Abstract
Di(2-ethylhexyl) phthalate (DEHP), renowned for its efficacy in enhancing the pliability of plastic products, serves predominantly as a plasticizer and additive in the plastics industry. A growing body of research in both animal models and human populations has elucidated that the maternal environment prior to birth exerts a significant influence on the growth and development of offspring. Consequently, our research is designed to investigate the effects of prenatal DEHP exposure on the pubertal reproductive development of female rat offspring and to decipher the underlying mechanisms. This study utilized Wistar rats as the experimental animal model, with exposure to varying doses of DEHP during pregnancy for group allocation and treatment. Transmission electron microscopy was used to examine ultrastructural changes in the hypothalamus of DEHP-exposed female offspring rats. High-performance liquid chromatography (HPLC) was employed to quantitatively analyze the amino acid levels in the hypothalamus of the female offspring. Enzyme-linked immunosorbent assay (ELISA) was used to quantitatively measure hormone levels in both the hypothalamus and peripheral blood. Immunohistochemistry was applied to quantify the expression of ERα and GnRH in the hypothalamus. Additionally, precise immunofluorescence analysis was conducted to assess the expression levels of IGF-1 and NKB in the rat hypothalamus. Spearman correlation analysis was employed to elucidate the associations between key factors in the hypothalamus. Our findings reveal that prenatal exposure to Di(2-ethylhexyl) phthalate (DEHP) precipitates the onset of puberty in female rat offspring, concurrently altering the expression of key puberty-regulating genes in the hypothalamus. This elucidation sheds light on the molecular mechanisms underpinning the role of hypothalamic ERα in modulating the IGF-1 and NKB pathways, contributing to DEHP-induced precocious puberty in females, and underscores the critical regulatory function of IGF-1/NKB crosstalk in this phenomenon. Consequently, our research posits that prenatal exposure to DEHP is likely transmitted from the mother to the embryo, precipitating developmental anomalies and the onset of precocious puberty in offspring. This underscores the significance of pregnancy as a pivotal period for endocrine disruption, offering a novel theoretical foundation for the prophylaxis and management of precocious puberty in female children.
Similar content being viewed by others
Introduction
Endocrine disrupting chemicals (EDCs) represent a diverse array of exogenous chemical agents or mixtures, which are known to interfere with the natural hormonal activities within the body, including the synthesis, secretion, transport, metabolism, and receptor binding of endogenous hormones1. Di-(2-ethylhexyl) phthalate (DEHP), a prevalent endocrine-disrupting chemical, is primarily employed as a plasticizer and additive in plastic products to enhance their flexibility. Notably, metabolic byproducts of phthalate compounds, including DEHP, have been detected in diverse population groups, encompassing both children and pregnant women2,3.
Individuals are susceptible to exposure to DEHP at all stages of their lifecycle, encompassing the critical intrauterine developmental period. The pathways of exposure are diverse, ranging from ingestion, inhalation, to dermal contact, with oral intake being the predominant route4. Exposure to DEHP has been implicated in inducing a spectrum of reproductive disturbances in female animals, including ovarian dysfunction, endometrial ectopia, and a discernible reduction in fertility rates5. Extensive research involving both animal models and human cohorts has established a significant impact of the prenatal maternal environment on offspring’s growth and development. Exposure to DEHP has been closely associated with an increased incidence of obesity, type 2 diabetes, and insulin resistance in adult offspring6,7,8. Prenatal exposure to DEHP has been shown to result in significant growth restriction in neonatal rat offspring9. A cohort study conducted in Borody revealed that prenatal exposure to phthalates in women is associated with the onset of lipid metabolism disorders in children post-birth3.
The central nervous system (CNS) constitutes a primary target for the action of EDCs, with exposure during pivotal developmental windows of the CNS potentially leading to significant disruptions in neural development10,11. The span from the embryonic stage through to adolescence represents a critical developmental window for the nervous system, wherein the brain exhibits heightened sensitivity to external environmental factors. Consequently, even low-level exposure to DEHP during this period can pose significant risks to neural development12,13. In an epidemiological study led by Akhgar Ghassabian, involving 775 mother-child dyads, it was discerned that prenatal exposure to phthalates is associated with a reduction in white matter volume in the brains of female children, subsequently leading to cognitive impairments2.
Exposure to EDCs during gestation and lactation periods has been found to precipitate the onset of puberty in female rat offspring. This exposure disrupts the epigenetic, molecular, and cellular architecture of the GnRH neuronal network in the hypothalamus, leading to enduring alterations in the reproductive axis and contributing to imbalances in female reproductive endocrinology14,15,16. Studies have elucidated that the IGF-1 and NKB signaling pathways are pivotal in orchestrating the endocrine activities of puberty. In pituitary cells, a synergistic action is observed between IGF-1 and NKB in upregulating NK3R expression, with a concomitant increase in NK3R mRNA levels in response to heightened IGF-1. Furthermore, the interplay between IGF-1 and NKB signaling systems plays a significant role in stimulating the secretion of growth prolactin from the pituitary gland17. Exposure to DEHP has been observed to expedite the timing of vaginal opening in adolescent female rats, concurrently leading to an upregulation in the expression of IGF-1, Kiss-1, GPR54, and GnRH in the hypothalamus18,19,20. Consequently, the possibility that DEHP induces precocious puberty in female offspring via interference with the IGF-1 and NKB signaling pathways necessitates more comprehensive and in-depth investigation to unravel the underlying molecular mechanisms.
Maternal exposure to environmental pollutants or aberrations in nutritional status, resulting in systemic physiological damage, may have transgenerational effects, potentially being transmitted to the embryo and leading to developmental anomalies in the offspring. This research entails a comprehensive observation of the impact of prenatal exposure to DEHP on puberty initiation markers in female rat offspring and establishes an induced model of female precocious puberty. It aims to elucidate the disruptive influence of DEHP on the initiation of female sexual development and its underlying molecular mechanisms, thereby offering novel perspectives and targets for the prevention and treatment of precocious puberty in female children.
Method and materials
Animal model
The experimental animals (Pregnant Wistar Rats) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The rats were maintained with free access to food and water at 25 °C and 60% humidity. The experiment was conducted at the Jilin University Laboratory Animal Centre (SYXK (JI) 2021-0006), supervised by the Animal Ethics and Welfare Committee of Jilin University (IACUC), with Ethics Approval No. 201,802,019. The experiment adhered to ARRIVE guidelines and the ethical guidelines of Jilin University and national regulations regarding the welfare of experimental animals, ensuring that the animals had access to water and food. The study adhered to the principles of the Helsinki Declaration.
Although a total of 60 pregnant Wistar rats were initially acquired for this study, only 48 met the inclusion criteria based on standardized assessments of body weight and overall health status. Following stratified randomization by body weight, these 48 rats were evenly assigned to four groups (n = 12 per group): a vehicle control group (corn oil) and three DEHP treatment groups (0.2, 1, and 5 mg/kg/day). The exclusion of 12 rats was due to significant deviations in baseline characteristics, which could have introduced confounding effects. This clarification ensures transparency and accurate interpretation of group sample sizes used in the experimental analyses.
In this study, the standard rodent mating protocol was employed to determine gestational day 0 (G0). A female rat was housed overnight with a male, and the presence of a vaginal plug the following morning was examined. The detection of a vaginal plug is widely accepted as an indication of successful mating. Alternatively, the day was also recorded as G0 if spermatozoa were identified in the vaginal smear. This approach is commonly used in reproductive research involving rodents and provides a reliable marker for the onset of pregnancy.
