Abstract
Ceftriaxone has shown promise as a neuroprotective agent through its modulation of glutamate transporters, yet its precise role in chronic pain remains underexplored. This study aimed to investigate the central mechanisms underlying the transition to chronic pain, focusing on glutamatergic activity in the primary somatosensory cortex hind limb (S1HL) and posterior intralaminar thalamic nucleus (PIL) in a rat model. Female Wistar rats (n = 36) were randomly assigned to six groups. Neuropathic pain was induced using paclitaxel (2 mg/kg, administered intraperitoneally on days 0, 2, 4, and 6). Starting on day 27, animals received daily intraperitoneal treatment for 10 days with either ceftriaxone (200 mg/kg), penicillin (400,000 U/kg), clonidine (2.5 µg/kg), morphine (0.1 mg/kg), or saline (control). Ceftriaxone treatment significantly increased pain thresholds. Voltammetry analysis showed that glutamate reuptake time (T80) in the S1HL cortex, which was prolonged to approximately 8 s in the paclitaxel group, returned to near-normal values (3–3.5 s) with ceftriaxone. Gene expression analyses revealed that ceftriaxone upregulated all assessed glutamate transporters and enzymes, including GLT-1, GLAST, EAAC1, EAAT4, GluL, and GLS. Notably, GLT-1 expression increased ~ 5-fold in the control group and only ~ 2-fold in the paclitaxel group. These findings suggest that ceftriaxone enhances glutamate reuptake and reduces excitotoxicity in the cortex, contributing to pain relief. The study highlights a potential role for glutamate regulation in chronic pain mechanisms and supports further exploration of ceftriaxone as a candidate for managing glutamate-mediated neuropathic pain. This aligns with the broader goals of improving neurological health and promoting innovative therapeutic strategies.
Similar content being viewed by others
Introduction
Neuropathic pain resulting from nervous system damage or dysfunction is not uncommon, and its pathobiology is not clear. Previous studies have suggested that nerve cell damage, altered nerve transmission, neurotransmitter imbalances, and nerve tissue inflammation could contribute to neuropathic pain1.
Paclitaxel-induced peripheral neuropathy affects approximately 97% of paclitaxel-treated patients2. Chronic paclitaxel-induced neuropathy significantly impairs patients’ long-term quality of life3 and may force dose reduction or discontinuation of chemotherapy, thereby limiting treatment effectiveness. Paclitaxel treatment may also cause acute encephalopathy4emotional distress, ataxia, and cognitive deficits5 despite its poor blood‒brain barrier penetration capacity6,7. Since paclitaxel is an effective chemotherapeutic agent, developing appropriate strategies to minimize its toxicity would result in better treatment of many cancers.
Ceftriaxone, a β-lactam antibiotic, increases GLT-1 expression in glial cells, thereby reducing synaptic glutamate levels. This mechanism contributes to the attenuation of neuropathic pain symptoms such as allodynia and hyperalgesia. Additionally, ceftriaxone prevents opioid-induced hyperalgesia and reduces glial activation8. Penicillins belong to the class of β-lactam antibiotics and exert their antibacterial effect by inhibiting bacterial cell wall synthesis. However, there is currently no direct scientific evidence indicating that penicillin increases the expression of the glutamate transporter GLT-1 in the CNS to alleviate neuropathic pain symptoms. Nevertheless, ampicillin, which is a member of the penicillin group, has been shown to induce GLT-1 expression in vitro, similar to ceftriaxone9.
Opioids are among the most potent analgesics used to manage both acute and chronic pain. However, prolonged use often leads to a reduction in pain-relieving effectiveness, necessitating increasing doses—a phenomenon known as tolerance—which poses a significant challenge to effective treatment. This development of tolerance not only complicates pain management but is also linked to heightened pain sensitivity, called hyperalgesia, which has been observed in both experimental studies and clinical settings following opioid administration10,11.
Clonidine, an α2-adrenergic receptor agonist, has anesthetic, sedative, anxiolytic, sympatholytic, and analgesic effects in the clinic12. It has been shown to have strong neuroprotective effects in various models of cerebral hypoxia-ischemia, where excessive extracellular glutamate accumulation plays a key pathological role13. These neuroprotective effects are mediated through α2-adrenergic receptors. Additionally, the activation of these receptors on the terminals of glutamatergic neurons inhibits glutamate release14. Therefore, the glutamatergic system and glutamate transporters may contribute to the neuroprotective and analgesic effects of clonidine15.
The transmission of pain signals from the periphery to the nucleus is glutamatergic16. Primary afferent neurons located in the dorsal root ganglia carry pain signals to the spinal cord. In the dorsal horn of the spinal cord, second-order neurons transmit these signals via the spinothalamic tract to the thalamus. The ventral posterolateral (VPL) nucleus of the thalamus processes these pain signals and relays them to the cerebral cortex17,18,19. High-affinity glutamate transporters (GTs) of the SLC1A family are responsible for the transmission of pain and noxious sensations from the periphery to the brain. The primary GTs involved in pain transmission are EAAT1 (GLAST), EAAT2 (GLT-1), and EAAT3 (EAAC1)20. These transporters are expressed in both neurons and glial cells and are responsible for the reuptake of glutamate from the synaptic cleft, thereby maintaining extracellular glutamate concentrations at nontoxic levels21. Inhibition or antisense downregulation of spinal GTs can trigger or exacerbate pain behaviors, whereas increasing the expression of GTs through viral gene transfer or positive pharmacological modulators can alleviate chronic pain22.
Glutamate, the predominant excitatory neurotransmitter, and glutamate receptors seem to be involved in the development of central hypersensitivity. Stimulation of NMDA receptors has been linked to pathological hyperalgesia and allodynia, suggesting that NMDA receptor antagonists are likely useful in the treatment of neuropathic pain. Increasing glutamate transporter 1 (GLT-1) activity, which reduces extracellular glutamate, may be an important target for pain management23. NMDA-type glutamate receptors are ligand-gated ion channels that mediate a significant component of excitatory neurotransmission in the central nervous system (CNS). They are widely distributed at all stages of development and play a critical role in normal brain functions, including neuronal development and synaptic plasticity24. The activation of NMDARs requires simultaneous binding by 2 different agonists, glutamate and glycine (Gly), which are therefore known as NMDAR coagonists25.
