Abstract
Type 2 diabetes mellitus (T2DM) and chronic periodontitis are interrelated chronic conditions that exacerbate each other’s progression. Despite their bidirectional relationship, oral health remains undervalued in diabetes management, particularly in underserved populations. The objective of this study was to assess whether a combined diabetes-periodontitis self-management intervention outperforms single-disease management in improving clinical outcomes, psychological well-being, and oral health in T2DM patients with chronic periodontitis. A randomized controlled trial was conducted across 12 community health centers in Nanjing, China, from June 2022 to June 2023. The participants (n = 784) were stratified into four groups, including G0 (control group, routine management), G1 (periodontal intervention), G2 (diabetes intervention), and G3 (comprehensive intervention), with 196 participants in each group. The control group (G0) received routine community-based diabetes management, while the intervention group (G1-G3) underwent a specialized self-management model-based intervention in addition to the routine management provided to the control group. The 3-month intervention included peer-led education on diabetes self-management, oral hygiene practices, dietary guidance, psychological support, and more. Outcomes [glycemic control, self-efficacy, quality of life (QoL), and oral health] were assessed at baseline (T0) and at 0 months (T1), 3 months (T2), and 6 months (T3) postintervention. Generalized estimating equations (GEEs) adjusted for covariates were used for longitudinal analysis. Compared with the other groups, the comprehensive intervention group (G3) had superior outcomes. At 3 months postintervention (T2), G3 showed the greatest improvements over G0 in terms of the fasting plasma glucose (FPG) control rate (B = 0.559, P = 0.027) and the glycated hemoglobin (HbA1c) control rate (B = 0.615, P = 0.007), alongside notable enhancements in self-efficacy (T1: B = 0.413, P = 0.020) and QoL (health utility: B = 0.025, P = 0.010). Specifically, compared with G0, G3 demonstrated marked improvements in oral health outcomes, including reduced gingival bleeding (B = − 0.751, P = 0.015), swelling (B = − 0.780, P = 0.018), tooth mobility (B= − 0.860, P = 0.007), chewing weakness (B = − 0.916, P = 0.015) and halitosis (B = − 0.763, P = 0.026). This study demonstrated that a community-based, integrated self-management intervention significantly improved glycemic control, self-efficacy, QoL, and oral health in T2DM patients with chronic periodontitis. By empowering patients through education and peer support, this model offers a scalable solution for underserved populations.
Similar content being viewed by others
Introduction
According to International Diabetes Federation 2021 statistics, the global population of individuals aged 20–79 years with diabetes reached 537 million, representing a 3.5-fold increase from 151 million in 2000 and is projected to rise to 783 million by 20451. Type 2 diabetes mellitus (T2DM) accounts for approximately 90% of all diabetes cases2. The rapid increase in T2DM incidence, coupled with its high morbidity and mortality from complications, poses a significant burden on individuals, families, and healthcare systems worldwide3,4. The World Health Organization reported that only 20–40% of diagnosed diabetes patients achieve optimal blood glucose control1.
Chronic periodontitis, a severe form of gum disease, is characterized by progressive attachment loss and alveolar bone resorption, leading to tooth loosening and loss, which significantly impacts overall health5. It is not only a common complication among diabetes patients but also a critical risk factor for poor glycemic control6,7,8,9,10. Studies have demonstrated11,12,13,14,15 that improving periodontal health can regulate glucose metabolism in patients with diabetes and periodontitis. The bidirectional relationship between these two conditions underscores the need for integrated management strategies that address both diseases simultaneously. However, oral health care services remain underemphasized in current diabetes management systems, and the awareness of oral health knowledge and the oral health status of diabetes patients are concerning16,17,18,19.
Oral health awareness plays a key role in diabetes self-management because chronic periodontitis is a prevalent and significant comorbidity that exacerbates glycemic control issues. Research has shown that poor oral health awareness among diabetes patients leads to under-recognition of periodontal disease, further impeding effective self-management16,19. Additionally, patients who are more aware of the interplay between their oral and systemic health are more likely to engage in preventive oral care practices, which can improve their overall health outcomes, including better glycemic control16,19. Thus, incorporating oral health awareness into diabetes self-management strategies may not only enhance the management of periodontal disease but also improve patients’ ability to control their blood sugar levels, reduce complications, and increase their overall quality of life (QoL).
The vast population of diabetes patients and the relatively limited health care resources necessitate that patients themselves take on the majority of disease management responsibilities. Self-efficacy, defined as an individual’s belief in their ability to manage their health, plays a pivotal role in diabetes self-management16,20. Higher self-efficacy has been associated with better glycemic control, improved adherence to treatment regimens, and enhanced overall well-being21,22,23,24,25. Diabetes patients often experience anxiety and concerns due to the need for strict medication adherence, lifestyle changes, and the risk of complications, which can significantly impact their QoL, as measured by health utility values and the EuroQol Visual Analogue Scale (EQ-VAS) scores26. For T2DM patients, particularly those with chronic periodontitis, enhancing self-efficacy and QoL is essential for achieving long-term health goals.
