Abstract
Despite the extensive applicability of cadmium oxide nanoparticles (CdO NPs) in diverse fields, there is a prominent gap in our understanding of their toxicological impacts, particularly when using invertebrate insect model organisms. This study aimed to instigate this research gap by investigating the oxidative stress and genotoxicity effects of CdO NPs using Galleria mellonella as an insect model. We evaluated the immune response, oxidative stress, stress response proteins, detoxification enzymes, DNA damage, and cell viability following exposure to a single dose of CdO NPs. A comprehensive approach was conducted, combining biochemical, molecular, ultrastructural, and morphological analyses. Interestingly, the most pronounced effect of CdO NPs on larval mortality was observed at the lowest dose of 0.01 mg/g body weight. Also, EDX analysis demonstrated a substantial accumulation of cadmium in larval midgut tissues exposed to a single injection of CdO NPs compared to the control, leading to oxidative stress, disruption of antioxidant and detoxification defense systems as well as DNA injury, and increased apoptotic and necrotic cell death lead to the destruction of the larval intestinal barrier. Moreover, pathohistological and ultrastructural investigations inspected prominent alterations and anomalies in larval midgut epithelium, including vacuolization, degeneration of cytoplasmic organelles, distorted microvilli, along with obvious necrotic signs. This study not only contributes to filling the prevailing knowledge gap concerning the toxicity of CdO NPs but also stipulates valuable insights into their broader environmental impact, potentially updating future risk assessments and monitoring decisions regarding nanomaterial applications.
Similar content being viewed by others
Introduction
In recent years, the use of heavy metals has significantly expanded across various industries, including incineration, battery production, and mining. Releasing these metals into the environment leads to contamination, posing serious concerns for ecological balance, food safety, and overall environmental health. By 2030, global industrial chemical production is expected to increase by 85%1. Among heavy metals, cadmium (Cd) is one of the most toxic, exerting harmful effects on animals, plants, and humans even at trace concentrations2,3,4,5. Recently, cadmium has been classified among the top seven hazardous pollutants due to its severe threats to biological systems.
A relatively new challenge for both the environment and human health is the increasing production and use of nanoparticles, including metallic nanoparticles. The extent of nanoparticle penetration into the environment, as well as their fate, remains largely unknown. However, researchers highlight the risks associated with the uncontrolled release of these structures into the environment at various stages of their lifecycle, from production, transportation, storage, and usage to disposal and recycling6,7,8. Cadmium-containing nanoparticles, such as cadmium sulfide (CdS) or cadmium oxide (CdO) nanoparticles, have significant potential applications in medicine, particularly in drug delivery, imaging, diagnostics, and various therapeutic approaches, including cancer treatment. Additionally, their antimicrobial activity presents an opportunity for combating resistant bacteria that do not respond to conventional medical treatments. Their unique properties also find applications in photovoltaics or solar cells, liquid crystal displays, and infrared (IR) detectors9,10,11,12.
Given the global shift toward renewable energy sources, it is expected that, in the coming decade, humanity will have to address the challenge of recycling and disposing of photovoltaic and solar cells and liquid crystal displays along with the potential release of hazardous substances, including nanoparticles, into ecosystems. While the scale of this phenomenon is difficult to estimate, nanoparticles will increasingly contribute to overall cadmium pollution, which is currently associated primarily with industrial processes, mining activities, and agricultural practices2. Therefore, understanding the effects of CdO NPs on organisms and elucidating their mechanisms of action is crucial for future preventive measures.
Cadmium toxicity is relatively well understood. Generally, it can cause irreversible physiological disturbances and severe tissue anomalies13. It is widely accepted that cadmium can lead to cell wall and membrane damage, and once inside the cell, it can indirectly generate a variety of reactive oxygen species (ROS), including superoxide (O⁻·2), nitric oxide (NO·), and hydroxyl radicals (OH∙)14. These ROS may overwhelm the cell’s antioxidant defense system, leading to oxidative stress. Due to Cd exposure, enzyme dysfunction, mitochondrial and lysosomal impairment, DNA damage, and protein degradation have been reported11. Additionally, cadmium has been found to induce epigenetic modifications, such as DNA methylation15.
The toxicity mechanism of metal nanoparticles may, in principle, be similar to that of their ionic counterparts. However, nanostructures exhibit distinct physicochemical properties compared to ionic cadmium (Cd2⁺), including high stability16. Due to various interactions with biological matrices (e.g., food components, hemolymph, and tissue fluids), metal nanoparticles may undergo agglomeration, potentially reducing their toxicity. On the other hand, it is well established that once nanoparticles enter tissues, they can gradually release metal ions over an extended period, contributing to prolonged and delayed toxic effects17. Therefore, directly extrapolating knowledge about ionic cadmium toxicity to the toxicity mechanisms of cadmium nanoparticles is neither straightforward nor ideal and requires further experimental validation.
The greater wax moth, Galleria mellonella, is widely known as an economically destructive honeybee pest. However, when used in the laboratory as an insect model18,19, this insect has another functional identity, representing an alternative in vivo model for ecotoxicological, immunological, and microbial investigations20. Specifically, its larvae rapidly gained popularity for their use in xenobiotics and toxins screening with numerous advantages, including their lesser ethical restrictions compared to other vertebrate models, low maintenance cost, ease of dosage applicability due to their comparatively large size (up to 2.5 cm), and their thermal tolerance (37 °C)20,21. Further, this species is characterized by its short time of completing larval development and its high fertility rate22.
Insect midgut represents a primary location for the accumulation of heavy metals18. The basic structure of insect midgut tissue can be compared with the human one due to their similarity, including arrangements of midgut epithelium and controlled permeability of septate junctions20. Further, the peritrophic matrix found in insect midgut has a functional similarity with the mucous layer found in mammals20. Interestingly, a precise description of G. mellonella structural and ultrastructural organs was previously conducted, which suggests it is a suitable model for pathohistological and toxicological studies22. Additionally, G. mellonella has an innate immune system like that of mammals, with both humoral and cellular responses. The cellular immune response comprises cells termed hemocytes that circulate within insect hemolymph21.
In this study, CdO NPs were investigated for their toxicity by analyzing the oxidative stress, genotoxicity effects, and cellular impairments following exposure to a single dose of CdO NPs using Galleria mellonella as a model insect. Biochemical, molecular, structural, and ultrastructural standard ecotoxicological assessments were carried out to assess potential toxicity on the midgut of G. mellonella. The results of the study fill the knowledge gap of Cd NPs toxicity. Thus, it provides insights into heavy metal usage management for a sustainable environment.
Materials and methods
Experimental model
G. mellonella larvae (last instar, 180 ± 10 mg, exhibiting vigorous movement and uniform cuticle coloration) were reared at 30 ± 1 °C, 65 ± 5% relative humidity, in darkness on an artificial diet23.
Cadmium oxide characterization
CdO NPs were commercially obtained from Nanotech Egypt for Photo-Electronics (Giza, Egypt). Immediately before use, their structural and morphological properties were thoroughly analyzed in our laboratory using various analytical techniques to ensure their quality and stability. The particle size distribution was determined using a Bettersizer 2600 Laser Diffraction particle size analyzer (Dandong Bettersize Instrument Co., Ltd., Liaoning, China). The crystalline structure and phase purity were examined by powder X-ray diffraction (XRD) using a MAC Science M03XHF diffractometer with CuKα radiation (λ = 1.5405 Å). The surface morphology was investigated using scanning electron microscopy (SEM; Hitachi S4800) coupled with energy-dispersive X-ray spectroscopy (EDS) for elemental composition analysis. Detailed particle size and morphological observations were conducted using transmission electron microscopy (TEM) with a JEOL-JEM 2100 instrument operating at 120 keV. The functional groups in the nanoparticles were identified using Fourier transform infrared spectroscopy (FTIR; Shimadzu-8400S) in the 400–4000 cm–1 range, employing the potassium bromide (KBr) pellet method.
