Abstract
Background
Bone nonunion is a major complication in fracture treatment. Despite considerable advancements in fracture care, 5–10% of fractures result in nonunion; This demonstrates the requirement for novel molecules to enhance fracture healing. Brain-derived neurotrophic factor (BDNF) has been increasingly recognized for its role in fracture healing; however, the underlying molecular mechanisms remain unclear. Osteoblast proliferation and migration are critical to this process. The nuclear casein kinase and cyclin-dependent kinase substrate 1 gene (NUCKS1), located on human chromosome 1 at 1q32.1, encodes a 27-kDa nuclear DNA-binding protein, which influences cell proliferation, migration, and apoptosis strongly. Although NUCKS1 is implicated in various diseases, its specific role in fracture healing remains unclear. Here, we explored whether NUCKS1 participates in the BDNF-mediated fracture-healing process.
Methods
We assessed NUCKS1 expression in MC3T3-E1 cells and callus from closed femoral fracture mice through quantitative reverse transcription polymerase chain reaction and Western blotting. NUCKS1 inhibition in MC3T3-E1 cells in terms of migration and proliferation changes was evaluated through transwell cell migration and Cell Counting Kit-8 assays. Fracture-healing status in mice was assessed through x-ray and micro-CT imaging 2 weeks after fracture.
Results
BDNF promoted NUCKS1 expression in both MC3T3-E1 cells and mouse callus tissues. NUCKS1 inhibition reduced MC3T3-E1 cell proliferation and migration and impaired fracture healing in mice. Finally, suppression of TrkB expression reduces both Akt phosphorylation and NUCKS1 expression, and inhibition of Akt phosphorylation reduces NUCKS1 expression.
Conclusion
BDNF may enhance NUCKS1 expression through the TrkB-mediated Akt pathway, promoting osteoblast proliferation and migration and facilitating fracture healing.
Similar content being viewed by others
Background
Brain-derived neurotrophic factor (BDNF), a pivotal member of the neurotrophic factor family, is primarily expressed in both central and peripheral nervous tissues and associated with neuronal differentiation, neurogenesis, and cognitive functions1,2,3. Notably, nonneuronal cells, including fibroblasts, endothelial cells, osteoblasts, and monocytes, also synthesize and release BDNF4,5,6. With advancements in research on BDNF, its roles in the fracture-healing process have become increasingly apparent. K. Asaumi demonstrated that osteogenic cells express BDNF and its receptors, suggesting the involvement of these molecules as autocrine and paracrine factors in the regulation of bone formation7. Studies have also corroborated the expression of BDNF and its receptors in human tissues during fracture healing8. The fracture-healing process is complex, involving the coordinated proliferation, migration, and differentiation of various cell types, including inflammatory cells, angioblasts, fibroblasts, chondrocytes, and osteoblasts9. An adequate number of new osteoblasts is essential for accelerating bone formation and calcification, as well as increasing bone volume and density10. Moreover, during bone remodeling, recruiting osteoblasts or their precursors to the bone surface is crucial; their arrival at the reconstruction site is a prerequisite for bone health and effective fracture repair11. In a rabbit ulna osteotomy model, Denise demonstrated that some osteoblasts involved in fracture healing are actively recruited to the fracture site rather than being infiltrated from the circulatory system passively12. Active osteoblast migration to the fracture site has also been validated in zebrafish fracture models13. Therefore, stimulation of osteoblast proliferation and migration may be a promising strategy. Further investigation on regulatory mechanisms underlying osteoblast proliferation and migration during fracture healing may provide newer therapeutic approaches for nonunion or delayed fracture healing, as well as novel tissue engineering applications.
Nuclear casein kinase and cyclin-dependent kinase substrate 1 (NUCKS1) is a highly conserved vertebrate-specific nuclear protein. NUCKS1 plays key roles in regulating chromatin structure and transcription, DNA repair, and the cell cycle14,15. NUCKS1 is a substrate for various kinases including cyclin-dependent kinases (CDK) and casein kinase 2 (CK2)16. Moreover, it is involved in cell proliferation and migration and cell-cycle regulation, particularly in rapidly growing cells17. For instance, In osteosarcoma, NUCKS1 promotes tumor cell growth and metastasis by enhancing asparagine synthesis18. in endometriosis, NUCKS1 enhances cell viability and migratory capacity through the PI3K and NF-κB pathways and inhibits apoptosis19; In non–small-cell lung cancer, NUCKS1 upregulates CDK1 expression, promoting cell proliferation and migration20;In contrast, the elimination of NUCKS1 in gastric cancer cells significantly induces apoptosis and reduces both cell proliferation and invasion16; Despite having established roles in cell proliferation and migration, the involvement of NUCKS1 in fracture healing and its underlying mechanisms remain unexplored. Therefore, we hypothesized that a regulatory network involving BDNF and NUCKS1 influences osteoblast proliferation and migration during the fracture-healing process.