Although glutamate changes in persistent pain have been described, their importance is not fully evident. It is necessary to determine whether glutamate activity contributes to central mechanisms involved in the development of chronic pain. Maladaptive plasticity in the somatosensory cortex and associated pain networks may result in neuropathic pain. However, the molecular or cellular mechanisms underlying this maladaptive plasticity are not clear. In the present study, we focused on the somatosensory cortex hind limb (S1HL) and posterior intralaminar thalamic nucleus (PIL) to compare paclitaxel-induced allodynia after different drug treatments and investigated the impact of varying expression levels of glutamate transporters and enzymes (glutaminase (GLS) and glutamine synthetase (GluL)). A review of the literature reveals that numerous studies have been conducted on various regions of the CNS26. These studies are particularly concentrated in the primary somatosensory cortex (S1), which plays a key role in pain processing. The thalamus, on the other hand, serves as a critical relay station for the transmission of pain signals to the cortex. Given the lack of previous studies utilizing in vivo voltammetry—a technique that enables real-time measurement of neurotransmitters—in these brain regions, we selected them for our experimental recordings.
Recent studies have highlighted the critical role of glutamate dysregulation in the pathophysiology of neuropathic pain26. Among the key mechanisms involved, the regulation of glutamate transporter-1 (GLT-1), which is responsible for clearing extracellular glutamate in the central nervous system, has gained attention as a promising therapeutic target. Interestingly, the β-lactam antibiotic ceftriaxone has been shown to upregulate GLT-1 expression at the transcriptional level, thereby reducing synaptic glutamate accumulation. Through this mechanism, ceftriaxone may help attenuate glutamate-mediated excitotoxicity and neuroinflammation, offering potential analgesic effects, particularly in neuropathic and inflammatory pain conditions9,27.
In the present study, we evaluated the role of glutamate transporters and the time taken for their reuptake (via in vivo voltammetry), and differences in the expression levels of glutamate transporters and enzymes were assessed.
Materials and methods
Experimental animals
In the present study, thirty-six female Albino Wistar rats, obtained from Atatürk University Medical Experimental Research and Application Center, weighing between 200 and 220 g were used. The animals were housed and kept in a natural day‒night environment at a room temperature of 22 °C. The study was conducted in accordance with ethical guidelines and regulations and approved by the “Atatürk University Health Sciences Institute Directorate Ethics Committee” and the “Atatürk University Animal Experiments Local Ethics Committee (AUHADYEK)” under reference numbers B.30.2.ATA.0.01.05/00/759 and B.30.2.ATA.023.85-10, respectively. All experiments were performed in accordance with the guidelines of the Institute (Care and Use of Laboratory Animals Guide (8th edition, National Academies Press) and the Turkish directive on the protection of animals used for experimental and other scientific purposes (Ministry of Food, Agriculture and Livestock, Regulation on the Welfare and Protection of Animals Used for Experimental and Other Scientific Purposes, 28141). After the completion of the study, the animals were euthanized via an anesthetic agent (thiopental 100 mg/kg, i.p.) as recommended by the Turkish Ministry of Food, Agriculture and Forestry Regulation (TM-FAFR), 28141. All the experiments were performed according to the TM-FAFR and ARRIVE guidelines and regulations.
Chemicals
Paclitaxel (CAS number 33069-62-4), penicillin G (CAS number 113-98-4), ceftriaxone (CAS number 104376-79-6), clonidine (CAS number 4205-90-7), and morphine (CAS number 57-27-2) were obtained from Sigma‒Aldrich Corporation (St. Louis, Missouri, USA).
Study plan
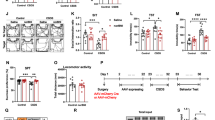
First, female rats were randomly divided into six groups. Baseline pain values were measured before paclitaxel was administered to these groups. Starting from day zero until day 6, with the exception of the saline group, the other five groups received 4 doses of paclitaxel intraperitoneally every other day. Between days 27 and 37, treatments with ceftriaxone, penicillin, clonidine, and morphine were administered. Between days 47 and 67, in vivo voltammetry measurements were conducted to determine the glutamate reuptake times in all the experimental groups. Rats that underwent voltammetry measurements between days 47 and 87 were decapitated, and their brain tissues were collected and marked for specific brain regions. mRNA isolation was subsequently performed from these brain regions, and the expression levels of the identified genes in all the tissues were measured. The data obtained from the tests were analyzed accordingly (Fig. 1).
Study plan. In this manner, the study plan and the tests and analyses conducted are summarized in an organized fashion. Throughout the research, pain threshold measurements were taken at specified intervals via the Randall‒Selitto method. The measurements and analyses indicated in the figure were carried out according to the chronological timeline provided.
Experimental groups and pain model
The experimental animals were divided into six groups of six animals in each group. Prior to paclitaxel injection (between 09:00 and 10:00 a.m.) to induce mechanical hyperalgesia and allodynia, baseline pain thresholds were determined from the hind paws of all the animals via the plantar Randall‒Selitto device to the analgesiometer test equipment (Ugo Basile, Italy) on different days. Mechanical hyperalgesia was measured using a Randall–Selitto plantar pressure device (Ugo Basile, Italy), which is a validated method for evaluating nociceptive thresholds in response to mechanical pressure stimuli. The stock drug (Paclitaxel®, 100 mg/17 ml) was subsequently diluted to 2 mg/mL. The experimental animals were administered 2 mg/kg paclitaxel intraperitoneally (i.p.) on days 0, 2, 4, and 6 to induce mechanical hyperalgesia and allodynia. Thus, all the animals received a total of 8 mg/kg paclitaxel. The animals that received paclitaxel were divided into five groups, of which four groups received 400,000 U/kg/day penicillin G28200 mg/kg/day ceftriaxone292.5 µg/kg/day clonidine, or 0.1 mg/kg/day morphine, whereas the control group received saline water i.p. The selected dosing of ceftriaxone (200 mg/kg/day, i.p., for 10 days) was based on previously published studies demonstrating that this dosage significantly upregulates GLT-1 expression in the CNS and yields analgesic effects in neuropathic pain models without inducing toxicity29. The mechanical hyperalgesia induced by paclitaxel was assessed via the plantar Randall Selitto (Ugo Basile, Italy) analgesiometer test equipment as described previously30. Prior to the test, the animals were allowed to adapt to the environmental conditions for 20–30 min to prevent potential sensitivity to painful stimuli in the hind paws due to test equipment pressure. All experiments in this study were performed exclusively on female Wistar rats, consistent with earlier paclitaxel-induced neuropathic pain models, which show higher reproducibility and stability in female animals31. Additionally, paclitaxel-induced neuropathy is notably more prevalent and severe in women, reflecting a higher translational value of female models3. We acknowledge that estrogen and progesterone can modulate glutamatergic neurotransmission and pain perception. However, to minimize variability, we used age- and weight-matched female rats and randomly assigned them to experimental groups to average out estrous cycle effects. To ensure accurate results, testing was not performed on consecutive days in the same group (Fig. 2A).