Currently, most research on interventions for glycemic control and chronic periodontitis in T2DM patients has focused on clinical measures. However, owing to high treatment costs and low oral health awareness among patients, exclusively relying on clinical interventions is insufficient to meet the oral health management needs of the vast community-based diabetes population. Strengthening self-management interventions may be an effective approach to improve both glycemic and periodontal outcomes in patients with diabetes and periodontitis27,28. There is an urgent need to widely implement oral health education programs for diabetes patients based on self-management models and integrate oral health care services into routine community-based diabetes management.
This randomized controlled trial (RCT), conducted under the National Essential Public Health Services Program, uses a multistage random cluster sampling method to select T2DM patients with chronic periodontitis managed by community-based public health services. This study aimed to evaluate the impact of a community-based comprehensive self-management intervention on glycemic control, self-efficacy, QoL, and oral health in T2DM patients with mild to moderate periodontitis. The findings provide clinical evidence for the development of feasible, effective, and scalable community-based oral health intervention models for T2DM patients.
Methods
Design and setting
This RCT was registered in the Chinese Clinical Trial Registry (ChiCTR2100052301) on 24/10/2021 and approved by the Ethics Committee of the Nanjing Center for Disease Control and Prevention (PJ2021-A001-02). This parallel, investigator-blind, controlled trial was conducted from June 2022 to June 2023 at community health service centers across 12 districts in Nanjing, China. The study adhered to the guidelines of the Helsinki Declaration, and its reporting followed the CONSORT standards. Informed consent was obtained from all participants.
From June to August 2022, a multistage stratified cluster random sampling method was employed to select 1,600 T2DM patients from communities enrolled in the National Essential Public Health Services Program in Nanjing. Eligible patients with mild to moderate chronic periodontitis were recruited via telephone invitations to participate in self-management activities and complete evaluation surveys. The classification criteria for chronic periodontitis and mild to moderate chronic periodontitis were based on previously published studies29,30.
Inclusion/exclusion criteria
Participants were included if they met the following criteria: (1) Aged ≥ 35 years; (2) Permanent residents of Nanjing (residing locally ≥ 6 months in the past year); (3) Diagnosed with T2DM according to the Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2020 edition)2, defined as fasting plasma glucose (FPG) ≥ 7.0 mmol/L, or glycated hemoglobin (HbA1c) ≥ 6.5%, or prior diagnosis by township/higher-level hospitals, or current use of antidiabetic medications/insulin; (4) Enrolled in the National Essential Public Health Services Program for T2DM management; (5) Clinically stable with no severe diabetes complications.
The exclusion criteria included the following: (1) History of infectious diseases (e.g., tuberculosis, syphilis, HIV); (2) Pregnancy; (3) Cognitive impairment or psychiatric disorders; (4) Use of medications affecting glucose metabolism or inducing gingival changes (e.g., immunosuppressants, antiepileptics, calcium channel blockers, steroids).
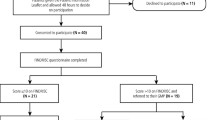
Postbaseline exclusion process: Of the 1,600 initially screened T2DM patients, 784 were enrolled after excluding those who failed to complete baseline surveys (n = 104), had severe periodontitis (n = 105) or the absence of periodontitis (confirmed via baseline periodontal examination; n = 411) or declined to participate in follow-up interventions (n = 196). The screening process is detailed in Fig. S1.
Sample size
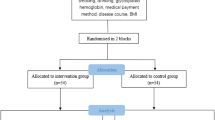
The sample size was estimated using the formula \(N=\frac{2\left({u}_{\alpha }+{u}_{\beta }\right)\times {\sigma }^{2}}{{d}^{2}}\), with a two-sided test α of 0.05, a power (1 − β) of 90%, and an expected HbA1c difference (d) of 0.5% between the intervention and control groups (7.20% vs. 6.70%)31. The estimated standard deviation (σ) was 1.4, and a 10% dropout rate was considered, resulting in a required sample size of 180 participants per group and a total of 720 participants across four groups. Ultimately, 784 T2DM patients with mild to moderate periodontitis were recruited for self-management intervention. The participants were randomly allocated by district, at a 1:1:1:1 ratio to one of four groups: the control group (G0, n = 196), periodontal intervention group (G1, n = 196), diabetes intervention group (G2, n = 196), and comprehensive diabetes and periodontal intervention group (G3, n = 196).
Interventions
For implementation, one community health center was randomly selected in each district to serve as the intervention site for all participants in that district. The three intervention groups formed self-management teams, each consisting of 12–18 T2DM patients with mild to moderate periodontitis. The intervention was based on a 3-month self-management program conducted from September to November 2022, which emphasized the principles of "peer support," "collaborative participation," and "self-management." Each team was co-led by two leaders: (1) a professional leader (e.g., physician, nutritionist) responsible for organizing evidence-based education and chronic disease management, and (2) a patient-elected deputy leader (a peer with T2DM and periodontitis) tasked with facilitating peer interactions, documenting group activities, and addressing members’ health needs. The deputy leader played a critical role in moderating small-group discussions, modeling self-management behaviors (e.g., glucose monitoring, oral hygiene practices), and maintaining peer accountability through regular reminders. Leaders underwent standardized training using the Self-Management Facilitator Manuals for Diabetes and Periodontitis Patients. Training included role-playing, simulated group sessions, and mentorship by the research team. Competency was assessed through trial presentations, and only leaders who passed this assessment were permitted to conduct group activities.
