Abstract
Up to this time, only five myxosporean species have been documented from fishes of the Barbonymus genus. Due to a limited number of myxozoan studies conducted in Southeast Asia, particularly in Malaysia, the diversity of this parasite group remains largely undiscovered. In this study, a comprehensive parasitology survey was conducted, revealing nine myxozoan parasites, including five different Myxobolus spp., three Thelohanellus sp. and one Myxidium sp. Using morphological and molecular data, we describe here five new species: Myxobolus gonionoti n. sp., found in the gill filaments; Myxobolus barbonymi n. sp., and Myxobolus faizahae n. sp. found in the muscle cells; Thelohanellus gonionoti n. sp. found in the fins; and Thelohanellus barbonymi n. sp. found in the gill arches. Additionally, we identified spores of the previously described Myxobolus dykovae in the gill lamellae of B. schwanefeldii and Thelohanellus zahrahae in the gill filaments of B. gonionotus. Furthermore, two undescribed species were documented solely based on morphology and morphometrics, a Myxobolus sp. from the muscle cells of B. schwanefeldii and a Myxidium sp. from the gallbladder of B. gonionotus.
Similar content being viewed by others
Introduction
The subphylum Myxozoa1 constitutes a diverse group of metazoan parasites characterized by a highly simplified morphology and a complex parasitic life cycle. These parasites play a significant role as pathogens in the wild and cultured fish stocks2. Among myxosporeans, the genus Myxobolus Bütschli, 1882 is the most diverse, with over 900 known species worldwide3. The genus Myxidium is the second most diverse, comprising more than 230 species4. Meanwhile, the genus Thelohanellus, ranking sixth in diversity, has more than 100 nominal species described to date5. Despite more than 3000 species having been described globally6, the biodiversity of this parasites is significantly understudied especially in Southeast Asia and Africa, where just a few studies on myxozoans have been conducted. This is largely due to limited numbers of experts specializing in myxozoan parasites. Data on myxosporeans, from countries in Southeast Asia such as Brunei, Cambodia, Laos, East Timor, Singapore, and the Philippines are still completely lacking. However, interest in myxozoan research has been steadily increasing worldwide, as evidenced by the growing amount of publications describing new species3,7,8,9,10,11,12.
The genus Barbonymus comprises ten species, including Barbonymus schwanefeldii Bleeker, 1854, Barbonymus gonionotus Bleeker, 1849, Barbonymus altus Günther, 1868, Barbonymus belinka Bleeker, 1860, Barbonymus balleroides Valenciennes, 1842, Barbonymus collingwoodii Günther, 1868, Barbonymus mahakkamensis Ahl, 1922, Barbonymus platysoma Bleeker, 1855, Barbonymus strigatus Boulenger, 1894, and Barbonymus sunieri Weber et Beaufort, 191613,14,15,16. These species are widely distributed across Southeast Asia17,18,19,20,21. In Malaysia, three species—B. schwanefeldii, B. gonionotus and B. altus are particularly common in freshwater ecosystems. These species are not only ecologically important but also hold significant commercial value, both as ornamental fish and as food, due to their high market price and consumer preference22,23. Given the economic importance of these species, conducting parasite surveys is crucial to mitigate the risk of pathogen infections that could affect both wild and cultured fish populations.
To our knowledge, only five myxozoan species have been reported from Barbonymus spp. in Malaysia so far. These include Thelohanellus zahrahae Székely, Shaharom-Harrison, Cech, Mohamed et Molnár 200924; Thelohanellus catlae Chakravarty et Basu, 1948; and Myxobolus macrocapsularis Reuss 1906, which infect the gills of Java barb, B. gonionotus25. Additionally, Myxobolus dykovae Székely, Shaharom-Harrison, Cech, Ostoros et Molnár 2009, has been documented infecting the gill lamellae of tinfoil barb, B. schwanefeldii26, while the recently described Ceratomyxa schwanefeldii Suhaimi, Székely, Cech, Sellyei et Borkhanuddin 2025, was found infecting the gallbladder of the same fish27. However, no myxozoan species have been already reported from red tailed tinfoil barb, B. altus. Considering the limited number of myxozoan species detected from Java barb, tinfoil barb and red-tailed tinfoil barb in Malaysia, a comprehensive survey was conducted on these species. In this study, we describe five novel myxosporean species: Myxobolus gonionoti n. sp., Myxobolus barbonymi n. sp., Myxobolus faizahae n. sp., Thelohanellus gonionoti n. sp., and Thelohanellus barbonymi n. sp. using morphological and molecular analyses. Additionally, we report on the occurrence of two previously known species, T. zahrahae and Myxobolus dykovae, in Malaysia and two unidentified myxozoan species, a Myxobolus sp. and a Myxidium sp.
Materials and methods
Ethical statement
The fish handling, sampling, and dissecting were approved by the Institutional Animal Care and Use Committee of the Veterinary Medical Research Institute, Budapest, Hungary. All research involving experiments on fish from genus Barbonymus was reviewed and approved by the Hungarian National Scientific Ethical Committee on Animal Experimentation under reference number: PE/EA/00081-4/2023. The authors complied with the ARRIVE guidelines (https://arriveguidelines.org).
Fish sampling and microscopic study
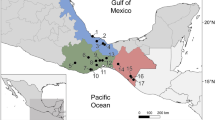
A total of twenty-eight fish samples (with a total length of 10.0–30.0 cm) from the rivers of Sungai Tong, Setiu and Sungai Nerus, Kuala Nerus were purchased from a local fish market in Kuala Terengganu, Terengganu, Malaysia (5° 18′ 44.703″ N 103° 7′ 40.332″ E). The procurement was conducted at two-week intervals over two periods: July to August 2023 and September to November 2024. The live fish were transported to the Marine Science Biodiversity Laboratory of the Faculty of Science and Marine Environment at Universiti Malaysia Terengganu in oxygenated plastic bags. Upon arrival, they were kept in a tank with continuous aeration until autopsy. Taxonomic identification of the fish specimens was based on their morphology. In the laboratory, the fish were euthanized by neural pithing, dissected, and all organs were thoroughly examined to detect plasmodia of myxosporean parasites. These studies were initially conducted with the naked eye, followed by observations under a dissecting microscope and a light microscope to confirm the presence of the plasmodia.
The plasmodia containing spores were isolated from infected organs and tissues, and examined on slide under an Olympus CX33 biological microscope (Olympus Corporation, Japan). Following microscopic observation, the remaining plasmodia and spores were collected into 1.5 mL Eppendorf tubes and preserved in 90% ethanol for molecular studies, and in 10% neutral buffered formalin for subsequent morphological analysis. All fixed samples were later transported to the Fish Pathology and Parasitology Laboratory at the HUN-REN Veterinary Medical Research Institute (Budapest, Hungary), where further analyses were conducted, including high-magnification examinations (100 × objective) and photographic documentation with an Olympus BX53 light microscope equipped with an Olympus DP74 digital camera (Olympus Corporation, Japan). The spores were measured according to the guidelines of Lom and Dyková28, using ImageJ software (http://imagej.nih.gov/ij). All measurements are presented as the mean and standard deviation (SD), followed by the range in parentheses in micrometers (µm). For histological analysis, infected organ and tissues fixed in 10% neutral buffered formalin (NBF) were embedded in paraffin, sectioned at 3–4 µm thin, stained with hematoxylin and eosin (H&E), and subsequently examined and photographed using an Olympus DP74 digital camera.
