Abstract
This study aimed to investigate the clinical effect of Xuebijing in alleviating acute kidney injury (AKI) after high-voltage electrical burns by inhibiting neutrophils, inflammatory cells, and its potential influence on reactive oxygen species (ROS). Ninety-six patients with AKI following high-voltage electrical burns, admitted to our hospital from February 2023 to December 2024, were included. They were randomized using a computer-generated random number sequence into two groups: a study group (Xuebijing, n = 48) and a control group (n = 48). This study was conducted in an open-label manner. Neutrophil, inflammatory cell, and ROS levels, as well as kidney function and oxidative stress factors (SOD, MDA), were compared at baseline (1 h before treatment) and 7 days post-treatment. Adverse reactions were recorded. The sample size was determined based on previous similar studies indicating clinically significant differences with comparable group sizes. Prior to treatment, there were no significant differences in BUN, SCr, or urinary protein between groups (P > 0.05). After 7 days, both groups showed significant improvements in BUN, SCr, and urinary protein levels, with the study group showing greater improvement (P < 0.05). Similarly, neutrophils, CRP, IL-18, and IL-6 levels decreased significantly in both groups, with the study group showing greater reductions (P < 0.05). Regarding oxidative stress markers, SOD levels decreased and MDA levels increased post-treatment in both groups. However, compared to the control group, the study group showed a more pronounced decrease in SOD levels and a more pronounced increase in MDA levels (P < 0.05), findings that are contrary to an expected direct antioxidant effect measured by these specific markers. Adverse reactions were mild in both groups, with the control group having a slightly higher rate (8.16% vs. 4.08%), but no significant differences were observed (P = 0.399). Xuebijing can alleviate AKI after high-voltage electrical burns by inhibiting neutrophils and inflammatory cells. While it improves kidney function and reduces inflammation, its effects on SOD and MDA levels suggest a complex interaction with the oxidative stress system that requires further investigation, as the observed changes in these markers (decreased SOD, increased MDA) in the Xuebijing group were more pronounced than in the control group. It shows potential as an effective treatment option for improving kidney function and reducing inflammation, warranting further clinical application.
Clinical trial registration This trial is registered with ClinicalTrials.gov under NCT07026227 (Date of registration: 18/06/2025).
Similar content being viewed by others
Introduction
Electrical burns refer to the injury caused by the direct passage of electrical current through the human body during an electrical shock. The electrical energy is converted into thermal energy within the body, leading to tissue and organ damage. Among burn injuries, acute kidney injury (AKI) is a common and severe complication, with an incidence rate as high as 30% and a mortality rate of up to 80%1,2. High-voltage electrical burns resulting in AKI are caused by multiple factors. Initially, severe trauma triggers an inflammatory cascade, with a significant accumulation of inflammatory cells and mediators in the bloodstream3. This results in increased vascular permeability, leading to local and systemic leakage and edema, exacerbated by the loss of body fluids due to the destruction of the skin, a natural barrier. Moreover, there is electrolyte imbalance and reduced renal blood flow. To ensure adequate blood supply to the brain and heart, the body enters a state of distributive shock and peripheral vasodilation. In parallel, stress responses, including hyperglycemia, occur, leading to mitochondrial dysfunction and oxidative stress, which in turn causes tubular injury to the kidneys. Systemic immune responses and oxidative stress, alongside renal microcirculation dysfunction, tubule damage, and tissue edema, all contribute to kidney cell injury and the onset of AKI4.
Renal interstitial fibrosis triggers the release of inflammatory chemokines from glomerular epithelial cells in the process of hypoxic injury, further exacerbating the inflammatory state. The C-reactive protein (CRP) level increases rapidly during acute injury and serves as a sensitive indicator of tissue damage. Interleukin-18 (IL-18), one of the most potent neutrophil chemotactic factors, induces the expression of various inflammatory mediators in endothelial cells, playing a critical role in damage5. Elevated IL-18 promotes the secretion of pro-inflammatory cytokines such as IL-6, further impairing glomerular perfusion. Neutrophils play a crucial role in the immune response; they adhere to endothelial cells, contributing to tissue damage. Following burns, neutrophils and platelets are central to the progression of stress-induced inflammatory responses5,6. The excessive generation of reactive oxygen species (ROS) is a critical factor in ischemia-reperfusion injury, leading to tissue oxidative damage. Insufficient production of superoxide dismutase (SOD) or excessive consumption of SOD leads to a significant increase in ROS, worsening tissue injury. ROS damage proteins, nucleic acids, and unsaturated fatty acids in biological membranes, forming lipid peroxides such as malondialdehyde (MDA), which serves as a marker for lipid peroxidation and reflects the extent of tissue damage7,8.
Currently, the primary treatment for AKI caused by burns is fluid resuscitation, along with correction of acid-base imbalances and electrolyte disturbances. For critically ill patients, renal replacement therapy may be required. However, these methods do not fully address the clinical challenges presented by AKI. Numerous studies have indicated that many traditional Chinese medicines (TCMs) have protective effects on the kidneys. Xuebijing, a traditional Chinese medicine preparation, is known for its ability to counteract bacterial toxins, reduce endotoxin levels, and modulate immune and inflammatory mediators. It has been widely used in the treatment of sepsis-induced AKI9, acute pancreatitis with AKI10, and other conditions. International studies have also begun to explore its mechanisms, suggesting effects on inflammatory pathways like NF-κB and Toll-like receptors11,12. Its constituent herbs, such as Salvia miltiorrhiza (Danshen) and Ligusticum chuanxiong, have been investigated for their antioxidant and anti-inflammatory properties in various experimental models13,14. However, its application in AKI following high-voltage electrical burns is relatively limited. Therefore, this study aimed to explore the potential clinical benefits of Xuebijing in alleviating AKI by inhibiting neutrophils, inflammatory cells, and investigating its impact on ROS following high-voltage electrical burns.