Preparation and administration of CdO NPs
CdO NPs were initially dispersed in sterile phosphate-buffered saline (PBS) to prepare a stock solution at a 1.26 mg/mL concentration. The stock solution was then serially diluted in PBS to obtain working solutions at various concentrations (0.18, 0.54, and 0.90 mg/mL), which were used for injection (~ 10 µL per individual). Before administration, the nanoparticle suspensions were sonicated for 10 min to ensure proper dispersion.
The toxicity assessment protocol was arranged according to Xu et al.,24. The CdO NP suspensions were administered to G. mellonella larvae via injection (~ 10 μL) using a 10-μL Hamilton microsyringe (Hamilton, USA) fitted with a 22-gauge needle. Injections were performed through the last left proleg of each larva. The working solution concentrations were adjusted (see section "Preparation and Administration of CdO NPs") to deliver dosages of 0.01, 0.03, 0.05, and 0.07 (stock solution) mg of CdO NPs per gram of larval body weight. This procedure aimed to determine the dose inducing approximately 50% insect mortality. The obtained data were then used to select concentration for further assessment in biochemical assays and histological observations. The control group was injected with PBS in a volume of 10 µL. After exposure, the larvae were transported to 15 cm diameter Petri dishes and maintained at 30 ± 1 °C, 65 ± 5% rh in darkness for 9 days. The larvae were fed their standard artificial diet throughout the experiment to maintain natural conditions and avoid starvation-related confounding effects. Daily observations were made to record larval mortality, with dead larvae identified by their immobile state and characteristic blackening. Each treatment group, including the control, consisted of 10 larvae, and all experiments were conducted in triplicate.
Based on the survival of G. mellonella larvae, a dose of 0.03 mg/g body weight was selected to evaluate the sublethal effects of CdO NPs after 48 h of exposure. Sixty larvae, weighing an average of 180 ± 10 mg, were randomly separated into two groups (30 larvae per group). The control group was injected with saline, while the experimental group received a single dose of CdO NPs (0.03 mg/g body weight). Injections were performed as previously described, and the larvae were incubated at 30 ± 1 °C, 65 ± 5% rh in darkness for 48 h before hemolymph collection and dissection. The sampling time (48 h) was selected based on the survival curve analysis for the 0.03 mg NPs/g body weight group and aimed to identify early defense mechanisms activated prior to the onset of lethal effects. The timing of sample collection and measurements (48 h) was selected to capture potential changes at the tissue, cellular, and molecular levels that may have underpinned approximately 50% of the mortality effect observed later (days 7–9 of the experiment).
Hemolymph collection and dissection of larvae
Following the 48-h incubation period, hemolymph was collected from the larvae. For collection, larvae were first cooled for 5 min on ice and then surface-sterilized with 95% ethanol. Pre-cooled Eppendorf tubes containing a small amount of phenylthiourea (PTU) crystals were prepared. Hemolymph was then extracted by carefully puncturing the base of the front portion of the hind leg using a fine insect needle. After that, the hemolymph was immediately collected into the prepared Eppendorf tubes and mixed with PTU crystals to ensure thorough distribution and prevent melanization. The hemolymph samples were subsequently stored at − 80 °C for further analysis. After hemolymph collection, G. mellonella larvae were dissected, and their midguts were collected for further analysis.
The homogenization of tissues
The insect’s midgut tissues were homogenized using 100 mg of tissue in 1 mL of PBS. Before freezing the supernatants at – 80 °C, homogenates were centrifuged at 13,000×g for 10 min at 4 °C to remove cellular debris25.
X-ray Detection of Heavy metals and Cd accumulation in the Midgut of G. mellonella
A Link-Isis EDX connected to a SEM (Jeol JSM-5300, Tokyo, Japan) at an accelerating voltage of 20 kV was used to analyze three samples from each group in order to determine the percentage of heavy metals deposited in the midgut tissues obtained from G. mellonella larvae. The EDX program automatically determined each peak’s identity based on element intensity relative to reference elements.
Biochemical assay determination
The total protein content of midgut tissue homogenates (mg/mg tissue) was measured according to Lowry et al.,26. The malondialdehyde (MDA) level was measured using the Ohkawa et al.,27 procedure to evaluate lipid peroxidation. The assay relies on the interaction of 2-thiobarbituric acid (TBA) with MDA at (95 °C), which produces a pink 2-thiobarbituric acid-reactive substance (TBARS) that can be detected with a spectrophotometer at 532 nm. The control was a reaction mixture devoid of a midgut sample.
To measure reduced glutathione (GSH) concentration, a volume of 600 µL of reaction buffer, 40 µL of 0.4% (w/v) DTNB, and 10–100 µL of CE were mixed, then Milli-Q water was added to reach a final volume of 1,140 µL. After gently mixing, GSH concentration was measured. Except for CE, all reagents were present in the blank solution28.
In order to ascertain the activities of detoxifying enzymes, glutathione S-transferase (GST) activity was estimated using the method previously reported by Carmagnol et al.,29 and β-carboxylesterase (CarE) activity was assessed using the procedure outlined by Thompson30. Further, the superoxide dismutase (SOD) level was evaluated utilizing the EliteTM SOD Activity Assay Kit (MBS433565, MyBioSource Co., San Jose, CA, USA) following the manufacturer’s procedures.
Quantification of heat shock proteins
ELISA tests from Enzo Life Sciences were used to perform quantitative HSP analysis. The commercial kits utilized were the HSP70 high-sensitivity ELISA kit and the HSP90 (human) ELISA kit. As directed by the manufacturer, three independent replicates of each test were conducted.
Estimation of metallothionein (MT)
Following the manufacturer’s instructions, the Metallothionein ELISA kit (Cat. no. CSB-E11315r) (CUSABIO Technology LLC., TX, USA) was used to assess the MT concentration quantitatively.
Assay for cytochrome P450 monooxygenase
The cytochrome P450 activity of insect homogenates was measured following Chang et al.,31. The NaOAc buffer was made from 800 mL of filtered water with 0.25 M sodium acetate (C2H2NaO2) dissolved. After that, acetic acid was added to reduce the pH to 5.0. After dissolving 20 mg of TMBZ in 25 mL of 100% methanol, 75 mL of 0.25 M NaOAc buffer (pH 5.0) was added. To create the oxidase positive control stock, 100 mL of 0.25 M NaOAc buffer (pH 5.0) was mixed with 10 mL of cytochrome-C.
The aliquot of 100 μL of KPO4 was added to the wells with positive and negative samples, filled with insect homogenates. Three wells were filled with 100 μL of the cytochrome-C positive control solution as positive controls, and each test well received 200 μL of TMBZ solution and 25 μL of 3% hydrogen peroxide (H2O2). Oxidase activity was measured by reading the optical density (OD) at 620 nm following a 5-min incubation period at room temperature. The cytochrome P450 activity measured by plate reading was represented in mUnits (mU) per mg of protein.
Total Hemocyte Count of THC
Following the procedure outlined by Jones32, the hemolymph samples were collected, and the Total Haemocyte Count (THC) was performed using a hemocytometer. Acidified physiological saline (0.1–2% acetic acid) was used to dilute hemolymph (1:20). Viable hemocytes were distinguished from non-viable ones using trypan blue staining. A hemocytometer was used to measure the total number of hemoglobin cells under a microscope. Hemocytes in five 1 mm squares X dilution X depth factor of chamber number of squares counted is the formula used to determine the hemocyte numbers per cubic mm. Generally, cells in the four corners and central squares are counted if the distribution is even.
