Abstract
TSG6 (Tumor necrosis factor stimulated gene-6) plays a critical role in modulating the tumor microenvironment by regulating inflammation, immune responses, and extracellular matrix remodeling. Hypoxia affects tumor growth, angiogenesis, and anti-cancer drug resistance in the tumor microenvironment, and TSG6 expression is known to influence HIF-1α expression in tumor tissues. In this study, we created TSG6 knockdown spheroids of canine mammary gland tumor (MGT) cells (CIPp and CIPm) to investigate the effects of TSG6 on angiogenesis and drug resistance in the tumor microenvironment. Using a siRNA transfection system, we induced TSG6 knockdown in canine MGT cells and formed TSG6 knockdown spheroids using an ultra-low adhesion plate. Cell viability and apoptosis were assessed by treating TSG6 knockdown spheroids with doxorubicin. Angiogenesis was evaluated by forming vascularized spheroids with canine MGT and endothelial cells (ECs). TSG6 knockdown led to reduced expression of tumor growth factors and multidrug resistance genes in canine MGT cells, as well as a significant reduction in hypoxic conditions within the spheroids. When treated with doxorubicin, TSG6 knockdown spheroids exhibited decreased viability and increased apoptosis. In a vascularized TSG6 knockdown spheroid model, TSG6 knockdown significantly reduced the expression of CD31 and tube formation in canine ECs. In conclusion, we created a TSG6 knockdown spheroid model to investigate the role of TSG6 in the tumor microenvironment. TSG6 knockdown significantly reduced anti-cancer drug resistance and angiogenesis in canine MGT cells. Therefore, TSG6 could be considered a potential therapeutic target for canine MGT.
Similar content being viewed by others
Introduction
The tumor microenvironment (TME) is an ecosystem created by cancer cells and comprises components including immune cells, stromal cells, blood vessels, and extracellular matrix1. TME elements collectively facilitate tumor growth, spread, and proliferation by providing an unbroken flow of nutrients and oxygen, obstructing immune surveillance, and effectively transporting drugs2. Hypoxia is an important feature of the TME3. Intratumoral hypoxia in cancer results from a complex interplay among cancer cell proliferation, aberrant angiogenesis, inadequate oxygen delivery, and altered metabolic states, affecting both malignant and stromal cells4. Intratumoral hypoxia is frequently associated with a poor prognosis of cancer5. Cancer cells and their surrounding environment may be hypoxic, which can reprogram the biology of the disease in a number of ways, including how it progresses, remains stem-like, and enters dormancy, as well as how it adapts to redox conditions, communicates with other cells, and resists treatment5,6. Hypoxia affects the TME and treatment effectiveness by activating vascular endothelial cells (ECs), upregulating the transcription of vascular endothelial growth factors (VEGFs), and promoting excessive angiogenesis7,8.
Monolayer 2D culture is the most common method for tumor cell experiments; however, this approach does not accurately reflect tumor biology associated with hypoxia9. It is well-documented that the genomic profiles and responses to treatment of tumor cells cultivated in 2D settings differ from those observed in solid tumors10,11. Furthermore, studying hypoxia in vivo is challenging due to the wide variation in oxygen tension across tumors12,13. To address these limitations, the suitability of the 3D culture method for studying tumor hypoxia is gaining recognition14. 3D spherical culture models offer advantages, such as precise control and reproducibility of tumor cells, rapid model generation, and minimizing the influence of non-tumor cell types15,16. Therefore, 3D spherical culture models are well-established methods used by researchers to replicate hypoxic TME. Cells in spheroid models are tightly clustered to mimic the specific aspects of a tumor14. The size of the spheroid and composition of the culture media, which can be adjusted to reliably predict viability and kinetic potential, are two factors that influence how well cell spheroid models mimic tumor hypoxia9.
Tumor necrosis factor-α (TNF-α) stimulated gene 6 (TSG6) is a secreted protein that belongs to the family of hyaluronan-binding proteins17. Although the role of TSG6 in inflammation has been well studied18, its role and mechanisms in tumors remain unclear. However, because inflammation is a major factor in tumor proliferation, the effect of TSG6 on tumor progression has been recently investigated19,20. In veterinary medicine, the association between TSG6 and TMEs, specifically tumor proliferation and metastasis in canine mammary gland tumors (MGT), has been studied21,22. A previous study identified the role of TSG6 in inducing hypoxia using CoCl221; however, TSG6 associated with TME has not been studied using 3D culture methods.
In this study, we aimed to induce TSG6 knockdown in canine MGT cells using a siRNA transfection system and subsequently create a TSG6 knockdown spheroid model using these cells. Using this TSG6 knockdown spheroid model, we investigated the impact of TSG6 on anti-cancer drug resistance and angiogenesis in canine MGT cells in a 3D culture environment. Our goal was to evaluate the potential of TSG6 as a therapeutic target for canine MGT.
Materials and methods
Cell culture
The Department of Veterinary Clinicopathology of Seoul National University provided canine malignant MGT cell lines (CIPp and CIPm). CIPp and CIPm cells originated from primary and metastatic canine MGTs respectively23. Both cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Welgene, Gyeong-San, Republic of Korea) using 10% fetal bovine serum (FBS; Gibco, Billings, MT, USA), 1% solution of 10,000 units/mL penicillin (Sigma-Aldrich, St. Louis, MO, USA), and 100 µg/mL streptomycin (Sigma-Aldrich) in 5% CO2 at 37 °C. The medium was changed every two to three days. The cells were subcultured until reaching 80–90% confluency.