From gestational day 0 (G0) to day 21 (G21), the pregnant rats underwent a consistent regimen of oral gavage dosing. The day following the birth of the offspring was designated as postnatal day 1 (PND1), during which fundamental observations and recordings were made. On postnatal day 21, the rat offspring were weaned, following which 20 female rats from each dosing group were selected for continued breeding in separate cages. The timing of vaginal opening in the offspring was monitored daily. At the age of 60 days, the rats were humanely euthanized, blood samples were collected, and their hypothalamuses were excised for further analysis (Fig. 1).
Animals were anesthetized with an overdose of isoflurane administered via inhalation until deep anesthesia was confirmed. Following anesthesia, euthanasia was performed by cervical dislocation to ensure immediate cessation of the heart and respiratory functions. This procedure was conducted with utmost care to ensure that the animals did not experience pain or distress. After euthanasia, the animals were monitored to confirm complete cessation of vital signs, and were immediately disposed of according to institutional guidelines for the disposal of biological waste.
Briefly, following euthanasia, the brains of the female offspring were rapidly removed and fixed in 4% paraformaldehyde at 4 °C for 24–48 h. After fixation, the brains were embedded in paraffin and coronally sectioned using a microtome. The hypothalamic region was identified based on the rat brain atlas (Paxinos and Watson), and 5 μm-thick sections were collected for subsequent histological and immunohistochemical analysis.
To better observe the toxic effects and mechanisms, the high-dose group of DEHP in this study was set at the highest exposure level in humans (5 mg/kg/day), with a fivefold interval between dose groups.
ERα and IGF-1/NKB downstream signaling in vivo intervention experiment
To elucidate the regulatory role and crosstalk mechanisms between ERα-mediated IGF-1 and NKB signaling pathways in DEHP-induced precocious puberty in female offspring, we selected 80 female Wistar rats exposed to DEHP, which were randomly divided into four groups: those receiving specific inhibitors for ERα, IGF-1R, and NK3R. Additionally, 20 normal female offspring rats were selected as a control group for in vivo intervention experiments. Utilizing a stereotaxic instrument, we precisely located the hypothalamus at the following coordinates: horizontal plane at −5 cm, coronal plane at 2.8 cm, and sagittal plane at 5.9 cm. This facilitated the accurate implantation of microinjection cannulas. Daily injections were administered, consisting of 5 µL of 10 mmol/L ERα inhibitor (Tamoxifen Citrate), 16 mmol/L IGF-1R inhibitor (BMS-754807), and 0.36 mmol/mL NK3R inhibitor (Pavinetant) (MCE, USA). The control group received an equivalent volume of saline solution. These injections were started on postnatal day 21 (PND21) in female offspring rats, immediately following weaning, and continued until the subjects reached 60 days of age, at which point they were euthanized. Animals were anesthetized with an overdose of isoflurane administered via inhalation until deep anesthesia was confirmed. Following anesthesia, euthanasia was performed by cervical dislocation to ensure immediate cessation of the heart and respiratory functions. This procedure was conducted with utmost care to ensure that the animals did not experience pain or distress. After euthanasia, the animals were monitored to confirm complete cessation of vital signs, and were immediately disposed of according to institutional guidelines for the disposal of biological waste.
Identification of the onset of sexual development in female rats
The vaginal opening in female rats is commonly used as an external indicator of the initiation of sexual maturation. In this study, the detection of vaginal opening was performed under adequate lighting conditions. During the procedure, the researcher gently restrained the female offspring and lifted the tail to visually examine the external genitalia. This method relies on the careful observation of whether the vaginal orifice has become patent and is widely recognized as a reliable external indicator of sexual maturation in rodents. Daily inspections were conducted during the expected period of vaginal opening, and the day when a clear opening was first observed was recorded as the onset of vaginal opening.
Ultrastructure of hypothalamus
The hypothalamic tissues of female offspring rats were collected immediately after euthanasia and processed for transmission electron microscopy (TEM) analysis following the steps below. Fixation: The hypothalamic tissues were rapidly dissected and placed in 0.1 M phosphate buffer (pH 7.4) at 4 °C. The samples were fixed in 2.5% glutaraldehyde for 12–24 h, rinsed with the same buffer, and subsequently post-fixed in 1% osmium tetroxide at room temperature for 1–2 h. Dehydration and Embedding: The samples were dehydrated through a graded ethanol series, cleared in propylene oxide, and infiltrated with epoxy resin. Polymerization was carried out at 60 °C for 48 h. Sectioning and Staining: Ultrathin sections approximately 70 nm in thickness were prepared using an ultramicrotome, mounted on copper grids, and stained sequentially with 2% uranyl acetate and lead citrate. Observation and Analysis: The sections were examined using a transmission electron microscope at an accelerating voltage of 80 kV. Ultrastructural features such as nuclear morphology, chromatin distribution, endoplasmic reticulum density, mitochondrial integrity, and lipofuscin accumulation were meticulously documented. Particular attention was given to DEHP-induced cytopathological alterations potentially associated with prenatal exposure, including nuclear condensation, cell shrinkage, mitochondrial swelling, and increased pericellular microglia. Structural changes related to DEHP exposure were quantitatively assessed through digital image acquisition and analysis.
Amino acid levels of hypothalamus
Female offspring rat hypothalamic tissues were rapidly dissected on ice and weighed. Subsequently, the tissues were placed in an ice-cold solution (e.g., 0.1 M perchloric acid) for homogenization, which precipitates proteins and releases free amino acids. The homogenate was centrifuged at 3500 r/min for 15 min at 4 °C, and the resulting supernatant was collected and passed through a 0.45 μm filter to remove suspended impurities. Quantitative analyses of the excitatory amino acids—glutamate (Glu) and aspartate (Asp)—and the inhibitory amino acid—γ-aminobutyric acid (GABA)—in the hypothalamus were performed using high-performance liquid chromatography (HPLC). The HPLC system was equipped with a C18 reversed-phase column and a detector suitable for amino acid detection (e.g., fluorescence or ultraviolet detector), and a gradient elution protocol was applied using a buffer (e.g., sodium acetate solution) and methanol. Standard curves for Glu, Asp, and GABA were constructed by preparing serial dilutions of each pure amino acid standard. Peak areas were plotted against the known concentrations to generate calibration curves with high linearity (R² > 0.99 for all). GABA concentrations in the samples were identified by comparing the retention time and peak area with those of the GABA standard under identical chromatographic conditions. Each sample was measured in triplicate, and the results were expressed in µmol/g tissue. This method ensures accurate and reproducible quantitative detection of amino acids in hypothalamic tissue.
Hormonal levels of hypothalamus
Following euthanasia, the hypothalamic tissues of female offspring rats were immediately dissected and homogenized in ice-cold lysis buffer containing protease inhibitors. The homogenates were centrifuged at 10,000 r/min for 15 min at 4 °C, and the supernatants were collected for further analysis. Quantitative measurements of IGF-1, NKB, and GnRH were performed using commercial ELISA kits (Thermo Fisher, USA), strictly following the manufacturer’s protocols. Samples and standards were each added in triplicate to antibody-precoated microplates. After incubation, washing, addition of detection antibodies and substrate reactions, absorbance was measured at 450 nm. Hormone concentrations were calculated based on standard curves and normalized to total protein content, which was determined using the BCA assay. Results were expressed as pg or ng hormone per milligram of protein.
Hormonal levels of serum
Serum levels of hormones, including luteinizing hormone (LH) and follicle-stimulating hormone (FSH), in female offspring rats were quantitatively analyzed using ELISA. Blood samples were collected from the tail vein of the female offspring at postnatal day 60 (PND60) under anesthesia. After blood collection, serum was separated by centrifugation at 3000 r/minfor 10 min at 4 °C. The serum samples were stored at −80 °C until further analysis.