Evaluation of cell viability
According to the manufacturer’s instructions, midgut tissues were subjected to flow cytometric analysis using the TACSTM Annexin-V-FITC apoptosis detection kit (TA4638, Germany). In short, the midgut tissues were homogenized in cold phosphate-buffered saline (PBS, pH 7.4) at 4 °C to extract the cell suspension. Before being resuspended in 195 µL of binding buffer, the cells were extracted and given two PBS washes. The cell suspensions were kept in the dark for 10 min after adding 5 µL of Annexin-V-FITC conjugate reagent. Following cell washing, 190 µL of binding buffer was used to resuspend the cells, and then 10 µL of propidium iodide solution was added. Then, the states of various cells were determined using flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA). The results were evaluated using Cell Quest Pro version 5.2.1, 2005 (Becton Dickinson, San Jose, CA, USA).
Evaluation of DNA damage
According to Tice et al.,33, the comet test was used to assess the genotoxicity of the cells isolated from G. mellonella midgut tissues. Before homogenizing in a cold buffer containing 0.075 M NaCl and 0.024 M Na2EDTA, the tissues were cut with tiny dissecting scissors. The cell pellet was produced by centrifuging the cell suspension for 10 min at 700 g and 4 °C, followed by two washes with the same buffer. Molten low-melting-point (LM) agarose was added to the cells, and the mixture was spread out over a frosted surface. Before starting the high pH (pH 13) electrophoresis, the slides were submerged in the lysis solution for 60 min.
After that, the slides spent fifteen minutes submerged in a neutralizing buffer. The samples were then dried, stained with ethidium bromide, and examined using a Leitz Orthoplan epi-fluorescent microscope fitted with a 590 nm barrier filter and an excitation filter ranging from 515 to 560 nm. A computer-based image analysis system (Comet Assay V software, Perspective Instruments) was linked to the microscope. 50–100 randomly chosen cells per slide were used to identify comet cells, and DNA damage was measured as tail length, tail DNA percentage, and tail moment correspondingly.
Morphological evaluation of G. mellonella midgut tissue
Midgut tissue samples were prepared for SEM evaluation according to the method described by Arafat et al.34 In brief, midgut tissues from each group were fixed in cold 4F1G buffer (4% formaldehyde – 1% glutaraldehyde fixative solution in PBS) and then post-fixed in 2% osmium tetroxide for two hours at 4 °C. The samples were subsequently washed in PBS (two hours at 4 °C), dehydrated in ascending grades of ethanol (50%, 70%, 90%, 95%, and 100% for 15 min per step; 4 °C), and dried using a critical point dryer (Minnesota, USA). Next, the samples were mounted on aluminum stubs. Finally, they were coated with gold–palladium using a sputter-coating device (JFC-1100 E) and examined under a SEM (JEOL JSM-5300, Japan).
Midgut tissue pathohistological and ultrastructural evaluations
Midgut histological sections obtained from control and CdO-treated larvae were prepared following the previously described procedure by Arafat et al.35 Briefly, following dissection, midgut samples were fixed for 24 h in 10% formaldehyde before processing. Then, dehydrated using a graded ethanol series (70–100%) for 10 min per step, 4 °C., samples were embedded in paraffin (Leica Microsystems, Heidelberg, Mannheim, Germany). Employing a Leica RM 2255 microtome, sections of 7 μm thickness were obtained. Finally, the sections were stained with hematoxylin and eosin (H&E) and examined under a light microscope (Olympus CX31, Tokyo, Japan).
For ultrastructural analysis, midgut tissues were fixed, dehydrated, and dried the same way as previously mentioned in section "Morphological evaluation of G. mellonella midgut tissue". Then, samples were sectioned into 60 nm-thick slices using an LKB ultramicrotome (LKB Bromma 2088 Ultrotome, Leica Instruments, USA) and placed onto 200-mesh naked copper grids. The sections were stained with uranyl acetate and lead citrate before being examined using a transmission electron microscope (TEM, JEM-1400 Plus, Tokyo, Japan) at an acceleration voltage of 80 kV.
Statistical procedures
Kaplan–Meier survival analysis was employed to generate survival curves for experimental groups treated with CdO NPs at concentrations of 0.01, 0.03, 0.05, and 0.07 mg/g of body weight. A log-rank test (Chi2, p < 0.05) was conducted to assess significant differences between the survival curves. Subsequently, pairwise comparisons using the log-rank test were performed to identify significant differences between the control and each treated group. Since the highest mortality was recorded on day 7, the LD50 for this time point was determined using an online calculator (Quest Graph™ LD50 Calculator, AAT Bioquest, Inc., Sunnyvale, CA, United States). Shapiro–Wilk normality test and Levene test for equal variances were conducted before selecting the appropriate statistical test to analyze significant differences between the control and the CdO NP-treated groups. As the results confirmed the assumptions of normal data distribution and equal variances, an unpaired t-test was used in the subsequent analysis. Principal Component Analysis (PCA) and hierarchical clustering were also performed for all parameters. All measurements were accomplished in triplicate, and the results are shown as mean ± SD. Statistical analyses were carried out using Statistica 13.3 and R version 4.2.2. Charts were created using BioRender.com.
Results
Characterization of CdO NPs
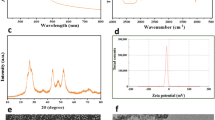
The structural and morphological characterization of the CdO NPs revealed several distinctive features. The particle size distribution analysis demonstrated a relatively narrow size range, with D50 and D90 values of 62 nm and 74 nm, respectively, indicating good size uniformity of the nanoparticles (Fig. S1A). The elemental composition, determined by EDS (Fig. S1B), confirmed the presence of Cd and O, with mass percentages of 71.91% and 26.71%, respectively, corresponding to atomic percentages of 28.09% for Cd and 73.29% for O, validating the formation of CdO NPs 23. The XRD pattern (Fig. S1C) confirmed the pure CdO with a cubic crystalline structure, exhibiting characteristic diffraction peaks. These peaks perfectly matched the standard JCPDS card (00-005-0640), indicating high phase purity. The FTIR spectrum (Fig. S1D) exhibited several characteristic absorption bands. The peaks at 449.09 cm-1 and 414.02 cm–1 were attributed to the Cd–O stretching vibration modes, confirming the presence of the metal oxide bond 27. The morphological analysis using SEM and TEM imaging (Fig. 1A,B) revealed oval- and spherical-shaped particles with a tendency toward agglomeration or clustering.
Cadmium oxide NPs characterization; (A) TEM image. (B) SEM image. (C) Kaplan–Meier survival analysis for G. mellonella exposed to CdO NPs. Insects (n = 30 per group) were exposed to nanoparticles for 9 days at the following doses: 0.01, 0.03, 0.05, and 0.07 mg/g of body weight. The Log-rank test results confirmed significant differences between the control and each treated group (p ≦ 0.0001 for CdO 0.01 and CdO 0.03 groups; p = 0.0006 for CdO 0.05 group, and p = 0.0052 for CdO 0.07 group).