Primary culture of canine aorta derived endothelial cells
All animal experiments were conducted in compliance with the ARRIVE guidelines and in accordance with relevant guidelines and regulations. The study was approved by the Institutional Animal Care and Use Committee of the Seoul National University (SNU), Republic of Korea (SUN-231016-3). ECs were isolated from the aorta of a 3 year-old male beagle dog (Raonbio, Yong-In, Republic of Korea). The dog was under general anesthesia with an intravascular injection of alfaxalone and inhalation of isoflurane. After the surgical removal of the aorta, the dog was euthanized using an intravascular injection of potassium chloride. The aorta was washed three times with Dulbecco’s phosphate-buffered saline (DPBS; Welgene) containing 1% penicillin/streptomycin (PS). The surrounding fatty tissue was removed and cut into pieces. Thereafter, 0.1% Collagenase type 1 A solution was added, and the aorta sample was incubated for 60 min at 5% CO2 and 37 °C. After digestion, Dulbecco’s modified Eagle’s medium (DMEM; Welgene) containing 10% FBS and 1% PS was added to the aorta sample to neutralize the collagenase solution. The aorta sample was centrifuged at 1200 x g for 5 min, the supernatant was removed, and the pellet was suspended by adding DMEM (Welgene) containing 10% FBS. The suspended aorta sample was passed through a 70 μm Falcon cell strainer (Thermo Fisher Scientific, Waltham, MA, USA) to remove debris, centrifuged again, and the supernatant was removed. To remove the erythrocytes, red blood cell lysis buffer (Sigma-Aldrich) was added, and the cells were incubated at room temperature (RT) for 10 min. The aorta sample was washed three times with DPBS, seeded in a cell culture dish, added to DMEM containing 10% FBS and 1% PS, and cultured at 37 °C and 5% CO2. All non-attached cells were removed the following day.
Characterization of canine aorta derived endothelial cell
Primary cultured ECs were treated with trypsin, harvested, and washed thrice with DPBS. ECs were then fixed with cold70% ethanol at -20 °C for at least 2 h. After fixation, ECs were washed three times with DPBS, stained with rabbit anti-CD31 (1:100; Novus Biologicals, Englewood, CO, USA; Cat# NB100-2284) for 1 h at 4 °C, and then stained with alexa fluor 488 conjugated goat anti-rabbit IgG antibody (Invitrogen, Carlsbad, CA, USA; Cat# A11008). Flow cytometry was performed within 1 h using FACS Aria II (BD Biosciences, Franklin Lakes, NJ, USA), and the results were analyzed using FlowJo software (BD Biosciences; RRID: SCR_008520).
TSG6 specific SiRNA transfection
Canine MGT cells were seeded in a 6-well plate at a density of 2 × 105 cells/well. Thereafter, the cells were transfected with TSG6 specific siRNA (si-TSG6) or scrambled siRNA (si-ctrl) (Santa Cruz Biotechnology, Dallas, TX, USA; Cat# sc-39819 and sc-37007, respectively) for 48 h using Lipofectamine RNAiMAX (Invitrogen; Cat# 56532), according to the manufacturer`s instructions.
Enzyme-linked immunosorbent assay
Culture media from canine MGT cells were obtained to confirm whether TSG6 knockdown occurred in canine MGT cells. A TSG6 ELISA kit (MyBioSource, San Diego, CA, USA; Cat# MBS7262296) was used according to the manufacturer’s instructions.
TSG6 knockdown spheroid formation using ultra-low adhesion well
To create TSG6 knockdown spheroids, canine MGT cells transfected with si-TSG6 or si-ctrl and non-transfected cells were harvested. Cells were seeded in an ultra-low attachment multiple-well plate (Corning, NY, USA; Cat# 7007) at a density of 5 × 104 cells/well. The cells were incubated in RPMI 1640 medium containing 20% FBS, 1% PS, and 5% Matrigel (Growth factor reduced; Corning; Cat#354230) at 5% CO2 and 37 °C. The spheroids were visualized every 12 h using an inverted microscope (CKX53; Olympus, Tokyo, Japan) at 4x magnification. TSG6 knockdown spheroids were obtained after 48 h of culture.
Vascularized spheroid formation
Canine MGT cells were transfected with si-TSG6 or si-ctrl and non-transfected cells. Canine ECs and MGT cells were seeded at a ratio of 1:3, with a total of 5 × 104 cells per well, in an ultra-low attachment multiple-well plate (Corning). The cells were incubated in RPMI 1640 medium containing 20% FBS, 1% PS, and 5% Matrigel (Growth factor reduced; Corning) at 5% CO2 and 37 °C. Vascularized spheroids were obtained after 48 h of culture.
Histological analysis of spheroids
After culturing for 48 h, spheroids were carefully pipetted. The spheroids were washed thrice with PBS and fixed with neutral buffered 10% formalin overnight at 4 °C. Fixed spheroids were prepared as blocks using Histogel (Epredia, Kalamazoo, MI, USA; Cat# HG-4000-012). These spheroid blocks were embedded in paraffin, sectioned, and stained with H&E (Thermo Fisher Scientific).