Materials and methods
Patient enrollment and study design
A total of 103 patients diagnosed with acute kidney injury (AKI) secondary to high-voltage electrical burns were initially screened for eligibility between February 2023 and December 2024. After rigorous assessment, seven patients were excluded: one due to pre-existing gastric cancer, four requiring long-term steroid therapy, one post-kidney transplant recipient, and one with chronic kidney disease. The remaining 96 eligible patients were randomized using a computer-generated sequence into two groups: the study group (n = 48), receiving Xuebijing injection combined with conventional treatment, and the control group (n = 48), receiving conventional treatment alone. All participants completed the study without loss to follow-up or discontinuation of the intervention. Baseline demographic and clinical characteristics showed no significant differences between groups (P > 0.05). The CONSORT flow diagram illustrating enrollment, allocation, follow-up, and analysis is provided in Supplementary Fig. 1. This open-label trial adhered to ethical standards approved by the Institutional Review Board of the Third Hospital of Hebei Medical University, with informed consent obtained from all participants or their guardians. This trial is registered with ClinicalTrials.gov under NCT07026227 (Date of registration: 18/06/2025).
Patients were randomized into two groups using a computer-generated random number sequence allocated by a central administrator not involved in patient recruitment or assessment. This study was conducted in an open-label manner due to the nature of the intervention (Xuebijing injection as an add-on therapy). However, laboratory personnel assessing outcomes were blinded to group allocation where feasible. A priori power analysis was not formally conducted for this specific study; however, the sample size of 48 patients per group was determined based on previous studies investigating Xuebijing in similar critical care settings9,10, which demonstrated statistically significant effects with comparable sample sizes, and was deemed sufficient to detect clinically relevant differences in the primary outcomes.
Inclusion and exclusion criteria
Inclusion criteria
-
1)
Burns caused by high-voltage electrical injury, with a serum creatinine (Cr) level increase of at least 26.5 µmol/L within 48 h, or urine output less than 0.5 mL/(kg•h) sustained for 6 h, or a Cr level rising to 1.5 times the baseline within 7 days.
-
2)
Burn area greater than 30% of the total body surface area (TBSA), or third-degree burns covering more than 10% of the TBSA.
-
3)
All patients were admitted within 24 h of the burn injury and survived for at least 14 days post-admission.
-
4)
All patients were aged ≥ 18 years.
Exclusion criteria
-
1.
Radiation exposure or nephrotoxic drug exposure within 5 days prior to admission.
-
2.
Pre-existing AKI at any stage, according to KDIGO criteria.
-
3.
Chronic kidney disease for any reason, with a glomerular filtration rate (GFR) ≤ 60 mL/(min·1.73 m²) for at least 3 months.
-
4.
Post-kidney transplant patients.
-
5.
Patients who had received or required continuous renal replacement therapy (CRRT) within 7 days.
-
6.
Anuria (urine output < 100 mL/d) preventing sample collection.
-
7.
Patients with malignant tumors.
-
8.
Patients with autoimmune diseases requiring long-term steroid therapy.
-
9.
Patients who had received high-dose steroid treatment within the past month.
Treatment protocol
The control group received conventional treatment, which included:
-
1)
General Treatment: Upon admission, the patient’s burn site and area were assessed, general vital signs monitored, and relevant laboratory tests and imaging were conducted.
-
2)
Wound Management: Wounds were cleaned as soon as possible, preferably within 6 h of injury. Small superficial second-degree blisters were left untreated, while large blisters crossing joints were drained with a small incision. For deeper third-degree necrotic epidermis, it was selectively preserved to prevent the drying and necrosis of remaining dermal tissue. Dressings were changed with biological active factor dressings (Changsha Hairun Biotechnology Co., Ltd.), followed by silver ion functional dressings (Changsha Hairun Biotechnology Co., Ltd.), cut to slightly exceed the wound margin and applied to the wound.
-
3)
Hyperkalemia Management: For patients with serum potassium > 6.5 mmol/L, calcium gluconate (10% calcium gluconate 20 mL IV) was administered to antagonize the myocardial toxicity of potassium ions. Insulin (10 units + 50% glucose 50 mL IV) was injected to drive potassium ions into the cells. Furosemide (20 mg IV) was also administered. Fluid resuscitation was performed according to the formula for burn shock in the first 24 h: burn area x 1.5 mL x body weight (kg) + water (5% glucose 2000 mL), with a crystalloid-to-colloid ratio of 2:1. For the second 24 h, crystalloid volume was reduced by half, while the water volume remained unchanged. The third 24 h adjusted according to the output of the second 24 h.
The study group received the same conventional treatment with the addition of Xuebijing injection (Tianjin Hongri Pharmaceutical Co., Ltd., National Drug Standard Z20040033). Xuebijing injection (50 mL) was diluted in 100 mL of 0.9% sodium chloride solution and administered via intravenous drip twice daily for 7 consecutive days.