The LH and FSH levels were measured using commercially available ELISA kits (Thermo Fisher, USA) according to the manufacturer’s protocols. Briefly, 50 µL of serum was added to the ELISA plates coated with specific antibodies for LH and FSH. After incubating for 1 h at room temperature, the plates were washed with phosphate-buffered saline (PBS) and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. The enzymatic reaction was developed using a TMB substrate solution, and the absorbance was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The concentrations of LH and FSH in the serum were calculated based on the standard curves generated using known concentrations of standards provided with the kits.
The intra-assay and inter-assay coefficients of variation (CV) were determined to assess the precision of the ELISA procedure. All analyses were performed in duplicate for each sample to ensure reliability and reproducibility.
Immunohistochemistry analysis
To quantitatively analyze the expression of ERα and GnRH in the hypothalamus, brain tissues were first fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at a thickness of 5 μm. After deparaffinization and rehydration, antigen retrieval was performed using a citrate buffer (pH 6.0) by microwave heating for 10 min. To minimize non-specific binding, the sections were then incubated at room temperature with 5% normal goat serum in phosphate-buffered saline (PBS) for 30 min. Subsequently, the sections were incubated overnight at 4 °C with a primary antibody against ERα (1:2000, Abcam, USA) and GnRH (1:500, Abcam, USA). After washing, the sections were incubated with a biotinylated goat anti-rabbit secondary antibody (1:1000, Abcam, USA), followed by streptavidin conjugated to horseradish peroxidase (HRP). Immunoreactivity was visualized using 3,3’-diaminobenzidine tetrahydrochloride (DAB; Sigma), and counterstaining was performed with hematoxylin. Finally, the stained sections were dehydrated through an ethanol gradient, cleared, and mounted. Images were captured using a light microscope, and quantitative analysis was conducted by calculating the integrated optical density (IOD) with image analysis software.
Immunofluorescence analysis
In parallel, quantitative assessment of IGF-1 and NKB expression levels in rat hypothalamus was performed using sophisticated immunofluorescence analysis. The hypothalamic tissue was fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for immunofluorescent labeling. Primary antibodies against IGF-1 (1:250, Abcam, USA) and NKB (1:100, Abcam, USA) were applied, followed by incubation with a Goat anti-rabbit IgG secondary antibody (Abcam, USA) at a 1:1000 dilution, yielding green or red fluorescence. Confocal microscopy elucidated distinct membranous and cytoplasmic staining in the hypothalamus, with DAPI (blue) (Beyotime, China) employed for nuclear counterstaining.
Extraction of total RNA and analysis of gene expression
Total RNA was extracted from hypothalamic tissues of adolescent female rats using Trizol reagent (Takara, Japan), according to the manufacturer’s instructions. Briefly, 50–100 mg of tissue was homogenized in 1 mL of Trizol on ice. After phase separation with chloroform, RNA was precipitated with isopropanol, washed with 75% ethanol, air-dried, and dissolved in RNase-free water. The purity and concentration of RNA were assessed spectrophotometrically, with an A260/A280 ratio of approximately 2.0 indicating high purity. Using 1 µg of total RNA as a template, complementary DNA (cDNA) was synthesized with a reverse transcription kit (Takara, Japan). Quantitative real-time PCR was subsequently performed on an ABI 7500 Real-Time PCR System using SYBR Green chemistry to detect mRNA expression levels of target genes including ERα, IGF-1, NKB, Kiss-1, GPR54, and GnRH, with β-actin serving as the internal reference. Table 1 shows the primer sequences used for amplification of each gene.
Western blot analysis
Hypothalamic tissues from adolescent female rats were homogenized in ice-cold RIPA buffer containing protease and phosphatase inhibitors. Protein concentrations were determined using the BCA assay. Equal amounts of protein (30–50 µg) were separated by 10–12% SDS-PAGE and transferred onto PVDF membranes. After blocking with 5% non-fat dry milk in TBST for 1 h at room temperature, the membranes were incubated overnight at 4 °C with primary antibodies against ERα (1:1000), IGF-1 (1:500), IGF-1R (1:1000), NKB (1:200), NK3R (1:100), and GnRH (1:1000), all purchased from Abcam (USA). Following washes, membranes were incubated with HRP-conjugated secondary antibodies (1:5000) for 1 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence (ECL) system and quantified using image analysis software, with β-actin serving as the internal loading control.
Furthermore, Western blot analysis was employed to evaluate the effects of specific inhibitors on the expression levels of ERα, IGF-1R, and NK3R in the hypothalamus. Following inhibitor treatment, systematic comparisons were conducted across groups to assess changes in ERα expression and in downstream signaling proteins within the IGF-1/NKB pathway.
Statistical analysis
In this study, one-way analysis of variance (ANOVA) was used for the preliminary statistical comparison of multiple groups. Data were analyzed using Analysis of Variance (ANOVA) via SPSS 23.0 (SPSS, Chicago, Illinois). Following the ANOVA analysis, pairwise comparisons between groups were further conducted using Tukey’s Honest Significant Difference (HSD) post hoc test. Statistical tests were conducted using GraphPad Prism software version 9.0 (GraphPad software, La Jolla, CA, USA). Values are expressed as mean ± standard deviation. Statistical significance was set at p < 0.05.
Results
Effects of exposure to DEHP during pregnancy on the timing of vaginal opening in female offspring
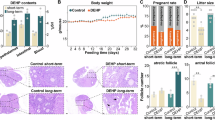
In the experimental groups exposed to DEHP prior to maternal gestation, a statistically significant intergroup effect was observed (p < 0.05). Figure 2 A illustrates that, compared to the control group and the 0.2 mg/kg/day DEHP group, the 1 and 5 mg/kg/day DEHP groups exhibited a statistically significant delay in the timing of vaginal opening in female offspring (p < 0.05). Specifically, the vaginal opening in the 1 mg/kg/day group was delayed by approximately 4 days compared to the control group (34.65 ± 0.51 vs. 30.35 ± 0.65), while in the 5 mg/kg/day DEHP group, the delay was about 9 days (34.65 ± 0.51 vs. 25.70 ± 0.72).
Pregnancy exposure to DEHP affects the vaginal opening time (A), body weight (B), levels of FSH (C) and LH (D) in the serum, levels of Asp (E), Glu (F), GABA (G), IGF-1 (H), NKB (I), and GnRH (J) in the hypothalamus of female offspring rats. (K) Ultrastructural analysis of neurons in the hypothalamus of female offspring rats. a: Control group; b: DEHP (5 mg/kg/day) exposed group, exhibiting hyperchromatic nuclei, increased heterochromatin, mitochondrial swelling, and an abundance of lipofuscin. θ: Nucleolus; ∅: Mitochondrial swelling; ♦: Increased lipofuscin; Nuc: Nucleus; M: Elevated number of microglial cells, in proximity to neurons. Scale bar = 2 μm. The levels were expressed as the mean value ± standard error. a Significant difference Compared with control (P < 0.05); b significant difference compared with 0.2 mg/kg/day group (P < 0.05); c significant difference compared with 1 mg/kg/day group (P < 0.05).
Effects of exposure to DEHP during pregnancy on the body weight of female offspring
We conducted a dynamic monitoring of the body weight of female offspring rats from birth to postnatal day 60 (PND60), and compared the DEHP-exposed groups with the control group at multiple time points. The results showed no significant differences in overall body weight changes between the 0.2 mg/kg/day and 1 mg/kg/day DEHP groups and the control group. However, the female offspring in the 5 mg/kg/day DEHP group exhibited a slight increase in body weight during the prepubertal period, with a faster rate of weight gain, although this difference did not reach statistical significance (Fig. 2B).