Immunohistochemistry analysis of spheroids
After culturing for 48 h, the spheroids were carefully pipetted. The spheroids were washed thrice with PBS and fixed with neutral buffered 10% formalin overnight at 4 °C. Fixed spheroids were prepared as blocks using Histogel (Epredia). Spheroid blocks were embedded in paraffin and sectioned. The sections were then deparaffinized and rehydrated. Antigen retrieval was performed using a 10 mM citrate buffer. The sections were washed thrice with cold DPBS (Welgene), blocked with 5% bovine serum albumin (BSA; Sigma-Aldrich) and 0.1% Triton X (Sigma-Aldrich) in DPBS (0.1% PBSTX), and stained with antibodies against TSG6 (1:100; Santa Cruz Biotechnology, Dallas, TX, USA; Cat# sc-377277) and CD31 (1:100; Novus Biologicals, Cat# NB100-2284) overnight at 4 °C. Thereafter, sections were washed thrice and incubated with a biotinylated universal antibody and avidin-biotin complex (ABC)-horseradish peroxidase (HRP) reagent (VECTASTAIN R.T.U Elite ABC Kit; Vector Laboratories, Burlingame, CA, USA), according to the manufacturer’s instructions. After incubation, the sections were treated with a peroxidase substrate (ImmPACT DAB Peroxidase Substrate; Vector Laboratories) for 5–10 min. Finally, the sections were washed with tap water for 5 min and counterstained with Harris hematoxylin.
In the spheroid section, the CD31-positive area was measured using ImageJ software (RRID: SCR_003070). Each relative CD31 + area was calculated as follows: [sample spheroid’s (CD31 + area / total area) / average of control groups’ (CD31 + area / total area)]
Immunofluorescence analysis using whole spheroid staining
To confirm hypoxic conditions, spheroids were incubated with pimonidazole (Hypoxyprobe RedAPC kit, Hypoxyprobe, Inc., Burlington, MA, USA; Cat# HP8-XXX) for 2 h before harvesting. Spheroids were washed thrice with cold DPBS and fixed with neutral buffered 10% formalin overnight at 4 °C. The fixed spheroids were then washed thrice with 0.1% PBSTX and permeabilized with 0.5% PBSTX for 2 h at RT. After permeabilization, spheroids were washed thrice with 0.1% PBSTX and blocked with 5% BSA in 0.1% PBSTX for 1 h at RT. Thereafter, spheroids were incubated overnight at 4 °C with an antibody against pimonidazole (1:50; Hypoxyprobe, Inc.). After incubation, the spheroids were washed thrice with DPBS, stained with Hoechst 33,342 (Thermo Fisher Scientific), and mounted in a mounting medium. Spheroids were before analyzed using a confocal laser scanning microscope (CLSM) (LSM 710; Zeiss, Oberkochen, Germany). The hypoxic cell area was measured using ImageJ software.
To confirm apoptosis conditions, the fixed spheroids were incubated with CellEvent Caspase 3/7 Green Detection Reagents (Invitrogen; Cat# C10423) for 30 min at 37 °C and then stained with Hoechst 33,342.
Furthermore, to confirm EC distribution in the vascularized spheroids, the fixed spheroids were stained with anti-CD31 (1:100; Novus Biologicals, Cat# NB100-2284) overnight at 4 °C and then stained with Alexa Fluor 488 conjugated anti-rabbit IgG secondary antibody and Hoechst 33,342.
Anti-cancer drug treatment
After being dissolved in tertiary distilled water, doxorubicin (DOX; Korea United Pharm, Inc., Cheong-Ju, Republic of Korea) was retained for a maximum of two weeks at 4 °C. Spheroids were treated with 0, 2.3, 4.6, and 9.2 µM of DOX.
Cell viability assay
Spheroids were treated with different concentrations of DOX for 24 h. After DOX treatment, the cells were incubated with CellTiter-Glo 3D (Promega, Madison, WI, USA; Cat# G968A) for 30 min at RT according to the manufacturer’s instructions. Luminescence signals were measured using a luminescence reader (BioTek Instruments, Winooski, VT, USA).
Quantitative reverse transcription polymerase chain reaction measurement
To confirm the changes in the mRNA expression levels following TSG6 knockdown in canine MGT cells, cells transfected with si-TSG6, si-ctrl, and non-transfected cells were harvested. Total RNA was extracted using an Easy-BLUE Total RNA Extraction Kit (iNtRON Biotechnology, Seongnam, Republic of Korea) according to the manufacturer’s instructions. A spectrometer (NanoPhotometer, Implen, Westlake Village, CA, USA) was used to assess the total RNA content and purity of each sample. CellScript All-in-One 5 × 1st cDNA Strand Synthesis Master Mix (CellSafe, Yong-In, Republic of Korea) was used to synthesize cDNA from each sample. First, 1 µL of cDNA and 400 nM of each forward and reverse primer (BIONICS, Seoul, Republic of Korea) were added to the samples for evaluation using AMPIGENE qPCR Green Mix Hi-ROX with SYBR Green dye (Enzo Life Sciences, Farmingdale, NY, USA). Thereafter, gene expression levels were calibrated against the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reference gene. Table 1 lists the primer sequences used in this study.
Western blot analysis
To confirm the changes in the protein expression levels following TSG6 knockdown in canine MGT cells, cells transfected with si-TSG6 or si-ctrl and non-transfected cells were harvested. The total protein was extracted from canine MGT cells using PRO-PREP Extraction Solution (iNtRON Biotechnology) according to the manufacturer’s protocol. The DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA) was used to measure the protein concentration in each sample. A 15 µg protein sample was loaded and separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to measure protein expression. Polyvinylidene difluoride membranes (EMD Millipore, Burlington, MA, USA) were used for protein transfer. After blocking the membranes with 5% BSA in Tris-buffered saline, the membranes were treated with primary antibodies against TSG6 (1:1000; Santa Cruz Biotechnology; Cat# sc-377277) and β-actin (1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA; Cat# sc-47778 HRP, RRID: AB_2714189) overnight at 4 °C. Goat anti-mouse HRP-labeled secondary antibody (Bethyl Laboratories, Montgomery, TX, USA; Cat# A90-116P) or goat anti-rabbit HRP-labeled secondary antibody (GenDEPOT, Barker, Texas, USA; Cat# SA002-500) was applied to the membranes after the initial incubation period and further incubated for 1 h at RT. Immunoreactive bands were detected by chemiluminescence (Advansta, San Jose, CA, USA). The bands were normalized to β-actin levels after being imaged with the ImageQuant Las4000 mini (GE Healthcare Life Sciences, Chicago, IL, USA).