Molecular and phylogenetic analyses
DNA was extracted from plasmodia and spores that had been preserved in 90% ethanol. Prior to extraction, the samples were washed and centrifuged following the method described by Suhaimi et al.29,30. Genomic DNA was then isolated using the Geneaid Tissue Genomic DNA Mini Kit (Geneaid Biotech Ltd., Taiwan), according to the manufacturer’s instructions for animal tissue samples. The 18S rDNA gene was subsequently amplified via direct PCR using various primer sets listed in Table 1. All polymerase chain reactions (PCRs) were performed in 25 μL reaction volumes, containing 2 μL of template DNA, 1 × DreamTaq buffer (10 × ; Thermo Scientific), 0.2 mM dNTP mix (10 mM; Thermo Scientific), 1.25 U DreamTaq polymerase (5 U; Thermo Scientific), 12.5 pmol of each primer, and molecular grade water. PCR amplifications using different primer pairs were conducted under various thermal cycling conditions. For the MyxospecF-18R and 18E-MyxospecR primers, the protocol of Liu et al.31 was followed, with a modified elongation time of 90 s. Amplifications using ERIB1-ACT1R primers followed the method described by Úngari et al.32. PCR products were analyzed by agarose gel electrophoresis. Bands of the expected size were excised, purified using the DNA Fragment Purification Kit (InViTek GmbH, Berlin, Germany), and sequenced in both directions using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Sequencing was performed on an ABI PRISM 3100 Genetic Analyzer, using the primers listed in Table 1.
The 18S rDNA sequences were analyzed using Geneious Prime v11.1 (Bio-matters; Auckland, New Zealand; https://www.geneious.com/)40 for visualization, editing, and assembly. Phylogenetic analysis included seven sequences generated in this study along with 33 related sequences obtained from the GenBank database, using Chloromyxum cyprini (AY604198) as the outgroup. Multiple sequence alignment was performed using the ClustalW algorithm41 in MEGA X (Molecular Evolutionary Genetics Analysis, 64-bit version; https://www.megasoftware.net/)42, and phylogenetic trees were constructed using both the Maximum Likelihood method with 1000 bootstrap replicates in MEGA X, and Bayesian Inference in MrBayes v3.2.443. Gaps were treated using partial deletion with 75% site coverage cut-off. The analyses were performed using the GTR + G + I model based on the Akaike information criterion (AIC) and Bayesian Information Criterion (BIC) executed in jModelTest 2.1.10 v2016030344. In the Bayesian analysis, Markov Chain Monte Carlo (MCMC) chains were executed for 1,000,000 generations, with the initial 25% of the generations were discarded to produce a consensus tree from the remaining topologies. Bootstrap values (BS) ≥ 70% for ML and posterior probabilities (PP) ≥ 0.90 for BI analyses were considered as well-supported clade. The resulting topologies were visualized in MEGA X and FigTree v. 1.4.445 and graphically edited with Inkscape (Free Software Foundation, Inc., MA, USA). The pairwise distances were performed using a distance matrix model p-distance for transitions and transversions in MEGA X software package.
Results
For the survey of myxosporean parasites in Malaysia, three Barbonymus species were selected. Among the six B. gonionotus examined, all were found to be infected (100%). In contrast, only one out of six B. schwanefeldii individuals was infected (16.6%). For B. altus, seven out of sixteen individuals found positive for myxosporean infections, resulting in a prevalence of 43.7%. Plasmodia were found in various body parts, including the gill filaments, gill lamellae, gill arches, muscle, fins, and gallbladder. Only mature spores were observed within the examined plasmodia. In total, five new myxozoans species were found, comprising three Myxobolus spp.—M. gonionoti n. sp., M. barbonymi n. sp., and M. faizahae n. sp. and two Thelohanellus species—T. gonionoti n. sp. and T. barbonymi n. sp. Additionally, two previously known species; T. zahrahae and M. dykovae were identified, along with an unidentified Myxobolus sp. and a Myxidium sp. In terms of myxosporeans prevalence, the novel T. gonionoti n. sp. showed the highest (50.0%, 3/6) and T. barbonymi n. sp. showed the lowest (12.5%, 2/16) prevalence. Furthermore, none of the infected fish collected from Sungai Tong, Setiu and Sungai Nerus, Kuala Nerus, Terengganu, Malaysia displayed any clinical symptoms.
Myxobolus gonionoti n. sp.
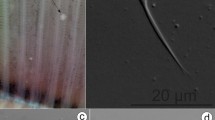
Plasmodia: Found on the gill filament, histozoic, big and round in shape (Fig. 1A–B), measuring 0.3 mm in length and 0.2 mm in width (n = 2).
Photomicrographs of Myxobolus gonionoti n. sp. from the gills of Barbonymus gonionotus. (A) Plasmodia (black arrow) of M. gonionoti n. sp. in the formalin-fixed gill filaments. (B) Round plasmodium located at the central part of a filament. (C) Spore in frontal view. (D) Spore in sutural view. Scale bars represent 10 µm, except (A) 1 mm and (B) 500 µm.
Description of myxospores. Fixed spores oval in both frontal and sutural views, tapering at the anterior ends (Figs. 1C–D, 9A) measuring 9.3 ± 0.3 (8.2–9.8) µm in length, 6.1 ± 0.3 (5.4–6.6) µm in width, and 4.7 ± 0.3 (4.4–5.1) µm in thickness (n = 9). Two pyriform, closely equal polar capsule sizes occupying half of the spore body cavity. Larger polar capsule measuring 5.2 ± 0.3 (4.3–6.0) µm in length and 2.1 ± 0.2 (1.6–2.4) µm in width and smaller polar capsule measuring 4.9 ± 0.3 (3.9–5.4) µm in length and 1.9 ± 0.2 (1.6–2.3) µm in width. No intercapsular appendix observed. Seven polar tubule coils in larger polar capsules and six in smaller polar capsule, positioned perpendicularly to the longitudinal axis of the polar capsules. Sporoplasm binucleate and containing an iodinophilous vacuole, but no observable mucous envelope. Measurements from 30 formalin-fixed spores from one host.
Taxonomic summary
Type host: Java barb, Barbonymus gonionotus.
Locality: Sungai Tong, Setiu, Terengganu, Malaysia.
Site of infection: Gill filament.
Prevalence: 16.6% (1/6).
Type material: Phototypes were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNMPCC-HNHM-PAR-72091.
Etymology: The name Myxobolus gonionoti n. sp. was derived from the name of the fish host, Barbonymus gonionotus.
18S rDNA sequence: Partial 18S rDNA sequence of M. gonionoti n. sp., consisting of 1,917 base pairs was deposited in GenBank under the accession number PV665937. The 18S rDNA sequence from the M. gonionoti n. sp. did not significantly match any of the myxozoan sequences available in GenBank. Pairwise distance estimation of the newly obtained 18S rDNA sequence indicated the highest similarities by 91.2% to M. barbonymi n. sp. (PV665939) (Table 6). Phylogenetic analysis revealed that M. gonionoti n. sp. clustered with maximum bootstrap support within a clade of muscle-infecting Myxobolus spp. that primarily infect cyprinids. However, its position is basal and separated from the rest of the clade (Fig. 10).