In this study, we assessed the effects of exogenous BDNF on NUCKS1 expression in both MC3T3-E1 cells in vitro and mouse callus tissue in vivo. We noted that NUCKS1 inhibition reduces cell proliferation and migration and that BDNF upregulates NUCKS1 expression through TrkB and AKT signaling, thereby promoting osteoblast proliferation and migration. This mechanism possibly enhances fracture healing and represents a novel therapeutic target for nonunion and delayed union fracture treatment.
Materials and methods
Cell culture and treatment
Murine MC3T3-E1 cells were sourced from Pricella Biotechnology (Wuhan, China) and cultured in Dulbecco’s modified Eagle Medium (KeyGEN, Jiangsu, China) supplemented with 10% fetal bovine serum in a humidified incubator at 37 °C with 5% CO2. BDNF (50 ng/mL; Proteintech, Wuhan, China) was added to the medium after 30-min serum starvation. After 12 h, NUCKS1 and p-Akt kinase expression was measured. In the experimental group, K252a (a TrkB inhibitor; 200 nmol/L; MCE, Shanghai, China) and LY294002 (an Akt inhibitor; 50 µmol/L; MCE) were added to the medium 1 h before BDNF treatment. The control group was treated with an equal volume of dimethyl sulfoxide as the solvent.
Western blotting
Cells were washed twice with phosphate-buffered saline, followed by lysis using RIPA buffer containing protease and phosphatase inhibitors, and then centrifugation at 13,000 rpm at 4 °C for 25 min. Next, the supernatant was collected for protein quantification. The tissue samples were minced into small fragments, rinsed with ice-cold PBS, and homogenized using a tissue homogenizer until no visible solid particles remained (or alternatively ground in liquid nitrogen). Subsequently, 1 mL of protein lysis buffer was added per 100 mg of tissue, followed by vigorous vortexing for 15 s and incubation on ice for 10 min. After another 5-second vortexing step, the samples were centrifuged at 16,000 × g for 25 min at 4 °C. The supernatant was then promptly transferred to a pre-chilled microcentrifuge tube and stored on ice or at − 80 °C for subsequent use. All samples were mixed with sodium dodecyl sulfate (SDS) loading buffer and then subjected to SDS polyacrylamide gel electrophoresis; next, the proteins were transferred onto polyvinylidene fluoride membranes. These membranes were then blocked with tris-buffered saline containing 5% nonfat milk and 0.1% Tween-20 TBS (TBST) at room temperature for 1 h. The blocked membranes were incubated with primary antibodies (1:1000) at 4 °C overnight and then with the secondary antibody (1:5000) at room temperature for 1 h. Primary antibodies against GAPDH, Akt, p-AktSre473, and NUCKS1 were obtained from Proteintech, whereas the secondary antibodies were sourced from Biosharp (Hefei, Anhui, China). The membranes were then washed three times with TBST for 10 min, each time. Protein bands were visualized under an enhanced chemiluminescence detection system and quantified using ImageJ.
Quantitative reverse transcription polymerase chain reaction
RNA was extracted using the guanidine isothiocyanate/phenol–chloroform method, and the A260/A280 ratio was measured to assess RNA concentrations. A portion of the extracted RNA was subjected to agarose gel electrophoresis to verify RNA quality. The extracted RNA was denatured at 65 °C for 5 min, and residual genomic DNA was removed using DNase. Reverse transcription (RT) reagents were then added to synthesize cDNA. Specific polymerase chain reaction (PCR) primers for target genes were designed using PrimerBank (http://pga.mgh.harvard.edu/primerbank/). The primers and reverse-transcribed cDNA were incorporated into a quantitative RT (qRT) PCR mixture for detection. The relative expression of the target gene mRNA was calculated statistically.