Xuebijing injection treatment
Xuebijing injection (Tianjin Hongri Pharmaceutical Co., Ltd., National Drug Standard Z20040033) was used in the study group as an adjunct to conventional treatment. The preparation and composition of Xuebijing injection are described as follows:
Composition and Preparation method
Xuebijing injection is a traditional Chinese medicine injection made from the following herbal ingredients:
-
Chishao (Paeoniae Radix Rubra) Primary active compounds: Paeoniflorin, paeonolide glycosides, and oxidized paeoniflorin.
-
Chuanxiong (Ligusticum wallichii) Primary active compounds: Ligustrazine, ferulic acid.
-
Danshen (Salvia miltiorrhiza) Primary active compounds: Danshensu, salvianolic acid.
-
Honghua (Carthamus tinctorius) Primary active compounds: Safflower yellow, hydroxy-safflower yellow A.
-
Danggui (Angelica sinensis) Primary active compounds: Ferulic acid, volatile oils.
The preparation process involves the following steps:
-
1)
Initial Extraction: The herbs are first ground and extracted using water or alcohol to obtain the active constituents.
-
2)
Purification: The extract is filtered and concentrated before undergoing further purification to remove impurities using methods such as macroporous resin adsorption or membrane separation.
-
3)
Final Processing: The purified extract is then adjusted to the desired concentration, sterilized, and filled into vials to produce the final Xuebijing injection.
Detailed component information
Supplementary Table 1 summarizes the detailed information of the plant species used in Xuebijing injection:
Side effects and safety evaluation
Xuebijing injection has been evaluated for its side effects and general safety. Known side effects may include:
-
Allergic Reactions: Rash, itching, erythema, and, in severe cases, respiratory distress or anaphylactic shock.
-
Gastrointestinal Reactions: Nausea, vomiting, abdominal pain, and diarrhea.
-
Cardiovascular Reactions: Chest tightness, palpitations, or elevated blood pressure.
-
Other Reactions: Dizziness, headaches, or fever.
Drug interactions
-
Potential Drug Interactions: Co-administration with certain antibiotics may alter the efficacy or increase the risk of adverse effects.
-
Combination with Similar Drugs: Concurrent use with other herbal medicines containing similar active compounds may increase the risk of excessive dosing and adverse reactions.
Safety assessment
-
General Safety: Xuebijing injection is generally safe when used appropriately under clinical supervision. However, due to the complexity of its composition and variability in individual responses, careful monitoring is required during treatment.
-
Special Populations:
-
Pregnant or Breastfeeding Women: Safety during pregnancy and lactation is not well established. Caution is advised.
-
Children: Safety in pediatric patients has not been fully evaluated, so use in this population should be approached with caution.
-
Patients with Liver or Kidney Impairment: Those with liver or kidney dysfunction may experience increased burden on these organs. Dose adjustment or cautious use is recommended for patients with impaired hepatic or renal function.
-
Administration protocol
The study group received 50 mL of Xuebijing injection diluted in 100 mL of 0.9% sodium chloride solution. It was administered intravenously twice daily for 7 consecutive days. The dosage regimen was consistent across all patients in the study group. The control group received only conventional treatment as described earlier.
Observational indicators for evaluation
The following parameters were measured to assess the efficacy and safety of Xuebijing injection in the study group:
-
Kidney Function: Blood urea nitrogen (BUN), serum creatinine (SCr), and 24-hour urinary protein. (Note: BUN/creatinine ratio and GFR percentage were not specifically calculated as primary endpoints in this protocol, as the study focused on direct markers of injury and inflammation and these derived ratios were planned for more extensive future investigation given data complexity. However, their value for post-hoc or future analyses is recognized.)
-
Inflammatory Markers: Neutrophils, inflammatory cytokines (IL-18, IL-6), and C-reactive protein (CRP).
-
Oxidative Stress Markers: Superoxide dismutase (SOD) and malondialdehyde (MDA).
-
Adverse Reactions: Any negative effects, including skin rashes, fever, and gastrointestinal symptoms.
These measures were assessed at baseline (1 h prior to treatment) and after 7 days of treatment to determine the effects of Xuebijing on kidney function, inflammation, and oxidative stress in patients with acute kidney injury following high-voltage electrical burns.
Observational indicators
The following parameters were measured in both groups before treatment (1 h prior) and 7 days after treatment: neutrophils, inflammatory cells, reactive oxygen species (ROS), kidney function (blood urea nitrogen [BUN], serum creatinine [SCr], 24-hour urinary protein), and oxidative stress markers (superoxide dismutase [SOD], malondialdehyde [MDA]). Adverse reactions, such as skin rashes, nausea, and fever, were also recorded.
Laboratory examination
Venous blood (3 mL) was collected from patients upon admission. The blood was centrifuged at 1500 rpm for 10 min, and the supernatant was stored at -20 °C in EP tubes for subsequent analysis of BUN, SCr, 24-hour urinary protein, C-reactive protein (CRP), IL-18, IL-6, SOD, and MDA levels.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software. Quantitative data were expressed as mean ± standard deviation (x̄ ± s) or median. Comparisons between two independent samples were performed using t-tests. Categorical data were presented as percentages (%) and analyzed using the χ² test. A p-value ≤ 0.05 was considered statistically significant, and p ≤ 0.01 indicated a highly significant difference.