Led by two team leaders, the activities included lectures, group discussions, experience sharing, live demonstrations, and Q&A sessions with community doctors. These activities aimed to equip patients with knowledge, skills, and communication techniques for effective self-management, thereby boosting their confidence. The interventions were held at community health centers or activity rooms, with sessions occurring every two weeks (six sessions total). Each session lasted 1.0–1.5 h and comprised 4–8 subactivities.
The control group (G0) received routine health management according to the National Essential Public Health Services guidelines, with no self-management activities. The intervention group (G1-G3) underwent a specialized self-management model-based intervention in addition to the routine management provided to the control group. The G1 group focused on oral health education, the relationship between periodontal disease and diabetes, periodontal disease treatments (surgical/non-surgical), and regular follow-up maintenance, etc. The detailed activities for the G1 group are listed in Supplementary Table S1. The G2 group focused on diabetes-related knowledge, diabetes self-management skills, psychological adjustment, physical exercise, a healthy diet, medication usage, blood glucose self-monitoring, the management of complications, the maintenance of good lifestyle habits, etc. Detailed activities for the G2 group are listed in Supplementary Table S2. The G3 group received a combined intervention including content from both the G1 and G2 groups, as described in Supplementary Table S3. In addition to the manuals, the intervention utilized tailored educational materials such as PowerPoint presentations and instructional videos. For each intervention group (G1, G2, and G3), specific lesson plans were created, with six different sets of materials aligned with the intervention focus. These videos were created by the project team and regularly updated to ensure accuracy.
Outcome measures and data collection
Trained researchers, blinded to group assignments, conducted assessments at baseline (T0), 0 months (immediately postintervention, T1), 3 months (T2), and 6 months (T3) postintervention, during the period from December 1, 2022, to June 1, 2023. Data collection included questionnaires (demographic information, chronic disease self-management, oral health status, etc.), physical measurements (height, weight, etc.), laboratory tests (FPG, HbA1c, etc.), and periodontal examinations. Tablets were used for real-time data collection and validation to ensure accuracy.
Glycemic control
FPG and HbA1c levels were measured according to the Chinese national standard for blood sample collection and processing (GB/T 38,576–2020)32. On the basis of the Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2020 edition), glycemic control status was classified as follows: FPG control status (met: FPG ≤ 7.0 mmol/L; not met: FPG > 7.0 mmol/L) and HbA1c control status (met: HbA1c < 7.0%; not met: HbA1c ≥ 7.0%)2.
Self-efficacy
Self-efficacy was assessed using the eight-item Chronic Disease Self-Management Research Scale, adapted from the Stanford Patient Education Research Center’s scale developed by Lorig et al.33. Each item was rated on a 10-point Likert scale ranging from 1 (not at all confident) to 10 (totally confident). The scale score was calculated as the average of the completed items, with higher scores indicating greater self-efficacy. The scale has demonstrated high reliability and validity in previous studies34.
QoL
QoL was evaluated using the EuroQol Five Dimensions Questionnaire (EQ-5D-5L), which includes five dimensions: mobility, self-care, usual activities (e.g., work, study, housework, family or leisure activities), pain/discomfort, and anxiety/depression). The scores were calculated using the Chinese population-based EQ-5D-5L utility value system, which has demonstrated good reliability and validity35. The health utility value ranges from -0.391 to 1.000, with higher scores indicating better health. An EQ-VAS score was also recorded, where patients self-rated their health on a scale of 0 to 100.
Oral health
Oral health status was assessed on the basis of the methods used in the Fourth National Oral Health Epidemiological Survey Report of China9, covering five items: gingival bleeding (Yes/No), gingival swelling (Yes/No), tooth mobility (Yes/No), chewing weakness (Yes/No), and halitosis (Yes/No).
Healthy lifestyle score
Healthy lifestyle behaviors included: (1) never smoking or having quit smoking; (2) moderate alcohol consumption (< 25 g/day for men, < 15 g/day for women)36; (3) adequate physical activity (≥ 150 min of moderate or ≥ 75 min of vigorous activity per week)37; and (4) a healthy diet, scored on the basis of the frequency of consuming six food groups (vegetables, fruits, whole grains, aquatic products ≥ 1 time/week, soy products ≥ 4 times/week, and red meat < 7 times/week)38. The diet score ranges from 0–6, with a score of 4 or higher considered a healthy diet. Each behavior was scored as 1 (met) or 0 (not met), with a total score ranging from 0 to 4 (higher scores indicating healthier lifestyles)39.