Transwell cell migration assay
A serum-free cell suspension was prepared and counted, typically at 105 cells per well (24-well plate). The cells were then seeded into the upper chamber of a transwell system. Next, each upper chamber was transferred to a new plate, and the medium in the upper chamber was removed, followed by addition of 100 µL of the cell suspension. In the lower chamber, we added 800 µL of culture medium containing 20% fetal bovine serum. The transwell system was then incubated at 37 °C for 24 h. After the chambers were inverted onto an absorbent paper to remove the medium, noninvading cells in the upper chamber were gently wiped away using a cotton swab. One or two drops of staining solution (0.1% crystal violet) were added to the lower surface of the membrane for 5 min, followed by phosphate buffered saline (PBS) multiple washings to remove excess dye and then by air-drying. Microscopic images were captured, and cells were counted using ImageJ.
Cell counting kit-8 assay
A 100-µL cell suspension was prepared in a 96-well plate. The plate was pre-incubated at 37 °C at 5% CO2 for 24 h. After appropriate incubation times (24, 48, 72, or 96 h), 10 µL of Cell Counting Kit-8 solution was added to each well, followed by incubation for 1 h. The absorbance of each well was measured at 450 nm on a microplate reader.
Cell transfection
Short interfering RNA (siRNA) was provided by Syngentech (Beijing, China). MC3T3-E1 cells were seeded in six-well plates and cultured at 37 °C overnight. After the cell density reached 40% confluence, transfection was performed. The culture medium was removed 2 h before transfection, and 400 µL of serum-free medium was added. A transfection complex (100 µL) was then added to the cells, which resulted in a final volume of 500 µL. After 4–6 h, the medium was replaced with a complete medium. The cells were incubated for 24–96 h and then sampled for analysis.
NUCKS1 ShRNA adeno-associated virus production
Adeno-associated viral vectors of mouse NUCKS1 short hairpin RNA (shRNA; i.e., pAAV-ITR-hU6-NUCKS1-shRNA mouse-CAG-EGFP-WPRE-ITR; denoted as Ad-NUCKS1-shRNA) and negative control (i.e., pAAV-ITR-hU6-NUCKS1-shRNA 03-CAG-EGFP-WPRE-ITR; denoted as Ad-NC) were constructed by Syngentech. Here, the target sequence was GTTGTGGATTACTCTCAGTTT.
Femur fracture animal model establishment and ethical approval
Twenty specific pathogen–free male C57/BL6 mice (SPF Biotech, Suzhou, Jiangsu, China), aged 12 weeks and weighing 23–25 g, were housed at five per cage with free access to food and water. These mice were used to establish a femur fracture animal model. In brief, the mice were anesthetized; then, a longitudinal incision of 5 mm was made on the lateral side of the left knee under sterile conditions. Next, the patella was dislocated, and a 0.5-mm-diameter hole was drilled into the intercondylar fossa of the femur. A 1-mL syringe needle was inserted into the marrow cavity, and the femoral shaft was cut transversely using a wire saw. Then, the patella was repositioned, and the incision was closed layer by layer. Antibiotic treatment was administered 3 days after surgery. The mice were randomly divided into four groups (n = 5 per group): blank control, BDNF, Ad-NC, and Ad-NUCKS1-shRNA. The BDNF and blank control groups were administered 0.1 mL of 1 µg/mL BDNF solution and the corresponding buffer every 2 days. In contrast, the Ad-NC and Ad-NUCKS1-shRNA groups were administered Ad-NC and Ad-NUCKS1-shRNA after surgery, respectively, along with 50 µL of 1 × 1012 vg/mL AAV9—based on previous reports21. All mice were euthanized by exposure to pentobarbital sodium 2 weeks after surgery, then take the femur on the fractured side.
All animal experimental procedures were approved by the Animal Ethics Committee of Nanjing Medical University Affiliated Nanjing Hospital (DWSY-23032336). All methods were carried out in accordance with relevant guidelines and regulations set forth by The Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International and are reported in accordance with ARRIVE guidelines.
Radiological examination
After 2 weeks of surgery, we used dual-energy x-ray bone densitometry to obtain x-ray images of the left femoral fracture area to assess fracture alignment and healing. Finally, we used the Lane–Sandhu X-ray scoring system to evaluate fracture healing.
