated that SASP derived from senescent hepatocytes exhibits stage-specific effects. In the early stages, SASP recruits protective myeloid cells to clear pre-malignant cells, thus suppressing tumor initiation, while in advanced stages, SASP promotes the accumulation of immunosuppressive immature myeloid cells that inhibit natural killer (NK) cell activity, fostering HCC progression17. Pribluda and colleagues further illustrated how senescence-associated inflammation transitions from tumor-suppressive to pro-tumorigenic depending on the integrity of the p53/p21 pathway in a colorectal cancer model33. P53-associated SASP promotes immune surveillance by recruiting immune cells such as macrophages, NK cells, and T cells, contributing to tumor regression34,35. In contrast, NF-κB-associated SASP fosters a pro-tumor environment by enhancing cell proliferation, metastasis, and immune suppression36. The balance of these opposing effects depends on key pathways, such as p53/p21 and NF-κB, and is influenced by tumor stage and microenvironment.
Notably, cancer cells can undergo senescence37, and oncogene-induced stress both activates p53 and stimulates SASP. P53 poses growth arrest in senescent cells, however, when p53 is mutated in HCC cells, these tumor cells resume proliferation, but the SASP cannot be suppressed despite reversion of the senescence growth arrest, in fact, an exaggerated SASP response is observed38,39,40,41. Our data reveal that NFATc1 expression correlates with NF-κB-associated SASP in senescent p53-mutant LM3 and Huh7 HCC cells. Suppression of NFATc1 significantly reduces NF-κB activation and SASP cytokine secretion, whereas its overexpression amplifies NF-κB-driven secretory profiles without affecting the growth arrest. These findings suggest that NFATc1 may enhance NF-κB-mediated SASP, thereby contributing to a pro-tumorigenic advantage in the context of p53-mutant HCC.
Persistent severe or irreparable DNA damage response (DDR) of sufficient magnitude to induce senescence is critical for the induction of the SASP42,43. NF-κB signalling can be activated by DDR44, which has been identified as the crucial regulatory factor for many SASP component levels in senescence45,46,47. A previous study showed that NFATc1 may regulate NF-κB subunit acetylation status and affect NF-κB function in cancers via histone deacetylase 18. Additionally, interleukin 1 β(IL1β) is a well-known key upstream pro-inflammatory cytokine of SASP components and interacts with IL-1 receptor, which could activate the NF-κB pathway, thereby inducing transcriptional activation additional SASP components during senescence48. NFATc1 has been reported as a crucial regulator of IL1β49, suggesting that NFATc1 may modulate NF-κB -mediated SASP in an IL1β-dependent manner. However, the specific mechanism require further exploration in future studies. Furthermore, our results demonstrated that NFATc1 significantly upregulates the expression of TMP21. TMP21, belonging to the p24 family, participates in transporting secretory proteins from endoplasmic reticulum into Golgi apparatus and is essential for keeping secretory pathway integrity within mammals50,51. It may play an essential role in directing the intracellular trafficking or secretion of SASP components. Thus, NFATc1 activation is associated not only with SASP production but also with its secretion and transport processes.
Collectively, this study provides valuable insights into the oncogenic role of NFATc1 in HCC, potentially through the promotion of SASP factor production and secretion. Targeting NFATc1 to inhibit NF-κB-associated SASP could offer a promising therapeutic strategy for anti-HCC treatment. However, several limitations should be acknowledged. While our findings offer initial mechanistic insights, the precise transcriptional regulation of NFATc1 on NF-κB/TMP21 or SASP components requires validation through ChIP-seq or luciferase assays to establish definitive causal relationships. Additionally, the H2O2-induced senescence model, although useful for studying senescence mechanisms, does not fully replicate the complexity of the HCC tumor microenvironment, particularly in terms of genomic instability, metabolic reprogramming, and immune interactions observed in clinical HCC progression. Future studies should incorporate patient-derived organoids and in situ HCC models to better capture native tumor-stroma interactions.We caution against drawing causal conclusions without further lineage-tracing studies and orthotopic tumor models. Lastly, the limited cohort size may constrain the generalizability of our findings and that further studies with larger clinical samples are needed to validate these results.
Data availability
The sequence data and some supporting raw data are provided in the supplementary information. Additional raw data generated and analyzed during this study can be requested by contacting Jing Zhang at Zhangjing8809@126.com.