Vascular tube formation assay
To determine the effect of TSG 6 knockdown on angiogenesis, ECs were seeded in Matrigel coated 24-well plates at 5 × 104 cells/well. ECs were cultured in a 1:1 mixture of culture medium and culture supernatant of canine MGT cells cultured as controls and transfected with si-ctrl or si-TSG6. Tube formation was confirmed using an inverted microscope 12 h after administration of the mixture and analyzed using an ImageJ angiogenesis analyzer.
Statistical analysis
Each experiment was performed at least three times. GraphPad Prism (version 9.3.1) (GraphPad Software, San Diego, CA, USA; RRID: SCR_002798) was used for statistical analyses. One-way and two-way analysis of variance (ANOVA) were used to analyze the data, and Tukey’s multiple comparison test was performed. The findings are shown as mean plus standard deviation (SD). Differences were considered statistically significant at p < 0.05.
Results
TSG6 affects the expression of tumor growth factors and multidrug resistance genes in canine MGT cells
TSG6 expression is reduced by TSG6 knockdown in canine MGT cells
To establish TSG6 knockdown spheroids, siRNAs were transfected into CIPp and CIPm cells, and the expression level of TSG6 was measured after 48 h. TSG6 mRNA expression was significantly decreased in CIPp and CIPm transfected with si-TSG6 compared with that in the control and si-ctrl transfection groups (Fig. 1A). After transfection, we collected the cell culture supernatants from CIPp and CIPm cells and confirmed the changes in TSG6 protein expression using ELISA. TSG6 expression was significantly reduced in CIPp and CIPm cells transfected with si-TSG6 compared to that in the control and si-ctrl transfection groups (Fig. 1B).
TSG6 knockdown in canine MGT cells. (A) Relative TSG6 mRNA expression in CIPp and CIPm cells cultured as controls and transfected with si-ctrl or si-TSG6. (B) TSG6 protein expression in cell culture supernatant of CIPp and CIPm cells cultured as controls and transfected with si-ctrl or si-TSG6. The results are presented as the mean ± SD and were from of triplicate samples from three independent experiments. TSG6, TNF-stimulated gene 6 protein; MGT, mammary gland tumor; ctrl, control. *p < 0.05, **p < 0.01, as determined by one-way analysis of variance (ANOVA).
Expression of tumor growth factor and multidrug resistance genes was decreased in TSG6 knockdown canine MGT cells
In CIPp and CIPm cells transfected with si-TSG6, the mRNA levels of tumor growth factors was observed, TGF-β, and VEGF decreased significantly compared to that in the control and si-ctrl groups TSG6. Additionally, the multidrug resistance genes MRP1 and P-gp were significantly reduced in the si-TSG6 transfection group compared to those in the control and si-ctrl groups (Fig. 2A, B).
TSG6 knockdown leads to reduced expression levels of tumor growth factors and multi-drug resistance genes in canine MGT cells. (A) Relative mRNA expression levels for of TGF-β, VEGF, MRP1, and P-gp in CIPp cells cultured as controls and transfected with si-ctrl or si-TSG6. (B) Relative mRNA expression levels for of TGF-β, VEGF, MRP1, and P-gp in CIPm cells cultured as controls and transfected with si-ctrl or si-TSG6. The results are presented as the mean ± SD and were from of triplicate samples from three independent experiments. TSG6, TNF-stimulated gene 6 protein; MGT, mammary gland tumor; TGF-β, Transforming growth factor β; VEGF, Vascular endothelial growth factor; MRP1, multi-drug resistance-related protein; P-gp, P-glycoprotein; ctrl, control. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as determined by one-way ANOVA.
TSG6 knockdown spheroid model of canine MGT cells
Establishment and confirmation of TSG6 knockdown spheroids of canine MGT cells
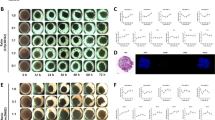
TSG6 knockdown CIPp and CIPm cells were seeded onto an ultra-low adhesion plate, and culture medium containing Matrigel was added (Fig. 3A). To establish the optimal culture time, we examined spheroid formation in CIPp and CIPm cells at 12-h intervals using a microscope (Fig. 3B, D). After 48 h of culture, spheroids were collected, and their histological structure was confirmed using Hematoxylin & Eosin (H&E) staining and TSG6 immunohistochemistry (IHC) (Fig. 3C, E). To determine whether TSG6 expression significantly differed during spheroid culture, we examined the changes in TSG6 protein expression in CIPp and CIPm TSG6 knockdown spheroids using western blottingTSG6. The results confirmed a significant reduction in TSG6 protein expression in the si-TSG6 knockdown group compared to that in the control and si-ctrl groups, even during spheroid culture (Fig. 3F, G).