Survival probability
Kaplan–Meier survival analysis demonstrated a significant impact of CdO NPs on the survivability of G. mellonella over the 9-day exposure period (Fig. 1C). Interestingly, the most pronounced effect was observed at the lowest dose of 0.01 mg/g body weight. As the concentration increased, the effect diminished; however, all investigation groups showed statistically significant differences compared to the control. For the 0.03 mg/g CdO group, 18 out of 30 individuals survived after 9 days of exposure. The LD50 value on day 7, when the highest mortality rate was recorded across all groups, was 0.0299. The following equation describes the relationship:
EDX evaluation of Cd agglomerated in midgut tissues of G. mellonella larvae
EDX analysis demonstrated a significant accumulation of cadmium in G. mellonella midgut tissues following a single injection of CdO NPs compared to the control (Fig. 2). Significantly, cadmium (Cd) perceived in the midgut tissue of the CdO NP-treated larvae (0.17 ± 0.02%) was the only difference from the other metals perceived in control tissues. The sulfur (S) concentration also reported a noticeable accumulation record in the midgut tissues dissected from the CdO NP-treated larvae (2.36 ± 0.03). These findings confirm the accumulation of Cd, which may result from the direct penetration of CdO NPs from the hemolymph into intestinal cells via membrane translocation. At the same time, the gradual release of Cd ions into the hemolymph cannot be excluded. Circulating hemolymph contacts all internal tissues, thereby facilitating the accumulation of Cd also within the intestinal tissue.
Impact of CdO NPs on physiological properties of G. mellonella larvae midgut
Hemocyte density in G. mellonella hemolymph was assessed in the control and CdO NP-treated larvae, as depicted in Fig. 4. Analysis of the total hemocyte count (THC; cell concentration 5 × 105 cells/mL) results reveal a significant intensification in the THC of the larvae exposed to CdO NPs compared to the control larvae (Fig. 3A). These findings propose that an inflammatory response was promoted following the exposure to CdO NPs.
The stress markers levels (mean ± SD) in midgut tissue of G. mellonella exposed to CdO NPs (0.03 mg/g body weight) for 48 h. (A) (THC)—total hemocyte count, (B) (MDA)—malondialdehyde, (C) (TP)—total protein, (D) (SOD —superoxide dismutase, (E) GSH—reduced glutathione, (F) (P450)—cytochrome P450, (G) (GST)—glutathione S-transferase, (H) CarE—carboxylesterase, (I) (MT)—metallothioneins, (J) (HSP 70)—70 kDa heat shock protein, and (K) (HSP 90)—90 kDa heat shock protein. *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant differences between the exposed group and the control group (unpaired t-test).
Remarkably, substantial dysregulations were documented in biochemical parameters of G. mellonella midgut tissue homogenate following exposure to CdO NPs. Compared to the control larvae, the MDA and TP levels were significantly increased following the adoption of CdO NPs, along with a substantial diminution in SOD activities and a notable rise in GSH level (Fig. 3B–E). Moreover, the detoxification parameters, cytochrome P450, GST, and CarE showed differing responses, including an increase in CYP450 and GST along with a reduction in CarE activities (Fig. 3F–H). Additionally, significant increases in MT, HSP70, and HSP90 levels were documented in the midgut of CdO NP-treated larvae (Fig. 3I–K). These findings suggest the physiological alteration in oxidative stress and detoxification biomarkers because of CdO NPs accumulation in midgut tissue, thus implying obstacles in antioxidant and detoxification defense systems.
Genotoxicity and cytotoxicity assessment in midgut tissues of G. mellonella
Comet assay results demonstrated a substantial elevation in comet assay parameters, involving a percentage of tailed cells, tail moment, tail length, and percentage of DNA in comet tail, along with a reduction in the untailed cells percentage (Figs. 4A-E and S2A,B). Consequently, DNA damage following the adoption of CdO NPs leads to critical cell death in the larval midgut tissues compared to the control larvae. Critically, the ratio of both late and early apoptotic cells was significantly raised along with a prominent reduction in viable cell percentage, as apparent from the quantification in (Figs. 4F–I and S2C,D). The comet and Annexin-V-FITC findings substantiate the deleterious genotoxic and cytotoxic effects, thus confirming oxidative stress results.
DNA damage and cell status in the midgut tissue of G. mellonella treated with CdO NPs (0.03 mg/g body weight) for 48 h, measured by the comet assay and annexin V apoptosis and necrosis assay, respectively. (A) Tail DNA—a percentage of DNA in the comet tail, (B) Tail length—comet tail length, (C) TM—tail moment, (D) Tailed cells, (E) Untailed cells, (F) Viable cells, (G) Early apoptotic cells, (H) Late apoptotic cells, (I) Necrotic cells. Principal component analysis (PCA) for all analyzed data (J) and dendrogram of hierarchical clustering (K). Abbreviations: Diagrams (J) and (K): HSP 70—70 kDa heat shock protein, HSP 90—90 kDa heat shock protein, MT—metallothioneins, GSH—reduced glutathione, GST—glutathione S-transferase, SOD—superoxide dismutase, MDA—malondialdehyde, P450—cytochrome P450, CarE—carboxylesterase, TP—total protein, THC—total hemocyte count, TDNA—a percentage of DNA in the comet tail, TL—comet tail length, TM—tail moment, TC—tailed cells, UC—untailed cells, VC—viable cells, EA—early apoptotic cells, LA—late apoptotic cells, and NC—necrotic cells. ***p < 0.001, ****p < 0.0001 denote significant differences between the exposed and control groups (unpaired t-test).
PCA description
The PCA analysis divided the data into two distinct groups, with Principal Component 1 (PC1) accounting for as much as 87.01% of the total variance. The parameters VC, UC, CarE, and SOD were correlated with each other and positively associated with PC1 (all showing higher values in the control group in comparison with the treated group). The remaining parameters, except for NC, were negatively associated with PC1 (displaying lower values in the control group compared to the treated group). Only the NC parameter was associated with Principal Component 2 (PC2), which explained just 6.09% of the data variance (Fig. 4J). Hierarchical clustering analysis confirmed strong and close relationships among most of the studied variables while also highlighting the distinct activity of CarE and SOD (Fig. 4K).
SEM investigation
Scanning electron microscope investigations were conducted to evaluate the morphological alterations of the midgut of G. mellonella following exposure to CdO NPs. Analysis of electron micrographs illustrated the altered morphology of midgut tissue following CdO NPs treatment compared to the characteristic morphology of midgut tissue obtained from control larvae (Fig. 5). In detail, midgut tissues of the control G. mellonella larvae displayed normal epithelium with a lumen characterized by various midgut folds with numerous cytoplasmic protrusions. Cytoplasmic protrusions appeared like a carpet overlaying the midgut epithelium, impeding the observation of the apical microvilli. Further, most cytoplasmic protrusions appeared with smooth surfaces (Fig. 5A,B). Further, higher magnifications of midgut tissues of the control larvae reveal obvious enterocyte boundaries along with microvilli projecting from apical surfaces. Also, the peritrophic matrix covering the epithelium was observed (Fig. 5C and C*). Conversely, the midgut tissue of larvae treated with CdO NPs appeared with general epithelial disorganization (Fig. 5A`) with ruptured plasma membranes of cytoplasmic protrusions (Fig. 5B` and B*). Additionally, altered morphology of the midgut lumen was noticed with signs of damage to the peritrophic matrix (Fig. 5C`).
Scanning electron micrographs illustrating the midgut morphology of G. mellonella obtained from control larvae (A-C) and CdO NP-treated group (A`- C`). (A) illustrate the lumen of midgut of the control group. (B) illustrates the typical cytoplasmic protrusions in between the midgut enterocytes. (C) exposes cell boundaries, microvilli, and peritrophic matrix. (C*) presents higher magnification of panel B. (A`) illustrates morphologically altered lumen of midgut of the treated group. (B`) shows rupture of plasma membranes of cytoplasmic protrusions (purple arrow). (B*) Privileged enlargement of the inset in A`. (C`) depicts the altered morphology of midgut lumen. (Blue circle) presents the damage of the peritrophic matrix. (A and A`) (scale bar = 50 µm and magnification X 500), (B and B`) (scale bar = 5 µm and magnification X 3000), (B*) (scale bar = 1 µm and magnification X 14,000),C and C` (scale bar = 5 µm and magnification X 5000), (C*) (scale bar = 1 µm and magnification X 10,000).