Our previous studies revealed that the most severe mechanical hyperalgesia and allodynia occur approximately 14 days after the first paclitaxel injection32,33,34. Hence, the animals were observed for 10 days (between the 30th and 40th days after the initiation of the study)28. Following the initiation of treatments (ceftriaxone, penicillin, clonidine, and morphine) on the 27th day, at least one test was conducted every week to monitor changes in pain thresholds in all four experimental groups (Fig. 1).
Microelectrode preparation and calibration
Microelectrode preparation and calibration were performed as described previously35,36.
Microelectrode placement
In vivo voltammetry recordings were conducted at the midpoint of the primer somatosensory cortex hind limb (S1HL) (AP + 2.6 mm, ML -1.92 mm, DV 1.6 mm from bregma) and posterior intralaminar thalamic nucleus (PIL) (AP + 2.4 mm, ML -5.8 mm, DV 4.6 mm from bregma) brain regions, following coordinates from the Rat Brain Atlas, Paxinos, 6th edition. The recording sites were determined, and the microelectrodes were fixed via a Stoelting Stereotaxic Instrument (Stoelting Co., Wood Dale, IL, USA) such that the desired brain region was recorded and received glutamate via the appropriate coordinates. The microelectrodes were calibrated in vitro35 to ensure accurate measurements of glutamate reuptake time.
In vivo recording (reuptake time (T80) measurement)
The rats were positioned in a stereotaxic frame and kept at a constant temperature of 37 °C via a heating pad. Microelectrodes were accurately positioned in the S1HL and PIL brain regions on the basis of coordinates from the stereotaxic atlas. Additionally, a miniature Ag/AgCl reference electrode was placed superficially on the cortex. The micropipette ends were connected to a Picospritzer III apparatus (Parker Hannifin, United States) to control the ejection volume, and 20 nL of 200 µmol/L glutamate was introduced into the synaptic cleft. The reuptake time (T80) was subsequently measured via the FAST-16 recording system, and glutamate reuptake was subsequently calculated36.
In vivo voltammetry data analysis
The microelectrode connected to the FAST16 recording system (Quanteon, Nicholasville, KY, USA) was used to measure the baseline glutamate (Glu) levels, the peak concentration of Glu (in µmol/L), and T80, which represents the time in seconds from the maximum peak increase to the 80% decay signal, indicating Glu clearance. The Glu signals measured in picoamperes (pAs) were converted to equivalent concentrations (µmol/L) via FAST16 system software following electrode calibrations. The T80 time obtained after the registration of all the groups was subsequently used for statistical comparisons between the groups35.
Tissue removal
Following the in vivo voltammetry recordings, the rats were euthanized via carbon dioxide (CO2) inhalation, and their brains were removed and preserved at -80 °C for subsequent study. The brain regions corresponding to S1HL and PIL were identified, carefully dissected, and removed for further analysis.
Gene expression analysis
Total RNA isolation
RNA isolation was performed from the S1HL and PIL brain regions, which were previously marked via a stereotaxic device and stored at − 80 °C. Approximately 5–10 mg of brain tissue was collected and placed into 2 cc tubes. To these tissues, 1 cc of QIAzol (Qiagen, Hilden, Germany) was added, and the samples were homogenized using a TissueLyser (Qiagen, Hilden, Germany) with mechanical shaking. Following homogenization, 200 µL of chloroform was added to the samples and incubated for 1 min. Subsequently, the homogenates were centrifuged at 12,000 × g for 15 min at 4 °C, and the supernatants were collected. RNA was isolated from the supernatants using the RNeasy Lipid Tissue Mini Kit 50 (Qiagen, Hilden, Germany) with the QIAcube system (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The RNA concentration was determined using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
cDNA synthesis
cDNA synthesis was carried out via the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. A LightCycler Nano real-time PCR system (Roche Diagnostics, Mannheim, Germany) was used for this process. The concentration of the synthesized cDNA was measured using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Relative quantification of gene expression
The expression levels of the Slc1a1 (EAAC1), Slc1a2 (GLT-1), Slc1a3 (GLAST), Slc1a6 (EAAT4), Gls (glutaminase), and GluL (glutamine synthetase, GS) genes were analyzed. TaqMan® probe-based technology was used for qPCR analysis using the LightCycler Nano real-time PCR system (Roche Diagnostics, Mannheim, Germany). The cDNA probes used for the analysis of various genes are listed in (Table 1).
The results obtained are expressed relative to the expression levels in the control animals. β-actin expression levels were used as the endogenous control for each brain region. The qPCRs were performed in triplicate for each target and reference gene via 2 µL of cDNA (300 ng), 1 µL of primer probe mix, and 10 µL of DNA probes master mix in a total volume of 20 µL per reaction. The tubes were subjected to heating at 50 °C for 2 min and 95 °C for 10 min, followed by 45 cycles of 15 s at 95 °C and 60 s at 60 °C. All the data obtained were expressed as proportional changes via the 2−ΔΔCt method36,37.
Statistical analyses
The data obtained are presented as the mean ± standard deviation (SD). Statistical analyses were performed via the SPSS 17.0 program. The normality of the data was tested via the Shapiro‒Wilks test. For normally distributed data, one-way ANOVA followed by the post hoc Tukey test was used, and a significance level of P < 0.05 was considered to indicate statistical significance.
Results
Chronic pain consequences
In this study, a total of thirty-six animals were assigned to each of the following groups: the control group, the paclitaxel group, and the four treatment groups.
Paclitaxel was administered intraperitoneally on days 0, 2, 4, and 6. Starting from the 27th day, different drug treatments were applied for a duration of 10 days. The efficacy of the drugs (ceftriaxone, penicillin, clonidine, and morphine) was assessed on the 1st (37th ), 10th (47th ), 30th (67th ), and 50th (87th ) days following the end of treatment. All measurements were recorded in grams. In Table 2, the average pain threshold values and statistical results obtained are given, while Fig. 2 illustrates the within-group pain threshold changes over time.
Pain threshold measurement values by day. To facilitate a better understanding of the pain threshold values presented in (Table 2), the changes in the pain threshold values at different time periods are presented in this figure. Paclitaxel was administered intraperitoneally on days 0, 2, 4, and 6. Beginning on day 27, treatments with ceftriaxone, penicillin, clonidine, or morphine were administered for 10 consecutive days.
The results shown in Fig. 2 clearly revealed that the pain levels in the paclitaxel-induced experimental animals were reduced after treatment with ceftriaxone. The analgesic effect of paclitaxel became evident 20 days after four doses were administered [F (5,30) = 172,49, p = 0,000]. Pain threshold tests were conducted on days 47, 67, and 87. The allodynic effect of paclitaxel persisted in all tests, and the analgesic effects of the drugs (ceftriaxone, penicillin, clonidine and morphine) continued on the 47th and 67th days when voltammetry studies were conducted [for the 47th and 67th days, respectively, F (5,30) = 36,79, p = 0,000; F (5,30) = 47,22, p = 0,000].