Remarks: The closest morphological resemblance to M. gonionoti n. sp. was found with M. dykovae26, although notable morphometric differences were present (Table 2). Myxospore of M. gonionoti n. sp. was smaller than that of M. dykovae but M. gonionoti n. sp. had unequal polar capsule sizes, whereas M. dykovae had equal polar capsule sizes. In terms of morphometric measurements, M. gonionoti n. sp. showed the greatest similarities to Myxobolus sangei Fomena, Lekeufack Folefack et Tang, 2007 particularly in spore width, large polar capsule width, and small polar capsule length and width. However, M. gonionoti n. sp. had smaller spore and polar capsule lengths. The number of turns in the larger polar capsule of M. gonionoti was similar to that of M. sangei, but it differed in the smaller polar capsule. Additionally, the spore width and larger polar capsule width of M. gonionoti n. sp. were similar to those of Myxobolus carlhubbsi McAllister, Cloutman, Leis, Camus et Robinson, 2023. Notably, Ky and Te25 in Chinh et al.46 reported Myxobolus macrocapsularis Reuss, 1849 from the gills of B. gonionotus, is a common parasite of the common bream (Abramis brama) and white bream (Blicca bjoerkna) in Europe. When comparing our spores with M. macrocapsularis as described by Ky and Te25, significant differences were observed particularly in the polar capsules; present spores have slightly unequal-sized polar capsules, while M. macrocapularis has equal-sized polar capsules. Additionally, the spores of M. macrocapularis differ from those of M. gonionoti n. sp. in the shape of their anterior ends; M. macrocapularis has slightly convergent or papilla-like anterior ends, whereas M. gonionoti n. sp. has tapered anterior ends. However, M. gonionoti n. sp. was compared with all available descriptions of M. macrocapularis in the literature47. The spores exhibited morphological and morphometric similarities to those of M. macrocapularis described by Donec and Shulman48. Notable, the schematic drawing by Donec and Shulman48 indicates that M. macrocapsularis has unequal-sized polar capsules, yet the descriptions only provide measurements for one size of polar capsule, suggesting similar sizes.
Myxobolus barbonymi n. sp.
Plasmodia: Found in the muscle, histozoic, elongated in shape (Fig. 2A), measuring 0.8 mm in length and 0.2 mm in width (n = 2).
Photomicrographs of Myxobolus barbonymi n. sp. from the skeletal muscle of Barbonymus gonionotus. (A) Plasmodia (white arrow) of M. barbonymi n. sp. in formalin-fixed muscle. (B) Spore in frontal view. (C) Spore in sutural view. (D) Sutural line of the spore. (E) Histological transverse section of muscle cells showing an intracellular plasmodium (P) of M. barbonymi n. sp. within a (skeletal) muscle cell of B. gonionotus, stained with hematoxylin and eosin (H&E). Scale bars represent 10 µm, except (A) 1 mm, (D) 20 µm and (E) 20 µm.
Description of myxospores: Fixed spores ellipsoidal, asymmetrical in frontal view, with flattened anterior end and lemon-shaped in sutural view (Figs. 2B–D, 9E) measuring 8.4 ± 0.5 (7.4–9.9) µm in length, 6.9 ± 0.5 (6.1–7.8) µm in width, and 5.2 ± 0.2 (4.9–5.4) µm in thickness (n = 5). Two pyriform and unequal polar capsules; larger polar capsule measuring 4.9 ± 0.3 (4.2–5.5) µm in length and 2.9 ± 0.3 (2.4–3.4) µm in width and smaller polar capsule measuring 3.8 ± 0.4 (3.0–4.7) µm in length and 2.4 ± 0.2 (2.0–2.8) µm in width. Strong and ‘U’-shaped intercapsular appendix between the anterior ends of the polar capsules. Polar tubule coiling five to six times in larger polar capsules and three to four times in smaller polar capsule, positioned perpendicularly to the longitudinal axis of the polar capsules. Sporoplasm binucleate, no iodinophilous vacuole and mucous envelope. Measurements from 30 formalin-fixed spores from two hosts.
Taxonomic summary
Type host: Java barb, Barbonymus gonionotus.
Locality: Sungai Tong, Setiu, Terengganu, Malaysia.
Site of infection: Intracelullarly in the muscle cell.
Prevalence: 33.3% (2/6).
Type material: Phototypes were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNMPCC-HNHM-PAR-72089.
Etymology: The name Myxobolus barbonymi n. sp. was derived from the genus name of the fish host, Barbonymus gonionotus.
Histology: Histopathological analysis revealed that the plasmodium of M. barbonymi n. sp. developed intracellularly within muscle cells (Fig. 2E). The lack of young sporogonic stages confirmed that the plasmodium was mature and completely filled with mature spores.
18S rDNA sequence: Partial 18S rDNA sequence of M. barbonymi n. sp., consisting of 1,849 base pairs was deposited in GenBank under the accession number PV665939. The 18S rDNA sequence from the M. barbonymi n. sp. did not significantly match any of the myxozoan sequences available in GenBank. Pairwise distance estimation of the newly obtained 18S rDNA sequence indicated the highest similarities by 92.7% to Myxobolus pseudodispar (KU340979) and 92.6% to Myxobolus musculi (JQ388891) (Table 6). Phylogenetic analysis revealed that M. barbonymi n. sp. was positioned in a monophyletic clade basally to other muscle-infecting Myxobolus spp. with high bootstrap support (Fig. 10).
Remarks: The morphology and morphometrics of M. barbonymi n. sp. differed from any previously described Myxobolus spp. with unequal polar capsule sizes. The closest morphological resemblance to M. barbonymi n. sp. was found with Myxobolus tauricus Miroshnichenko, 1979 and Myxobolus hakyi Landsberg et Lom, 1991. Furthermore, M. barbonymi n. sp. can be distinguished by its flattened anterior end, a feature not previously reported in any Myxobolus spp. nor any muscle-infecting Myxobolus species. The morphometric measurements of M. barbonymi n. sp. were almost similar to Myxobolus faizahae n. sp., with M. barbonymi n. sp. being larger in size. Notably, the wider polar capsule and shorter polar capsule of M. barbonymi n. sp. were similar to those of M. pseudodispar55,56 (Table 3).
Myxobolus faizahae n. sp.
Plasmodia: Found between muscle cells, histozoic, elongated and oval in shape (Fig. 3A), measuring 92.9 ± 62.7 µm in length and 59.3 ± 29.9 µm in width (n = 4).
Photomicrographs of Myxobolus faizahae n. sp. from the muscle of Barbonymus altus. (A) Plasmodia (black arrow) of M. faizahae n. sp. in formalin-fixed muscle. (B) Spore in frontal view. (C) Spore in sutural view. (D) Histological longitudinal section of muscle cells showing plasmodia (P) located among (skeletal) muscle cells (m) of B. altus, surrounding connective tissue (arrowhead), stained with hematoxylin and eosin (H&E). Scale bars represent 10 µm, except (A) 200 µm and (D) 20 µm.