Results
Comparison of general data between groups
According to the inclusion criteria, a total of 103 patients with acute kidney injury (AKI) following high-voltage electrical burns were enrolled. Seven patients were excluded: one patient had gastric cancer, four patients required long-term steroid use, one patient underwent kidney transplantation, and one patient had chronic kidney disease. Finally, 96 patients with acute kidney injury following high-voltage electrical burns were included in the study. The baseline data (including age, gender, BMI, alcohol consumption, smoking, hypertension, hyperlipidemia, and diabetes) showed no statistically significant differences between the two groups (P > 0.05) (Table 1).
Comparison of BUN, scr, and 24-h urine protein levels between groups
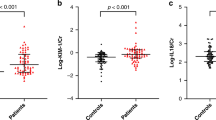
One hour before treatment, there were no statistically significant differences between the two groups regarding BUN, SCr, or 24-hour urine protein levels (P > 0.05). Seven days after treatment, BUN, SCr, and 24-hour urine protein levels in both groups were significantly lower than before treatment, and the study group showed significantly lower levels than the control group, with statistically significant differences (P < 0.05) (Table 2; Fig. 1).
Comparison of BUN, SCr, and 24-h urine protein levels between groups. This figure shows the changes in blood urea nitrogen (BUN, mmol/L), serum creatinine (SCr, µmol/L), and 24-h urine protein (mg) levels before (1 h before treatment) and after 7 days of treatment in both the study and control groups. The levels of BUN, SCr, and 24-hour urine protein were significantly reduced in both groups after treatment compared to baseline (#P < 0.05). However, the study group exhibited significantly lower levels of BUN, SCr, and 24-h urine protein than the control group after 7 days of treatment (*P < 0.05). Data are presented as mean values. #P < 0.05 compared to the same group before treatment; *P < 0.05 for the comparison between the study group and the control group after 7 days of treatment.
Comparison of neutrophils, inflammatory cells, and inflammatory cytokines between groups
One hour before treatment, there were no statistically significant differences between the two groups in terms of neutrophils, CRP, IL-18, or IL-6 levels (P > 0.05). Seven days after treatment, the levels of neutrophils, CRP, IL-18, and IL-6 in both groups were significantly lower than before treatment, and the study group showed significantly lower levels than the control group, with statistically significant differences (P < 0.05) (Table 3; Fig. 2).
Comparison of neutrophils, inflammatory cytokines (CRP, IL-18, IL-6) between groups. This figure displays the changes in neutrophil count (109/L), C-reactive protein (CRP, mg/L), interleukin-18 (IL-18, ng/mL), and interleukin-6 (IL-6, ng/mL) levels before (1 h before treatment) and after 7 days of treatment in both groups. All inflammatory markers were significantly reduced in both groups after treatment compared to baseline (#P < 0.05). The study group showed significantly lower levels of neutrophils, CRP, IL-18, and IL-6 than the control group after 7 days of treatment (*P < 0.05). Data are presented as mean values. #P < 0.05 compared to the same group before treatment; *P < 0.05 for the comparison between the study group and the control group after 7 days of treatment.
Comparison of oxidative stress levels between groups
One hour before treatment, there were no statistically significant differences between the two groups in terms of SOD and MDA levels (P > 0.05). Seven days after treatment, SOD levels in both groups were significantly lower than before treatment (P < 0.05), and MDA levels were significantly higher than before treatment (P < 0.05). Comparing the groups post-treatment, the study group showed significantly lower SOD levels (98.21 ± 7.06 U/mL vs. 106.29 ± 7.45 U/mL, P < 0.001) and significantly higher MDA levels (12.09 ± 1.02 nmol/mL vs. 10.18 ± 1.03 nmol/mL, P < 0.001) compared to the control group (Table 4; Fig. 3). These findings indicate that while both groups experienced increased oxidative stress markers post-burn, this effect was more pronounced in the study group for SOD and MDA.
Comparison of oxidative stress levels between groups. This figure illustrates the changes in superoxide dismutase (SOD, U/mL) and malondialdehyde (MDA, nmol/mL) levels before (1 h before treatment) and after 7 days of treatment in both groups. After treatment, SOD levels were significantly lower, and MDA levels were significantly higher in both groups compared to baseline (#P < 0.05). The study group exhibited significantly lower SOD levels and significantly higher MDA levels compared to the control group after 7 days of treatment (*P < 0.05). Data are presented as mean values. #P < 0.05 compared to the same group before treatment; *P < 0.05 for the comparison between the study group and the control group after 7 days of treatment.
Comparison of adverse reactions between groups
During treatment, the control group experienced 4 cases of adverse reactions: 2 cases of mild abdominal pain, 1 case of mild rash, and 1 case of nausea. All symptoms disappeared after stopping the medication and did not require special treatment. The incidence of adverse reactions in the control group was 8.16% (4/49). In the study group, there were 2 cases: 1 case of mild abdominal pain and 1 case of mild rash, with an incidence of adverse reactions of 4.08% (2/49). The comparison of adverse reactions between the two groups showed no statistically significant difference (χ² = 0.399, P = 0.528).
Typical case descriptions
Case 1: acute kidney injury following 380-V high-voltage electrical burn
A 45-year-old male patient presented with a 380-volt high-voltage electrical burn to the trunk, resulting in third-degree burns. Upon admission, the patient exhibited signs of acute kidney injury (AKI), with elevated levels of BUN, SCr, and 24-hour urine protein. The patient was assigned to the study group and underwent the prescribed treatment.
Figure 4 shows the treatment process for this case:
-
a.