Statistical analysis
Baseline differences between groups were analyzed using ANOVA or the Kruskal‒Wallis H test for continuous variables and the chi-square test for categorical variables. Normality of continuous data was assessed using the Shapiro‒Wilk test. If the data were normally distributed, parametric tests were used (ANOVA); otherwise, non-parametric tests (Kruskal‒Wallis H test) were applied. To assess potential attrition bias, baseline characteristics of completers and dropouts were compared in G0 and G1 using t-tests or Mann‒Whitney U tests for continuous variables and chi-square tests or Fisher’s exact tests for categorical variables. For G2 and G3, descriptive statistics are reported without formal testing due to the small number of dropouts (n ≤ 3). Generalized Estimating Equations (GEEs) were used to analyze longitudinal data, accounting for within-subject correlations, time-varying covariates, and missing data40,41. To address potential intra-district correlations, an unstructured working correlation matrix was utilized within the GEE framework. The GEE model was used to assess the changes over time at different evaluation points for all indicators and to compare the changes in outcome variables between the intervention and control groups. If the group × time interaction was significant (P < 0.05), further analysis was performed to evaluate the main effects of group and time. The model was adjusted for age, gender, area, education level, marital status, occupational status, sleep duration, healthy lifestyle score, duration of T2DM, disease history and medication history. All analyses were performed using IBM SPSS version 23 with a two-sided α of 0.05.
Results
A total of 784 T2DM patients with chronic periodontitis were enrolled in the study. These participants were randomized into four groups: G0, G1, G2, and G3, with 196 participants in each group. In accordance with the per-protocol set (PPS) principle, only data from participants who completed the entire intervention process were included in the final statistical analysis. An additional 24 participants in G0, 8 in G1, 3 in G2, and 2 in G3 were excluded from the final analysis due to incomplete intervention evaluations. Consequently, data from 747 participants were analyzed: 172 from G0, 188 from G1, 193 from G2, and 194 from G3. The participant flowchart is shown in Fig. S1.
The participants had a mean age of 65.26 ± 7.72 years, and 39.2% were male. Among them, 78.6% had a disease duration of ≥ 5 years, and 81.7% were taking diabetes medications. The baseline characteristics were similar across the control and intervention groups (P > 0.05) (Table 1).
The control rates for FPG and HbA1c in the study participants were 48.2% and 57.0%, respectively. The mean self-efficacy score, health utility value, and EQ-VAS score were 8.09 ± 1.41, 0.97 ± 0.07, and 79.01 ± 16.92, respectively, with no significant differences among the four groups (P > 0.05). The proportions of participants reporting oral health issues were as follows: gingival bleeding (36.3%), gingival swelling (34.0%), tooth mobility (47.8%), chewing weakness (24.1%), and halitosis (26.1%). No significant differences in these outcomes were observed between the control and intervention groups at baseline (P > 0.05) (Table 1). No significant differences in baseline characteristics (all P > 0.05) were observed between completers and dropouts within any study group (Supplementary Tables S4-5).
Glycemic control
Using FPG and HbA1c control status as dependent variables, the GEE model revealed significant group × time interaction effects (P < 0.001), indicating that the trends in FPG and HbA1c control rates over time differed between the intervention and control groups. Further analysis of the group effects revealed significant differences in FPG control rates between the intervention and control groups at T1 (P = 0.022) and T2 (P = 0.002). Similarly, significant differences in HbA1c control rates were observed at T1 (P < 0.001). A further analysis of the time effects demonstrated significant differences in FPG (P < 0.001, P = 0.001, and P = 0.002) and HbA1c (all P < 0.001) control rates across follow-up periods in G1, G2, and G3 (Table 2).
The parameter estimates in Table 5, in conjunction with those in Fig. 1A–B, revealed that at T2, the FPG control rates for G2 (B = 0.667, P = 0.010) and G3 (B = 0.559, P = 0.027) were significantly higher than those for G0, with G3 showing the highest FPG control rate. Additionally, at T1 and T2, the HbA1c control rates for G2 (T1: B = 0.494, P = 0.034; T2: B = 0.718, P = 0.003) and G3 (T1: B = 1.150, P < 0.001; T2: B = 0.615, P = 0.007) were significantly greater than those of G0, with the greatest improvement observed at T2. At T3, the HbA1c control rate in G2 remained significantly greater than that in G0 (B = 0.786, P = 0.001).
Changes in glycemic control status, self-efficacy and QoL-related indicators between groups at different time points. (A) FPG control rate. (B) HbA1c control rate. (C) Health utility values. (D) EQ-VAS scores. (E) Self-efficacy scores. (A–B) Data are presented as percentages (%). (C–E) Data are presented as the mean.
Self-efficacy and QoL
When the self-efficacy score was used as the dependent variable, the results of the GEE model revealed no significant group × time interaction effect (P = 0.413) (Table 3). However, as shown in Table 5 and Fig. 1E, the self-efficacy scores in the G3 group were significantly higher than those in the G0 group at both T1 (B = 0.413, P = 0.020) and T3 (B = 0.402, P = 0.019). Notably, the greatest improvement in self-efficacy was observed in the G3 group at T1 compared with the other intervention groups.