Micro-computed tomography analysis
After soft tissue removal and internal fixation, micro-computed tomography (CT, ThermoFisher Scientific) was used for three-dimensional scanning and reconstruction of the left femur of the mice. The left femur was wrapped in a saline-soaked gauze and placed on the specimen holder during scanning. After scanning, three-dimensional reconstruction and microstructural analysis of the femoral calluses were performed using a reconstruction software program. The fracture line was considered the starting point, with an additional 100 layers added distally and proximally for analysis. The callus analysis parameters included bone volume fraction [bone volume (BV)/total volume (TV)], trabecular number (Tb.N), trabecular separation (Tb.Sp), and trabecular thickness (Tb.Th).
Statistical analysis
All statistical data were analyzed using GraphPad Prism (version 10.0), with results expressed as the mean ± standard deviation. Student’s t-test was applied to the comparison between the two groups. Analysis of variance (ANOVA) was used with Tukey’s post hoc test to examine the statistical significance of the differences among multiple independent groups, with p < 0.05 considered to indicate statistical significance.
Results
BDNF promotes NUCKS1 expression in cells and tissues
After BDNF stimulation for 12 h, the Western blot and qRT-PCR results demonstrated an increase in NUCKS1 expression in MC3T3-E1 cells (Fig. 1A–C). Similarly, 2 weeks after exogenous BDNF administration, NUCKS1 expression was observed in callus tissues, corroborating the results of our cell analysis (Fig. 1D and E).
Effects of BDNF on NUCKS1 expression in MC3T3-E1 cells and fracture-healing tissues (n = 3). (A) Western blots of NUCKS1 expression in MC3T3-E1 cells treated with 50 ng/mL BDNF for 12 h. (B) Statistical analysis of results in (A). (C) Statistical analysis of the results qRT-PCR analysis of NUCKS1 expression in MC3T3-E1 cells treated with 50 ng/mL BDNF for 12 h. (D) Western blots of NUCKS1 expression in the callus tissue after local administration of 100 ng/mL BDNF to the fracture site every 2 days for 2 weeks. E Statistical analysis of results in (D). Data in (B), (C), and (E) were analyzed using independent-samples t test; *p < 0.05, **p < 0.01, ***p < 0.001.
NUCKS1 Inhibition reduces MC3T3-E1 cell migration and proliferation
To confirm siRNA inhibition efficacy, we selected two siRNA groups and measured NUCKS1 expression levels through qRT-PCR and Western blot. The results demonstrated significant NUCKS1 knockdown in both the NUCKS1-siRNA1 and NUCKS1-siRNA2 groups compared with the control group (Fig. 2A–C). In a 24-h transwell cell migration assay, the number of migrating cells in the siNUCKS1-1 and siNUCKS1-2 groups decreased significantly compared with the control group (all p < 0.05; Fig. 2D and E). Moreover, the Cell Counting Kit-8 assay revealed that at 48–96 h, cell proliferation in the siNUCKS1-1 and siNUCKS1-2 groups was significantly diminished compared with the control group (all p < 0.05; Fig. 2F). Taken together, these results suggested that NUCKS1 inhibition reduces both cell migration and proliferation.
Effects of NUCKS1 inhibition on MC3T3-E1 cell migration and proliferation. (A) qRT-PCR analysis for NUCKS1 downregulation after transfection with two siRNAs. (B) Western blots of NUCKS1 downregulation after transfection with two siRNAs. (C) Statistical analysis of results in (B). D MC3T3-E1 cells were stimulated with BDNF (50 ng/ml) for 24 h after transfection, and migration measured using transwell cell migration assay. (E) Statistical analysis of results in (D). F CCK-8 assay for transfected cell proliferation 0, 24, 48, 72, and 96 h after stimulation with 50 ng/mL BDNF. Each experiment was repeated three times; *p < 0.05, **p < 0.01, ***p < 0.001.
BDNF regulates NUCKS1 expression via TrkB and Akt signaling
We first pretreated our cells with the TrkB receptor inhibitor K252a. Western blotting revealed that NUCKS1 expression significantly decreased after the addition of K252a compared with the control group. Furthermore, we pretreated the cells with the Akt inhibitor LY294002; this resulted in a significant decrease in both NUCKS1 expression and Akt phosphorylation compared with the control group. However, no significant increase was noted in NUCKS1 expression or Akt phosphorylation after BDNF was added (Fig. 3A–E). Moreover, K252a pretreatment significantly inhibited Akt phosphorylation (Fig. 3F–H). Therefore, BDNF affects NUCKS1 expression through the TrkB and Akt pathways.