Pre-treatment: The patient’s burn wounds were classified as third-degree, with extensive tissue necrosis and exposed fat.
-
b.
Treatment Phase: The surgical team performed debridement to remove necrotic tissue and exposed fat, followed by continuous negative pressure wound therapy (NPWT) to promote healing.
-
c.
Treatment Phase: After one week of NPWT, significant progress was observed in the wound bed.
-
d.
Treatment Phase: After NPWT, the device was removed, and preparation for a second-stage skin grafting was completed.
-
e.
Post-treatment: One week after the skin graft, the grafts were successfully integrated, and the wound area showed complete closure.
Treatment process for a 45-year-old male patient with acute kidney injury following a 380-volt high-voltage electrical burn (Case 1). (a) Pre-treatment: A 45-year-old male patient with third-degree burns to the trunk caused by a 380-volt electrical injury. The burn wound exposed underlying fat tissue. (b) Treatment Phase: Surgical debridement of necrotic tissue and exposed fat was performed, followed by continuous negative pressure wound therapy (NPWT). (c) Treatment Phase: After one week of NPWT, the wound bed showed significant progress with improved tissue granulation. (d) Treatment Phase: Removal of the NPWT device, preparing for a second-stage skin grafting procedure. (e) Post-treatment: One week after skin grafting, the grafts were successfully integrated, and the wound was completely closed.
Following treatment, the patient’s kidney function improved significantly. BUN, SCr, and 24-hour urine protein levels decreased substantially, with the study group showing significantly lower levels than the control group (P < 0.05) (Table 2; Fig. 1). Additionally, inflammatory markers such as CRP, IL-18, and IL-6 showed significant reductions (P < 0.05) (Table 3; Fig. 2).
Case 2: acute kidney injury following 10,000-v high-voltage electrical burn
A 32-year-old male patient presented with a 10,000-volt electrical burn to the left upper limb, resulting in third-degree burns. The patient developed acute kidney injury upon admission, with elevated neutrophil count, inflammatory markers, and BUN levels. He was assigned to the study group for the treatment protocol.
Figure 5 shows the treatment process for this case:
-
a.
Pre-treatment: The burn area was extensive, with noticeable swelling and a third-degree burn pattern. Immediate surgical intervention was initiated, including incision and drainage to relieve pressure.
-
b.
Treatment Phase: The patient underwent debridement of necrotic tissue followed by continuous NPWT for one week. Despite initial improvement, some muscle and tendon exposure remained.
-
c.
Treatment Phase: After one week of NPWT, a skin graft procedure was performed, alongside the placement of artificial dermis to promote better integration of the graft.
-
d.
Treatment Phase: Two weeks after the initial graft, the patient underwent a second-stage operation to remove remaining necrotic tissue.
-
e.
Treatment Phase: One week post-second surgery, the patient’s wound exhibited marked healing and improved tissue viability.
-
f.
Post-treatment: One month after treatment, the patient showed complete wound closure, and the kidney function had improved significantly. BUN, SCr, and 24-hour urine protein levels were notably lower. The changes in markers of oxidative stress, SOD and MDA, were as described in Table 4; Fig. 3, showing a more pronounced decrease in SOD and increase in MDA in the study group compared to the control group (P < 0.05).
Treatment process for a 32-year-old male patient with acute kidney injury following a 10,000-volt high-voltage electrical burn (Case 2). (a) Pre-treatment: A 32-year-old male patient with third-degree burns to the left upper limb caused by a 10,000-volt electrical injury. The burn area was swollen, and surgical intervention was required immediately, including incision and drainage. (b) Treatment Phase: Necrotic tissue was debrided, followed by continuous negative pressure wound therapy (NPWT) for one week. Some tendon exposure remained. (c) Treatment Phase: After one week of NPWT, the patient underwent skin grafting and artificial dermis placement. (d) Treatment Phase: Two weeks after the first grafting procedure, a second surgery was performed to remove remaining necrotic tissue. (e) Treatment Phase: One week post-second surgery, the wound showed marked healing with improved tissue viability. (f) Post-treatment: One month after treatment, the wound was fully closed, and kidney function had significantly improved.
In this case, the patient experienced mild abdominal pain and a rash, which were resolved after medication discontinuation. The treatment protocol demonstrated efficacy in improving kidney function and reducing inflammation, alongside facilitating wound healing.
Discussion
The damage to the body caused by high-voltage electricity is mainly due to electrical thermal effects and non-thermal effects. Both effects can lead to damage to tissues and organs, with electrical thermal effects potentially causing progressive necrosis of tissues and organs. Acute renal failure is one of the important complications of high-voltage electrical burns and contributes to an increased mortality rate. Therefore, actively exploring effective methods for treating acute kidney injury (AKI) following high-voltage electrical burns is a key focus of clinical research.
Xuebijing (a traditional Chinese medicine injection composed of Radix Paeoniae Rubra, Ligusticum chuanxiong, Salvia miltiorrhiza, Carthamus tinctorius, and Angelica sinensis) has been reported to have significant effects such as promoting blood circulation, removing blood stasis, unblocking meridians, and dispersing toxins. Current studies suggest that Xuebijing has a notable anti-endotoxin effect, and clinical observations have shown that Xuebijing can significantly protect renal function in patients with burns complicated by kidney injury. It can effectively reduce serum creatinine (Cr) levels. Some studies suggest that the therapeutic effects of Xuebijing are related to various cytokines11,12. Internationally, components of Xuebijing have been recognized for their pharmacological activities; for instance, salvianolic acids from Salvia miltiorrhiza are known for antioxidant and anti-inflammatory properties15, and ligustrazine from Ligusticum chuanxiong has shown protective effects in ischemia-reperfusion injury models16. Based on this, we hypothesize that Xuebijing alleviates acute kidney injury after high-voltage electrical burns by inhibiting neutrophils, inflammatory cells, and influencing oxidative stress levels, thereby clarifying its potential in the treatment of acute kidney injury post-high-voltage electrical burns.