Ultrastructural observation of the hypothalamus by electron microscopy
Under electron microscopy, the neuronal nuclei in the hypothalamus of the offspring in the control group were observed to be relatively large, predominantly circular, with minimal heterochromatin and predominantly euchromatin, featuring prominent and clear nucleoli. The perinuclear cytoplasm was rich in rough endoplasmic reticulum (Fig. 2Ka).
In the 5 mg/kg/day DEHP-exposed offspring group, neurons demonstrated a notable decrease in cell volume, increased electron density, hyperchromatic nuclei, and augmented heterochromatin. The perinuclear area exhibited a reduced rough endoplasmic reticulum, and some mitochondria were swollen. Additionally, an increase in lipofuscin deposits was evident. Concurrently, an increased number of microglial cells, often in close proximity to neurons, was observed (Fig. 2Kb).
Effects of exposure to DEHP during pregnancy on the expression levels of LH and FSH in the serum of female offspring rats
The ELISA results showed that in the 1 mg/kg/day and 5 mg/kg/day DEHP-exposed groups, the serum levels of LH and FSH in female offspring were significantly higher compared to the control group and the low-dose group (0.2 mg/kg/day). Notably, the levels of LH and FSH in the 5 mg/kg/day DEHP group were markedly higher than those in all other groups (p < 0.05) (Figs. 2 C and 2D).
Effects of exposure to DEHP during pregnancy on excitatory and inhibitory amino acid levels in the hypothalamus of female offspring rats
The results indicated that the 1 mg/kg/day DEHP-exposed offspring group had significantly higher levels of Glu and Asp in the hypothalamus compared to the control group and the 0.2 mg/kg/day DEHP group. Notably, the levels in the 5 mg/kg/day DEHP group were substantially higher than in all other groups (p < 0.05) (Fig. 2E F). Compared to the control group and the 0.2 mg/kg/day DEHP group, the GABA concentration in the hypothalamus of female offspring exposed to 1 mg/kg/day DEHP was significantly reduced. More importantly, the GABA levels in the 5 mg/kg/day DEHP group were significantly lower than those in all other groups (p < 0.05) (Fig. 2G).
Effects of exposure to DEHP during pregnancy on the expression levels of IGF-1, NKB, and GnRH in the hypothalamus of female offspring rats
The ELISA results revealed that DEHP exposure upregulated the expression of IGF-1 in the hypothalamus of female offspring, demonstrating a clear dose-response relationship, with higher doses correlating with increased levels of IGF-1 expression (Fig. 2H). In the 1 and 5 mg/kg/day DEHP groups, the expression of NKB and GnRH was significantly higher than in the control group and the 0.2 mg/kg/day group (p < 0.05) (Fig. 2I J).
Immunohistochemical staining of ERα and GnRH expression in the hypothalamus of female offspring rats
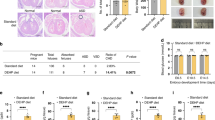
Positive immunohistochemical staining for ERα and GnRH was observed in the cell membranes and nuclei of neuronal and glial cells in the hypothalamus, indicated by the presence of brown-yellow granules. When compared to the control group, the DEHP-treated group showed a marked increase in the intensity of immunohistochemical staining in hypothalamic tissues, with a significant elevation in Integrated Optical Density (IOD) values (p < 0.05) (refer to Figs. 3 A,3D and 3E).
Immunofluorescence analysis of IGF-1 and NKB expression in the hypothalamus of female offspring rats
This study employed advanced immunofluorescence techniques to examine the expression of IGF-1 in the hypothalamus of female progeny rats, as depicted in Fig. 3B. The investigation revealed that the nuclei and cytoplasm of hypothalamic neurons and neuroglial cells exhibited positive staining, evidenced by distinct green fluorescence. Notably, NKB-positive cells were localized in the cytoplasm and cell membranes of both neuronal and neuroglial cells, characterized by prominent red fluorescence (Fig. 3 C).
In a significant finding, when compared with the control group, the rats treated with 1 and 5 mg/kg/day DEHP demonstrated a marked increase in the fluorescence intensity of IGF-1 and NKB in the hypothalamic tissues (p < 0.05) (Figs. 3 F and 3G).
(A) Immunohistochemical staining of ERα and GnRH expression in the hypothalamus of female offspring rats (×400). Immunofluorescence staining of IGF-1 (B) and NKB (C) expression in the hypothalamus of female offspring rats (×400). Effects of DEHP on expression of ERα(D), GnRH (E), IGF-1 (F) and NKB (G), in the hypothalamus of female offspring rats. The levels were expressed as the mean value ± standard error. a Significant difference Compared with control (P < 0.05); b significant difference compared with 0.2 mg/kg/day group (P < 0.05); c significant difference compared with 1 mg/kg/day group (P < 0.05).
Effects of DEHP on mRNA and protein expression of relevant genes in the hypothalamus of female offspring rats
Our results showed that the mRNA levels of ERα, IGF-1, IGF-1R, NKB, NK3R, Kiss-1, GPR54, and GnRH in the hypothalamus of the DEHP exposed group were significantly higher than the control group (p < 0.05) (Fig. 4). Western blot results indicated that the expression level of ERα in the 5 mg/kg/day DEHP group was significantly higher than the control and 0.2 mg/kg/day DEHP group. The expression level of IGF-1 in the 5 mg/kg/day DEHP group was significantly higher than the other three groups. The expression of NKB protein in the 1 and 5 mg/kg/day DEHP groups was significantly higher than the control and 0.2 mg/kg/day DEHP groups. The expression level of GnRH protein in the DEHP exposed group was significantly higher than the other three groups, showing a dose-response relationship (p < 0.05) (Fig. 5).
Effects of exposure to DEHP during pregnancy on mRNA expression levels of ERα (A), IGF-1 (B), IGF-1R (C), NKB (D), NK3R (E), Kiss-1 (F), GPR54 (G) and GnRH(H) in the hypothalamus of female offspring rats. The mRNA levels were expressed as the mean value ± standard error. a Significant difference Compared with control (P < 0.05); b significant difference compared with 0.2 mg/kg/day group (P < 0.05); c significant difference compared with 1 mg/kg/day group (P < 0.05).
Protein expression of ERα (B), IGF-1 (C), IGF-1R(E), NKB(D), NK3R(F), and GnRH(G) in the hypothalamus of female offspring rats. (A) Protein bands and (B-G) protein levels were expressed as the mean value ± standard error. a Significant difference Compared with control (P < 0.05); b significant difference compared with 0.2 mg/kg/day group (P < 0.05); c significant difference compared with 1 mg/kg/day group (P < 0.05).
Prenatal DEHP exposure delineates its profound impact on the interactions among neurodevelopmental factors within the hypothalamus of female offspring
In our research, Spearman correlation analysis was employed to elucidate the associations between key factors in the hypothalamus. As depicted in Figs. 6 A and 6B, in the control group, there was a significant positive correlation between IGF-1R and NK3R/GnRH, and between ERα and NKB (P < 0.05). In the 5 mg/kg/day DEHP group, a notable positive correlation was observed between ERα and NKB, and between IGF-1 and IGF-1R, as well as between IGF-1/IGF-1R and GnRH (P < 0.05).
Analysis of the interaction between neutral developmental factors in the hypothalamus (A: Control group, B: 5 mg/kg/day DEHP group). Correlation heatmap. Correlation analysis using Spearman’s correlation analysis. Red and blue indicate positive (0 < ρ < 1) and negative (− 1 < ρ < 0) correlations, respectively. * Significant difference compared with control (P < 0.05). Expression levels of hypothalamic related proteins in offspring female rats treated with ERα, IGF-1, and NKB inhibitors. (C) Representative protein bands; (D) ERα, (E) IGF-1R, (F) NK3R, and (G) GnRH protein levels were expressed as the mean value ± standard error, n = 12. a Significant difference compared with control (P < 0.05).