Effects of BDNF on NUCKS1 expression via TrkB-mediated Akt signaling. (n = 3) (A) Western blots of MC3T3-E1 cells pretreated with K252a (200 nmol/L, TrkB inhibitor) for 1 h before BDNF stimulation showing NUCKS1 expression after 12 h. (B) Quantitative analysis of results in (A). (C) Western blots of MC3T3-E1 cells pretreated with LY294002 (50 µmol/L, Akt inhibitor) for 1 h before BDNF stimulation showing NUCKS1, Akt, and p-Akt expression after 12 h. (D) and (E) Quantitative analysis of result in (C). (F) Western blots of MC3T3-E1 cells pretreated with K252a (200 nmol/L) and LY294002 (50 µmol/L) for 1 h before BDNF stimulation showing NUCKS1, Akt, and p-Akt expression after 12 h. (G) and (H) Quantitative analysis of results in (F). *p < 0.05, **p < 0.01, ***p < 0.001.
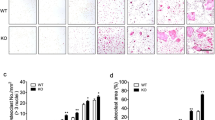
BDNF improves fracture healing by modulating NUCKS1 expression
We compared our x-ray and micro-CT results and noted that 2 weeks after surgery, the fracture line was less distinct, with healing tissue appearing denser, in the exogenous BDNF group than in the control group. Moreover, the Lane–Sandhu x-ray scores were higher in the BDNF group than in the control group (Fig. 4A and B). Moreover, BV/TV, Tb.N, and Tb.Th were significantly higher, but Tb.Sp was significantly lower, in the BDNF group than in the control group (all p < 0.05; Fig. 4C and D). Furthermore, Western blotting of extracted tissue proteins revealed an increase in NUCKS1 expression in the fracture callus of the BDNF group (Fig. 1C and D). To validate our results further, we infected the fracture site of mice with adeno-associated viruses. Thereafter, Western blotting demonstrated a considerable decrease in NUCKS1 expression in the infected healing tissues (Fig. 4E and F). The results demonstrate that 2 weeks after surgery, the fracture line was less distinct, the healing tissue was larger, and the Lane–Sandhu x-ray scores were higher in the Ad-NC group than in the Ad-NUCKS1-shRNA group (Fig. 4G and H). Moreover, BV/TV, Tb.N, and Tb.Th were significantly higher but Tb.Sp was significantly lower in the Ad-NC group than in the Ad-NUCKS1-shRNA group (all p < 0.05; Fig. 4I and J). These findings suggested that BDNF influences fracture healing by modulating NUCKS1 expression.
Radiological analysis of mouse femur fractures 2 weeks after exogenous BDNF administration and adeno-associated virus transfection. (A) and (B) Representative X-ray images and Lane–Sandhu X-ray scores in the exogenous BDNF (100 ng/mL) and control groups 2 weeks after fracture. (C) Representative three-dimensional micro-CT images of the exogenous BDNF (100 ng/mL) and control groups 2 weeks after fracture. D Statistical analysis of BV/TV, Tb.N, Tb.Th, and Tb.Sp in (C). (E) Western blots showing NUCKS1 expression in mouse callus tissue 2 weeks after adeno-associated virus transfection. (F) Statistical analysis of results in (E). (G) and (H) Representative X-ray images and Lane–Sandhu X-ray scores in the groups 2 weeks after fracture. I Representative three-dimensional, coronal, and transverse micro-CT images of the Ad-NC and Ad-NUCKS1-shRNA groups 2 weeks after fracture. (J) Statistical analysis of BV/TV, Tb.N, Tb.Th, and Tb.Sp in (I). Each group included four mice. *p < 0.05, **p < 0.01, ***p < 0.001.
Dicussion
Fractures are a major global public health challenge because they are associated with high incidence, disability, and mortality rates; approximately 10% of the affected patients experience delayed healing or nonunion11. Fracture repair is a regenerative process that encompasses angiogenesis, stem cell differentiation, osteogenesis, and chondrogenesis22. Effective bone formation and remodeling require precise regulation of the proliferation, migration, and differentiation of osteoblasts, the primary bone-forming cells. These cells are derived from bone marrow mesenchymal stem cells and primarily produce bone matrix components, such as alkaline phosphatase, osteoprotegerin, and bone sialoprotein—all of which are crucial for fracture healing23,24. BDNF has diverse roles in osteoblasts. For instance, Hiroko reported that BDNF stimulates MC3T3-E1 cell differentiation, promoting new bone formation and maturation25. Additionally, Zhang reported that BDNF/TrkB enhances the expression of vascular endothelial growth factor (VEGF) in osteoblasts26. However, research on the effects of BDNF on osteoblast proliferation and migration is limited, and the underlying molecular mechanisms remain unclear.