Establishment and confirmation of TSG6 knockdown spheroids. (A) Method used for TSG6 knockdown spheroid formation. Canine MGT cells were transfected with si-TSG6 and seeded in an ultra-low adhesion plate containing cell culture medium with Matrigel. (B) and (C) panels depict CIPp and (D) and (E) panels depict CIPm. (B, D) Microscopic images of spheroid formation at different culture times. (C, E) Histological images of spheroids with by H&E staining and TSG6 immunohistochemistry (black bar = 500 μm). (F) Protein expression of TSG6 in CIPp and CIPm cells cultured as controls and transfected with si-ctrl or si-TSG6 as measured by western blotting. TSG6 band density was estimated using Image J software and normalized to that of β-actin. (G) Relative TSG6 protein expression in CIPp and CIPm cells cultured as controls and transfected with si-ctrl or si-TSG6. The results are presented as the mean ± SD and were of three independent experiments. TSG6, TNF-stimulated gene 6 protein; MGT, mammary gland tumor; H&E, hematoxylin, and eosin; ctrl, control. *p < 0.05, **p < 0.01, as determined by one-way ANOVA.
Hypoxia is reduced in TSG6 knockdown spheroids of canine MGT cells
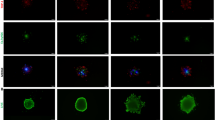
We evaluated hypoxia using confocal imaging to assess the effect of TSG6 on hypoxia induced by CIPp and CIPm in a 3D environment (Fig. 4A). A significant reduction in the hypoxic area was observed in the si-TSG6 transfection groups of CIPp and CIPm compared with that in the control and si-ctrl groups (Fig. 4B).
TSG6 knockdown reduces hypoxic conditions in spheroids of canine MGT cells. (A) Confocal images of spheroids stained with APC dye conjugated IgG1 mouse monoclonal anti-pimonidazole antibody (red) and Hoechst 33,342 (blue) (scale bar = 100 μm). (B) Relative hypoxic cell area in spheroids of CIPp and CIPm cells cultured as controls and transfected with si-ctrl or si-TSG6. The results are presented as the mean ± SD and were of three independent experiments. TSG6, TNF-stimulated gene 6 protein; MGT, mammary gland tumor; APC, Allophycocyanin; IgG1, Immunoglobulin G1; ctrl, control. *p < 0.05, **p < 0.01, ***p < 0.001, as determined by one-way ANOVA.
Anti-cancer drug resistance is reduced in TSG6 knockdown spheroids of canine MGT cells
To evaluate the influence of TSG6 on anti-cancer drug resistance induced by CIPp and CIPm in a 3D environment, we assessed anti-cancer drug resistance by measuring cell viability and apoptosis. CIPp and CIPm spheroids were exposed to doxorubicin (0, 2.3, 4.6, and 9.2 µM) for 24 h. After doxorubicin treatment, a significant reduction in cell viability was observed in the si-TSG6 transfection groups of CIPp and CIPm compared to that in the control and si-ctrl transfection groups (Fig. 5A). To assess the extent of apoptosis induced in CIPp and CIPm spheroids upon doxorubicin treatment, we evaluated the expression of caspases 3/7 using immunofluorescence imaging (Fig. 5B). We observed asignificant increase in the mean fluorescence intensity of caspase 3/7 in the si-TSG6 transfection groups of CIPp and CIPm compared to that in the control and si-ctrl transfection groups (Fig. 5C).
Increased anti-cancer drug resistance in TSG6 knockdown spheroids of canine MGT cells. (A) Cell viability of spheroids comprising of CIPp and CIPm cells cultured as controls and transfected with si-ctrl or si-TSG6 under doxorubicin (0, 2.3, 4.6, and 9.2 µM) over 24 h. (B) Immunofluorescence images of spheroids o CIPp and CIPm cells cultured as controls and transfected with si-ctrl or si-TSG6 under doxorubicin (0, 2.3, 4.6, and 9.2 µM) over 24 h. Spheroids were stained with CellEvent Caspase 3/7 Green Detection Reagents (green) and Hoechst 33,342 (blue) (scale bar = 1000 μm). (C) Mean fluorescence intensity of Caspase 3/7 in spheroids of CIPp and CIPm cells cultured as controls and transfected with si-ctrl or si-TSG6 under doxorubicin (0, 2.3, 4.6, and 9.2 µM) for 24 h. The results are presented as the mean ± SD and were of three obtained independent experiments. TSG6, TNF-stimulated gene 6 protein; MGT, mammary gland tumor; ctrl, control. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as determined by two-way ANOVA.
Vascularized TSG6 knockdown spheroid model of canine MGT cells with canine endothelial cells
Establishment and confirmation of vascularized TSG6 knockdown spheroids of canine MGT cells with canine endothelial cells
To investigate the impact of TSG6 on angiogenesis when culturing CIPp and CIPm in a 3D environment, we established a vascularized spheroid model by combining canine MGT cells and primary cultured canine ECs obtained from the canine aorta. We characterized these ECs by culturing them and confirmed CD31 expression using flow cytometry (Fig. 6B). Subsequently, we seeded canine ECs and MGT cells in a 1:3 ratio in an ultralow adhesion plate and cultured them for 48 h to form vascularized spheroids (Fig. 6A). We confirmed the hypoxic conditions and CD31 expression in the vascularized CIPp and CIPm spheroids using confocal imaging (Fig. 6C).