Description of myxospores: Fixed spores ellipsoidal, asymmetrical in frontal view and lemon-shaped in sutural view (Figs. 3B–C, 9B) measuring 9.3 ± 0.3 (8.7–9.9) µm in length, 5.8 ± 0.2 (5.4–6.1) µm in width, and 4.4 ± 0.3 (3.5–4.8) µm in thickness. Two pyriform and unequal polar capsules; larger polar capsule measuring 4.4 ± 0.3 (3.8–5.0) µm in length and 2.8 ± 0.3 (2.3–3.3) µm in width and smaller polar capsule measuring 3.3 ± 0.2 (2.9–3.9) µm in length and 1.9 ± 0.1 (1.6–2.2) µm in width. No intercapsular appendix observed. Polar tubule coiling four to five times in larger polar capsules and three to four times in smaller polar capsule, positioned perpendicularly to the longitudinal axis of the polar capsules. Sporoplasm binucleate, but iodinophilous vacuole not visible, and no mucous envelope observable. Measurements from 30 formalin-fixed spores from two host.
Taxonomic summary
Type host: Red tailed tinfoil barb, Barbonymus altus.
Locality: Sungai Tong, Setiu and Sungai Nerus, Kuala Nerus, Terengganu, Malaysia.
Site of infection: Associated with intramuscular connective tissues.
Prevalence: 66.6% (4/6).
Type material: Phototypes were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNMPCC-HNHM-PAR-72090.
Etymology: The name Myxobolus faizahae n. sp. is dedicated to Professor Emeritus Dr. Faizah Shaharom, a renowned fish parasitologist from Malaysia.
Histology: Histopathological analysis revealed that the plasmodium was encapsulated by a thin layer of connective tissue and associated with the intramuscular connective tissue among muscle cells (Fig. 3D).
18S rDNA sequence: Partial 18S rDNA sequence of M. faizahae n. sp., consisting of 1,783 base pairs was deposited in GenBank under the accession number PV665938. The 18S rDNA sequence from the M. faizahae n. sp. did not significantly match any of the myxozoan sequences available in GenBank. Pairwise distance estimation of the newly obtained 18S rDNA sequence indicated the highest similarities of 94.4% to Myxobolus bhadrensis (AF378343) and 94.2% to Myxobolus kingchowensis (MH521302) (Table 6). Phylogenetic analysis revealed that M. faizahae n. sp. formed a close relationship with Myxobolus terengganuensis and clustered within well-supported clade of muscle-infecting Myxobolus spp. with high bootstrap support (Fig. 10).
Remarks: The closest morphological resemblance to M. faizahae n. sp. was found to M. pseudodispar55,56 although notable morphometric differences were observable (Table 3). Myxospore of M. faizahae n. sp. was smaller than M. pseudodispar, they were similar only in the width of the larger polar capsule and in the number of polar tubule coils. In terms of morphometric measurements, M. faizahae n. sp. showed the greatest similarity to M. bhadrensis Székely, Cech, Chaudhary, Borzák Singh et Molnár, 2015 particularly in spore thickness, smaller polar capsule width and the number of coils in both polar capsules. However, overall, M. faizahae n. sp. was smaller than M. bhadrensis. The M. barbonymi n. sp. was between the Myxobolus sp. described here and M. faizahae n. sp. in size. Additionally, M. faizahae n. sp. showed the highest similarity to the Myxobolus sp. in this study, particularly in both widths of larger and smaller polar capsules.
Thelohanellus gonionoti n. sp.
Plasmodia: Ellipsoidal shape plasmodium found under the dermis covering the interlepidotrichial ligament and the fin rays Fig. 4A).
Photomicrographs of Thelohanellus gonionoti n. sp. from the fin of Barbonymus gonionotus. (A) Plasmodium (white arrow) of T. gonionoti under the dermis partially covering the fin ray and the interlepidotrichial ligament. (B) Spore in frontal view with the presence of an iodinophilous vacuole (iv) within the sporoplasm. (C) Spore in sutural view. (D) Histological transverse section of the tail fin showing a plasmodium (P) located between the interlepidotrichial ligament (ie) and the dermis (d), and bulging toward the epidermis (ed). (E) Higher magnification of the plasmodium (P) filled with mature spores. Stained with hematoxylin and eosin (H&E). Scale bars represent 10 µm, except (D) 50 µm, and (E) 20 µm.
Description of myxospores: Fixed spores elongate-pyriform, with tapered and truncated anterior ends in both frontal and sutural views (Fig. 4B–C, 9C) measuring 18.4 ± 1.0 (16.3–18.3) µm in length, 8.9 ± 0.8 (7.2–9.2) µm in width, and 6.9 ± 0.4 (6.4–6.9) µm in thickness (n = 5). A single pyriform polar capsule measuring 9.2 ± 1.0 (7.3–10.3) µm in length and 4.8 ± 0.5 (3.8–5.6) µm in width and occupied more than half of the spore body cavity. Polar tubule coiling eight to nine times, positioned perpendicularly to the longitudinal axis of the polar capsules. Sutural line straight, smooth and thick in the middle of spore body. Sporoplasm binucleate measuring 1.1 ± 0.2 (0.7–0.9) in diameter and containing an iodinophilous vacuole measuring 3.8 ± 0.5 (2.8–3.9) in diameter. No mucous envelope observable. Measurements from 30 formalin-fixed spores from one host.
Taxonomic summary
Type host: Java barb, Barbonymus gonionotus.
Locality: Sungai Tong, Setiu, Terengganu, Malaysia.
Site of infection: Under the dermis and above the interlepidotrichial ligament.
Prevalence: 50.0% (3/6).
Type material: Phototypes were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNMPCC-HNHM-PAR-72093.
Etymology: The name Thelohanellus gonionoti n. sp. was derived from the name of the fish host, Barbonymus gonionotus.
Histology: Histopathological analysis of the tail fin revealed a plasmodium with mature spores (Fig. 4E), which located under the dermis and above the interlepidotrichial ligament. The plasmodium was bulging toward the epidermis (Fig. 4D). The dermis and interlepidotrichial tissue are also visible in the histological section.
18S rDNA sequence: Partial 18S rDNA sequence of T. gonionoti n. sp., consisting of 1,897 base pairs was deposited in GenBank under the accession number PV665940. The 18S rDNA sequence from the T. gonionoti n. sp. did not significantly match any of the myxozoan sequences available in GenBank. Pairwise distance estimation of the newly obtained 18S rDNA sequence indicated the highest similarities by 98.9% to T. barbonymi n. sp. (PV665941) (Table 6). Phylogenetic analysis revealed that T. gonionoti n. sp. was clustered within a monophyletic clade together with other gill-infecting Thelohanellus spp., that parasitize cyprinids such as B. altus, B. gonionotus, and Mystacoleucus marginatus Valenciennes, 1842, with maximum bootstrap support (Fig. 10).