Abbreviations
- HCC:
-
hepatocellular carcinoma
- NFAT:
-
nuclear factor of activated T cells
- SASP:
-
senescence-associated secretory phenotype
- DDR:
-
DNA damage response
- EMT:
-
epithelial-mesenchymal transition
- NF-κB:
-
nuclear factor kappa B
- IL:
-
interleukin
- TNF-α:
-
tumor necrosis factor-α
- AFP:
-
alpha-fetoprotein
- FBS:
-
fetal bovine serum
- shRNA:
-
short hairpin RNA
- CCK-8:
-
cell counting kit-8
- PI:
-
propidium iodide
- RT-qPCR:
-
quantitative reverse transcription polymerase chain reaction
- IHC:
-
immunohistochemistry
- SA-β-gal:
-
senescence-associated β-galactosidase
- H&E:
-
hematoxylin and eosin
- H2O2 :
-
hydrogen peroxide
- TME:
-
tumor microenvironment
References
Yang, C. et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 20, 203–222 (2023).
Vogel, A., Meyer, T., Sapisochin, G., Salem, R. & Saborowski, A. Hepatocellular carcinoma. Lancet 400, 1345–1362 (2022).
Anwanwan, D., Singh, S. K., Singh, S., Saikam, V. & Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer. 1873, 188314 (2020).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Shou, J. et al. Nuclear factor of activated T cells in cancer development and treatment. Cancer Lett. 361, 174–184 (2015).
Tripathi, M. K. et al. Nuclear factor of activated T-cell activity is associated with metastatic capacity in colon cancer. Cancer Res. 74, 6947–6957 (2014).
Hasselluhn, M. C., Schmidt, G. E., Ellenrieder, V., Johnsen, S. A. & Hessmann, E. Aberrant NFATc1 signaling counteracts TGFβ-mediated growth arrest and apoptosis induction in pancreatic cancer progression. Cell. Death Dis. 10, 446 (2019).
Song, Y. et al. NFAT2-HDAC1 signaling contributes to the malignant phenotype of glioblastoma. Neuro Oncol. 22, 46–57 (2020).
Xue, Y. et al. Cav2. 2-NFAT2-USP43 axis promotes invadopodia formation and breast cancer metastasis through cortactin stabilization. Cell. Death Dis. 13, 812 (2022).
Heim, L. et al. NFATc1 promotes antitumoral effector functions and memory CD8 + T-cell differentiation during non-small cell lung cancer development. Cancer Res. 78, 3619–3633 (2018).
Chang, Y. et al. USP7-mediated JUND suppresses RCAN2 transcription and elevates NFATC1 to enhance stem cell property in colorectal cancer. Cell. Biol. Toxicol. 39, 3121–3140 (2023).
Shen, T. et al. NFATc1 promotes epithelial-mesenchymal transition and facilitates colorectal cancer metastasis by targeting SNAI1. Exp. Cell. Res. 408, 112854 (2021).
Tripathi, P. et al. Activation of NFAT signaling establishes a tumorigenic microenvironment through cell autonomous and non-cell autonomous mechanisms. Oncogene 33, 1840–1849 (2014).
Capece, D. et al. Cancer secretome and inflammation: the bright and the dark sides of NF-κB. Semin Cell. Dev. Biol. 78, 51–61 (2018).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Huang, Y. et al. The hepatic senescence-associated secretory phenotype promotes hepatocarcinogenesis through Bcl3-dependent activation of macrophages. Cell. Biosci. 11, 173 (2021).
Eggert, T. et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell. 30, 533–547 (2016).
Yamagishi, R. et al. Gasdermin D-mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci. Immunol. 7, eabl7209 (2022).
Li, F. et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat. Cell. Biol. 22, 728–739 (2020).
Martin, N., Zhu, K., Czarnecka-Herok, J., Vernier, M. & Bernard, D. Regulation and role of calcium in cellular senescence. Cell. Calcium. 110, 102701 (2023).
Ma, X. et al. The nuclear receptor RXRA controls cellular senescence by regulating calcium signaling. Aging Cell. 17, e12831 (2018).
Zhang, J. et al. The p66shc-mediated regulation of hepatocyte senescence influences hepatic steatosis in nonalcoholic fatty liver disease. Med. Sci. Monit. 26, e921887 (2020).
Oikawa, T. et al. Acquired expression of NFATc1 downregulates E-cadherin and promotes cancer cell invasion. Cancer Res. 73, 5100–5109 (2013).
Yang, M. H., Wang, Y. S., Shi, X. Q., Zhao, X. W. & Li, B. Arsenic trioxide restrains lung cancer growth and metastasis by blocking the calcineurin-NFAT pathway by upregulating DSCR1. Curr. Cancer Drug Targets. 22, 854–864 (2022).