Neurotrophic factors such as BDNF, nerve growth factor, neurotrophin 3, and substance P play a role in bone formatio27. BDNF, a member of the neurotrophic factor family, has garnered considerable attention due to its broad biological activities. BDNF can stimulate periodontal ligament cell proliferation and differentiation into periodontal tissue cells, which induces early periodontal regeneration in the bone defect repair process28. In ovariectomized rat models, BDNF was noted to enhance osteogenesis and improve bone microstructure by influencing osteoblast differentiation27. Moreover, BDNF promotes osteoblast migration by upregulating integrin β1, thereby enhancing fracture healing29. Although numerous studies have described the positive effects of BDNF on cell differentiation and migration, few have addressed its impact on cell proliferation. For instance, BDNF treatment can enhance human periodontal ligament and MLO-Y4 cell proliferation but not that of human mesenchymal stem or ST-2 cells27,30. These discrepancies may be due to variations in the BDNF concentrations and cell types used. In the current study, BDNF was noted to influence MC3T3-E1 cell proliferation and migration positively. As such, we next elucidated the yet-unclear mechanisms underlying the effects of BDNF on osteoblast proliferation and migration; in particular, we focused on the effects of BDNF and its TrkB receptor in osteoblast proliferation and migration.
NUCKS1, one of the most substantially modified proteins in the human proteome, plays roles in chromatin structure regulation, transcription, DNA repair, cell-cycle control, and cell proliferation and migration15. Based on the aforementioned findings, we hypothesized that NUCKS1 is essential in osteoblast proliferation and migration. To test this, we inhibited NUCKS1 expression in MC3T3-E1 cells by using siRNA, which resulted in decreased proliferation and migration. Moreover, we found that BDNF promotes NUCKS1 expression in these cells. This study is the first to report that BDNF influences osteoblast proliferation and migration via NUCKS1. To elucidate the mechanism by which BDNF regulates NUCKS1, we initially validated the involvement of the TrkB receptor. The functional effects of BDNF/TrkB signaling on osteoblasts are multifaceted. The rs6256 polymorphism in BDNF may modulate osteoblast activity, and TrkB may be associated with apoptosis31,32. Furthermore, BDNF and its TrkB receptor are essential at all bone formation stages and are critical in fracture healing27. Western blotting revealed that K252a, a TrkB inhibitor, impeded BDNF-induced NUCKS1 expression in MC3T3-E1 cells, suggesting that BDNF influences NUCKS1 expression via the TrkB receptor. On binding to BDNF, TrkB undergoes dimerization due to conformational changes and autophosphorylation of tyrosine residues within the intracellular domain, thereby activating the mitogen-activated protein kinase, phosphatidylinositol 3-kinase (PI3K), and phospholipase C γ pathways33. Notably, the PI3K/Akt pathway is implicated in cell migration and proliferation34,35. Further investigation revealed that Akt kinase is crucial in BDNF/TrkB signaling. By using the Akt inhibitor LY294002, we demonstrated that inhibiting Akt phosphorylation reduces BDNF-induced NUCKS1 expression. Our findings also revealed that K252a inhibits Akt phosphorylation, reducing NUCKS1 expression. In a femoral fracture mouse model, exogenous BDNF administration resulted in increased NUCKS1 expression in callus tissues, consistent with our cell culture results. To assess the role of NUCKS1 in fracture healing, we locally administered the mouse femur fracture areas with adeno-associated viruses to inhibit NUCKS1 expression; this suppression hindered fracture healing compared with the control group. Taken together, our findings suggested that BDNF influences NUCKS1 expression via TrkB and Akt signaling, thereby affecting osteoblast proliferation and migration and ultimately fracture healing (Fig. 5).