Remarks: The morphology and morphometrics of T. gonionoti n. sp. were distinct from those of any previously described Thelohanellus spp., characterized by truncated anterior ends (Table 4). The closest morphological resemblance to T. gonionoti n. sp. was found to T. zahrahae (both previously described and present study) and the newly described T. barbonymi n. sp., although notable morphometric differences were present. Thelohanellus gonionoti n. sp. can be distinguished from both species by the polar capsule position, which leans towards the side of the spore body. In terms of morphometric measurements, T. gonionoti n. sp. differed significantly from the previously described Thelohanellus spp. It shared similarities only with T. barbonymi n. sp. in terms of the length of the polar capsule and the number of coils.
Thelohanellus barbonymi n. sp.
Plasmodia: Found in the gill arches, histozoic, round to oval in shape (Fig. 5A), measuring 0.22 mm in diameter (n = 1).
Photomicrographs of Thelohanellus barbonymi n. sp. from the gill of Barbonymus altus. (A) Plasmodium (black arrow) of T. barbonymi n. sp. in formalin-fixed gill arches. (B) Mature spores of T. barbonymi n. sp. released from plasmodium. (C) Spore in frontal view with the presence of single nucleus (black arrow) and an iodinophilous vacuole (iv) within the sporoplasm. (D) Spore in sutural view. Scale bars represent 10 µm, except (A) 500 µm.
Description of myxospores: Fixed spores elongate-pyriform, with tapered and truncated anterior ends in both frontal and sutural views (Figs. 5B–D, 9D) measuring 22.0 ± 0.7 (20.3–23.2) µm in length, 9.9 ± 0.6 (8.1–10.8) µm in width, and 7.7 ± 0.5 (6.7–8.3) µm in thickness (n = 15). A single pyriform polar capsule measuring 9.1 ± 0.5 (8.2–10.1) µm in length and 5.4 ± 0.3 (4.9–5.9) µm in width, occupying ¼ of the spore body cavity. Polar tubule coiling eight times, positioned perpendicularly to the longitudinal axis of the polar capsules. Sutural line straight, smooth and thin in the middle of spore body. Sporoplasm binucleate and containing an iodinophilous vacuole measuring 4.4 ± 0.4 (3.4–4.9) µm in diameter. No mucous envelope observable. Measurements from 30 formalin-fixed spores from one host.
Taxonomic summary
Type host: Red tailed tinfoil barb, Barbonymus altus.
Locality: Sungai Tong, Setiu and Sungai Nerus, Kuala Nerus, Terengganu, Malaysia.
Site of infection: Gill arches.
Prevalence: 12.5% (2/16).
Type material: Phototypes were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNMPCC-HNHM-PAR-72092.
Etymology: The name Thelohanellus barbonymi n. sp. was derived from the genus name of the fish host, Barbonymus altus.
18S rDNA sequence: Partial 18S rDNA sequence of T. barbonymi n. sp., consisting of 1,907 base pairs was deposited in GenBank under the accession number PV665941. The 18S rDNA sequence of T. barbonymi n. sp. did not significantly match any other myxozoan sequences available in GenBank. Pairwise distance estimation of the newly obtained 18S rDNA sequence indicated the highest similarities by 98.9% to T. gonionoti n. sp. (PV665940) (Table 6). Phylogenetic analysis revealed that T. barbonymi n. sp. was positioned in a monophyletic clade together with other gill-infecting Thelohanellus spp., along with T. gonionoti n. sp. that parasitize cyprinids such as B. altus, B. gonionotus, and M. marginatus, with maximum bootstrap support (Fig. 10).
Remarks: The morphology and morphometrics of T. barbonymi n. sp. were distinct from any previously described Thelohanellus spp., characterized by truncated anterior ends (Table 4). The closest morphological and morphometric resemblance to T. barbonymi n. sp. was found with T. zahrahae, both in previous and present study, although notable morphometric differences were present. Myxospore of T. barbonymi n. sp. was smaller than T. zahrahae from previous study by Székely et al.24 but larger than T. zahrahae from the present study. However, T. barbonymi n. sp. was wider than both T. zahrahae from previous study and the present study. They can be distinguished by having a different number of coils (8 vs. 7). Regarding the spore width, T. barbonymi n. sp. showed the highest similarities to T. catlae, Chakravarty et Basu, 1948. When comparing the newly described T. gonionoti n. sp. and T. zahrahae from the present study, T. barbonymi n. sp. was the largest in size among these species.
Thelohanellus zahrahae Székely et Molnár, 2009
Plasmodia: Found in the gill filaments, histozoic, elongated in shape (Fig. 6A–B), measuring 1.27 ± 7.2 (1.26–1.28) mm in length and 0.29 ± 7.8 (0.28–0.29) mm (n = 2).
Photomicrographs of Thelohanellus zahrahae from the gill of Barbonymus gonionotus. (A) Large elongated shape plasmodium (white arrow) located inside a filament. (B) Higher magnification of the isolated plasmodium of T. zahrahae. (C) Spore in frontal view. (D) Spore in sutural view. Scale bars represent 10 µm, except (B) 200 µm.
Redescription of myxospores: Fixed spores elongate-pyriform, with tapered and truncated anterior ends in both frontal and sutural views (Fig. 6C–D) measuring 20.5 ± 0.5 (19.4–21.6) µm in length, 9.1 ± 0.6 (8.1–10.6) µm in width, and 7.6 ± 0.2 (7.3–7.8) µm in thickness (n = 7). A single pyriform polar capsule measuring 8.8 ± 0.8 (6.6–10.2) µm in length and 5.5 ± 0.5 (4.7–6.8) µm in width, occupied half of the spore body cavity. Polar tubule coiling seven times, positioned perpendicularly to the longitudinal axis of the polar capsules. Sutural line straight, smooth and thick in the middle of spore body. Sporoplasm binucleate measuring 1.0 ± 0.3 (0.6–1.7) in diameter (n = 12) and contains an iodinophilous vacuole measuring 3.9 ± 0.5 (3.1–4.8) in diameter. No mucous envelope observable. Measurements from 30 formalin-fixed spores from one host.
Taxonomic summary
Type host: Java barb, Barbonymus gonionotus.
Locality: Sungai Tong, Setiu, Terengganu, Malaysia.
Site of infection: Gill filaments.
Prevalence: 33.3% (2/6).
Type material: Series of phototypes were deposited in the collection of the Fish Pathology and Parasitology Research Team, Veterinary Medical Research Institute, Budapest, Hungary.
18S rDNA sequence: Partial 18S rDNA sequence of T. zahrahae, consisting of 1,901 base pairs was deposited in GenBank under the accession number PV647345. The 18S rDNA sequence from the present T. zahrahae matched with the sequence of T. zahrahae (EU643622) available in GenBank showing 99.7% (Table 6). Phylogenetic analysis revealed that T. zahrahae sequences showed a close relationship with an undescribed Thelohanellus species (MK332024) infecting M. marginatus, with high bootstrap support (Fig. 10).