Expression levels of hypothalamic related proteins in offspring female rats treated with ERα, IGF-1, and NKB inhibitors
Utilizing stereotactic brain technology for in vivo inhibitor intervention, our findings indicate that inhibition of hypothalamic ERα effectively blocks DEHP’s activation of IGF-1 and NKB. The inhibitors used were Tamoxifen Citrate for ERα, BMS-754,807 for IGF-1R, and ESN-364 for NK3R. Post-treatment with IGF-1R-specific inhibitor, there was a significant reduction in the expression levels of IGF-1R, NK3R, and GnRH proteins in the hypothalamus, a trend similarly observed following NK3R inhibitor treatment (P < 0.05; Figs. 6 C-G).
Discussion
DEHP, a commonly used plasticizer, which is extensively incorporated into a myriad of plastic materials to augment their pliability. The propensity of DEHP to leach from these plastic products precipitates contamination of critical resources such as drinking water and food, culminating in its pervasive exposure to humans. Insights gleaned from animal research and epidemiological studies illuminate DEHP’s capacity to inflict toxicological harm on an array of organs, encompassing the brain, reproductive systems, blood, liver, lungs, and kidneys. Notably, DEHP exhibits estrogen-mimicking and anti-androgenic properties, thereby disrupting the intricate balance of the endocrine system. Intriguingly, recent scholarly inquiries have brought to light the gender-specific neurotoxic repercussions of DEHP, particularly pronounced during the formative phases of life, suggesting potential long-standing and even transgenerational ramifications21,22,23,24. Previous studies have shown that DEHP exerts differential effects on the male and female brain, particularly during critical windows of neurodevelopment. For example, in male offspring, DEHP exposure has been associated with alterations in testosterone production, impaired sexual behavior, and changes in synaptic plasticity. In contrast, female offspring tend to exhibit disrupted hypothalamic regulation of gonadotropin-releasing hormone (GnRH), earlier onset of puberty, and increased vulnerability to anxiety- and cognition-related impairments. These sex-specific differences are believed to arise from divergent hormonal milieus and the differential sensitivity of male and female brains to endocrine disruption during development. Consequently, an exhaustive exploration of DEHP’s deleterious effects on progeny holds paramount importance in the realm of human reproductive health.
Our research investigated the impact of maternal exposure to DEHP during gestation on the developmental milestones of female offspring rats. The findings demonstrate that, in contrast to the control and 0.2 mg/kg/day DEHP-exposed groups, the onset of vaginal opening in offspring subjected to 1 mg/kg/day and 5 mg/kg/day DEHP was notably protracted.
The timing of vaginal opening serves as a crucial biological indicator of sexual maturation in female rats. Our investigation revealed that DEHP exposure markedly postpones the onset of this developmental milestone, a phenomenon consistently observed across various dosing regimens. In experiments where maternal rats were administered a daily dose of 300 mg/kg of DEHP, it was observed that their offspring exhibited a significant delay in vaginal opening, suggesting a profound disruption in the sexual maturation process25. In a parallel study, analogous outcomes were documented, with maternal rats subjected to DEHP exposure during gestation and lactation periods. This exposure resulted in a discernible delay in the vaginal opening of their offspring, alongside notable alterations in ovarian steroid levels26. In their seminal study, Grande SW and colleagues observed a notable delay in the vaginal opening time in rats when administered with DEHP at doses of 15 mg/kg/day or higher, thereby revealing a pronounced dose-response correlation27. Furthermore, in female rats treated with DEHP, there was a tendency for delayed vaginal opening, and DEHP exposure may alter the expression of genes in the hypothalamic arcuate nucleus, which are pertinent to the regulation of pubertal functions14. Our findings are in alignment with previous research, indicating that DEHP may disrupt the endocrine system, thereby impacting the reproductive development process in animals and precipitating early onset of puberty in offspring rats.
Research has revealed that DEHP can disrupt the homeostasis of the nervous system, adversely affecting cognitive functions28, and it exerts a significant influence on brain development stages from the prenatal period through to postnatal and adolescent phases29,30.
The hypothalamus is crucial in maintaining hormonal balance and the process of sexual maturation. In our study, ultrastructural examination of the hypothalamic tissue in offspring female rats was conducted. Electron microscopy revealed that neurons in the DEHP-exposed offspring displayed abnormal cellular structures, including reduced volume, increased electron density, hyperchromatic nuclei, increased heterochromatin, and reduced rough endoplasmic reticulum. Additionally, these neurons exhibited mitochondrial swelling and increased lipofuscin deposition. An augmented number of microglial cells, often in proximity to neurons, was also observed. These findings suggest that DEHP exposure may adversely affect neuronal energy metabolism, oxidative stress responses, inflammatory reactions, synaptic formation, and neurotransmitter release, leading to disrupted inter-neuronal communication and impacting the sexual development process in offspring female rats.
Excitatory amino acids (EAA), including glutamate (Glu) and aspartate (Asp), serve as excitatory neurotransmitters in the central nervous system. These amino acids are influenced by sex hormones, thereby playing a pivotal role in regulating brain development. During the early stages of brain development, an excessive trophic action of the excitatory amino acid system may lead to an inappropriate increase in neuronal numbers, potentially resulting in excitotoxicity within the nervous system. In our study, exposure to DEHP resulted in a significant increase in the levels of aspartate and glutamate in the hypothalamus of offspring rats, leading to neuronal toxicity and an accelerated growth and development process. This finding is consistent with previous research, which has shown that prenatal and perinatal DEHP exposure can lead to a significant increase in the levels of aspartate and gamma-aminobutyric acid in prepubertal female offspring rats, along with a concomitant rise in serum gonadotropin levels31. These findings further corroborate the hypothesis that DEHP, as an endocrine disruptor, may affect the regulation of neurotransmitters and hormones in the hypothalamus of offspring female rats, potentially contributing to the onset of precocious puberty.
Although γ-aminobutyric acid (GABA) primarily acts as an inhibitory neurotransmitter in the mature central nervous system, it exerts excitatory effects during early developmental stages. This “switch” in GABA function from excitatory to inhibitory is crucial for neural development and is primarily regulated by the expression of chloride transporters such as NKCC1 and KCC2.While the main focus of this study was on excitatory amino acids (Glu and Asp), we recognize that DEHP may interfere with the GABAergic system, particularly during this critical developmental period. Literature has shown that environmental endocrine disruptors, such as DEHP, can affect the expression or function of these chloride transporters, thereby delaying the excitatory-to-inhibitory transition of GABA. This disruption can lead to an imbalance in the excitation-inhibition balance within the hypothalamic neural network. Such changes may impact the onset of puberty and neuronal plasticity.
IGF-1, a critical regulator of reproductive and neuroendocrine functions, plays a pivotal role in the growth and development during puberty32. It is capable of inducing the release of GnRH, thus facilitating sexual maturation33. The onset of central precocious puberty is associated with an increase in peripheral blood levels of IGF-134,35. Both peripheral and central sources of IGF-1 can interact with hypothalamic IGF-1 receptors (IGF-1R), activating the PI3K/Akt/mTOR pathway, and consequently upregulating the expression levels of Kiss-1/GPR45 mRNA in neurons of the hypothalamus in rats36,37,38. During puberty, high expression levels of IGF-1 are observed in the hypothalamus of rats, mice, monkeys, and humans. IGF-1 is known to elevate the GnRH mRNA content in primary hypothalamic cells of juvenile mice32. Additionally, the specific knockout of IGF-1R in mouse GnRH neurons has been shown to result in delayed puberty39.