According to the results in Table 2, the analgesia measurements in the control group remained unchanged, whereas the pain threshold values in the five groups injected with paclitaxel decreased significantly [F (5,30) = 112,67, p = 0,000]. This decrease was most pronounced on the 37th day after the injection of paclitaxel [F (5,30) = 112,67, p = 0,000]. The administration of drugs during the treatment process was expected to return pain to the prepaclitaxel level. Table 2 indicates that pain sensitivity thresholds returned to prepaclitaxel levels after 10 days of drug (ceftriaxone, penicillin, clonidine, and morphine) treatment starting on day37 [F (5,30) = 36,79, p = 0,000]. On the 50th (87th) day after treatment, the pain threshold of the treatment groups returned to the values before treatment [F (5,30) = 149,08, p = 0,000].
Voltammetry results
Glutamate reuptake times were determined in the S1HL and PIL brain regions. These reuptake times were determined to be approximately 8 s in the paclitaxel group, where a pain model was created in the S1hl rat brain region, whereas this period was measured to be approximately 3 s in the control group. In the treatment groups, this reuptake time was significantly accelerated, although it did not decrease to the level of the control group (Fig. 3D). The reuptake times measured in the PIL brain region are shown in (Fig. 3E).
Reuptake times of brain regions (T80). (A) Sagittal section of the rat S1HL and PIL brain regions. (B) Schematic of the coronal section of the S1HL region, while (C) represents the PIL region. (D) and (E) depict the glutamate reuptake times (T80) obtained from in vivo voltammetry analyses of the S1HL and PIL brain regions. The uptake times are presented as the means ± standard deviations. To evaluate the results, one-way ANOVA with Tukey’s test was used, and p < 0.05 was considered to indicate statistical significance. (*) The results for the control group are provided, while (#) the results for the paclitaxel group are shown.
Real-time PCR
The gene expression levels of glutamate transporters and enzymes related to glutamate metabolism in the S1HL and PIL brain regions were assessed via the 2−ΔΔCt method (Fig. 2).
Paclitaxel significantly slowed glutamate reuptake in the S1HL region compared with that in the control group, with reuptake times of approximately 8 s in the S1HL region [F (5,87) = 25.27, p = 0,000] and approximately 5 s in the PIL region [F (5,82) = 7,97, p = 0,000]. However, treatment with these drugs (ceftriaxone, penicillin, clonidine, and morphine) significantly accelerated glutamate reuptake in the S1HL region. The reuptake time in the morphine-treated group was 3.67 s, whereas in the other treatment groups, it ranged between 5 and 6 s. In the PIL brain region, the reuptake time was approximately 4 s in all the treatment groups except for the clonidine treatment group. Interestingly, there was no significant difference in glutamate reuptake between the paclitaxel group and the other treatment groups in the PIL brain region.
Gene expression in all groups by brain region (S1HL, PIL). (A) SLC1A2 gene expression analysis of the GLT-1 glutamate transporter. (B) Results of SLC1A3 gene expression analysis for the GLAST glutamate transporter. (C) SLC1A1 gene expression analysis of the EAAC1 glutamate transporter. (D) SLC1A6 gene expression analysis of the EAAT4 glutamate transporter. (E) Glul gene expression analysis of the glutamine synthetase enzyme. (F) Results of Gls gene expression analysis for glutaminase. All the results are presented as the means +/- standard deviations (SDs) for the reuptake times (T80). One-way ANOVA with Tukey’s post hoc test was used for the analyses. The ANOVA results for the S1HL brain region were as follows: GLT-1, F (5,12) = 280,11, p = 0,000; GLAST, F (5,12) = 302,59, p = 0,000; EAAC1, F (5,12) = 1374,98, p = 0,000; EAAT4, F (5,12) = 546,59, p = 0,000; glutamine synthetase, F (5,12) = 290,23, p = 0,000; and glutaminase, F (5,12) = 115,51 p = 0,000. The PIL brain regions were as follows: GLT-1, F (5,12) = 78,67, p = 0,000; GLAST, F (5,12) = 158,96, p = 0,000; EAAC1, F (5,12) = 78,47, p = 0,000; EAAT4, F (5,12) = 151,07, p = 0,000; glutamine synthetase, F (5,12) = 239,38, p = 0,000; and glutaminase, F (5,12) = 24,47 p = 0,000. Differences were considered significant at * P < 0.05 compared with the control and # P < 0.05 compared with paclitaxel.
As shown in Fig. 4A, the expression of GLT-1 increased in the S1HL region due to the effect of paclitaxel, and the expression of GLT-1 in the S1HL region was more similar to that in the control group after treatment with clonidine, penicillin, or morphine. However, paclitaxel did not significantly alter GLT-1 expression in the PIL region, whereas penicillin and ceftriaxone administration increased these rates.
As shown in Fig. 4B, paclitaxel led to a decrease in GLAST gene expression in both the S1HL and PIL regions. Clonidine treatment did not significantly affect these regions. Ceftriaxone caused a significant increase (P < 0.05) in the S1HL region and partially reversed the decrease caused by paclitaxel in the PIL region toward control values. Morphine treatment reversed the changes in the expression of both the S1HL and PIL regions.
As shown in Fig. 4C, there was a significant (P < 0.05) decrease in EAAC1 glutamate transporter expression in the PIL region in the paclitaxel group compared with the control group, whereas no significant change was observed in the S1HL region. Compared with that in the paclitaxel group, the PIL brain region in the treatment groups was increased. There was no significant difference in these parameters between the clonidine treatment group and the other treatment groups (P < 0.05). In the S1HL brain region, a significant (P < 0.05) increase was observed in the ceftriaxone treatment group, whereas the penicillin and morphine treatment groups presented a significant decrease (P < 0.05).
As shown in Fig. 4D, the rate of change in EAAT4 expression in the S1HL brain region was approximately 2.5 times greater in the paclitaxel-treated group than in the control group. Clonidine, penicillin, and morphine treatments decreased these rates to the values of the control group. In the ceftriaxone-treated group, this percentage further increased up to 5-fold. In the PIL brain region, no significant difference was observed between the paclitaxel-treated group and the control group. This change increased approximately 1.7-fold in the penicillin and ceftriaxone treatment groups.