Remarks: The morphology and morphometric data of T. zahrahae were consistent with previously described T. zahrahae from Java barb in a fish farm at Machang, Kelantan (Table 4). Although minor differences in size were observed in all measurements, myxospore of T. zahrahae from the previous study were generally larger than those from the present study. Notably, T. catlae was reported by Ky and Te25 in Chinh et al.46 from the gills and skin of B. gonionotus, a common parasite of the common carp (Cyprinus carpio). However, significant morphological and morphometric differences were observed, with T. catlae is larger in size and possesses pear shaped and spherical polar capsules, in contrast to the present T. zahrahae, which has elongated-pyriform spores with truncated anterior ends and a pyriform polar capsule.
Myxobolus dykovae Székely et Molnár, 2009
Plasmodia: Found in the gill lamellae, histozoic, small and oval in shape (Fig. 7A), measuring 0.1 (0.1–0.2) mm in both length and width (n = 10).
Photomicrographs of Myxobolus dykovae from the gill of Barbonymus schwanefeldii. (A) Plasmodium (black arrow) located in the gills. (B) Spore in frontal view. (C) Histological transverse section of filament showing plasmodium (P) located between gill lamella of B. schwanefeldii, stained with hematoxylin and eosin (H&E). Scale bars represent 10 µm, except (C) 50 µm.
Redescription of myxospores: Fixed spores oval in both frontal and sutural views, tapering at the anterior ends (Fig. 7B) measuring 11.7 ± 0.6 (10.3–12.8) µm in length, 6.8 ± 0.4 (5.9–7.7) µm in width, and 5.4 ± 0.3 (5.1–5.7) µm in thickness (n = 4). Two equal, pyriform polar capsules, measuring 5.8 ± 0.5 (4.7–7.0) µm in length and 2.2 ± 0.2 (1.8–2.6) µm in width, and occupied half of the spore body cavity. No intercapsular appendix observed. Polar tubule coil six to seven times and positioned perpendicularly to the longitudinal axis of the polar capsules. Sporoplasm binucleate, containing an iodinophilous vacuole, but no mucous envelope observable. Measurements from 30 formalin-fixed spores from one host.
Taxonomic summary
Type host: Tinfoil barb, Barbonymus schwanefeldii.
Locality: Sungai Tong, Setiu, Terengganu, Malaysia.
Site of infection: Secondary gill lamellae.
Prevalence: 16.6% (1/6).
Type material: Series of phototypes were deposited in the collection of the Fish Pathology and Parasitology Research Team, Veterinary Medical Research Institute, Budapest, Hungary.
Histology: Histopathological analysis showed that an oval-shaped plasmodia filled with mature spores were found intralamellarly within the gills (Fig. 7C).
18S rDNA sequence: Partial 18S rDNA sequence of M. dykovae, consisting of 1,643 base pairs was deposited in GenBank under the accession number PV647344. The 18S rDNA sequence from the present M. dykovae matched with the sequence of M. dykovae available in GenBank. Pairwise distance estimation of the newly obtained 18S rDNA sequence indicated the highest similarities by 99.4% to M. dykovae (EU643627) (Table 6). According to the phylogenetic analysis, M. dykovae sequences were positioned in a clade comprising many gill-infecting Myxobolus spp. and various Myxobolus spp. that infect others organs besides gills (Fig. 10).
Remarks: The morphology and morphometric data of M. dykovae were consistent with previously described M. dykovae from tinfoil barb in Tasik Kenyir (Table 2). Minor differences in size were observed in all characters, but these remained within the established range of variation. Notably, the morphometrics of the present M. dykovae showed the highest similarity to Myxobolus alvarezae Cech, Molnár et Székely, 2012 in all measurements except spore thickness and polar capsule length.
Myxidium sp.
Description of myxospores. Fixed dispersal spores, fusiform shaped in both frontal and sutural views with tapered ends (Fig. 8A–B), measuring 12.1 ± 0.6 (11.0–13.7) µm in length, 5.9 ± 0.3 (5.2–6.4) µm in width, and 4.8 ± 0.4 (4.2–5.2) µm in thickness (n = 4). Two equal, slightly pyriform polar capsules, measuring 3.4 ± 0.2 (3.0–3.7) µm in length and 2.9 ± 0.2 (2.5–3.5) µm in width. Distance between two polar capsules, measuring 4.5 ± 0.6 (3.4–6.0) µm in distance. Polar tubules coiling five times and positioned perpendicular to the longitudinal axis of the polar capsules. Straight sutural line, and the valves exhibiting seven longitudinal striations. Sporoplasm binucleate, filling the spore body cavity between the two polar capsules. Measurements from 30 formalin-fixed spores from two host.
Photomicrographs of Myxidium sp. from the gallbladder of Barbonymus gonionotus and Myxobolus sp. from the muscle cells of B. schwanefeldii. (A) Spore of Myxidium sp. in frontal view. (B) Spore of Myxidium sp. in sutural view. (C) Spore of Myxobolus sp. in frontal view. (D) Spore of Myxobolus sp. in sutural view. Scale bars represent 10 µm.
Taxonomic summary
Type host: Java barb, Barbonymus gonionotus.
Locality: Sungai Tong, Setiu, Terengganu, Malaysia.
Site of infection: Gallbladder.
Prevalence: 33.3% (2/6).
Type material: Series of phototypes were deposited in the collection of the Fish Pathology and Parasitology Research Team, Veterinary Medical Research Institute, Budapest, Hungary.
Remarks: The morphology and morphometrics of Myxidium sp. did not match any previously described Myxidium spp. (Table 5). The closest morphological resemblance to Myxidium sp. was found with Myxidium macropodus Chen et Hsieh, 1984 and Myxidium aristichthysi Chen in Chen and Ma62 although notable morphometric differences were present. In terms of morphometric measurements, Myxidium sp. showed the greatest similarities to Myxidium chiluense Ma in Chen and Ma62 in most measurements. Notably, the spore thickness of Myxidium sp. was similar to that of Myxidium mapienense Ma et Zhao in Chen and Ma62. Throughout this study, several attempts at molecular analyses were performed; however, these attempts were unsuccessful due to the low number of spores available.
Myxobolus sp.
Plasmodia: Found in the muscle cells, histozoic, elongated in shape.
Description of myxospores: Fixed spores oval in frontal view and lemon-shaped in sutural view (Fig. 8C–D), measuring 11.7 ± 0.5 (11.0–12.5) µm in length, 6.4 ± 0.5 (5.8–7.2) µm in width, and 4.8 ± 0.8 (4.3–5.4) µm in thickness (n = 2). Two pyriform and unequal polar capsules; larger polar capsule, measuring 6.0 ± 0.5 (5.3–6.9) µm in length and 2.8 ± 0.3 (2.2–3.2) µm in width and smaller polar capsule measuring 4.8 ± 0.4 (4.2–5.4) µm in length and 2.2 ± 0.3 (1.7–2.9) µm in width. No intercapsular appendix observed. Polar tubule coil five times in larger polar capsules and four times in smaller polar capsule, positioned perpendicularly to the longitudinal axis of the polar capsules. Sporoplasm binucleated, but iodinophilous vacuole not visible and no mucous envelope observable. Measurements from 12 formalin-fixed spores from one host.