For health utility values, the results of the GEE model demonstrated a significant group × time interaction effect (P = 0.049), indicating that the trends in health utility values over time differed between the intervention and control groups (Table 3). Further analysis of the group effects revealed significant differences in health utility values between the intervention and control groups at T1 (P < 0.001) and T3 (P = 0.006). Time effect analysis revealed significant differences in health utility values across follow-up periods in the G2 (P = 0.005) and G3 (P < 0.001) groups (Table 2).
The parameter estimates from Table 5, in conjunction with those in Fig. 1C-D, show that the health utility values in the G3 group were significantly higher than those in the G0 group at T1 (B = 0.025, P = 0.010) and T3 (B = 0.021, P = 0.027). The greatest improvement in health utility values was observed in the G3 group at T1 compared with the other intervention groups.
Oral health
The results of the GEE model revealed significant group × time interaction effects for tooth mobility (P = 0.030) and chewing weakness (P = 0.037), indicating that the trends in the proportions of patients with these conditions over time differed between the intervention and control groups. However, no significant interaction effects were observed for gingival bleeding (P = 0.262), gingival swelling (P = 0.266), or halitosis (P = 0.673) (Table 4). Further analysis of the group effects revealed significant differences in tooth mobility (all P < 0.001) and chewing weakness (T2: P = 0.005; T3: P < 0.001) between the intervention and control groups at T2 and T3. Analysis of the time effects revealed statistically significant differences in tooth mobility across different follow-up times for G1 (P < 0.001) and G3 (P < 0.001), and statistically significant differences in chewing weakness for G3 across different follow-up times (P < 0.001) (Table 4).
The parameter estimates from Table 5, in conjunction those in Fig. 2, show that at T2, the proportion of patients with gingival bleeding in the G3 group (B = − 0.751, P = 0.015) was significantly lower than that in the G0 group, with the greatest reduction observed in G3. Similarly, at T2, the proportions of patients with gingival swelling in the G1 (B = -0.788, P = 0.023) and G3 (B = − 0.780, P = 0.018) groups were significantly lower than those in the G0 group, with the greatest reduction observed in G3. At T2 and T3, the proportions of patients with tooth mobility in the G1 (T2: B = − 0.798, P = 0.010; T3: B = − 0.685, P = 0.021) and G3 (T2: B = − 0.860, P = 0.007; T3: B = -0.644, P = 0.028) groups were significantly lower than those in the G0 group, with the greatest reduction observed in G3 at T2. At T2 and T3, the proportions of patients with chewing weakness in the G3 group (T2: B = − 0.916, P = 0.015; T3: B = − 1.209, P = 0.001) were significantly lower than those in the G0 group. At T2, the proportion of patients with halitosis in the G3 group (B = − 0.763, P = 0.026) was significantly lower than that in the G0 group.
Discussion
This randomized controlled trial demonstrated that a community-based comprehensive intervention integrating self-management activities for diabetes and periodontitis significantly improved glycemic control, self-efficacy, QoL, and oral health in patients with T2DM and chronic periodontitis. The findings extend the literature by emphasizing the feasibility of addressing both conditions through a scalable, community-driven model.
Compared with the control group (G0), the intervention groups, particularly G2 and G3, achieved superior glycemic control. The significant group × time interactions for FPG and HbA1c (P < 0.001) suggest that the intervention effects were sustained over time. Notably, G3 presented the highest FPG control rate at T2 (B = 0.559, P = 0.027) and the most pronounced HbA1c improvements at T1 (B = 1.150, P < 0.001) and T2 (B = 0.615, P = 0.007). These results reinforce the importance of combining diabetes self-management with periodontal care, as improved oral health may increase systemic inflammation and insulin sensitivity, thereby facilitating glycemic regulation10,42. The gradual decline in HbA1c control rates at T3 in G2 highlights the need for ongoing reinforcement to sustain long-term benefits.
Although the overall group × time interaction for self-efficacy was nonsignificant (P = 0.413), G3 showed marked improvements at T1 and T3 (T1: B = 0.413, P = 0.020; T3: B = 0.402, P = 0.019), suggesting that personalized, intensive interventions may better enhance patients’ confidence in disease management. The results of this study align with those of previous studies, which, however, focused on single interventions43,44,45,46. This study innovatively demonstrated that a combined intervention for diabetes and periodontal disease is more effective in enhancing self-efficacy than individual diabetes or periodontal interventions were. Enhancing self-efficacy is a key mechanism for improving health behaviors in chronic disease patients47,48. The significant improvements in self-efficacy and glycemic control observed in this study may result from better self-management behaviors, such as blood glucose monitoring, lifestyle changes, and medication adherence, leading to more effective glycemic control44,45,46.
Our results indicate that comprehensive self-management interventions are effective in improving QoL, yielding better outcomes than single diabetes interventions. The favorable QoL outcomes may be attributed to several key components of the intervention. First, the inclusion of psychological support and stress management strategies likely alleviated negative emotions such as anxiety, depression, and frustration, which are common among patients managing dual chronic conditions49,50. Second, the provision of oral health education and self-care practices empowered patients to address oral symptoms (e.g., gingival bleeding, tooth mobility) that directly impact daily functioning and self-esteem. Third, dietary guidance tailored to both diabetes and periodontal health helped patients navigate dietary restrictions while maintaining nutritional balance, reducing the burden of managing conflicting dietary recommendations51.