Liu17 and colleagues pointed out that effective preventive and therapeutic measures can significantly improve the clinical symptoms of acute kidney injury patients. Jin18 and others emphasized that effective interventions could improve the prognosis of patients with sepsis complicated by acute kidney injury. Rutter19 indicated that effective interventions can treat acute kidney injury and improve renal function. Fan Xiaoyuan20 found that after applying active and effective treatments to sepsis patients, renal function improved, and inflammation markers were reduced. Our research shows that after 7 days of treatment, the study group had significantly lower levels of BUN, serum creatinine (SCr), and 24-hour urine protein compared to the control group (P < 0.05), suggesting that Xuebijing can improve renal function in patients with acute kidney injury after high-voltage electrical burns. Although not pre-specified as primary endpoints for this initial investigation, which focused on direct markers of renal injury and inflammation, future studies would benefit greatly from incorporating detailed analyses of BUN/creatinine ratios and changes in estimated GFR to provide a more holistic view of renal functional recovery dynamics.
Many studies21,22 have found that acute kidney injury after high-voltage electrical burns triggers the release of various inflammatory factors, such as neutrophils, CRP, IL-18, and IL-6, leading to a series of inflammatory changes. These factors are key contributors to systemic inflammation and also induce renal inflammation. Clinical evidence confirms that the elevation of neutrophils, CRP, IL-18, and IL-6 can induce immune-inflammatory responses, causing kidney function damage. Sklienka23 and others observed that IL-6 and other inflammatory factors were highly expressed. Zou24 et al. suggested that effective interventions could lower inflammation markers in patients with impaired kidney function. Our results showed that after 7 days of treatment, the study group had significantly lower levels of neutrophils, CRP, IL-18, and IL-6 compared to the control group (P < 0.05), indicating that Xuebijing can reduce these inflammatory markers in patients with acute kidney injury after high-voltage electrical burns. This is likely due to Xuebijing’s regulatory effect on inflammatory response mechanisms. Xuebijing, a national class II new drug, contains active ingredients such as Danshensu, catechins, ligustrazine, ferulic acid, and carthamin, which reduce capillary permeability, decrease exudation, improve local blood circulation, lower endotoxin levels, counteract the mononuclear macrophage system, release endogenous inflammatory mediators, regulate immune function, and alleviate sepsis-induced inflammatory stress responses. The carotenoids in Carthamus tinctorius exhibit platelet-activating factor inhibitory activity, exerting anti-inflammatory effects. Xuebijing injection protects endothelial cells, antagonizes endotoxins, effectively clears inflammatory mediators, and inhibits the levels of cytokines such as TNF-α and IL-6, improving microcirculation25,26. These anti-inflammatory effects are supported by international research indicating that herbal components can modulate signaling pathways such as NF-κB, which is central to inflammation6.
Oxidative stress imbalance is critically involved in the pathogenesis of AKI and burn injuries. Typically, AKI is associated with decreased levels of antioxidant enzymes like SOD and increased levels of lipid peroxidation products like MDA7,8. A therapeutic intervention aimed at reducing oxidative stress would be expected to increase SOD levels and decrease MDA levels. However, in our study, while both groups showed a decrease in SOD and an increase in MDA post-treatment compared to baseline (indicative of ongoing oxidative stress post-burn), the study group treated with Xuebijing exhibited significantly lower SOD levels and significantly higher MDA levels compared to the control group after 7 days (P < 0.05). This finding is counterintuitive if Xuebijing is hypothesized to act primarily as an antioxidant in this context. While the individual components of Xuebijing have shown antioxidant properties in preclinical settings15,16,29, the observed paradoxical effects on SOD and MDA in our critically ill cohort highlight the challenges of translating these findings to complex clinical scenarios involving severe systemic stress. Other studies investigating herbal interventions in critical illness have occasionally reported similar discordant findings or complex modulations of oxidative stress markers, suggesting that the net effect in vivo can differ from predictions based on isolated component activities or simpler models. Du et al.27 pointed out that effective interventions can improve microcirculation in sepsis-induced acute kidney injury and alleviate oxidative stress damage, thus protecting kidney function. Gao et al.28 found that traditional Chinese medicine decoctions could effectively treat patients with chronic kidney disease (stages 2–4) complicated by acute kidney injury and adjust oxidative stress (presumably by increasing SOD and decreasing MDA, though specific outcomes from this study are not detailed here). The results from our study regarding SOD and MDA suggest that Xuebijing’s interaction with the oxidative stress system in severe high-voltage electrical burn-induced AKI may be more complex than a simple direct antioxidant effect. Several factors could contribute to this observation: (1) The timing of measurement (7 days post-treatment) might reflect a specific phase of a dynamic oxidative stress response where these paradoxical changes occur. (2) Xuebijing might exert its primary beneficial effects through potent anti-inflammatory actions (as observed with CRP, IL-18, IL-6, and neutrophils), which might indirectly influence oxidative stress pathways in ways not captured by SOD and MDA alone. (3) The severe nature of high-voltage electrical burns could induce an overwhelming oxidative insult, and Xuebijing might modulate specific components of this response differently, or the decrease in SOD could reflect increased consumption in an attempt to manage ROS, while increased MDA reflects ongoing lipid peroxidation that is not fully mitigated by Xuebijing at this dose or duration. (4) Some components in complex herbal mixtures can have biphasic or even pro-oxidant effects under certain conditions, although this is speculative for Xuebijing. Therefore, further research with a broader panel of oxidative stress and antioxidant markers, alongside time-course analyses, is essential to comprehensively elucidate Xuebijing’s impact on redox balance in this setting.