Pathohistological assessment
Examination of midgut tissues of G. mellonella control larvae, as illustrated in (Fig. 6A–C) revealed that it consisted of regular cellular arrangements of numerous columnar cells with goblet cells and their cavities in between them in addition to nidi of regenerative cells, all distributed entirely throughout its extension. Furthermore, various cytoplasmic protrusions were noticed on the apical surface of the midgut columnar cells, confirming the SEM findings. By contrast, exposure of G. mellonella larvae to CdO NPs resulted in significant structural anomalies in the midgut epithelium with general disorganization throughout its whole extension. Most notably, histopathological features were identified approximately along the intestinal cells. In addition, variable levels of midgut tissue damage and degeneration were observed, including sloughed midgut epithelium, vacuolation, necrotic signs, and decreased presence of columnar and regenerative cells, as shown in (Fig. 6A`–C`).
Photomicrographs illustrating the histological structures of G. mellonella larvae midgut tissue obtained from control (A–C) and CdO NPs-treated group (A`–C`). Lumen (L), columnar cell (CC), cytoplasmic protrusion (CP), regenerative cells (R), vacuole (V), the asterisk (*) signifies goblet cell cavity. Note that the rectangle in (A`–C`) denotes the most prominent damage in the slide. In all photos, the scale bar = 50 µm and magnification 400.
Ultrastructure assessment
TEM was used to evaluate the damage of the midgut epithelium of G. mellonella following their exposure to CdO NPs with reference to the control midgut tissue. Examination of the control midgut epithelium revealed well-organized cells presenting the typical structure of the midgut epithelium of G. mellonella, as illustrated in (Fig. 7A–C). Numerous digestive cells in the form of columnar cells with large nuclei and well-organized apical microvilli were observed. Also, enterocyte apical cytoplasm was noticed with various organelles. Further, intact basal lamina was observed with mitochondria-rich basal cytoplasm. By contrast, midgut tissues of CdO NP-treated larvae showed severe tissue injury lacking the representative construction of the normal tissue, including prominent vacuolization, degeneration of cytoplasmic organelles, distorted microvilli, lysis of cytoplasm, the disintegration of digestive cells, necrosis of goblet cells. Further, ruptured cell membranes were noticed, which were mainly provoked through a necrotic form of cell death, as shown in (Fig. 7A`–C`).
Transmission electron micrographs illustrate the ultrastructure construction of G. mellonella larvae midgut from the control (A–C) and CdO NP-treated group (A`- C`). (A and B) showing typical digestive cells with large nuclei and apical cytoplasm with numerous organelles. (C) presenting the basal cytoplasm of the midgut digestive cells with normal basal lamina. (A`) illustrating degenerating digestive cells lacking the characteristic organelles of the apical cytoplasm with obvious vacuolation, and distorted microvilli (purple braces). (B`- C`) demonstrating the necrotic damage of midgut cells (asterisk *), rupture of cell membranes, and cytoplasmic lysis. Lumen (L), digestive cell (DC), microvilli (Mv), nucleus (N), autophagic structure (au), basal lamina (Bl), vacuole (V). In all photos, the scale bar = 2.0 µm and magnification X 2000.
Discussion
The outcomes of the present study revealed a noteworthy impact of CdO NPs on G. mellonella larvae survival, immune response, genotoxicity, and cytotoxicity, along with substantial physiological alterations and structural injuries. Interestingly, the application of CdO NPs on G. mellonella larvae at a single dose of 0.01 mg/g body showed the highest mortality in larvae. By contrast, the mortality rates of larvae significantly declined with the increase of CdO NPs concentration. This could be related to the magnetic properties of NPs, which predominantly contribute to their agglomeration within insect tissues36. Accordingly, higher concentrations can result in larger accumulation, consequently reducing their uptake within insect tissues. Bioaccumulation of NPs is achieved by their small characteristic size, which is combined with a large surface area, facilitating their passage through cells and their interaction with biomolecules, including proteins and nucleic acids, leading to toxicity37 , clarifying the attenuated toxicity of the higher concentrations of CdO NPs documented in this study. Therefore, the circulation of lower concentrations of CdO NPs can influence their accumulation in multiple organs because of their smaller agglomeration. Thus, impacts the overall insect homeostasis and potentially causing death38. Remarkably, high xenobiotic doses can lead to insect resistance due to the increase in mutation rate39. Accordingly, we presume that larvae injected with a high concentration of CdO NPs could adapt to NPs, consistent with34, as they demonstrated that an intermediate dose of 0.03 mg/g of NiF22O4 NPs was the lowest and most effective dose against male Blaps polychresta with 67% mortality after 48 h of exposure.
Notably, the bioaccumulation of CdO NPs in the midgut tissue of G. mellonella was evinced using SEM–EDX analysis, revealing a substantial accumulation of Cd compared to the control larvae. Our study employed injection as the exposure method to maintain complete control over the administered dose. Nevertheless, cadmium was detected in the midgut tissue. This implies that CdO NPs possess the capacity to traverse the cellular barriers of G. mellonella. Given their nanoscale dimensions, the ability to migrate across cell membranes and biological barriers may extend to various tissues and potentially lead to the widespread distribution of nanoparticles throughout the organism. This, in turn, may result in toxic effects, including in the larval midgut tissue, and contribute to the observed lethality. An alternative hypothesis that should also be considered involves the migration of cadmium ions. It is well established that metal-based nanoparticles tend to release metal ions gradually. Therefore, even if a portion of the injected nanoparticles initially settled in the body cavity, it is highly plausible that Cd ions may be released and distributed systemically via the hemolymph over time. However, there was little variation in the lethality of G. mellonella larvae exposed to okadaic acid between force-feeding and injection40. Additionally, along with Cd, a noticeable increase in sulfur content in the midgut tissue of G. mellonella was recorded. Sulfur has been reported to mitigate metal toxicity. Specifically, it can alleviate Cd toxicity41. Further42, documented that sulfur might restrain Cd uptake. Consequently, in the present study, we suggest that sulfur elevation in midgut tissue following the exposure of CdO NPs could be attributed to the increase in sulfur-containing compounds, such as GSH and GST, as a defense response against Cd toxicity.
Hemocytes are responsible for important immune functions regarding humoral and cellular responses43. Our results have shown that the administration of CdO NPs led to significant hemocyte activation and increased THC. As mentioned above, this may represent either a direct effect resulting from the migration of CdO NPs into the hemocytes or an indirect effect associated with the migration of cadmium ions into these cells. Consistent with our findings, previous studies on Locusta migratoria, Bombyx mori, and G. mellonella demonstrated an elevation in THC following exposure to Al2O3 NPs43, ZnO NPs44, and CuO NPs45. Further, it interpreted that increase in THC as an immune response in the form of induction of hematopoiesis through hemocyte mitotic division activation in response to heavy metals accumulation43. Conversely22, reported a reduction in viable cell counts of G. mellonella larvae midgut after 48 h of exposure to polypropylene. Authors of this study related that decrease to physiological alterations that occur during larval pupal transformation. Also, Liao et al.46 documented a significant reduction in G. mellonella larvae hemocytes after their exposure to insecticidal proteins of Enterobacter cloacae, mainly because of the inhibition and destruction of cellular immune response. Another contrary effect was reported by Iwański et al.,47 in G. mellonella larvae following treatment with Pseudomonas aeruginosa exotoxin A. Authors of this study implied a substantial decrease in THC as a consequence of inhibited hemocyte proliferation or cytotoxic activity.