Critically, the structured peer support embedded in the intervention further enhanced these outcomes. Patient-elected deputy leaders, who shared similar health challenges, played a pivotal role in fostering trust and relatability. By modeling practical self-management behaviors (e.g., adapting meal plans to cultural preferences and demonstrating oral hygiene techniques), these leaders normalized health-promoting actions and provided contextually relevant solutions to barriers such as medication adherence. Peer-led discussions also created a supportive environment where participants collaboratively addressed shared challenges, reducing feelings of isolation and enhancing social accountability. This aligns with evidence that peer support interventions improve chronic disease outcomes through enhanced self-efficacy and sustained behavior change52.
These findings underscore the importance of a holistic approach to chronic disease management. By integrating psychological, dietary, and oral health components into a unified intervention, this study demonstrated that comprehensive care can effectively mitigate these challenges, leading to meaningful improvements in QoL. Future interventions should continue to emphasize these multidimensional strategies to address the complex needs of this patient population.
The intervention significantly reduced tooth mobility and chewing weakness (group × time P = 0.030 and P = 0.037), with G3 showing the greatest reduction in gingival bleeding (B = − 0.751, P = 0.015), swelling (B = − 0.780, P = 0.018), tooth mobility (B = − 0.860, P = 0.007) and halitosis (B = − 0.763, P = 0.026) at T2. These improvements may reflect enhanced oral hygiene practices and reduced periodontal inflammation, which are critical for stabilizing glycemic levels53,54,55. Poor oral health, particularly in the form of tooth loss or impaired chewing function, often leads to dietary restrictions and suboptimal nutrient intake, exacerbating glycemic instability56,57. Reducing tooth mobility and chewing weakness directly supports patients’ ability to maintain a nutritious diet, which is essential for effective diabetes management.This study has several limitations. First, the 6-month follow-up may underestimate long-term outcomes; extended observation is needed to assess durability. Second, reliance on self-reported oral health status introduces potential recall bias. Third, the exclusion of participants with incomplete evaluations (e.g., 24 in G0) may limit generalizability. Additionally, although attrition rates were higher in the control group, the lack of baseline differences between completers and dropouts mitigated concerns about attrition bias. Nevertheless, the possibility of residual confounding from unmeasured factors cannot be excluded. This study focused on a population from Nanjing, China, which may limit the generalizability of the findings to other geographic regions or populations with different cultural or socio-economic backgrounds. While Nanjing is a representative city in Eastern China, future research should explore the applicability of this intervention in other regions with diverse demographic characteristics. Additionally, further studies are needed to investigate the long-term effects and the potential scalability of this intervention across different healthcare systems and cultural contexts.
Conclusion
This trial demonstrated that a community-based, integrated self-management intervention effectively improved glycemic control, self-efficacy, QoL, and oral health in T2DM patients with chronic periodontitis. By empowering patients through education and peer support, this model offers a feasible, scalable solution for underserved populations. However, given the relatively short follow-up period and sample size, further studies with larger cohorts and longer follow-up are necessary to confirm the long-term effects and broader applicability of this intervention. Policymakers and healthcare providers should consider integrating oral health into diabetes management protocols, but further research is needed to assess the cost-effectiveness and long-term impact of such an integrated approach in mitigating the dual burden of these globally prevalent diseases.
Data availability
Data are available from the corresponding author on reasonable request.
References
Magliano, D.J., Boyko, E.J. In IDF DIABETES ATLAS 10th edn (Brussels, 2021).
Association. DSotCM: China Type 2 Diabetes Prevention and Treatment Guidelines (2020 Edition). Chin. J. Diabetes 2021(04), 315–409.
Collaborators GBDCoD: Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403(10440), 2100–2132.
Diseases, G. B. D. & Injuries, C. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403(10440), 2133–2161 (2024).
Xin, W. The Fourth National Oral Health Epidemiological Survey Report (2018).
Llambes, F., Arias-Herrera, S. & Caffesse, R. Relationship between diabetes and periodontal infection. World J. Diabetes 6(7), 927–935 (2015).
Wang, T. F., Jen, I. A., Chou, C. & Lei, Y. P. Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: A meta-analysis. Medicine (Baltimore) 93(28), e292 (2014).
Oberti, L., Gabrione, F., Nardone, M. & Di Girolamo, M. Two-way relationship between diabetes and periodontal disease: A reality or a paradigm?. J. Biol. Regul. Homeost. Agents 33(3 Suppl. 1), 153–159 (2019).
Arora, N. et al. Periodontal infection, impaired fasting glucose and impaired glucose tolerance: Results from the continuous national health and nutrition examination survey 2009–2010. J. Clin. Periodontol. 41(7), 643–652 (2014).