Wang, S. et al. Calcineurin/NFATc1 pathway contributes to cell proliferation in hepatocellular carcinoma. Dig. Dis. Sci. 57, 3184–3188 (2012).
Xu, S. et al. NFAT c1 is a tumor suppressor in hepatocellular carcinoma and induces tumor cell apoptosis by activating the FasL-mediated extrinsic signaling pathway. Cancer Med. 7, 4701–4717 (2018).
Wang, J. et al. NFAT2 overexpression suppresses the malignancy of hepatocellular carcinoma through inducing Egr2 expression. BMC Cancer. 20, 966 (2020).
Shinmen, N. et al. Activation of NFAT signal by p53-K120R mutant. FEBS Lett. 583, 1916–1922 (2009).
Singh, S. K. et al. Antithetical NFATc1-Sox2 and p53-miR200 signaling networks govern pancreatic cancer cell plasticity. EMBO J. 34, 517–530 (2015).
Takasugi, M., Yoshida, Y. & Ohtani, N. Cellular senescence and the tumour microenvironment. Mol. Oncol. 16, 3333–3351 (2022).
Huda, N. et al. Hepatic senescence, the good and the bad. World J. Gastroenterol. 25, 5069–5081 (2019).
Rao, S. G. & Jackson, J. G. SASP: tumor suppressor or promoter?? Yes! Trends Cancer. 2, 676–687 (2016).
Pribluda, A. et al. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell. 24, 242–256 (2013).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Coppé, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Chang, B. D. et al. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 18, 4808–4818 (1999).
Coppé, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Coppé, J. P. et al. Tumor suppressor and aging biomarker p16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 286, 36396–36403 (2011).
Moiseeva, O., Deschênes-Simard, X., Pollak, M. & Ferbeyre, G. Metformin, aging and cancer. Aging (Albany NY). 5, 330–331 (2013).
Moiseeva, O. et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF‐κ B activation. Aging Cell. 12, 489–498 (2013).
Rodier, F. et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell. Biol. 11, 973–979 (2009).
Malaquin, N., Martinez, A. & Rodier, F. Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 82, 39–49 (2016).
Wang, B., Han, J., Elisseeff, J. H. & Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell. Biol. 25, 958–978 (2024).
Kang, C. et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349, aaa5612 (2015).
Chien, Y. et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 25, 2125–2136 (2011).
Salminen, A., Kauppinen, A. & Kaarniranta, K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell. Signal. 24, 835–845 (2012).
Espitia-Corredor, J. A. et al. Resolvin E1 attenuates doxorubicin-induced cardiac fibroblast senescence: A key role for IL-1β. Biochim. Biophys. Acta Mol. Basis Dis. 1868, 166525 (2022).
Giblin, M. J. et al. Nuclear factor of activated T-cells (NFAT) regulation of IL-1β-induced retinal vascular inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 166238 (2021).
Blum, R. & Lepier, A. The luminal domain of p23 (Tmp21) plays a critical role in p23 cell surface trafficking. Traffic 9, 1530–1550 (2008).
Chen, F. et al. TMP21 is a presenilin complex component that modulatesγ-secretase but not ɛ-secretase activity. Nature 440, 1208–1212 (2006).
Acknowledgements
We extend our profound gratitude to the members of the Department of Pathology at the Affiliated Hospital of Jining Medical University for their invaluable support. Moreover, we are profoundly appreciative of all the participants who contributed to this study. We would also like to express our sincere thanks to Editeg for their exceptional assistance with language editing.
Funding
This work was supported by Shandong Province Natural Science Foundation (ZR2024QH076), Key Research and Development Program of Jining City (2024YXNS039, 2024YXNS108), Talent Program Fund of Affiliated Hospital of Jining Medical University (2022-yxyc-003, 2022-yxyc-013), and Postdoctoral Fund of Affiliated Hospital of Jining Medical University (JYFY364860).
Author information
Authors and Affiliations
Contributions
JZ, JS and BW were involved in study design; ZC, JZ, LH, LD, CZ and YL performed the experiments; ZC, LH, LD and YL analyzed the data; JZ and ZC wrote the manuscript. All authors revised and approved the final version of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Z., Huang, L., Ding, L. et al. NFATc1 facilitates hepatocellular carcinoma progression by regulating the senescence-associated secretory phenotype. Sci Rep 15, 24824 (2025). https://doi.org/10.1038/s41598-025-07585-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07585-3