Modern pharmacological studies29 indicate that gallic acid in Radix Paeoniae Rubra has superoxide dismutase-like activity and protects cell membranes. Danshensu can scavenge free radicals and has a strong ability to inhibit lipid peroxidation. These known properties of individual components contrast with our overall findings for SOD and MDA with the composite Xuebijing injection in this specific clinical scenario, highlighting the complexity of multi-component herbal interventions. Zheng et al.30 reported that Xuebijing has few adverse reactions, and when used according to the drug instructions, side effects are rare. Our study showed no significant difference in adverse reactions between the two groups (P > 0.05), consistent with previous findings, indicating that Xuebijing does not increase adverse reactions in patients with acute kidney injury following high-voltage electrical burns and has a high safety profile.
This study has several limitations. Firstly, the sample size, while based on previous similar studies, was relatively small, which may limit the generalizability of the findings. Secondly, the follow-up period was short (7 days), preventing assessment of long-term outcomes. Thirdly, the open-label nature of the study is a significant limitation. Although necessary due to the characteristics of the Xuebijing intervention as an add-on therapy, and while laboratory personnel assessing biochemical outcomes were blinded where feasible, the lack of blinding for clinicians and patients could have introduced performance or detection bias, potentially influencing subjective assessments of clinical improvement or reporting of adverse events. This might have particularly affected the interpretation of overall efficacy, though key renal and inflammatory markers were objectively measured.
Conclusion
In conclusion, Xuebijing can improve renal function in patients with acute kidney injury after high-voltage electrical burns and significantly reduce neutrophil counts and levels of inflammatory markers such as CRP, IL-18, and IL-6. However, its effect on oxidative stress as measured by SOD and MDA in this study was unexpected, with the Xuebijing group showing a more pronounced decrease in SOD and increase in MDA compared to the control group. This suggests that while Xuebijing provides renal and anti-inflammatory benefits, its impact on these specific oxidative stress markers requires further investigation to be fully understood. The treatment does not increase adverse reactions, making it a safe intervention. Its potential for clinical application in improving kidney function and reducing inflammation is significant, though the mechanism related to oxidative stress modulation needs more detailed exploration. Future research should expand the sample size, extend the follow-up duration, incorporate a broader range of oxidative stress markers, address the limitations of an open-label design through robust blinding strategies where possible, and potentially explore dose-response relationships to better understand these complex interactions.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Georgeto, S. M. et al. Electric burn of the skull: treatment by applying trepanations and wound dressing. Illustrative case. J. Neurosurg. Case Lessons. 7 (12), CASE23635 (2024).
Manas, R. K. & Chatterji, S. K. Management of post-electric burn microstomia by free radial artery forearm flap in a 1-year-old child. Indian J. Plast. Surg. 56 (6), 535–539 (2023).
Chen, B. et al. Clinical characteristics and risk factors for severe burns complicated by early acute kidney injury. Burns 46 (5), 1100–1106 (2020).
Folkestad, T. et al. Acute kidney injury in burn patients admitted to the intensive care unit: a systematic review and meta-analysis. Crit. Care. 24 (1), 2 (2020).
Ihim, S. A. et al. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: biological role in induction, regulation, and treatment. Front. Immunol. 13, 919973 (2022).
George, B., Suchithra, T. V. & Bhatia, N. Burn injury induces elevated inflammatory traffic: the role of NF-κB. Inflamm. Res. 70 (1), 51–65 (2021).
El Ayadi, A., Salsbury, J. R., Enkhbaatar, P., Herndon, D. N. & Ansari, N. H. Metal chelation attenuates oxidative stress, inflammation, and vertical burn progression in a Porcine brass comb burn model. Redox Biol. 45, 102034 (2021).
Ito, Y. et al. Heat-Not-Burn cigarette induces oxidative stress response in primary rat alveolar epithelial cells. PLoS One. 15 (11), e0242789 (2020).
Wu, L. et al. Meta-analysis of the efficacy of Xuebijing injection in the treatment of sepsis complicated by acute kidney injury. Zhongguo Linchuang Yanjiu. 36 (10), 1474–1480 (2023).
Li, Y. et al. Analysis of the renal protective effect of Xuebijing in patients with acute pancreatitis complicated by acute kidney injury. Yiyao Qianwei. 13 (03), 112–114 (2023).
Zhang, C. et al. Xuebijing alleviates LPS-induced acute lung injury by downregulating pro-inflammatory cytokine production and inhibiting gasdermin-E-mediated pyroptosis of alveolar epithelial cells. Chin. J. Nat. Med. 21 (8), 576–588 (2023).