Branch CMAE, Branch CMAD, Branch CMDAEaMP: Expert consensus on adult prediabetes intervention in China (2023 edition). Chin. J. Diabetes 2023(06), 484–494.
Sanz, M. et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the international diabetes federation and the European federation of periodontology. J. Clin. Periodontol. 45(2), 138–149 (2018).
Preshaw, P. M. & Bissett, S. M. Periodontitis and diabetes. Br. Dent. J. 227(7), 577–584 (2019).
Kim, H. K., Kim, Y. G., Cho, J. H., Lee, S. K. & Lee, J. M. The effect of periodontal and prosthodontic therapy on glycemic control in patients with diabetes. J. Adv. Prosthodont. 11(5), 247–252 (2019).
Sundar, C., Ramalingam, S., Mohan, V., Pradeepa, R. & Ramakrishnan, M. J. Periodontal therapy as an adjunctive modality for HbA1c reduction in type-2 diabetic patients. J. Educ. Health Promot. 7, 152 (2018).
Tsobgny-Tsague, N. F. et al. Effects of nonsurgical periodontal treatment on glycated haemoglobin on type 2 diabetes patients (PARODIA 1 study): A randomized controlled trial in a sub-Saharan Africa population. BMC Oral Health 18(1), 28 (2018).
Poudel, P. et al. Oral health knowledge, attitudes and care practices of people with diabetes: A systematic review. BMC Public Health 18(1), 577 (2018).
Bahammam, M. A. Periodontal health and diabetes awareness among Saudi diabetes patients. Patient Prefer Adherence 9, 225–233 (2015).
Herman, W. H., Taylor, G. W., Jacobson, J. J., Burke, R. & Brown, M. B. Screening for prediabetes and type 2 diabetes in dental offices. J. Public Health Dent. 75(3), 175–182 (2015).
Nazir, M. A. et al. The burden of diabetes, its oral complications and their prevention and management. Open Access Maced. J. Med. Sci. 6(8), 1545–1553 (2018).
Bandura, A. Social cognitive theory: An agentic perspective. Annu. Rev. Psychol. 52, 1–26 (2001).
Syrjälä, A. M., Kneckt, M. C. & Knuuttila, M. L. Dental self-efficacy as a determinant to oral health behaviour, oral hygiene and HbA1c level among diabetic patients. J. Clin. Periodontol. 26(9), 616–621 (1999).
Lorig, K., Ritter, P. L., Ory, M. G. & Whitelaw, N. Effectiveness of a generic chronic disease self-management program for people with type 2 diabetes: A translation study. Diabetes Educ. 39(5), 655–663 (2013).
Lorig, K., Ritter, P. L., Villa, F. J. & Armas, J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ. 35(4), 641–651 (2009).
Dongbo, F., Ding, Y., McGowan, P. & Fu, H. Qualitative evaluation of chronic disease self management program (CDSMP) in Shanghai. Patient Educ. Couns. 61(3), 389–396 (2006).
Fu, D. et al. Implementation and quantitative evaluation of chronic disease self-management programme in Shanghai, China: Randomized controlled trial. Bull. World Health Organ. 81(3), 174–182 (2003).
Polonsky, W. H. Emotional and quality-of-life aspects of diabetes management. Curr. Diab. Rep. 2(2), 153–159 (2002).
Nishihara, U. et al. A periodontal disease care program for patients with type 2 diabetes: A randomized controlled trial. J. Gen. Fam. Med. 18(5), 249–257 (2017).
Davis, J. et al. 2022 National standards for diabetes self-management education and support. Diabetes Care 45(2), 484–494 (2022).
Page, R. C. & Eke, P. I. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 78(7 Suppl), 1387–1399 (2007).
Chinese Expert Consensus on the Diagnostic Criteria for Severe Periodontitis and Treatment Principles for Periodontal Diseases in Special Populations. Chin. J. Stomatol. 52(2), 67–71 (2017).
Huo, X. et al. Effects of mobile text messaging on glycemic control in patients with coronary heart disease and diabetes mellitus: A randomized clinical trial. Circ. Cardiovasc. Qual. Outcomes 12(9), e005805 (2019).
Medicine TSAHoGUoC, Shanghai BCNERCo, Hospital ZPC, University F, Shanghai XinChao Biotechnology Co. L, Hospital NG, Center FUSC, Assessment CNASfC, Hospital TMUCIa, Hospital ZT et al. Human Blood Sample Collection and Processing. In., vol. GB/T 38576–2020: State Administration for Market Regulation; National Standardization Management Committee 24 (2020).
Meterko, M. Outcome measures for health education and other health care interventions. Int. J. Qual. Health Care 9(1), 65–66 (1997).
Dongjie, X. Development and application of the self-management behavior assessment scale for breast cancer patients undergoing PICC chemotherapy. Nurs. Contin. Educ. J. 18, 1712–1715 (2014).
Chunmei, W. A Study on the Applicability of the EQ-5D Utility Value Index System for the Chinese Population. Huazhong University of Science and Technology (HUST) (2017).