Figure 4E shows a significant (P < 0.05) increase in glutamine synthetase gene expression in both the S1HL and PIL brain regions due to the effect of paclitaxel. No significant changes were detected in the S1HL brain regions in the penicillin and ceftriaxone treatment groups. However, a significant (P < 0.05) decrease was observed in the clonidine and morphine treatment groups. In the PIL brain region, a significant (P < 0.05) increase was observed in the ceftriaxone-treated group compared with the paclitaxel-treated group, whereas a significant (P < 0.05) decrease was observed in the penicillin-treated group compared with the paclitaxel group.
Finally, as shown in Fig. 4F, paclitaxel reduced the rate of glutaminase expression in both regions (S1HL and PIL). However, penicillin, ceftriaxone, and morphine treatments abolished this proportional decrease.
Discussion
This study aimed to investigate the roles of glutamate transporters in the molecular mechanisms of chronic pain and the effects of drugs targeting this transport system for pain treatment. Neuropathic pain is caused by nerve damage that disrupts signal transmission. Various factors, such as diabetes, trauma, infections, or neurological disorders, are associated with neuropathy38. The relationship between pain and glutamate is crucial for the development of reasonable therapeutic approaches to manage neuropathic pain and chronic pain.
Glutamate plays a significant role in normal neuronal function, but increased levels of glutamate can be harmful. Extracellular glutamate levels are regulated by the glutamate transporters GLT1/EAAT2 located on astrocyte cell membranes. In chronic pain conditions, including neuropathic pain, downregulation of GLT1 and loss-of-function variants have been reported21.
In the present study, drugs that enhance glutamate removal from the extracellular environment were tested for their potential to accelerate the clearance of glutamate from the synaptic gap (Fig. 3). These drugs, which increase glutamate transporter activity, had positive clinical effects on Randall-Selitto pain tests, as they raised pain thresholds and approached normal levels, similar to those in the control group (Table 2). Functionally, the clearance time of glutamate from the synaptic gap (T80) was extended by at least 50% in the paclitaxel-treated groups (Fig. 3). This functional delay in glutamate clearance could contribute to the persistence of elevated glutamate levels in the extracellular environment, resulting in a decrease in pain threshold values (allodynia) in experimental animals approximately 20 days after paclitaxel administration (Table 2), as shown by the Randall‒Selitto test. These findings suggest that paclitaxel-induced allodynia is associated with increased levels of synaptic glutamate, which could be attributed to nerve damage contributing to neuropathic pain31.
β-lactam antibiotics, such as penicillin, increase the expression of the glutamate transporter GLT-1 in the central nervous system. An increase in GLT-1 reduces excitotoxicity by reducing synaptic glutamate levels and is effective in pain modulation9. However, this effect may take time; the increase in GLT-1 levels may have continued after treatment ended and may have become effective on day 47. Therefore, while the pain threshold was still low on day 37, a significant improvement may have been observed on day 47. Another possible explanation could be related to the pharmacological properties of penicillin. Penicillins do not readily cross the blood‒brain barrier; however, they may exert indirect central effects by modulating the expression of transporter proteins in brain endothelial and glial cells39. These indirect mechanisms typically require time to become effective, which could explain the delayed therapeutic response observed after the treatment period. Similarly, ceftriaxone, like penicillin, is a β-lactam antibiotic that can increase the expression of the glutamate transporter GLT-1 in the central nervous system, thereby reducing synaptic glutamate levels. However, the translation of this molecular effect into clinical analgesia may require time. In the case of prolonged morphine use, the development of opioid tolerance followed by rebound effects after drug discontinuation may transiently enhance the analgesic response (Fig. 2). On day 87, except for the penicillin-treated group, the pain thresholds decreased in the other treatment groups, including those receiving morphine, ceftriaxone, and clonidine. This decline may indicate the loss of the drugs’ therapeutic effects over time.
To understand the molecular mechanisms involved in the alleviation of neuropathic pain, we conducted gene expression analyses, which revealed that upregulation of the GLT-1 transporter in the somatosensory cortex (S1HL) and thalamic nuclei results in increased glutamate uptake by astrocytes. This results in an increase in glutamate levels in the intercellular fluid, resulting in an increase in the expression of glutamine synthetase and leading to a reduction in extracellular glutamate levels. As a consequence, increased glutamine is transported to presynaptic neurons. However, we observed a suppression of the conversion of glutamine back to glutamate, as evidenced by the decreased expression of the glutaminase enzyme (Fig. 4F) by paclitaxel in the S1HL region. Thus, paclitaxel appears to increase the expression of GLT-1 in the S1HL region, causing astrocytes to take up excess glutamate (Fig. 2A), with no significant change in the PIL region, which may aid in the conversion of excess glutamate to be taken up by astrocytes (Fig. 4E).
The glial glutamate transporter GLT1 plays a crucial role in neuropathic pain. Nerve damage is responsible for changes in glutamatergic transmission in the spinal cord and various supraspinal regions40. At sites of inflammation, the initial increase in GLT1 activity depends on the degree of injury and contributes to slowing the development of hyperalgesia41. However, this initial upregulation is followed by downregulation of GLT1, which promotes the facilitation of reduced network transmission as the injury persists, leading to persistent pain26. Studies revealed that GLT1 expression in the superficial dorsal horn was reduced, suggesting that targeted restoration of GLT1 expression may constitute a promising approach for the treatment of chronic neuropathic pain42suggesting that GLT-1 could serve as a potential therapeutic target for pain management42,43,44,45.
The anti-neuropathic pain action of ceftriaxone is associated with the upregulation of GLT1 in the spinal cord. Previous studies have demonstrated that repeated intraperitoneal (i.p.) injections of ceftriaxone (200 mg/kg) for five days significantly alleviate both mechanical and thermal pain hypersensitivity42. Ceftriaxone also relieves trigeminal neuropathic pain (TNP) by suppressing plasticity42. In the present study, an increase in the expression of these genes (GLT-1, GLAST, EAAC1 and EAAT4) led to increased expression of all the analyzed GLT-1 glutamate transporters, with a 4.5-fold increase in expression; GLAST, with a 1.5-fold increase; EAAC1, with a 1.6-fold increase; and EAAT4, with an approximately 5-fold increase, compared with those in the control group (Fig. 4A). These increases were more pronounced in the ceftriaxone-treated group than in the other treatment groups. In the ceftriaxone group, excess glutamate induced an approximately 2-fold increase in the expression of GS, which resulted in increased glutamine levels in presynaptic neurons, leading to a 1.3-fold increase in glutaminase expression. Thus, ceftriaxone treatment increases the levels of all glutamate transporters and the levels of glutamine synthetase and glutaminase in a glutamate-dependent manner.