It is broadly determined that insect midgut normal physiology is necessary for passing through reproduction, development, and growth48. Midgut tissue of G. mellonella demonstrated critical injury on the biochemical and cytological levels within 24 to 48 h following injection of CdO NPs, leading to sufficient impairment to activate the defense systems by inducing GSH, CYP450, and GST. However, we noticed a decrease in SOD and CarE activities with elevation in MT, HSP70, and HSP90 levels. Similar findings regarding the activation of the immune and repair systems through the significant rise in hemocytes number and the induction of GST and SOD, respectively, were reported by Emery et al.,20 within 4 to 24 h following the intrahaemocoelic injection of G. mellonella larvae with indomethacin, which evidenced the damage in the midgut. They documented deteriorated midgut tissue as sloughed epithelium within the lumen, vacuolations, apoptotic bodies, and nuclear damage. Moreover, previous findings by Ogasawara et al. (2014)49 suggested that Cd toxicity induces GSH synthesis as a response to its detoxification. They specified that the instant rise in GSH is a defense response and subsequent activation of MT with chelating potential for Cd accumulated in cells, thereby suppressing Cd toxicity. However, cells might undergo injury when GSH and MT are overwhelmed due to elevated Cd accumulation within cells15 . Further, Cd could accumulate in intestinal tissue through binding with metal transporters such as MT, according to Ohta and Ohba,50 findings. Nevertheless, we propose that SOD antioxidant function toward detoxification of free radicals might be affected as a result of Cd exposure in the same manner as other metalloproteins dysfunction due to heavy metal exposure15.
Stress proteins, including HSPs and Mt in Cadmium-low-tolerance insects, are known to be key protective against Cd toxicity51. In this study, the levels of HSPs and Mt were significantly increased following Cd intoxication. These findings are consistent with the report of Tarnawska51 they documented an increase in stress protein levels, including HSPs and Mt, in Spodoptera exigua exposed to high Cd concentrations, suggesting that HSPs and MT play a protective function in cadmium-low-tolerance insects. Consistent with a previous report by34, the significant proliferation in HSP70 expression might be a defensive mechanism to reinstate larval cellular integrity.
Genomic instability can also be expected due to exposure to Cd15. The genotoxic effects of cadmium are multifactorial and involve the induction of oxidative stress and DNA damage mediated by reactive oxygen species (ROS), the inhibition of DNA repair mechanisms, binding with DNA, and/or interference with DNA-stabilizing proteins1,52,53,54. The increased DNA fragmentation observed in our study matches previous observations by Mahmood et al. (2024)1. Authors of this study reported a significant spike in the entire comet parameters, in association with elevated lipid peroxidation levels, alongside a reduction in antioxidant enzymes, including SOD.
Furthermore, in our research, exposure of G. mellonella larvae to CdO NPs notably affects the health status of larval midgut cells. Similarly, previous studies showed increased apoptosis and necrosis combined with decreased viable cells as a result of heavy metals toxification55,56 or pathogens infections47. Consequently, various histological abnormalities were observed in the midgut epithelium of CdO NP-treated larvae (Figs. 5, 6 and 7). The general morphology of the control midgut epithelium of G. mellonella larvae is analogous to that previously described for many lepidopterans such as Diatraea saccharalis57 and Manduca sexta 58. Also, it is ultrastructurally similar to the control midgut tissue of G. mellonella larvae depicted by Rost-Roszkowska et al.22. Numerous apoptotic cells among columnar cells, and autophagic structures were noticed in the Spodoptera exiqua midgut epithelium of various groups and generations reared on Cd-contaminated food59. Similarly, consuming polypropylene by G. mellonella larvae leads to notable cytoplasm vacuolization, which is considered a necrotic symptom at the beginning of the cytopathological process22. Consistent with our findings, Dabour et al.,60 documented apparent cytological alterations in the midgut epithelium of Apis millefera workers following their exposure to CdO and PbO NPs, including vesiculation, chromatin condensation and irregular distribution, mitochondrial lysis, dilation of endoplasmic reticulum, and fragmentation. Also, on the ultrastructure level, Elhenawy et al.,55 documented a notable distortion of Musca domestica larval midgut following exposure to CdS NPs. Further, a previous report by Shi et al.,48 documented destruction in silkworm midgut normal functions following exposure to lead because of the reduction in microvilli, which might affect larval development. Along with deformed columnar cells and dissolved intestinal cell walls. This consequently reduced the function of the intestinal barrier and increased pathogen invasion. The studies cited above, as well as our results, reveal the high sensitivity of midgut tissue to various toxins, including CdO NPs. Even if a toxic substance does not enter the organism directly via the digestive tract, it may still migrate to this tissue and induce all the described adverse effects, as illustrated in (Fig. 8).
Conclusion
In conclusion, this study is the first to investigate the detrimental effects of CdO NPs on the larval midgut tissue of Galleria mellonella. Our findings demonstrate that CdO NPs induce significant adverse effects, including oxidative stress, disruption of detoxification defense systems, DNA damage, and an increase in apoptotic, necrotic, and dead cells—ultimately compromising the integrity of the intestinal barrier. These results contribute to a better understanding of cadmium toxicity and its specific impact on midgut tissues.
However, the study has some limitations. While injection ensures precise control over the administered dose, it does not reflect natural exposure routes. Therefore, future research should aim to assess the toxicity of CdO NPs following oral exposure. Additionally, it would be valuable to investigate the effects of CdO NPs on other tissues and to conduct studies that elucidate the pathways of nanoparticle distribution and migration within the organism.
Data availability
Data is contained within the article.
References
Mahmood, S., Parwez, H., Siddique, Y. H., Amir, M. & Javed, S. Assessing the multi-dimensional impact of lead-induced toxicity on collembola found in maize fields: From oxidative stress to genetic disruptions. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 898, 503789. https://doi.org/10.1016/j.mrgentox.2024.503789 (2024).
Ankush, Ritambhara, Lamba, S., Deepika & Prakash, R. in Cadmium Toxicity in Water (eds A.K. Jha & N. Kumar) 3–20 (Springer, 2024).
Kaushik, S., Ranjan, A., Sidhu, A., Singh, A. K. & Sirhindi, G. Cadmium toxicity: its’ uptake and retaliation by plant defence system and ja signaling. Biomet. Int. J. Role Metal Ions Biol. Biochem. Med. 37, 755–772. https://doi.org/10.1007/s10534-023-00569-8 (2024).
Shao, Y., Zheng, L. & Jiang, Y. Cadmium toxicity and autophagy: A review. Biomet. Int. J. Role Metal Ions Biol. Biochem. Med. 37, 609–629. https://doi.org/10.1007/s10534-023-00581-y (2024).
Soni, S., Jha, A. B., Dubey, R. S. & Sharma, P. Mitigating cadmium accumulation and toxicity in plants: The promising role of nanoparticles. Sci. Total. Environ. 912, 168826. https://doi.org/10.1016/j.scitotenv.2023.168826 (2024).
Donia, D. T. & Carbone, M. Fate of the nanoparticles in environmental cycles. Int. J. Environ. Sci. Technol. 16, 583–600. https://doi.org/10.1007/s13762-018-1960-z (2019).