To date, the bone regeneration molecule pathways primarily include factors (e.g., bone morphogenetic proteins 2 and 7) directly influencing bone formation or increasing the number of osteogenic cells35. In addition to these common osteogenic pathways, promising research directions have indicated that bone healing includes inflammation, vascularization, and cell recruitment to the fracture site. BDNF not only affects various bone-forming cells directly but also promotes VEGF expression to induce neovascularization, suppresses various proinflammatory factors, and increases neurogenesis indirectly to enhance osteogenesis26,36,37. Because of the poor pharmacokinetics of BDNF, many recent studies have focused on identifying small molecule mimetics; however, some have indicated that these mimetics may not induce TrkB phosphorylation, suggesting that BDNF remains the most promising option33,38. Moreover, NUCKS1 is implicated in not only cell proliferation, migration, and apoptosis but also inflammation and VEGF regulation mediatio19. Therefore, whether NUCKS1 plays a role in the influence of BDNF on VEGF and proinflammatory factors during fracture healing warrants further investigation.
In summary, this is the first study to describe the role of NUCKS1 in osteoblast proliferation and migration and elucidate the mechanisms by which BDNF facilitates fracture healing. Thus, NUCKS1 may be a novel therapeutic target for enhancing BDNF-mediated fracture healing.
Conclusion
Our results indicated that through the TrkB-mediated Akt signaling pathway, BDNF could potentially upregulate NUCKS1 expression, thereby stimulating osteoblast proliferation and migration to accelerate fracture healing. BDNF may play a regulatory role in osteoblast proliferation and migration, which could provide additional insights into its potential contributions to fracture healing processes. (Fig. 5).
Data availability
All data generated or analyzed during this study are included in this manuscript.
References
Huang, E. J., Reichardt, L. F. & Neurotrophins Roles in neuronal development and function. Annu. Rev. Neurosci. 24 (1), 677–736 (2001).
Miranda, M. et al. Brain-Derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 13, 363 (2019).
Li, Y. et al. The role of brain derived neurotrophic factor in central nervous system. Front. Aging Neurosci., 14. (2022).
Kurihara, H. et al. Neurotrophins in cultured cells from periodontal tissues. J. Periodontol. 74 (1), 76–84 (2003).
Donovan, M. et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Dev., (127): 4531–4540. (2000).
Cartwright, M., Mikheev, A. M. & Heinrich, G. Expression of neurotrophin genes in human fibroblasts: Differential regulation of the brain-derived neurotrophic factor gene. Int. J. Dev. Neurosci. 12 (8), 685–693 (1994).
Asaumi, K. et al. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone 26 (6), 625–633 (2000).
Kilian, O. et al. BDNF and its TrkB receptor in human fracture healing. Annals Anat. - Anatomischer Anzeiger. 196 (5), 286–295 (2014).
Wang, H. et al. Advances in the role and mechanism of fibroblasts in fracture healing. Front. Endocrinol. 15, 1350598 (2024).
Liu, D. et al. Bone marrow mesenchymal stem cell-derived exosomes promote osteoblast proliferation, migration and inhibit apoptosis by regulating KLF3-AS1/miR-338-3p. BMC Musculoskelet. Disord. 25 (1), 122 (2024).
Dirckx, N., Van Hul, M. & Maes, C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res. Part. C: Embryo Today: Reviews. 99 (3), 170–191 (2013).
Shirley, D. et al. Systemic recruitment of osteoblastic cells in fracture healing. J. Orthop. Res. 23 (5), 1013–1021 (2005).
Geurtzen, K. et al. Mature osteoblasts dedifferentiate in response to traumatic bone injury in the zebrafish fin and skull. Development 141 (11), 2225–2234 (2014).
Huang, P. et al. Roles of NUCKS1 in diseases: Susceptibility, potential biomarker, and regulatory mechanisms. Biomed. Res. Int., : 1–7. (2018).
Østvold, A. C., Grundt, K. & Wiese, C. NUCKS1 is a highly modified, chromatin-associated protein involved in a diverse set of biological and pathophysiological processes. Biochem. J. 479 (11), 1205–1220 (2022).
Huang, Y. et al. NUCKS1 promotes gastric cancer cell aggressiveness by upregulating IGF-1R and subsequently activating the PI3K/Akt/mTOR signaling pathway. Carcinogenesis 40 (2), 370–379 (2018).
Liu, T. et al. Increased NUCKS expression is a risk factor for poor prognosis and recurrence in endometrial cancer. Am. J. Cancer Res. 5 (12), 3659–3667 (2015).