In the present study, all the treatments were administered to the experimental groups for a duration of 10 days (27–37 days). In the clonidine treatment group, the pain threshold decreased to 42 g on the 27th day and decreased to 30 g on the 37th day. The beneficial effect of clonidine was significant only 20 days after the start of treatment, specifically on the 47th day. However, this therapeutic effect of the drug completely disappeared in the other treatment groups, and the pain threshold decreased again (Table 2). On the 87th day, pain persisted in the paclitaxel group. We propose that clonidine directly reduces the effect of glutaminase by increasing the production and release of glutamate, as its generation decreases in neurons. Owing to the decreased glutamate level in the synaptic cleft, GLT-1 expression decreases; thus, the expression of glutamine synthetase is likely to decrease. Similarly, the levels of the glutamate transporter EAAT4, which is predominantly located in postsynaptic neurons46are decreased 3-fold (Fig. 4D) in response to paclitaxel. GLT-1 expression is increased in the S1HL region, and astrocytes effectively take up excess glutamate (Fig. 4A), with no change in the PIL region in response to paclitaxel treatment.
GLAST, the astrocytic glutamate transporter47has been implicated in the regulation of excitatory synaptic activity and nociceptive responses in inflammation-induced paw pain48. Decreased GLAST expression has been associated with reduced CSF glutamate concentrations and antinociceptive effects48. In the present study, paclitaxel administration led to a decrease in the GLAST gene expression rate. However, compared with the control, ceftriaxone treatment increased this expression rate to normal levels and elevated it by 50% in the S1HL brain region. While the GLAST gene expression rates reached normal levels in the penicillin and morphine treatment groups, the expression levels did not reach normal levels in the clonidine treatment group.
Additionally, excessive glutamate in the S1HL region might contribute to a 3-fold increase in the expression level of the postsynaptic glutamate transporter EAAT4, potentially accelerating postsynaptic transmission. We propose that the effect of EAAT4 on pain transmission could be related to glutamate release into postsynaptic neurons. According to the voltammetry analysis results, the synaptic clearance time (T80) for glutamate in the S1HL region was 3.38 s in the control group and 8.18 s in the paclitaxel group, indicating a significant delay. This could be a key factor in the development of neuropathic pain caused by paclitaxel, which returned to near-normal values in the treatment groups.
In the present study, penicillin had no effect on glutamate levels but increased the expression of glutamine synthetase and decreased the expression of glutaminase (Fig. 4D,E). As a result, synaptic glutamate levels are reduced to physiological levels, leading to decreased expression of GLT-1, GLAST, EAAC1, and EAAT4 (during the pathological study conducted between the 47th and 67th days).
The effectiveness of morphine treatment was evident, as it restored the synaptic clearance time of glutamate to physiological levels (Fig. 3). The expression levels of excitatory amino acid transporters were also found to be at the physiological level. Morphine treatment successfully reversed the abnormalities caused by paclitaxel, reverting them to normal. According to the voltammetry results, the glutamate clearance time, which was 8.18 ± 1.33 s after paclitaxel administration, decreased to 3.67 ± 0.82 s, which was close to the physiological level following morphine treatment. Although morphine may not directly affect the glutamatergic system, it appears to indirectly influence it. The reduction in glutamate activity following morphine treatment may have contributed to pain alleviation. Morphine exerts its pain-relieving effects, at least in part, through the Sigma-1 receptor, which interacts with other targets, such as the NMDA receptor49. Thus, morphine may decrease presynaptic glutamate release, leading to reduced glutamate transport needs and a return to normal clearance times that contribute to its pain-relieving action50.
Glutaminase converts glutamine to glutamate51. In neuropathic pain, elevated glutamate levels are observed in the synaptic cleft52. Wang et al.. (2016) reported that glutaminase-1 (GLS1) expression is significantly elevated in the CPSP group compared with the non-CPSP group53. Binns et al.. reported that spinal nerve ligation (SNL) led to reduced glutamate uptake activity extending into the deep dorsal and ventral horns54.
In the present study, we observed that ceftriaxone increased the pain threshold by increasing the reuptake time (T80) in the S1HL cortex region, which upregulated the gene expression of glutamate transporters and related enzymes in astrocytes, especially in the S1HL region. Interestingly, in the ceftriaxone treatment group, the expression of GLT-1, the primary glutamate transporter, was increased compared with that in the paclitaxel group. This increase in gene expression corresponded to the restoration of the reuptake time. These findings support the findings of previous studies36suggesting that ceftriaxone could be employed as a new therapeutic strategy to alleviate glutamate-induced pain. However, the long-term use of ceftriaxone, particularly for nonantibacterial indications, carries significant risks, such as potential adverse effects and the development of antibiotic resistance. Therefore, its application in the treatment of chemotherapy-induced neuropathic pain should be carefully evaluated. Although its ability to enhance GLT-1 expression presents a potential therapeutic advantage, a cautious approach should be adopted in clinical settings, considering the lack of long-term safety data and the importance of a thorough benefit-risk assessment.
In conclusion, the effects of different pharmacological agents on the glutamate transport system were investigated at both the molecular and behavioral levels in this study, and the central role of GLT-1 in the regulation of neuropathic pain was revealed. The significant therapeutic effect of Ceftriaxone supports the evaluation of this transporter as a potential target. However, the limitations of long-term antibiotic use and the limited efficacy of other agents reveal the need to develop new and specific GLT-1 modulators. Future studies to confirm these mechanisms in human-based models and clinical settings are important.
Limitations and future directions
While the study comprehensively integrates functional and transcriptional data, key limitations include the reliance on mRNA levels without protein confirmation (e.g., via Western blot or IHC) and the lack of causal evidence linking transporter expression directly to pain reversal. Hence, additional studies are warranted in this direction.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Treede, R. D. et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70, 1630–1635. https://doi.org/10.1212/01.wnl.0000282763.29778.59 (2008).
Tanabe, Y. et al. Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int. J. Clin. Oncol. 18, 132–138. https://doi.org/10.1007/s10147-011-0352-x (2013).
da Costa, R. et al. Taxane-induced neurotoxicity: pathophysiology and therapeutic perspectives. Br. J. Pharmacol. 177, 3127–3146. https://doi.org/10.1111/bph.15086 (2020).
Ziske, C. G. et al. Acute transient encephalopathy after Paclitaxel infusion: report of three cases. Annals Oncology: Official J. Eur. Soc. Med. Oncol. 13, 629–631. https://doi.org/10.1093/annonc/mdf025 (2002).
Thornton, L. M., Carson, W. E. 3, Shapiro, C. L., Farrar, W. B., Andersen, B. L. & rd, & Delayed emotional recovery after taxane-based chemotherapy. Cancer 113, 638–647. https://doi.org/10.1002/cncr.23589 (2008).