Establishment and confirmation of vascularized TSG6 knockdown spheroids of canine MGT cells with canine ECs. (A) Method of forming vascularized TSG6 knockdown spheroids comprising of canine ECs and MGT cells. Cells were seeded onto an ultra-low adhesion plate with a 1:3 ratio. (B) Characterization of canine ECs by flow cytometry. Canine ECs were positive for CD31. (C) Confocal imaging of vascularized TSG6 knockdown spheroids. The spheroids were stained with APC dye-conjugated IgG1 mouse monoclonal anti-pimonidazole antibody (red), anti-CD31 rabbit monoclonal antibody (green), and Hoechst 33,342 (blue) (scale bar = 100 μm). (D) Immunohistology images of vascularized TSG6 knockdown spheroids. The spheroids were stained with anti-CD31 rabbit monoclonal antibody (brown) (scale bar = 20 μm). (E) Relative CD31-positive area in vascularized TSG6 knockdown spheroids. The results are presented as the mean ± SD and were of three obtained independent experiments. TSG6, TNF-stimulated gene 6 protein; MGT, mammary gland tumor; EC, endothelial cell; APC, Allophycocyanin; IgG1, Immunoglobulin G1; ctrl, control. *p < 0.05, **p < 0.01, as determined by one-way ANOVA.
Expression of CD31 is reduced in vascularized TSG6 knockdown spheroid model of canine MGT cells with canine endothelial cells
To investigate the changes in CD31 expression based on TSG6 expression, we examined CD31 through IHC in the vascularized spheroids of CIPp and CIPm. The results revealed a significant reduction in CD31 expression in the si-TSG6 transfection groups of CIPp and CIPm compared to that in the control and si-ctrl transfection groups (Fig. 6D, E).
Vascular tube formation in canine ECs is reduced as TSG6 expression decreases
Canine ECs were seeded on Matrigel-coated plates to assess the effect of TSG6 on vascular tube formation in canine ECs. Subsequently, the cells were treated with a 1:1 mixture of cell culture supernatants from CIPp and CIPm spheroids and regular culture medium. We examined vascular tube formation using a microscope (Fig. 7A, C). Using the Angiogenesis Analyzer in ImageJ, we evaluated the key indicators of vascular tube formation, including the number of junctions, number of segments, total length, and total branching length. The results revealed indicated that when canine ECs were treated with the cell culture supernatant from the CIPp and CIPm si-TSG6 transfection groups, these indicators TSG6weresignificantly decreased compared to the canine ECs treated with the cell culture supernatant from the control and si-ctrl transfection groups (Fig. 7B, D).
Vascular tube formation reduced in canine ECs cultured with supernatant containing of TSG6 knockdown canine MGT cells. A and B panels represent CIPp and C and D panels represent CIPm. (A, C) Angiogenesis analyzer of canine ECs cultured with supernatant of CIPp and CIPm cultured as controls and transfected with si-ctrl or si-TSG6. (scale bar = 20 μm) (B, D) Indicators of vascular tube formation (umber of junctions, Number of segments, total length, total branching length) were reduced in canine ECs cultured with CIPp and CIPm supernatant for use cultured as controls or transfected with si-ctrl or si-TSG6. The results are presented as the mean ± SD and were of three independent experiments. TSG6, TNF-stimulated gene 6 protein; MGT, mammary gland tumor; ctrl, control. *p < 0.05, **p < 0.01, as determined by one-way ANOVA.
Discussion
In this study, a TSG6 knockdown spheroid model of canine MGT cells was constructed using a siRNA transfection system to determine the effect of TSG6 on hypoxia in a TME-like environment, and the resulting changes in anti-cancer drug resistance and angiogenesis. To ensure that a proper spheroid model was formed, it was necessary to determine the number of cells, sphericity, spheroid size, and culture time. Therefore, we seeded CIPp and CIPm cells at 1 × 105, 5 × 104, and 2.5 × 104 cells per well on ultra-low adhesion plates and adopted the conditions (cell number: 5 × 104 cells per well, culture time:48 h) that formed spheroids suitable for use in the experiment when monitored every 12 h (Supplementary Figs. 1 and 2). In a previous study, when creating a spheroid model using CIPp and CIPm, necrotic cores were identified due to poor oxygen and nutrient delivery inside the spheroid, and the resulting decrease in internal cell density caused the spheroid to be damaged when used in later experiments28. In this study, we added growth factor-reduced Matrigel to form a spheroid to reinforce cell density. Although a necrotic core was confirmed by H&E staining, the spheroid damage rate was reduced compared to the previously reported model. Furthermore, to confirm that the TSG6 knockdown status was maintained during 3D spheroid culture, we confirmed that TSG6 expression was significantly reduced in the TSG6 knockdown spheroid of CIPp and CIPm using IHC and western blottingTSG6TSG6TSG6 and ensured that TSG6 knockdown spheroid models were properly formed.
3D spheroid cultures induce a hypoxic state similar to that observed in tumors9. This leads to the upregulation of hypoxia-inducible factors (HIFs), which subsequently influence tumor cell proliferation by inhibiting apoptosis and regulating metabolic reprogramming by expressing various cytokines and growth factors29,30. It has also been reported that there is a spatial correlation among hypoxia markers, proliferation, and DNA damage repair signaling inside spheroids31. Therefore, in this study, we aimed to confirm the effect of TSG6 on hypoxia in TSG6 knockdown spheroids and evaluate the resistance of canine MGT cells to anti-cancer drugs by changing the hypoxic condition of the TSG6 knockdown spheroids. First, we identified changes in the expression of tumor growth factors and multidrug resistance genes in canine MGT cells upon TSG6 knockdown. TSG6 knockdown significantly decreased MRP1 and P-gp expression in both CIPp and CIPm cells. We also confirmed the hypoxic conditions in the TSG6 knockdown model confirmed using a hypoxyprobe and indicating found that the significantly hypoxic area was significantly reduced in the TSG6 knockdown CIPp and CIPm spheroids. In addition, when comparing the viability of the TSG6 knockdown spheroids of canine MGT cells after doxorubicin treatment, both CIPp and CIPm were significantly reduced upon TSG6 knockdown. Similarly, the increased expression of caspase 3/7, which is associated with apoptosis, was also observed. A previous study has shown that the expression of HIF-1a is increased in 3D spheroid cultures of CIPp and CIPm, and the expression of MRP1 and P-gp, which are multidrug resistance genes, is also increased28. In another study, when hypoxia was induced using CoCl2 in CIPp and CIPm, the expression of HIF-1a was significantly inhibited in TSG6 knockdown cells, and their metastatic and proliferative ability was reduced21. Thus, it is suggested that TSG6 affects HIF-1a expression in canine MGT cells and that the inhibition of TSG6 expression significantly reduces hypoxia in the TME. In addition to hypoxia, TSG6 affects the expression of multidrug resistance genes, and TSG6 inhibition significantly reduces doxorubicin treatment-induced viability and increases apoptosis in canine MGT cells.