Arienzo, M. & Ferrara, L. Environmental fate of metal nanoparticles in estuarine environments. Water 14, 1297. https://doi.org/10.3390/w14081297 (2022).
Mishra, S. & Sundaram, B. Fate, transport, and toxicity of nanoparticles: An emerging pollutant on biotic factors. Process Saf. Environ. Prot. 174, 595–607. https://doi.org/10.1016/j.psep.2023.04.037 (2023).
Rather, A. et al. Recent progress in the green fabrication of cadmium sulfide and cadmium oxide nanoparticles: Synthesis, antimicrobial and cytotoxic studies. Mater. Sci. Eng. B 286, 116022. https://doi.org/10.1016/j.mseb.2022.116022 (2022).
Ebrahim, F., Al-Hartomy, O. & Wageh, S. Cadmium-based quantum dots alloyed structures: Synthesis, properties, and applications. Materials (Basel, Switzerland) 16, 5877. https://doi.org/10.3390/ma16175877 (2023).
Ghasempour, A. et al. Cadmium sulfide nanoparticles: Preparation, characterization, and biomedical applications. Molecules 28, 3857. https://doi.org/10.3390/molecules28093857 (2023).
Patel, K. et al. Unveiling new frontiers in advancements of bio-inspired fabrication of cadmium-based nanomaterials for biomedical and catalytic applications—A review. Inorg. Chem. Commun. 170, 113457. https://doi.org/10.1016/j.inoche.2024.113457 (2024).
Amr, A. et al. Liquid chromatography–mass spectrometry profiling of propolis and royal jelly and their ameliorative effects on cadmium-instigated pathological consequences in ovarian tissues of rats. Microchem. J. 207, 111800. https://doi.org/10.1016/j.microc.2024.111800 (2024).
Wang, Y. L. et al. Oxidative stress and potential effects of metal nanoparticles: A review of biocompatibility and toxicity concerns. Environ. Pollut. 346, 123617. https://doi.org/10.1016/j.envpol.2024.123617 (2024).
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M. R. & Sadeghi, M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. 12, 643972. https://doi.org/10.3389/fphar.2021.643972 (2021).
Nivetha, A., Mangala Devi, S. & Prabha, I. Fascinating physic-chemical properties and resourceful applications of selected cadmium nanomaterials. J. Inorg. Organomet. Polym. 29, 1423–1438. https://doi.org/10.1007/s10904-019-01141-z (2019).
El-Samad, L. M., El-Gendy, A. H., Abdel-Moneim, A. M., El-Ashram, S. & Augustyniak, M. CuO NPs-induced damage to testes and deregulation of the antioxidant system in wild terrestrial organism Blaps sulcata (Coleoptera: Tenebrionidae). Environ. Nanotechnol. Monit. Manag. 18, 100751. https://doi.org/10.1016/j.enmm.2022.100751 (2022).
Chen, R. Y. & Keddie, B. A. The Galleria mellonella-Enteropathogenic Escherichia coli model system: Characterization of pathogen virulence and insect immune responses. J. Insect. Sci. https://doi.org/10.1093/jisesa/ieab046 (2021).
Aamer, N. A., El-Moaty, Z. A., Augustyniak, M., El-Samad, L. M. & Hussein, H. S. Impacts of combining Steinernema carpocapsae and Bracon hebetor parasitism on Galleria mellonella Larvae. Insects 15, 588. https://doi.org/10.3390/insects15080588 (2024).
Emery, H., Johnston, R., Rowley, A. F. & Coates, C. J. Indomethacin-induced gut damage in a surrogate insect model, Galleria mellonella. Arch. Toxicol. 93, 2347–2360. https://doi.org/10.1007/s00204-019-02508-4 (2019).
Campbell, J. S. et al. Characterising phagocytes and measuring phagocytosis from live Galleria mellonella larvae. Virulence 15, 2313413. https://doi.org/10.1080/21505594.2024.2313413 (2024).
Rost-Roszkowska, M. et al. Consumption of polypropylene caused some ultrastructural and physiological changes in some tissues of Galleria mellonella (Lepidoptera: Pyralidae) larvae. Eur. Zool. J. 91, 213–234. https://doi.org/10.1080/24750263.2024.2308529 (2024).
Moya-Andérico, L., Admella, J. & Torrents, E. A clearing protocol for Galleria mellonella larvae: Visualization of internalized fluorescent nanoparticles. N. Biotechnol. 60, 20–26. https://doi.org/10.1016/j.nbt.2020.08.002 (2021).
Xu, M. N. et al. Zinc oxide nanoparticles prime a protective immune response in Galleria mellonella to defend against candida albicans. Front. Microbiol. 12, 766138. https://doi.org/10.3389/fmicb.2021.766138 (2021).
Höring, M. et al. Accurate lipid quantification of tissue homogenates requires suitable sample concentration, solvent composition, and homogenization procedure-a case study in murine liver. Metabolites 11, 365. https://doi.org/10.3390/metabo11060365 (2021).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Ohkawa, H., Ohishi, N. & Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. https://doi.org/10.1016/0003-2697(79)90738-3 (1979).
Salbitani, G., Bottone, C. & Carfagna, S. Determination of reduced and total glutathione content in extremophilic microalga Galdieria phlegrea. Bio Protoc. 7, e2372. https://doi.org/10.21769/BioProtoc.2372 (2017).
Carmagnol, F., Sinet, P. M., Rapin, J. & Jerome, H. Glutathione-S-transferase of human red blood cells; assay, values in normal subjects and in two pathological circumstances: Hyperbilirubinemia and impaired renal function. Clin. Chim. Acta. 117, 209–217. https://doi.org/10.1016/0009-8981(81)90040-1 (1981).
Thompson, H. M. Esterases as markers of exposure to organophosphates and carbamates. Ecotoxicology 8, 369–384. https://doi.org/10.1023/A:1008934505370 (1999).
Chang, K. S., Kim, H. C., Klein, T. A. & Ju, Y. R. Insecticide resistance and cytochrome-P450 activation in unfed and blood-fed laboratory and field populations of Culex pipiens pallens. J. Pest. Sci. 90, 759–771. https://doi.org/10.1007/s10340-016-0820-1 (2017).
Jones, J. C. Current concepts concerning insect hemocytes. Am. Zool. https://doi.org/10.1093/icb/2.2.209 (1962).
Tice, R. R. et al. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35, 206–221. https://doi.org/10.1002/(sici)1098-2280(2000)35:3%3c206::aid-em8%3e3.0.co;2-j (2000).
Arafat, E. A. et al. Fabrication of biosynthesized nickel ferrites nanoparticles and evaluation of their insecticidal efficacy on beetles (Blaps polychresta) testicular integrity. Sci. Rep. 15, 7214. https://doi.org/10.1038/s41598-025-90496-0 (2025).
Arafat, E. A. et al. Toxicological investigations of biosynthesized nickel ferrites nanoparticles on midgut epithelium of Blaps polychresta as nanopesticides: Structural damages and oxidative stress. Pestic. Biochem. Physiol. 208, 106314. https://doi.org/10.1016/j.pestbp.2025.106314 (2025).
Ma, Y. et al. Structural and magnetic properties of cadmium oxides with different annealing temperatures. J. Alloy. Compd. 998, 174988. https://doi.org/10.1016/j.jallcom.2024.174988 (2024).
Abbasi, R., Shineh, G., Mobaraki, M., Doughty, S. & Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanopart Res. 25, 43. https://doi.org/10.1007/s11051-023-05690-w (2023).