Fellner, S. et al. Transport of Paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Investig. 110, 1309–1318. https://doi.org/10.1172/jci15451 (2002).
Lange, M. et al. Decline in cognitive function in older adults with Early-Stage breast Cancer after adjuvant treatment. Oncologist 21, 1337–1348. https://doi.org/10.1634/theOncologist.2016-0014 (2016).
Nicholson, K. J., Gilliland, T. M. & Winkelstein, B. A. Upregulation of GLT-1 by treatment with ceftriaxone alleviates radicular pain by reducing spinal astrocyte activation and neuronal hyperexcitability. J. Neurosci. Res. 92, 116–129. https://doi.org/10.1002/jnr.23295 (2014).
Rothstein, J. D. et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433, 73–77. https://doi.org/10.1038/nature03180 (2005).
Célèrier, E., Laulin, J. P., Corcuff, J. B., Le Moal, M. & Simonnet, G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J. Neuroscience: Official J. Soc. Neurosci. 21, 4074–4080. https://doi.org/10.1523/jneurosci.21-11-04074.2001 (2001).
Mao, J., Sung, B., Ji, R. R. & Lim, G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J. Neuroscience: Official J. Soc. Neurosci. 22, 8312–8323. https://doi.org/10.1523/jneurosci.22-18-08312.2002 (2002).
Kamibayashi, T. & Maze, M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology 93, 1345–1349. https://doi.org/10.1097/00000542-200011000-00030 (2000).
Laudenbach, V. et al. Effects of alpha(2)-adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and Dexmedetomidine. Anesthesiology 96, 134–141. https://doi.org/10.1097/00000542-200201000-00026 (2002).
Kamisaki, Y., Hamahashi, T., Okada, C. M. & Itoh, T. Clonidine Inhibition of potassium-evoked release of glutamate and aspartate from rat cortical synaptosomes. Brain Res. 568, 193–198. https://doi.org/10.1016/0006-8993(91)91397-j (1991).
Woo, J. H., Han, J. I., Baik, H. J. & Lee, H. Effects of clonidine on the activity of the rat glutamate transporter EAAT3 expressed in Xenopus oocytes. Korean J. Anesthesiol. 62, 266–271. https://doi.org/10.4097/kjae.2012.62.3.266 (2012).
Ang, S. T., Ariffin, M. Z. & Khanna, S. The forebrain medial septal region and nociception. Neurobiol. Learn. Mem. 138, 238–251. https://doi.org/10.1016/j.nlm.2016.07.017 (2017).
Hudson, A. J. Pain perception and response: central nervous system mechanisms. Can. J. Neurol. Sci. 27, 2–16. https://doi.org/10.1017/s0317167100051908 (2000).
Palkovits, M. [The brain and the pain: neurotransmitters and neuronal pathways of pain perception and response]. Orv Hetil. 141, 2231–2239 (2000).
Millan, M. J. The induction of pain: an integrative review. Prog Neurobiol. 57, 1–164. https://doi.org/10.1016/s0301-0082(98)00048-3 (1999).
Malik, A. R. & Willnow, T. E. Excitatory amino acid transporters in physiology and disorders of the central nervous system. Int. J. Mol. Sci. 20 https://doi.org/10.3390/ijms20225671 (2019).
Sung, B., Lim, G. & Mao, J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J. Neuroscience: Official J. Soc. Neurosci. 23, 2899–2910. https://doi.org/10.1523/jneurosci.23-07-02899.2003 (2003).
Gegelashvili, G. & Bjerrum, O. J. High-affinity glutamate transporters in chronic pain: an emerging therapeutic target. J. Neurochem. 131, 712–730. https://doi.org/10.1111/jnc.12957 (2014).
Stephens, R. L. Jr. Glutamate transporter activators as anti-nociceptive agents. Eurasian J. Med. 43, 182–185. https://doi.org/10.5152/eajm.2011.39 (2011).
Hansen, K. B., Yi, F., Perszyk, R. E., Menniti, F. S. & Traynelis, S. F. NMDA receptors in the central nervous system. Methods Mol. Biology (Clifton N J). 1677, 1–80. https://doi.org/10.1007/978-1-4939-7321-7_1 (2017).
Flores-Soto, M. E. et al. [Structure and function of NMDA-type glutamate receptor subunits]. Neurologia 27, 301–310. https://doi.org/10.1016/j.nrl.2011.10.014 (2012).
Temmermand, R., Barrett, J. E. & Fontana, A. C. K. Glutamatergic systems in neuropathic pain and emerging non-opioid therapies. Pharmacol. Res. 185, 106492. https://doi.org/10.1016/j.phrs.2022.106492 (2022).
Khan, A. J., Husain, Q., Choudhuri, G. & Parmar, D. Association of polymorphism in alcohol dehydrogenase and interaction with other genetic risk factors with alcoholic liver cirrhosis. Drug Alcohol Depend. 109, 190–197. https://doi.org/10.1016/j.drugalcdep.2010.01.010 (2010).
Gepdiremen, A., Hacimüftüoglu, A., Düzenli, S., Oztaş, S. & Süleyman, H. Effects of Salicylic acid in glutamate- and Kainic acid-induced neurotoxicity in cerebellar granular cell culture of rats. Pharmacol. Res. 42, 547–551. https://doi.org/10.1006/phrs.2000.0717 (2000).
Ramandi, D., Elahdadi Salmani, M., Moghimi, A., Lashkarbolouki, T. & Fereidoni, M. Pharmacological upregulation of GLT-1 alleviates the cognitive impairments in the animal model of Temporal lobe epilepsy. PLoS One. 16, e0246068. https://doi.org/10.1371/journal.pone.0246068 (2021).
Pereira-Silva, R., Teixeira-Pinto, A., Neto, F. L. & Martins, I. µ-Opioid receptor activation at the dorsal reticular nucleus shifts diffuse noxious inhibitory controls to hyperalgesia in chronic joint pain in male rats. Anesthesiology 140, 1176–1191. https://doi.org/10.1097/aln.0000000000004956 (2024).
Meacham, K., Shepherd, A., Mohapatra, D. P. & Haroutounian, S. Neuropathic pain: central vs. Peripheral mechanisms. Curr. Pain Headache Rep. 21, 28. https://doi.org/10.1007/s11916-017-0629-5 (2017).
Okkay, U. et al. Achillea millefolium alleviates testicular damage in paclitaxel-intoxicated rats via Attenuation of testicular oxido-inflammatory stress and apoptotic responses. Andrologia 53, e14028. https://doi.org/10.1111/and.14028 (2021).
Hacimuftuoglu, A. et al. The analgesic effect of Metformin on paclitaxel-induced neuropathic pain model in rats: by considering pathological results. J. Cancer Res. Ther. 16, 34–39. https://doi.org/10.4103/jcrt.JCRT_1455_16 (2020).