Liao, X. & Rello, J. Efficacy of Xuebijing injection for Sepsis (EXIT-SEP): lost in translation. Anaesth. Crit. Care Pain Med. 42 (4), 101257 (2023).
Wang, X. et al. Evaluation of Herb-Drug interaction between Danshen and Rivaroxaban in rat and human liver microsomes [published correction appears in front pharmacol. 2022;13:1039267]. Front. Pharmacol. 12, 776891 (2021).
Ran, X., Ma, L., Peng, C., Zhang, H. & Qin, L. P. Ligusticum Chuanxiong hort: a review of phytochemistry and Pharmacology. Pharm. Biol. 49 (10), 1079–1087 (2011).
Liao, W. et al. A review of the mechanism of action of Dantonic® for the treatment of chronic stable angina. Biomed. Pharmacother. 109, 690–700. https://doi.org/10.1016/j.biopha.2018.10.013 (2019).
Ran, X., Ma, L., Peng, C., Zhang, H. & Qin, L. P. Ligusticum Chuanxiong hort: a review of chemistry and Pharmacology. Pharm. Biol. 49 (11), 1180–1189. https://doi.org/10.3109/13880209.2011.576346 (2011).
Liu, C. et al. Incidence and risk factors of acute kidney injury in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front. Immunol. 14, 1173952 (2023).
Jin, B. et al. Early high-dose continuous veno-venous hemofiltration alleviates the alterations of CD4 + T lymphocyte subsets in septic patients combined with acute kidney injury. Artif. Organs. 46 (7), 1415–1424 (2022).
Rutter, W. C., Burgess, D. R., Talbert, J. C. & Burgess, D. S. Acute kidney injury in patients treated with Vancomycin and piperacillin-tazobactam: A retrospective cohort analysis. J. Hosp. Med. 12 (2), 77–82 (2017).
Fan, X. et al. Clinical observation on the effect of early continuous blood purification therapy in patients with gastric cancer complicated by acute kidney injury. Practical Cancer J. 39 (12), 2061–2063 (2024).
Wang, F. et al. Inflammatory kidney injury in trichloroethylene hypersensitivity syndrome mice: possible role of C3a receptor in the accumulation of Th17 phenotype. Ecotoxicol. Environ. Saf. 186, 109772 (2019).
Li, Y. et al. Kidney injury in a murine hemorrhagic shock/resuscitation model is alleviated by sulforaphane’s Anti-Inflammatory and antioxidant action. Inflammation 47 (6), 2215–2227 (2024).
Sklienka, P. et al. Physiologic risk factors for early acute kidney injury in severely injured patients. Bratisl Lek Listy. 121 (11), 779–785 (2020).
Zou, F. et al. Treatment efficacy of continuous renal replacement therapy on symptoms, inflammatory mediators, and coagulation function in patients with sepsis-associated acute kidney injury. Arch. Esp. Urol. 75 (9), 746–752 (2022).
Ding, W. C. et al. Mechanism of Xuebijing injection in treatment of sepsis-associated ARDS based on network Pharmacology and in vitro experiments. Zhongguo Zhong Yao Za Zhi. 48 (12), 3345–3359 (2023).
Tu, Y. L. et al. Quality markers prediction of Xuebijing injection based on fingerprint, network pharmacology, and content determination. Zhongguo Zhong Yao Za Zhi. 49 (18), 4885–4897 (2024).
Du, H., Wang, Z. & Zhu, W. Effect of Ulinastatin combined with CRRT on renal function, HMGB1, KIM-1, and PCT in patients with septic acute kidney injury. Chin. J. Integr. Kidney Disease. 25 (05), 424–426 (2024).
Gao, Y. & Luo, T. Effects of Levosimendan combined with CRRT on renal blood flow parameters, inflammatory factors, and recovery progress in patients with septic shock-induced acute kidney injury. Chin. Med. Innov. 21 (35), 1–6 (2024).
Ding, W., He, R. & Wang, J. Effect of Xuebijing on serum inflammatory factors and oxidative free radicals in patients with acute pancreatitis. Contemp. Med. 21 (23), 134–135 (2015).
Zheng, R. et al. Areal-world study on adverse drug reactions to Xuebijing injection: hospitalintensive monitoring based on 93 hospitals (31,913 cases). Ann. Transl Med. 7 (6), 117 (2019Mar).
Funding
This study was funded by Research Fund of Hebei Provincial Health and Family Planning Commission.
Author information
Authors and Affiliations
Contributions
Author contributionXiaopei He: conceptualization and design of this study, data analysis and interpretation, drafting the manuscriptNa Li: conceptualization and design of this study, data analysis and interpretationLeilei Ma: data analysis and interpretation, drafting the manuscriptMeng Yang: data analysis and interpretationLiqing Ren: data analysis and interpretationJiawen Hao: data analysis and interpretationXin Xue: conceptualization and design of this study, drafting and revising the manuscriptYinghui Pang: conceptualization and design of this study, drafting and revising the manuscriptAll authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
The research protocol was in compliance with the relevant requirements of the Declaration of Helsinki and was approved by the Ethics Committee of the Third Hospital of Hebei Medical University. The patient or patient guardian provided informed consent to participate in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, X., Li, N., Ma, L. et al. Xuebijing alleviates high-voltage electrical burn-induced acute kidney injury by inhibiting neutrophils and inflammation. Sci Rep 15, 30410 (2025). https://doi.org/10.1038/s41598-025-11977-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11977-w