Tumor cells respond to reduced oxygen levels by stimulating angiogenesis32. HIF transcription factors target proangiogenic factors and their receptors, such as VEGF, VEGF receptor-1 and − 2 (VEGFR-1 and -2), basic fibroblast growth factor (bFGF), platelet-derived growth factor B (PDGF), insulin-like growth factor II (IGF2), adrenomedullin, and epidermal growth factor (EGF). This mechanism provides insights into how hypoxia regulates angiogenesis at the molecular level33,34,35. Therefore, various studies have been conducted on hypoxia-induced 3D culture models to study angiogenesis and vascularized tumor spheroids that combine ECs with various cancer cell lines from the breast, lung, and colon36,37. In the present study, we used canine ECs to form a vascularized spheroid model of canine MGT cells to determine the effect of TSG6 on angiogenesis in the TME. When we evaluated the expression of the EC marker CD31 in the vascularized CIPp and CIPm spheroids, we found that CD31 expression was significantly reduced in TSG6 knockdown spheroids. HIF-1α has been demonstrated to influence CD31 expression in different cancers, such as breast cancer38, renal cell carcinoma39, and uveal melanoma40. Hypoxia inducible factors, such as VEGF and TGFβ, have been implicated in tumor angiogenesis41,42. Similarly, in this study, TSG6 knockdown significantly decreased the expression of VEGF and TGFβ in canine MGT cells. When the culture supernatants of TSG6 knockdown canine MGT cells were treated with canine ECs, vascular tube formation was reduced in response to TSG6 reduction. These results suggest that TSG6 affects tumor angiogenesis.
In conclusion, we established a TSG6 knockdown spheroid model of canine MGT cells to analyze the association between TSG6, hypoxia, cancer drug resistance, and angiogenesis. TSG6 knockdown in canine MGT cells reduces hypoxia in 3D spheroid cultures and decreases the expression of multidrug resistance genes and growth factors. This inhibition induces anti-cancer drug resistance and decreases angiogenesis in canine MGT cells. Therefore, TSG6 in canine MGT could be considered a potential therapeutic target, and the TSG6 knockdown spheroid model may be applied as a new method for future tumor research.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Balkwill, F. R., Capasso, M. & Hagemann, T. The tumor microenvironment at a glance. J. Cell. Sci. 125, 5591–5596 (2012).
Shelton, S. E., Nguyen, H. T., Barbie, D. A. & Kamm, R. D. Engineering approaches for studying immune-tumor cell interactions and immunotherapy. iScience 24, 101985 (2020).
Li, Y., Zhao, L. & Li, X. F. Hypoxia and the tumor microenvironment. Technol. Cancer Res. Treat. 20, 15330338211036304 (2021).
Jin, M. Z. & Jin, W. L. The updated landscape of tumor microenvironment and drug repurposing. Signal. Transduct. Target. Ther. 5, 166 (2020).
Qiu, G. Z. et al. Reprogramming of the tumor in the hypoxic niche: The emerging concept and associated therapeutic strategies. Trends Pharmacol. Sci. 38, 669–686 (2017).
Doktorova, H., Hrabeta, J., Khalil, M. A. & Eckschlager, T. Hypoxia-induced chemoresistance in cancer cells: The role of not only HIF-1. Biomed. Pap Med. Fac. Univ. Palacky Olomouc 159, 166–177 (2015).
Palazon, A. et al. An HIF-1alpha/VEGF-A Axis in cytotoxic T cells regulates tumor progression. Cancer Cell. 32, 669–683e5 (2017).
Ribatti, D. Tumor refractoriness to anti-VEGF therapy. Oncotarget 7, 46668–46677 (2016).
Riffle, S. & Hegde, R. S. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. J. Exp. Clin. Cancer Res. 36, 102 (2017).
Mehta, G., Hsiao, A. Y., Ingram, M., Luker, G. D. & Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control Release 164, 192–204 (2012).
Ghosh, S. et al. Three-dimensional culture of melanoma cells profoundly affects gene expression profile: A high density oligonucleotide array study. J. Cell. Physiol. 204, 522–531 (2005).
Hammond, E. M. et al. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin. Oncol. R. Coll. Radiol. 26, 277–288 (2014).
Dewhirst, M. W. & Birer, S. R. Oxygen-enhanced MRI is a major advance in tumor hypoxia imaging. Cancer Res. 76, 769–772 (2016).
Nunes, A. S., Barros, A. S., Costa, E. C., Moreira, A. F. & Correia, I. J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 116, 206–226 (2019).
Hirschhaeuser, F. et al. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 148, 3–15 (2010).
Baker, B. M. & Chen, C. S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell. Sci. 125, 3015–3024 (2012).
Wisniewski, H. G. & Vilcek, J. TSG-6: An IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor. Rev. 8, 143–156 (1997).