Exposure to DEHP during pregnancy and prepuberty has been associated with elevated serum IGF-1 levels in girls aged 8 to 1440, leading to the onset of precocious puberty. Our preliminary research suggests that DEHP may activate the hypothalamic IGF-1 signaling pathway, thereby facilitating the release of GnRH and initiating female sexual development19. Exposure to DEHP has been observed to cause neurobehavioral damage from birth through adulthood, as well as memory loss and irreversible neurological damage during pregnancy and infancy30. In this study, ELISA assay results corroborated similar conclusions, indicating that exposure to DEHP upregulates the expression of IGF-1 in the hypothalamus of offspring female rats. Moreover, a distinct dose-response relationship was observed, whereby higher doses of DEHP correlated with increased levels of IGF-1 expression.
NKB is identified as another critical factor in regulating puberty and adult reproductive functions. NKB/NK3R, by stimulating Kisspeptin, exerts its regulatory influence on GnRH neurons in the hypothalamus for the secretion of GnRH; the interaction of NKB with receptors on the axons of GnRH neurons affects their function, and ultrastructural examination reveals that NKB neuronal fibers in the hypothalamic arcuate nucleus can make direct contact with the axons of GnRH neurons41,42. Studies have found that a signaling network between NKB and kisspeptin neurons in the hypothalamus of female pubertal rhesus monkeys accelerates the release of GnRH43. During puberty in female rats and mice, increased expression levels of hypothalamic NKB (Tac2) and NK3R (Tacr3) have been observed. In vivo experiments confirm that NK3R agonists increase LH secretion and advance the onset of puberty, while inhibition of NK3R expression delays pubertal development44,45. However, there are no reports yet on how DEHP affects NKB or its receptors. ELISA assay results from this study indicate that the expression levels of NKB and GnRH in the 1 and 5 mg/kg/day DEHP dose groups were significantly higher than those in the control and 0.2 mg/kg/day groups. These findings suggest that DEHP may accelerate the growth and development of offspring female rats and induce precocious puberty by affecting the expression of IGF-1 and NKB in the hypothalamus, thereby promoting GnRH secretion.
DEHP, containing a benzene ring and structurally similar to estradiol, exhibits estrogen-like effects46. In the mammalian brain, estrogen plays a critical role in regulating GnRH neurons via estrogen receptors (ERα and ERβ)47. ERα, functioning as a transcription factor, predominates in the neuroendocrine-gonadal system. It can specifically bind to the IGF-1 promoter, thereby transactivating the transcription of the IGF-1 gene and upregulating the expression level of IGF-1. In contrast, ERβ does not exhibit this function48,49. Similarly, estrogen can lead to an increase in NKB mRNA expression in the hypothalamic arcuate nucleus, but this effect is not observed in mice with ERα knockout, indicating that ERα mediates estrogen’s regulation of NKB gene expression, thereby influencing GnRH secretion50,51.While estrogen, through ERα, exerts beneficial effects such as neuroprotection, mood improvement, and enhancement of memory and cognitive functions during normal physiological processes, disruption of ERα signaling during critical brain developmental stages can lead to adverse outcomes. In this study, we observed an increased expression of ERα in the hypothalamus of female offspring exposed to DEHP, suggesting that DEHP may aberrantly activate the estrogen signaling pathway. This indicates a disruption in estrogen homeostasis, which is not only associated with reproductive system abnormalities, such as precocious puberty, but may also lead to impaired cognitive function.
GnRH acts on gonadotropin-secreting cells in the anterior pituitary gland and stimulates secretion of the gonadotropins LH and FSH. Gonadotropic hormones act on the ovaries to cause follicular maturation and ovulation. In the present study, ELISA results of serum samples demonstrated that DEHP exposure led to an increase in the secretion of gonadotropins, supporting the hypothesis that DEHP may accelerate the onset of precocious puberty by disrupting normal neuroendocrine regulatory mechanisms. In particular, higher doses of DEHP exposure (1 mg/kg/day and 5 mg/kg/day) appeared to cause an excessive elevation in sex hormone levels, thereby promoting the premature initiation of puberty.
Building on preliminary studies, this research further examines the effects of prenatal DEHP exposure on the sexual development of offspring female rats, particularly investigating whether DEHP induces precocious puberty in females via the hypothalamic ERα-mediated IGF-1 and NKB signaling pathways. Prenatal exposure to DEHP may disrupt gene encoding in the hypothalamus, and the effects are likely to be permanent52. Perinatal exposure to DEHP can alter the expression levels of ERα and ERβ in the pituitary gland of prepubescent and adult female rats, as well as increase the proportion of lactotrophs in adulthood53. However, the impact of DEHP on ERα in the hypothalamus remains unclear. In this study, immunohistochemical staining of ERα in the hypothalamus of offspring female rats revealed positive staining in the neuronal and neuroglial cell membranes and nuclei, with visible brown-yellow granules. Compared to the control group, the DEHP-exposed group showed an intensified degree of immunohistochemical staining, with significant increases in mean optical density (MOD) and integrated optical density (IOD) values. These results suggest that DEHP exposure may act by activating ERα in the cell membranes and nuclei of hypothalamic cells in offspring female rats, thereby influencing the timing and process of sexual maturation.
Immunofluorescence studies in this research revealed that DEHP exposure increases the expression levels of IGF-1, NKB, and GnRH in the hypothalamic tissue of offspring female rats. Western Blot protein analysis also showed that in the 5 mg/kg/day DEHP exposure group, the levels of ERα, IGF-1, NKB, and GnRH were significantly higher than in the control group. Real-time quantitative PCR results further indicated that the hypothalamic ERα, IGF-1, IGF-1R, N KB, NK3R, Kiss-1, GPR54, and GnRH mRNA levels were notably higher in the DEHP-exposed group compared to the control. These findings suggest that DEHP exposure may induce precocious puberty in offspring female rats by activating ERα, thereby upregulating the protein expression levels of IGF-1 and NKB in the hypothalamus and promoting GnRH secretion.
Our study results indicate that DEHP exposure leads to dose-dependent changes in the expression of key genes and proteins associated with precocious puberty in the hypothalamus. In the 5 mg/kg/day group, molecular-level changes were most pronounced, accompanied by significant phenotypic differences, such as a notable delay in vaginal opening. In the 1 mg/kg/day group, although the molecular changes were milder compared to the higher dose groups, certain phenotypic differences were still observed compared to the control group. It is noteworthy that in the 0.2 mg/kg/day group, although the expression levels of some genes and proteins related to precocious puberty were also elevated, these molecular changes did not translate into obvious phenotypic differences. One possible explanation is that the endocrine disruption effects induced by low-dose DEHP exposure did not reach the threshold required to cause significant phenotypic changes. In other words, while subtle gene expression changes may occur under low-dose conditions, these did not lead to noticeable physiological or developmental abnormalities due to potential compensatory mechanisms in the body or insufficient activation of downstream effectors. This aligns with the concepts of threshold effects and compensation mechanisms. Other possible explanations include non-monotonic dose-response effects and time dynamics. Endocrine disruptors often exhibit non-monotonic dose-response curves, where low doses may cause molecular-level changes without immediately leading to obvious phenotypic alterations, while higher doses induce significant developmental disruptions. The gene and protein expression changes observed in the low-dose group may represent early molecular events leading to phenotypic changes, which may only manifest as observable phenotypic differences after the time points measured in our study.
We employed Spearman correlation analysis to associate key factors in the hypothalamus. In the control group, a positive correlation between IGF-1R and NK3R, GnRH, may reveal an endocrine feedback mechanism. The positive correlation between ERα and NKB indicates the role of estrogen and its receptor in regulating neurokinin expression.