Arafat, E. A. et al. Toxicological investigations of biosynthesized nickel ferrites nanoparticles on midgut epithelium of Blaps polychresta as nanopesticides: Structural damages and oxidative stress. Pestic Biochem. Physiol. 208, 106314. https://doi.org/10.1016/j.pestbp.2025.106314 (2025).
Muniz-Junior, G., Roque, F. D. O., Pires, A. P. F. & Guariento, R. D. Are lower pesticide doses better? An evolutionary perspective on integrated pest management. Ecol. Model. 482, 110408. https://doi.org/10.1016/j.ecolmodel.2023.110408 (2023).
Coates, C. J. et al. The insect, Galleria mellonella, is a compatible model for evaluating the toxicology of okadaic acid. Cell Biol Toxicol 35, 219–232. https://doi.org/10.1007/s10565-018-09448-2 (2019).
Li, Y. et al. Effects of sulfur on the toxicity of cadmium to Folsomia candida in red earth and paddy soil in southern Fujian. J. Hazard. Mater. 387, 121683. https://doi.org/10.1016/j.jhazmat.2019.121683 (2020).
Zhang, W. et al. Spatial distribution and toxicity of cadmium in the joint presence of sulfur in rice seedling. Environ. Toxicol. Pharmacol. 36, 1235–1241. https://doi.org/10.1016/j.etap.2013.10.007 (2013).
Arafat, E. A. et al. Entomotherapeutic Role of Periplaneta americana extract in alleviating aluminum oxide nanoparticles-induced testicular oxidative impairment in migratory locusts (Locusta migratoria) as an ecotoxicological model. Antioxidants (Basel, Switzerland) 12, 653. https://doi.org/10.3390/antiox12030653 (2023).
Mir, A. H., Qamar, A., Qadir, I., Naqvi, A. H. & Begum, R. Accumulation and trafficking of zinc oxide nanoparticles in an invertebrate model, Bombyx mori, with insights on their effects on immuno-competent cells. Sci. Rep. 10, 1617. https://doi.org/10.1038/s41598-020-58526-1 (2020).
Tunçsoy, B., Sugeçti, S., Büyükgüzel, E., Özalp, P. & Büyükgüzel, K. Effects of copper oxide nanoparticles on immune and metabolic parameters of Galleria mellonella L.. Bull. Environ. Contam. Toxicol. 107, 412–420. https://doi.org/10.1007/s00128-021-03261-0 (2021).
Liao, C. et al. Effects of insecticidal proteins of Enterobacter cloacae NK on cellular immunity of Galleria mellonella larvae. Front. Microbiol. 14, 1154811. https://doi.org/10.3389/fmicb.2023.1154811 (2023).
Iwański, B., Mizerska-Kowalska, M. & Andrejko, M. Pseudomonas aeruginosa exotoxin A induces apoptosis in Galleria mellonella hemocytes. J. Invertebr. Pathol. 197, 107884. https://doi.org/10.1016/j.jip.2023.107884 (2023).
Shi, Y. et al. Effect of lead exposure on silkworm midgut: Insights into oxidative gene expression, digestive function, and microbial community structure. Process Saf. Environ. Prot. 190, 481–494. https://doi.org/10.1016/j.psep.2024.07.048 (2024).
Ogasawara, Y. et al. Significance of the rapid increase in GSH levels in the protective response to cadmium exposure through phosphorylated Nrf2 signaling in Jurkat T-cells. Free Radic. Biol. Med. 69, 58–66. https://doi.org/10.1016/j.freeradbiomed.2014.01.005 (2014).
Ohta, H. & Ohba, K. Involvement of metal transporters in the intestinal uptake of cadmium. J. Toxicol. Sci. 45, 539–548. https://doi.org/10.2131/jts.45.539 (2020).
Tarnawska, M., Kafel, A., Augustyniak, M., Rost-Roszkowska, M. & Babczyńska, A. Microevolution or wide tolerance? Level of stress proteins in the beet armyworm Spodoptera eqigua hübner (Lepidoptera: Noctuidae) exposed to cadmium for over 150 generations. Ecotoxicol. Environ. Saf. 178, 1–8. https://doi.org/10.1016/j.ecoenv.2019.04.017 (2019).
Bertin, G. & Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88, 1549–1559. https://doi.org/10.1016/j.biochi.2006.10.001 (2006).
Đukić-Ćosić, D., Baralić, K., Javorac, D., Djordjevic, A. B. & Bulat, Z. An overview of molecular mechanisms in cadmium toxicity. Curr. Opin. Toxicol. 19, 56–62. https://doi.org/10.1016/j.cotox.2019.12.002 (2020).
Badawi, K., El Sharazly, B. M., Negm, O., Khan, R. & Carter, W. G. Is Cadmium genotoxicity due to the induction of redox stress and inflammation? A systematic review. Antioxidants 13, 932. https://doi.org/10.3390/antiox13080932 (2024).
Elhenawy, H. I. et al. Assessing the toxicity of green Agaricus bisporus-based Cadmium Sulfide nanoparticles on Musca domestica as a biological model. Sci. Rep. 14, 21519. https://doi.org/10.1038/s41598-024-70060-y (2024).
El-Samad, L. M. et al. Biomonitoring of heavy metal toxicity in freshwater canals in Egypt using creeping water bugs (Ilyocoris cimicoides): Oxidative stress, histopathological, and ultrastructural investigations. Antioxidants (Basel, Switzerland) 13, 1039. https://doi.org/10.3390/antiox13091039 (2024).
Pinheiro, D. O., Quagio-Grassiotto, I. & Gregório, E. A. Morphological regional differences of epithelial cells along the midgut in Diatraea saccharalis Fabricius (Lepidoptera: Crambidae) larvae. Neotrop. Entomol. 37, 413–419. https://doi.org/10.1590/s1519-566x2008000400009 (2008).
Windfelder, A. G. et al. An enteric ultrastructural surface atlas of the model insect Manducasexta. iScience 27, 109410. https://doi.org/10.1016/j.isci.2024.109410 (2024).
Babczyńska, A. et al. Adaptation by death? Cell death-based tolerance to cadmium in 150-generation exposure of Spodoptera exiqua Hübner (Lepidoptera: Noctuidae). Environ. Entomol. 52, 1057–1070. https://doi.org/10.1093/ee/nvad077 (2023).
Dabour, K., Al Naggar, Y., Masry, S., Naiem, E. & Giesy, J. P. Cellular alterations in midgut cells of honey bee workers (Apis millefera L.) exposed to sublethal concentrations of CdO or PbO nanoparticles or their binary mixture. Sci. Total Environ. 651, 1356–1367. https://doi.org/10.1016/j.scitotenv.2018.09.311 (2019).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
L.M.E.-S.: conceptualization, project administration, investigation, data curation, formal analysis, writing, reviewing and editing the manuscript. E.A.A.: investigation, data curation, visualization, formal analysis, writing—original draft, reviewing and editing the manuscript. H.A.A.: investigation, data curation, visualization, formal analysis, writing—original draft and editing the manuscript. A.M.A: data curation, formal analysis and writing—original draft. E.H.H.: formal analysis and writing—original draft. A.A.Al.: data curation, formal analysis and writing—original draft. M.A.: data curation, visualization, formal and statistical analysis, writing—original draft, reviewing and editing the manuscript. N.A.A.: conceptualization, investigation, data curation, visualization, formal analysis, and writing—original draft. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
El-Samad, L.M., Arafat, E.A., Aamer, H.A. et al. Insights into the pathophysiological, molecular, and ultrastructural alterations induced by cadmium oxide nanoparticle toxicity in the midgut of Galleria mellonella. Sci Rep 15, 41452 (2025). https://doi.org/10.1038/s41598-025-08578-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08578-y