Day, A. J. & Milner, C. M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 78–79, 60–83 (2019).
Chan, T. C. et al. High TNFAIP6 level is associated with poor prognosis of urothelial carcinomas. Urol. Oncol. 37, 293e11–293e24 (2019).
Liu, B. et al. TSG-6 promotes Cancer cell aggressiveness in a CD44-Dependent manner and reprograms normal fibroblasts to create a Pro-metastatic microenvironment in colorectal Cancer. Int. J. Biol. Sci. 18, 1677–1694 (2022).
Kim, T. H. et al. Hypoxia increases the proliferative and metastatic ability of canine mammary tumor cells via up-regulation of TSG-6. Anticancer Res. 42, 5803–5812 (2022).
Lee, J. H., An, J. H. & Youn, H. Y. Tumour necrosis factor stimulated gene 6 intrinsically regulates PD-L1 expressions in breast cancer cells, leading to modulation of tumour microenvironment. Vet. Comp. Oncol. 21, 255–269 (2023).
Uyama, R. et al. Establishment of four pairs of canine mammary tumour cell lines derived from primary and metastatic origin and their E-cadherin expression. Vet. Comp. Oncol. 4(2), 104–113 (2006).
Kim, S. M. et al. Enhanced angiogenic activity of dimethyloxalylglycine-treated canine adipose tissue-derived mesenchymal stem cells. J. Vet. Med. Sci. 81, 1663–1670 (2019).
Yi, Z., Stunz, L. L. & Bishop, G. A. CD40-mediated maintenance of immune homeostasis in the adipose tissue microenvironment. Diabetes 63, 2751–2760 (2014).
Kuk, M., Kim, Y., Lee, S. H., Kim, W. H. & Kweon, O. K. Osteogenic ability of canine adipose-derived mesenchymal stromal cell sheets in relation to culture time. Cell. Transpl. 25, 1415–1422 (2016).
Zandvliet, M., Teske, E., Schrickx, J. A. & Mol, J. A. A longitudinal study of ABC transporter expression in canine multicentric lymphoma. Vet. J. 205, 263–271 (2015).
Lim, G. H. et al. Macrophage induces anti-cancer drug resistance in canine mammary gland tumor spheroid. Sci. Rep. 13, 10394 (2023).
Menrad, H. et al. Roles of hypoxia-inducible factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of hepatocellular tumor spheroids. Hepatology 51, 2183–2192 (2010).
Pescador, N. et al. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLOS ONE 5, e9644 (2010).
Riffle, S., Pandey, R. N., Albert, M. & Hegde, R. S. Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids. BMC Cancer 17, 338 (2017).
Seo, B. R., DelNero, P. & Fischbach, C. In vitro models of tumor vessels and matrix: engineering approaches to investigate transport limitations and drug delivery in cancer. Adv. Drug Deliv. Rev. 69–70, 205–216 (2014).
Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005).
Vaupel, P. & Harrison, L. Tumor hypoxia: Causative factors, compensatory mechanisms, and cellular response. Oncologist 9, 4–9 (2004).
Carmeliet, P. et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998).
Ehsan, S. M., Welch-Reardon, K. M., Waterman, M. L., Hughes, C. C. & George, S. C. A three-dimensional in vitro model of tumor cell intravasation. Integr. Biol. 6, 603–610 (2014).
Brassard-Jollive, N., Monnot, C., Muller, L. & Germain, S. Vitro 3D systems to model tumor angiogenesis and interactions with stromal cells. Front. Cell. Dev. Biol. 8, 594903 (2020).
Qin, L. et al. NCOA1 promotes angiogenesis in breast tumors by simultaneously enhancing both HIF1alpha- and AP-1-mediated VEGFa transcription. Oncotarget 6, 23890–23904 (2015).
Biswas, S. et al. CD31 angiogenesis and combined expression of HIF-1alpha and HIF-2alpha are prognostic in primary clear-cell renal cell carcinoma (CC-RCC), but HIFalpha transcriptional products are not: implications for antiangiogenic trials and HIFalpha biomarker studies in primary CC-RCC. Carcinogenesis 33, 1717–1725 (2012).
Mouriaux, F. et al. Increased HIF-1alpha expression correlates with cell proliferation and vascular markers CD31 and VEGF-A in uveal melanoma. Invest. Ophthalmol. Vis. Sci. 55, 1277–1283 (2014).
Lv, X. et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 4, 19–24 (2017).
Mallikarjuna, P., Zhou, Y. & Landström, M. The synergistic Cooperation between TGF-beta and hypoxia in cancer and fibrosis. Biomolecules 12, 635 (2022).
Acknowledgements
We wish to express our gratitude to the staff and crew of Seoul National University CLSM and FACS Facility (NCIRF) for their efforts and perseverance.
Funding
This study was partially supported by the VIP animal medical center, the BK 21 Plus Program for Creative Veterinary Science Research and the Research Institute for Veterinary Science at Seoul National University. Also, This research was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (Grant No. RS-2023-00237922).
Author information
Authors and Affiliations
Contributions
G.H.L. participated in the conception, design, acquisition, analysis, and interpretation of data and wrote the main manuscript text. J.H.A. and J.H.L. conceived and interpreted the data. K.W.S. interpreted the data. H.Y.Y. supervised the study. All of the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lim, GH., An, JH., Lee, JH. et al. TSG6 affects anti cancer drug resistance and angiogenesis in 3D spheroid model of canine mammary gland tumor cells. Sci Rep 15, 30852 (2025). https://doi.org/10.1038/s41598-025-12950-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12950-3