Zheng, S. et al. NUCKS1, a LINC00629-upregulated Gene, Facilitated Osteosarcoma Progression and Metastasis by Elevating Asparagine Synthesis 14 (Cell Death & Disease, 2023). 8.
Li, B. et al. Roles of increased NUCKS1 expression in endometriosis. BMC Women’s Health. 23 (1), 432 (2023).
Zhao, S. et al. NUCKS1 promotes proliferation, invasion and migration of Non-Small cell lung Cancer by upregulating CDK1 expression. Cancer Manage. Res. 12, 13311–13323 (2020).
Kwong, J. Q. et al. The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight. 3 (22), e121689 (2018).
Zhang, L. et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther., 11(1). (2020).
Tao, K. et al. Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway. Toxicol. Lett. 240 (1), 68–80 (2016).
Liu, Y. et al. Pentraxin 3 promotes the osteoblastic differentiation of MC3T3-E1 cells through the PI3K/Akt signaling pathway. Biosci. Rep. 40 (6), 1–10 (2020).
Ida-Yonemochi, H. et al. Locally produced BDNF promotes sclerotic change in alveolar bone after nerve injury. PLOS ONE. 12 (1), e0169201 (2017).
Zhang, Z. et al. BDNF regulates the expression and secretion of VEGF from osteoblasts via the TrkB/ERK1/2 signaling pathway during fracture healing. Mol. Med. Rep. 15 (3), 1362–1367 (2017).
Park, E. J. et al. Brain-Derived neurotrophic factor (BDNF) enhances osteogenesis and May improve bone microarchitecture in an ovariectomized rat model. Cells 13 (6), 518 (2024).
Kiyota, M. et al. Periodontal tissue regeneration with cementogenesis after application of brain-derived neurotrophic factor in 3‐wall inflamed intra‐bony defect. J. Periodontal Res. 59 (3), 530–541 (2024).
Zhang, Z. et al. BDNF promoted osteoblast migration and fracture healing by up-regulating integrin β1 via TrkB‐mediated ERK1/2 and AKT signalling. J. Cell. Mol. Med. 24 (18), 10792–10802 (2020).
Loy, T. L. et al. Effects of BDNF and PEC nanoparticles on osteocytes. Molecules 25 (18), 4151 (2020).
Deng, F-Y. et al. SNP rs6265 regulates protein phosphorylation and osteoblast differentiation and influences BMD in humans. J. Bone Miner. Res. 28 (12), 2498–2507 (2013).
Pinski, J. et al. Trk receptor Inhibition induces apoptosis of proliferating but not quiescent human osteoblasts. Cancer Res. 62 (4), 986–989 (2002).
Liu, C., Chan, C. B. & Ye, K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Translational Neurodegeneration, 5(1). (2016).
Tian, W. et al. TIM-3 regulates the proliferation by BDNF-mediated PI3K/AKT axis in the process of endometriosis. Mol. Med. 29 (1), 170 (2023).
Hankenson, K. D., Gagne, K. & Shaughnessy, M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 94, 3–12 (2015).
Kauschke, V. et al. Effects of a pasty bone cement containing Brain-Derived neurotrophic Factor-Functionalized mesoporous bioactive glass particles on metaphyseal healing in a new murine osteoporotic fracture model. Int. J. Mol. Sci. 19 (11), 3531 (2018).
Liu, Q. et al. Effect of Brain-Derived neurotrophic factor on the neurogenesis and osteogenesis in bone engineering. Tissue Eng. Part A. 24 (15–16), 1283–1292 (2018).
Boltaev, U. et al. Multiplex quantitative assays indicate a need for reevaluating reported small-molecule TrkB agonists. Sci. Signal. 10 (493), eaal1670 (2017).
Acknowledgements
Not applicable.
Funding
National Natural Science Foundation of China (81871762).
Author information
Authors and Affiliations
Contributions
MWH and ZZT conceived and designed the experiments. MWH and HCH performed the experiments. MWH, MX, LYH and QXW contributed sample collection and statistical analysis. MWH wrote the manuscript. ZZT revised it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meng, W., Meng, X., Han, C. et al. BDNF improves fracture healing through promoting osteoblasts proliferation and migration via trkb/akt regulated NUCKS1 expression. Sci Rep 15, 27107 (2025). https://doi.org/10.1038/s41598-025-11594-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11594-7