Wang, S. S., Lay, S., Yu, H. N. & Shen, S. R. Dietary guidelines for Chinese residents (2016): Comments and comparisons. J. Zhejiang Univ. Sci. B 17(9), 649–656 (2016).
China Adult Physical Activity Guidelines (Excerpt). J. Nutr. 2012(2), 105–110.
Society CN: Chinese Dietary Guidelines 2022 (2022).
Zhang, X. et al. Healthy lifestyle behaviours and all-cause and cardiovascular mortality among 0.9 million Chinese adults. Int J Behav Nutr Phys Act 18(1), 162 (2021).
Duenas, M., Salazar, A., Ojeda, B., Arana, R. & Failde, I. Generalized estimating equations (GEE) to handle missing data and time-dependent variables in longitudinal studies: An application to assess the evolution of health related quality of life in coronary patients. Epidemiol. Prev. 40(2), 116–123 (2016).
Brobbey, A. et al. Repeated measures discriminant analysis using multivariate generalized estimation equations. Stat. Methods. Med. Res. 31(4), 646–657 (2022).
Sgolastra, F., Severino, M., Pietropaoli, D., Gatto, R. & Monaco, A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: A meta-analysis of randomized clinical trials. J. Periodontol. 84(7), 958–973 (2013).
Cheng, L. et al. The effects of an empowerment-based self-management intervention on empowerment level, psychological distress, and quality of life in patients with poorly controlled type 2 diabetes: A randomized controlled trial. Int. J. Nurs. Stud. 116, 103407 (2021).
Gamboa Moreno, E. et al. Efficacy of a self-management education programme on patients with type 2 diabetes in primary care: A randomised controlled trial. Prim. Care Diabetes 13(2), 122–133 (2019).
Mohebbi, B., Tol, A., Sadeghi, R., Mohtarami, S. F. & Shamshiri, A. Self-management intervention program based on the health belief model (HBM) among women with gestational diabetes mellitus: A quazi-experimental study. Arch. Iran Med. 22(4), 168–173 (2019).
Tsokani, S. et al. Exploring the effectiveness of self-management interventions in type 2 diabetes: A systematic review and network meta-analysis. Healthcare (Basel) 12(1), 27 (2023).
Jang, Y. & Yoo, H. Self-management programs based on the social cognitive theory for Koreans with chronic disease: A systematic review. Contemp. Nurse 40(2), 147–159 (2012).
Yao, J. et al. The association between self-efficacy and self-management behaviors among Chinese patients with type 2 diabetes. PLoS ONE 14(11), e0224869 (2019).
Wang, Z. et al. Causal relationship between diabetes and depression: A bidirectional Mendelian randomization study. J. Affect. Disord. 351, 956–961 (2024).
Warren, K. R., Postolache, T. T., Groer, M. E., Pinjari, O. & Kelly, D. L. Role of chronic stress and depression in periodontal diseases. Periodontol 64(1), 127–138 (2014).
Munshi, M. N. et al. Management of diabetes in long-term care and skilled nursing facilities: A Position statement of the American diabetes association. Diabetes Care 39(2), 308–318 (2016).
Kong, L. N., Hu, P., Yang, L. & Cui, D. The effectiveness of peer support on self-efficacy and quality of life in adults with type 2 diabetes: A systematic review and meta-analysis. J. Adv. Nurs. 75(4), 711–722 (2019).
Baeza, M. et al. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. 28, e20190248 (2020).
Preshaw, P. M. et al. Periodontitis and diabetes: A two-way relationship. Diabetologia 55(1), 21–31 (2012).
Simpson, T. C. et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst. Rev. 4(4), CD004714 (2022).
Genco, R. J., Graziani, F. & Hasturk, H. Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus. Periodontol 83(1), 59–65 (2020).
Vu, G. T., Little, B. B., Lai, P. C. & Cheng, G. L. Tooth loss and uncontrolled diabetes among US adults. J. Am. Dent. Assoc. 153(6), 542–551 (2022).
Acknowledgements
We would like to express our sincere appreciation to the physicians at the community health service centers of Nanjing for their significant assistance and support in patient recruitment and the implementation of the intervention. This study was funded by Nanjing Medical Science and Technology Development Project of China (ZKX20045).
Author information
Authors and Affiliations
Contributions
X.H. formulated and evoluted the goals and aims of overarching research. X.H., C.W. and N.Z. supervised, investigated, and managed the project. Y.Z. conceptualized, surveyed, developed the methodology, analyzed the data, and wrote original draft. Y.C., N.Z. and C.W. designed the methodology, interpreted the data, and performed the data analysis. H.X. interpreted the data and performed the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This RCT was approved by the Ethics Committee of the Nanjing Center for Disease Control and Prevention (PJ2021-A001-02). The study adhered to the guidelines of the Helsinki Declaration, and its reporting followed the CONSORT standards. Informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Chen, Y., Wang, C. et al. Community interventions improve diabetes management and oral health in type 2 diabetes patients with chronic periodontitis. Sci Rep 15, 24395 (2025). https://doi.org/10.1038/s41598-025-09034-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09034-7