Schematic drawings of myxosporean parasites from Barbonymus species. (A) Myxobolus gonionoti n. sp. in frontal and sutural views. (B) Myxobolus faizahae n. sp. in frontal and sutural views. (C) Thelohanellus gonionoti n. sp. in frontal and sutural views. (D) Thelohanellus barbonymi n. sp. in frontal and sutural views. (E) Myxobolus barbonymi n. sp. in frontal, sublateral and sutural views. Scale bars represent 10 µm.
Taxonomic summary
Type host: Tinfoil barb, Barbonymus schwanefeldii.
Locality: Sungai Tong, Setiu, Terengganu, Malaysia.
Site of infection: Intracellularly of skeletal muscle cells.
Prevalence: 16.6% (1/6).
Type material: Series of phototypes were deposited in the collection of the Fish Pathology and Parasitology Research Team, Veterinary Medical Research Institute, Budapest, Hungary.
Remarks: The morphology and morphometrics of Myxobolus sp. did not match any previously described Myxobolus spp. (Table 3). The closest morphological and morphometric resemblance to Myxobolus sp. was found with M. pseudodispar although notable morphometric differences were present. Notably, the morphometrics of Myxobolus sp. showed the greatest similarities to M. musculi Keysselitz, 1908 in terms of spore length, and to M. bhadrensis in all measurements except for the width of the larger polar capsule. Unfortunately, molecular analysis of this sample was unsuccessful due to low number of spores available.
Discussion
This study identified five novel myxozoan species (three Myxobolus spp., two Thelohanellus spp.), detected two previously described species (M. dykovae, and T. zahrahae) and recorded two unidentified species (Myxobolus sp. and Myxidium sp.) from Barbonymus spp., based on analyses of the 18S rDNA gene sequences and morphological characteristics. The species’ tissue preferences were different, with three Myxobolus spp. infecting the muscle cells, a Myxobolus and two Thelohanellus species—the gills, a Thelohanellus species—the fins and a Myxidium sp.—the gallbladder.
Due to the high similarity of Myxobolus spp. spores, species identification based solely on morphological criteria is challenging. While host specificity and the predilection site of plasmodia within organs or tissues can assist in distinguishing morphologically identical or similar spores, accurate differentiation often requires molecular analyses. This approach is particularly critical in cases where the host species are closely related, such B. gonionotus, B. schwanefeldii, and B. altus, which fishes may be susceptibility to the same myxosporean species.
Organ and tissue specificity is also a crucial diagnostic criterion for myxosporean species55. Besides gills, muscle cells are also a common infection site for various myxosporean parasites in fish63. As of 2021, approximately 75 of 979 Myxobolus spp. have been described from muscle tissue3,58,64. The majority of these muscle-dwelling species, such as M. cyprini, M. musculi and M. pseudodispar, belong to the ‘pseudodispar’ morphological type and form intracellular plasmodia. The Myxobolus spp. (M. barbonymi n. sp., M. faizahae n. sp. and Myxobolus sp.) found in this study also exhibit ‘pseudodispar’-like morphology. Notably, one of the three species, M. faizahae, formed intramuscular plasmodia, whereas M. barbonymi n. sp. and Myxobolus sp. developed intracellular plasmodia, resembling those of M. pseudodispar. Phylogenetic analysis and pairwise distance calculations confirmed the validity of M. barbonymi n. sp. and M. faizahae n. sp. as new species. Our results indicate that the phylogenetic relationships among Myxobolus spp. in this study show some correlation with their predilection sites in the host. Specifically, the newly identified Myxobolus spp. that infect muscle tend to cluster together in one clade (Fig. 10). This finding is consistent with previous studies that highlight the importance of tissue tropism as a phylogenetic criterion for myxosporean species36,65,66. Notably, the two unequal polar capsules also appear to be relevant for the phylogenetic relationships of Myxobolus species in this study. The newly identified M. barbonymi n. sp. and M. faizahae n. sp., along with other Myxobolus species having two unequal polar capsules (except for Myxobolus stanlii Iwanowicz, Iwanowicz, Howerth, Schill, Blazer et Johnson, 2013, which has equal polar capsule sizes), were clustered within muscle-infecting Myxobolus spp. (Fig. 10). Conversely, those with two equal polar capsules tended to cluster within a smaller group of Myxobolus spp. (Fig. 10).
Maximum likelihood phylogenetic tree of 18S rDNA sequences of Myxobolus gonionoti n. sp., Myxobolus barbonymi n. sp., Myxobolus faizahae n. sp., Thelohanellus gonionoti n. sp., Thelohanellus barbonymi n. sp., Myxobolus dykovae, Thelohanellus zahrahae, and related species. Chloromyxum cyprini was chosen as outgroup. Nodal supports are indicated for maximum likelihood (ML) at 1000 replicates and Bayesian Inference (BI). Only values with ≥ 70% bootstrap (BS) and ≥ 0.90 posterior probabilities (PP) support are presented. The sequences from the present study is in bold. The scale bar indicates the expected number of substitutions per site.
The gills are the most commonly affected organs by myxosporean parasites67. This study identified two newly described gill-infecting myxosporeans: M. gonionoti n. sp. and T. barbonymi n. sp., alongside two previously described species M. dykovae and T. zahrahae. Myxobolus gonionoti n. sp. was compared with all known gill-infecting Myxobolus species from the literature, and exhibits the closest morphological resemblance to M. dykovae. Additional comparisons with related species, including M. sangei and M. carlhubbsi, also revealed distinguishing features that support the designation of M. gonionoti n. sp. as a new species. Interestingly, M. gonionoti n. sp. may be confused with M. macrocapsularis, a species previously reported from B. gonionotus by Ky and Te25 in Chinh et al.46. However, molecular analysis clearly differentiates M. gonionoti n. sp. from M. macrocapsularis, revealing only 77% sequence similarity (Table 6), which strongly supports its recognition as a distinct species. It is important to note that earlier identifications of M. macrocapsularis were based solely on morphological features, as molecular data were not widely used at the time. Therefore, the spores reported by Ky and Te25 may have been misidentified, and it is plausible that they were actually observing M. gonionoti n. sp.
Plasmodia of T. barbonymi n. sp. developed in the gill arches, an unusual location for Thelohanellus species, which typically develop in gill lamellae and gill filaments. Although T. barbonymi n. sp. shares similarities with T. zahrahae, which also infects the gills of different host within the same genus, it represents a distinct taxon based on the genetic characteristics and host species. Notably, only a few Thelohanellus species, such as Thelohanellus valeti Fomena et Bouix, 1987, have been reported from both stomach and gill arches of Barbus jae and Barbus aspilus from Cameroon, corroborated the unusual site preference observed in T. barbonymi n. sp. These findings highlight the diversity of tissue tropism among Thelohanellus species and support the acceptance of T. barbonymi n. sp. as a novel taxon.
This study also identified previously described gill-infecting myxozoan species including T. zahrahae and M. dykovae. Both species were identified based on morphological characteristics consistent with earlier descriptions and then further validated by molecular analysis. Although other Thelohanellus species, such as T. catlae, have previously been reported from B. gonionotus, as noted by Ky and Te25 in Chinh et al.46, our findings indicate that the present T. zahrahae differs notably in key features. Additionally, the most significant difference is with the shape of the plasmodia; T. zahrahae has elongated plasmodia (Fig. 6A–B), while T. catlae has spherical-shaped plasmodia confirming the distinct identity of T. zahrahae. Furthermore, molecular analysis of 18S rDNA sequences confirmed the identity of our samples as T. zahrahae and M. dykovae, supporting their classification despite the slight morphological differences.