In the 5 mg/kg/day DEHP group, the positive correlation between ERα and NKB remained unchanged, suggesting that DEHP exposure does not affect this specific endocrine pathway. Thus, it can be inferred that DEHP exerts its endocrine-disrupting effects via this route. However, the strengthened positive correlations between IGF-1 and IGF-1R, as well as IGF-1, IGF-1R, and GnRH, indicate that DEHP further affects the functionality of the reproductive axis through the IGF-1 system.
To ascertain the regulatory role and crosstalk mechanism of ERα-mediated IGF-1 and NKB signaling pathways in DEHP-induced precocious puberty in offspring female Wistar rats, we randomly divided the DEHP-exposed female offspring into four groups: those receiving specific inhibitors of ERα, IGF-1R, and NK3R. Additionally, 20 female offspring rats were selected as the control group for this in vivo intervention experiment. Through the assessment of ERα, IGF-1R, NK3R, and downstream GnRH protein expression, it was found that inhibiting hypothalamic ERα could block the activation of IGF-1 and NKB by DEHP. Post-treatment with IGF-1R specific inhibitors led to a significant decrease in the expression levels of IGF-1R, NK3R, and GnRH proteins in the hypothalamus, a trend similarly observed with NK3R inhibitors. This suggests that DEHP disrupts the hypothalamic IGF-1 and NKB signaling pathways by activating ERα, and through inter-pathway crosstalk regulates Kiss-1/GPR54, promoting GnRH release. This, in turn, affects the reproductive endocrine regulation function of the hypothalamic-pituitary-ovarian axis, ultimately leading to the onset of precocious puberty in female offspring.
Despite the valuable insights gained from this work, several limitations should be acknowledged. First, the study primarily focused on central neuroendocrine mechanisms, the neuroanatomical localization of these molecules in neurons and glial cells has not been investigated in detail. Second, although molecular and phenotypic changes were observed, histological evaluations of ovarian tissue were not included, which would have strengthened our understanding of the reproductive outcomes. Third, while this study demonstrated changes in gene and protein expression, mechanistic studies involving gene knockdown or receptor antagonists would provide stronger causal evidence. Lastly, long-term behavioral and fertility outcomes beyond puberty onset were not evaluated and should be explored in future studies to assess the lasting impacts of DEHP exposure.
Conclusion
This study, by observing the impact of prenatal DEHP exposure on sexual initiation markers in offspring female rats, elucidates DEHP’s disruptive role in the onset of female sexual development and its molecular mechanisms. It reveals the molecular mechanisms of hypothalamic ERα-regulated IGF-1 and NKB pathways in elevating GnRH, inducing precocious puberty in the DEHP-exposed animal model with inhibitor intervention, and the regulatory role of IGF-1/NKB crosstalk. This research sheds light on the effects of DEHP exposure on the offspring’s reproductive endocrine system, providing new insights and targets for the prevention and treatment of precocious puberty in girls.
Data availability
The data generated and/or analyzed during the current study are available from the corresponding author, Te Liu, upon reasonable request. Please contact Te Liu at iamliute@jlu.edu.cn for further information.
References
Thacharodi, A. et al. Water a major source of endocrine-disrupting chemicals: an overview on the occurrence, implications on human health and bioremediation strategies[J]. Environ. Res. 231 (Pt 1), 116097 (2023).
Ghassabian, A. et al. Prenatal exposure to common plasticizers: a longitudinal study on phthalates, brain volumetric measures, and IQ in youth[J]. Mol. Psychiatry. 28(11), 4814–4822 (2023).
Ashrap, P. et al. Maternal urinary phthalate metabolites are associated with lipidomic signatures among pregnant women in Puerto Rico[J]. J. Expo Sci. Environ. Epidemiol. 32 (3), 384–391 (2022).
Ge, X. et al. Prenatal exposure to the phthalate DEHP impacts reproduction-related gene expression in the pituitary[J]. Reprod. Toxicol. 108, 18–27 (2022).
Yi, H. et al. Phthalate exposure and risk of ovarian dysfunction in endometriosis: human and animal data[J]. Front. Cell. Dev. Biol. 11, 1154923 (2023).
Su, H. Y. et al. Prenatal exposure to low-dose di-(2-ethylhexyl) phthalate (DEHP) induces potentially hepatic lipid accumulation and fibrotic changes in rat offspring[J]. Ecotoxicol. Environ. Saf. 269, 115776 (2023).
Radke, E. G. et al. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence[J]. Environ. Int. 132, 104768 (2019).
Chen, Y. et al. Phthalate mixtures and insulin resistance: an item response theory approach to quantify exposure burden to phthalate mixtures[J]. J. Expo Sci. Environ. Epidemiol. 34(4), 581–90 (2024).
Lucaccioni, L. et al. Perinatal exposure to phthalates: from endocrine to neurodevelopment Effects[J]. Int. J. Mol. Sci. 22(8), 4063 (2021).
Patisaul, H. B. Endocrine disrupting chemicals (EDCs) and the neuroendocrine system: beyond estrogen, androgen, and thyroid[J]. Adv. Pharmacol. 92, 101–150 (2021).
Naffaa, V., Laprevote, O. & Schang, A. L. Effects of endocrine disrupting chemicals on Myelin development and diseases[J]. Neurotoxicology 83, 51–68 (2021).
Robles-Matos, N. et al. Environmental exposure to endocrine disrupting chemicals influences genomic imprinting, growth, and Metabolism[J]. Genes (Basel). 12(8), 1153 (2021).
O’Shaughnessy, K. L., Fischer, F. & Zenclussen, A. C. Perinatal exposure to endocrine disrupting chemicals and neurodevelopment: how articles of daily use influence the development of our children[J]. Best Pract. Res. Clin. Endocrinol. Metab. 35 (5), 101568 (2021).
Roepke, T. A. et al. Regulation of arcuate genes by developmental exposures to endocrine-disrupting compounds in female rats[J]. Reprod. Toxicol. 62, 18–26 (2016).
Boberg, J. et al. Comparison of female rat reproductive effects of pubertal versus adult exposure to known endocrine disruptors[J]. Front. Endocrinol. (Lausanne). 14, 1126485 (2023).
Lopez-Rodriguez, D. et al. Cellular and molecular features of EDC exposure: consequences for the GnRH network[J]. Nat. Rev. Endocrinol. 17 (2), 83–96 (2021).
Hu, G., He, M. & Wong, A. O. Novel functional role of NK3R expression in the potentiating effects on somatolactin alpha autoregulation in grass carp pituitary cells[J]. Sci. Rep. 6, 36102 (2016).
Liu, T. et al. Di-(2-ethylhexyl) phthalate induces precocious puberty in adolescent female rats[J]. Iran. J. Basic. Med. Sci. 21 (8), 848–855 (2018).
Shao, P. et al. The interference of DEHP in precocious puberty of females mediated by the hypothalamic IGF-1/PI3K/Akt/mTOR signaling pathway[J]. Ecotoxicol. Environ. Saf. 181, 362–369 (2019).
Liu, T. et al. Effects of Di-(2-ethylhexyl) phthalate on the Hypothalamus-Uterus in pubertal female Rats[J]. Int. J. Environ. Res. Public. Health. 13(11), 1130 (2016).
Liu, Y. et al. An insight into sex-specific neurotoxicity and molecular mechanisms of DEHP: A critical review[J]. Environ. Pollut. 316 (Pt 2), 120673 (2023).
Zhang, X. et al. The role of Estrogen receptors (ERs)-Notch pathway in thyroid toxicity induced by Di-2-ethylhexyl phthalate (DEHP) exposure: population data and in vitro studies[J]. Ecotoxicol. Environ. Saf. 269, 115727 (2023).