Cetin, D., Hacımuftuoglu, A., Tatar, A., Turkez, H. & Togar, B. The in vitro protective effect of Salicylic acid against Paclitaxel and cisplatin-induced neurotoxicity. Cytotechnology 68, 1361–1367. https://doi.org/10.1007/s10616-015-9896-3 (2016).
Hacimuftuoglu, A. et al. Astrocyte/neuron ratio and its importance on glutamate toxicity: an in vitro voltammetric study. Cytotechnology 68, 1425–1433. https://doi.org/10.1007/s10616-015-9902-9 (2016).
Taspinar, N. et al. Differential effects of inhibitors of PTZ-induced kindling on glutamate transporters and enzyme expression. Clin. Exp. Pharmacol. Physiol. 48, 1662–1673. https://doi.org/10.1111/1440-1681.13575 (2021).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego Calif). 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Finnerup, N. B., Kuner, R. & Jensen, T. S. Neuropathic pain: from mechanisms to treatment. Physiol. Rev. 101, 259–301. https://doi.org/10.1152/physrev.00045.2019 (2021).
Haddad, N. et al. The Blood–Brain barrier and pharmacokinetic/pharmacodynamic optimization of antibiotics for the treatment of central nervous system infections in adults. Antibiotics 11, 1843 (2022).
Zhao, Z., Hiraoka, Y., Ogawa, H. & Tanaka, K. Region-specific deletions of the glutamate transporter GLT1 differentially affect nerve injury-induced neuropathic pain in mice. Glia 66, 1988–1998. https://doi.org/10.1002/glia.23452 (2018).
Guo, W. et al. Altered glial glutamate transporter expression in descending circuitry and the emergence of pain chronicity. Mol. Pain. 15, 1744806918825044. https://doi.org/10.1177/1744806918825044 (2019).
Luo, X. et al. Ceftriaxone relieves trigeminal neuropathic pain through suppression of Spatiotemporal synaptic plasticity via restoration of glutamate transporter 1 in the medullary dorsal Horn. Front. Cell. Neurosci. 14, 199. https://doi.org/10.3389/fncel.2020.00199 (2020).
Alotaibi, G. & Rahman, S. Effects of glial glutamate transporter activator in formalin-induced pain behaviour in mice. Eur. J. Pain. 23, 765–783. https://doi.org/10.1002/ejp.1343 (2019).
Nozad, A., Hamidi, N. & Amani, M. The role of glutamate transporter-1 in firing activity of locus coeruleus neurons and nociception in rats. Exp. Brain Res. 239, 1287–1294. https://doi.org/10.1007/s00221-021-06065-0 (2021).
Putatunda, R., Hala, T. J., Chin, J. & Lepore, A. C. Chronic at-level thermal hyperalgesia following rat cervical contusion spinal cord injury is accompanied by neuronal and astrocyte activation and loss of the astrocyte glutamate transporter, GLT1, in superficial dorsal Horn. Brain Res. 1581, 64–79. https://doi.org/10.1016/j.brainres.2014.05.003 (2014).
Nakagawa, T. & Kaneko, S. SLC1 glutamate transporters and diseases: psychiatric diseases and pathological pain. Curr. Mol. Pharmacol. 6, 66–73. https://doi.org/10.2174/18744672113069990033 (2013).
Unger, T., Lakowa, N., Bette, S. & Engele, J. Transcriptional regulation of the GLAST/EAAT-1 gene in rat and man. Cell. Mol. Neurobiol. 32, 539–547. https://doi.org/10.1007/s10571-011-9790-2 (2012).
Niederberger, E. et al. The glutamate transporter GLAST is involved in spinal nociceptive processing. Biochem. Biophys. Res. Commun. 346, 393–399. https://doi.org/10.1016/j.bbrc.2006.05.163 (2006).
Fu, K. et al. 2,6-diazaspiro[3.4]octan-7-one derivatives as potent sigma-1 receptor antagonists that enhanced the antinociceptive effect of morphine and rescued morphine tolerance. Eur. J. Med. Chem. 249, 115178. https://doi.org/10.1016/j.ejmech.2023.115178 (2023).
Sánchez-Fernández, C. et al. Potentiation of morphine-induced mechanical antinociception by σ₁ receptor inhibition: role of peripheral σ₁ receptors. Neuropharmacology 70, 348–358. https://doi.org/10.1016/j.neuropharm.2013.03.002 (2013).
Song, M., Kim, S. H., Im, C. Y. & Hwang, H. J. Recent development of small molecule glutaminase inhibitors. Curr. Top. Med. Chem. 18, 432–443. https://doi.org/10.2174/1568026618666180525100830 (2018).
Chen, S., Kadakia, F. & Davidson, S. Group II metabotropic glutamate receptor expressing neurons in anterior cingulate cortex become sensitized after inflammatory and neuropathic pain. Mol. Pain. 16, 1744806920915339. https://doi.org/10.1177/1744806920915339 (2020).
Wang, H. et al. Glutaminase 1 is a potential biomarker for chronic post-surgical pain in the rat dorsal spinal cord using differential proteomics. Amino Acids. 48, 337–348. https://doi.org/10.1007/s00726-015-2085-z (2016).
Binns, B. C., Huang, Y., Goettl, V. M., Hackshaw, K. V. & Stephens, R. L. Glutamate uptake is attenuated in spinal deep dorsal and ventral Horn in the rat spinal nerve ligation model. Brain Res. 1041, 38–47. https://doi.org/10.1016/j.brainres.2005.01.088 (2005).
Author information
Authors and Affiliations
Contributions
N.T: Data curation, Investigation, Conceptualization, Formal analysis, Visualization, Writing—original draft, Writing—review & editing, Supervision. A.H: Formal analysis, Writing—original draft, Writing—review & editing, Supervision. D.B: Formal analysis, Visualization. E.F.K: Formal analysis. N.G.B: Formal analysis. H.B: Formal analysis. A.M.A: formal analysis, validation, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Approval for the study was obtained from the “Atatürk University Health Sciences Institute Directorate Ethics Committee” and the “Atatürk University Animal Experiments Local Ethics Committee (AUHADYEK)” under reference numbers B.30.2.ATA.0.01.05/00/759 and B.30.2.ATA.023.85-10, respectively.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Taspinar, N., Hacimuftuoglu, A., Binnetoglu, D. et al. Ceftriaxone suppresses the severity of paclitaxel-induced glutamate-mediated chronic pain in experimental animals. Sci Rep 15, 34487 (2025). https://doi.org/10.1038/s41598-025-08119-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08119-7