Zhang, X. et al. Di(2-ethylhexyl) phthalate (DEHP) and thyroid: biological mechanisms of interference and possible clinical implications[J]. Environ. Sci. Pollut Res. Int. 29 (2), 1634–1644 (2022).
Lee, H. J. et al. Untargeted metabolomics analysis reveals toxicity based on the sex and sexual maturity of single Low-Dose DEHP Exposure[J]. Toxics. 11(9), 794 (2023).
Nardelli, T. C. et al. In utero and lactational exposure study in rats to identify replacements for Di(2-ethylhexyl) Phthalate[J]. Sci. Rep. 7 (1), 3862 (2017).
Shanmugam, D. A. S. et al. Maternal exposure to di(2-ethylhexyl) phthalate (DEHP) causes multigenerational adverse effects on the uterus of F(1) and F(2) offspring rats[J]. Reprod. Toxicol. 115, 17–28 (2023).
Grande, S. W. et al. A dose-response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development[J]. Toxicol. Sci. 91 (1), 247–254 (2006).
Kang, J. S. et al. Long-term exposure changes the environmentally relevant bis(2-ethylhexyl) phthalate to be a neuro-hazardous substance disrupting neural homeostasis in emotional and cognitive functions[J]. Environ. Pollut. 324, 121387 (2023).
Ren, W. Q. et al. Subchronic exposure to di-(2-ethylhexyl) phthalate (DEHP) elicits blood-brain barrier dysfunction and neuroinflammation in male C57BL/6J mice[J]. Toxicology 499, 153650 (2023).
Safarpour, S. et al. Effects of Di-2-Ethylhexyl phthalate on central nervous system functions: A narrative Review[J]. Curr. Neuropharmacol. 20 (4), 766–776 (2022).
Carbone, S. et al. Different effects by sex on hypothalamic-pituitary axis of prepubertal offspring rats produced by in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP)[J]. Neurotoxicology 33 (1), 78–84 (2012).
Wolfe, A., Divall, S. & Wu, S. The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1)[J]. Front. Neuroendocrinol. 35 (4), 558–572 (2014).
Dees, W. L., Hiney, J. K. & Srivastava, V. K. IGF-1 influences Gonadotropin-Releasing hormone regulation of Puberty[J]. Neuroendocrinology 111 (12), 1151–1163 (2021).
Liu, Y. et al. Analysis of serum insulin-like growth factor-1, fibroblast growth factor 23, and Klotho levels in girls with rapidly progressive central precocious puberty[J]. Eur. J. Pediatr. 182 (11), 5007–5013 (2023).
Escagedo, P. D. et al. Insulin-like growth factor 1, but not Insulin-Like growth factor-Binding protein 3, predicts central precocious puberty in girls 6–8 years old: A retrospective Study[J]. Horm. Res. Paediatr. 94 (1–2), 44–51 (2021).
Bar-Lev, T. H. et al. Role of PI4K and PI3K-AKT in ERK1/2 activation by GnRH in the pituitary gonadotropes[J]. Mol. Cell. Endocrinol. 415, 12–23 (2015).
Srivastava, V. K., Hiney, J. K. & Dees, W. L. Manganese-Stimulated Kisspeptin is mediated by the IGF-1/Akt/Mammalian target of Rapamycin pathway in the prepubertal female Rat[J]. Endocrinology 157 (8), 3233–3241 (2016).
Hiney, J. K. et al. Regulation of Kisspeptin synthesis and release in the preoptic/anterior hypothalamic region of prepubertal female rats: actions of IGF-1 and Alcohol[J]. Alcohol Clin. Exp. Res. 42 (1), 61–68 (2018).
Qiu, X. et al. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells[J]. Endocrinology 154 (3), 1337–1348 (2013).
Watkins, D. J. et al. Relating phthalate and BPA exposure to metabolism in peripubescence: the role of exposure timing, sex, and Puberty[J]. J. Clin. Endocrinol. Metab. 101 (1), 79–88 (2016).
Avendano, M. S., Vazquez, M. J. & Tena-Sempere, M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty[J]. Hum. Reprod. Update. 23 (6), 737–763 (2017).
Skorupskaite, K. et al. Neurokinin B regulates gonadotropin secretion, ovarian follicle growth, and the timing of ovulation in healthy Women[J]. J. Clin. Endocrinol. Metab. 103 (1), 95–104 (2018).
Garcia, J. P. et al. Kisspeptin and neurokinin B signaling network underlies the pubertal increase in GnRH release in female Rhesus Monkeys[J]. Endocrinology 158 (10), 3269–3280 (2017).
True, C. et al. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice[J]. Endocrinology 156 (4), 1386–1397 (2015).
Qiu, J. et al. High-frequency stimulation-induced peptide release synchronizes arcuate Kisspeptin neurons and excites GnRH neurons[J]. Elife 5, e16246 (2016).
Ye, H., Dudley, S. Z. & Shaw, I. C. Intimate Estrogen receptor-alpha/ligand relationships signal biological activity[J]. Toxicology 408, 80–87 (2018).
Novaira, H. J. et al. Impairments in the reproductive axis of female mice lacking Estrogen receptor beta in GnRH neurons[J]. Am. J. Physiol. Endocrinol. Metab. 315 (5), E1019–E33 (2018).
Ogo, Y. et al. IGF-1 gene expression is differentially regulated by Estrogen receptors alpha and beta in mouse endometrial stromal cells and ovarian granulosa cells[J]. J. Reprod. Dev. 60 (3), 216–223 (2014).
Loutchanwoot, P. & Vortherms, T. Effects of puerarin on estrogen-regulated gene expression in gonadotropin-releasing hormone pulse generator of ovariectomized rats[J]. Steroids 135, 54–62 (2018).
Skorupskaite, K. et al. Interactions between neurokinin B and Kisspeptin in mediating Estrogen feedback in healthy Women[J]. J. Clin. Endocrinol. Metab. 101 (12), 4628–4636 (2016).
Wang, L. et al. Glutamatergic transmission to hypothalamic Kisspeptin neurons is differentially regulated by estradiol through Estrogen receptor alpha in adult female Mice[J]. J. Neurosci. 38 (5), 1061–1072 (2018).
Tran, M. et al. Prenatal DEHP exposure predicts neurological disorders via transgenerational epigenetics[J]. Sci. Rep. 13 (1), 7399 (2023).
Perez, P. A. et al. The phthalate DEHP modulates the Estrogen receptors alpha and beta increasing lactotroph cell population in female pituitary glands[J]. Chemosphere 258, 127304 (2020).
Funding
This work was supported by the Jilin Scientific and Technological Development Program (Grant No.20240404029ZP), Jilin Province Special Program for Health Scientific Research Talents (Grant No.2024scz20) and the construction project of Chun Lei plan of China-Japan Union Hospital of Jilin University (Grant No. 2023CL06), and the Young Clinical Research Fund of the Chinese Stemmatological Association (CSA-02022-04).
Author information
Authors and Affiliations
Contributions
All authors had full access to the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. XYL and CYL wrote the main manuscript text, they have contributed equally to this work and share first authorship; Resources: TL, HL, BZ, and YZW. Visualization: MDY, ZSZ, QJL and TL. Writing-Review&Editing:YYX and TL. YYX and TL are the co-corresponding authors.All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Approval statement
This study was approved by the Animal Care and Use Committee of Jilin University (Approval No. 201802019).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Li, C., Li, Q. et al. Impact of prenatal Di(2-ethylhexyl) phthalate exposure on pubertal development in female offspring rats: A focus on ERα-Mediated IGF-1/NKB crosstalk in the hypothalamus. Sci Rep 15, 22255 (2025). https://doi.org/10.1038/s41598-025-08253-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08253-2