The phylogenetic relationships among Myxobolus spp. and Thelohanellus spp. in this study show some correlation with their predilection sites in the host. It is noteworthy that M. dykovae clustered with other predominantly gill-infecting Myxobolus spp., as well as some species of Myxobolus spp. that infect other organs such as pharynx and hepatopancreas (Fig. 10). However, M. gonionoti n. sp., in contrast, is clustered with muscle-infecting Myxobolus spp. (Fig. 10). Its unique position can be explained by the absence of closely related species that also infect gills in current genetic databases, suggesting that such species from that geographic area are still undiscovered or undescribed. It is also plausible that these parasites evolved in parallel with muscle parasites but later adapted to infect gills68. Regarding the Thelohanellus lineage, T. barbonymi n. sp. and T. zahrahae cluster with other gill-infecting Thelohanellus spp. (Fig. 10). Thelohanellus barbonymi n. sp. forms a close relationship with T. gonionoti n. sp., which infects the fins of B. barbonymus. Meanwhile, T. zahrahae forms a sister group with Thelohanellus sp. (MK332024) from M. marginatus. This pattern highlights the complex phylogenetic connections within Thelohanellus, which often exhibit polyphyly and intermix with Myxobolus clades.
The morphometrics of T. gonionoti n. sp. were distinct from those of any previously described Thelohanellus spp., including those characterized by truncated anterior ends (Table 4). Thelohanellus gonionoti n. sp. possesses a unique feature; the polar capsule position leans towards the side of the spore body, in contrast to T. barbonymi n. sp. and T. zahrahae. There are relatively several fin-infecting Thelohanellus spp., including Thelohanellus assambai Fomena, Marqués, Bouix et Njine, 1994; Thelohanellus avijiti Basu et Haldar, 2003; Thelohanellus caudatus Pagarkar et das, 1993; Thelohanellus disporomorphus Basu, Modak et Haldar, 2006; Thelohanellus globulosa Singh et Kaur, 2012; Thelohanellus habibpuri Acharya et Dutta, 2007; Thelohanellus kalavatae Singh et Kaur, 2013; Thelohanellus leshanensis Zhao et Ma, 1992; Thelohanellus nikolskii Akhmerov, 1955; Thelohanellus potaili Lalitha Kumari, 1969; Thelohanellus sanagaensis Fomena, Marqués, Bouix et Njine, 1994; Thelohanellus shaochingensis Chen in Chen et Ma, 1998; Thelohanellus shortii Qadri, 1967; Thelohanellus wusihensis Chen in Chen et Ma, 1998; Thelohanellus deri Singh et Kaur, 2012; Thelohanellus haldari Singh et Kaur, 2012; Thelohanellus rohi Singh et Kaur, 20155,69. Among these, T. assambai from Labeo sp., is the only species that possesses truncated anterior ends. Unfortunately, no nucleotide sequence of T. assambai is available for comparison with T. gonionoti n. sp. to identify its position on the phylogenetic tree. Furthermore, 18S rDNA sequence and pairwise distance analyses have confirmed that T. gonionoti n. sp. is a valid new species (Table 6). Phylogenetic analyses have revealed that T. gonionoti n. sp. clusters within the gill-infecting Thelohanellus spp. clade consisting T. barbonymi n. sp., Thelohanellus sp. and T. zahrahae (Fig. 10). Geographical location can also serve as an important criterion for phylogenetic relationships. Notably, all Thelohanellus species in this study, which are collected from Southeast Asia, formed a cohesive cluster. Specifically, T. gonionoti n sp., T. barbonymi n. sp., T. zahrahae were identified in Malaysia, while Thelohanellus sp. was found in Thailand.
Approximately 79 freshwater Myxidium species have been reported from Asia, including India, Japan and China4. However, there are only three known Myxidium spp. in Southeast Asia, including Myxidium cf. notopterum from the gallbladder of Notopterus notopterus70, Myxidium sp. from the gallbladder of Osteochilus hasselti71, and Myxidium cuneiforme from the gallbladder of B. gonionotus72. Myxidium sp. found in the gallbladder of B. gonionotus in this study does not match any previously described Myxidium spp. Previously, Thumvittayakul et al.72 reported M. cuneiforme, a common parasite of the common carp (Cyprinus carpio) from the gallbladder of B. gonionotus in Thailand. When comparing our spores with M. cuneiforme, significant differences were observed in both morphology and morphometric measurements. Myxidium cuneiforme is larger and possesses crescent-shaped spores with teardrop-shaped polar capsules and 6 to 8 longitudinal striations. In contrast, the present Myxidium sp. has fusiform spores with a slightly pyriform polar capsule and seven longitudinal striations. Although, the morphology and morphometrics of the present Myxidium sp. are distinct from all previously described Myxidium spp., it cannot be described as a new species due to a lack of molecular data.
In conclusion, this study identified nine myxozoan parasites, including five new myxosporeans in Barbonymus species collected from freshwater ecosystems in Malaysia. These findings highlight the underexplored diversity of myxosporeans in Barbonymus spp. within Malaysian freshwater ecosystems. This research underscores the need for further research to explore more myxosporean parasites from other fish species in Malaysia, contributing to a more comprehensive understanding of the diversity of Malaysian myxozoan fauna.
Data availability
The datasets generated and/or analysed during the current study are available in GenBank database under the accession numbers of PV647344, PV647345, PV665937, PV665938, PV665939, PV665940, and PV665941. Type materials have been deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, under collection numbers HNMPCC-HNHM-PAR-72089 to HNMPCC-HNHM-PAR-72093, and are available upon reasonable request through the Head of the Zoological Department.
References
Grassé, P. P. Embranchement des myxozoaires. Précis. Zool. 1, 107–112 (1970).
Lom, J. & Arthur, J. R. A guideline for the preparation of species descriptions in Myxosporea. J. Fish Dis. 12, 151–156 (1989).
Eiras, J. C., Cruz, C. F., Saraiva, A. & Adriano, E. A. Synopsis of the species of Myxobolus (Cnidaria, Myxozoa, Myxosporea) described between 2014 and 2020. Folia Parasitol. 68, 012 (2021).
Eiras, J. C., Saraiva, A., Cruz, C. F., Santos, M. J. & Fiala, I. Synopsis of the species of Myxidium Bütschli, 1882 (Myxozoa: Myxosporea: Bivalvulida). Syst. Parasitol. 80, 81–116 (2011).
Zhang, J. Y. et al. Synopsis of the species of Thelohanellus Kudo, 1933 (Myxozoa: Myxosporea: Bivalvulida). Syst. Parasitol. 86, 235–256 (2013).
Whipps, C. W., Atkinson, S. D. & Hoeksema, B. W. World List of Myxozoa. https://www.marinespecies.org/myxozoa (2025).
Whipps, C. M. & Zhao, Y. Synopsis of the species of the genus Sphaeromyxa Thelohan, 1892 (Myxosporea: Bivalvulida: Variisporina: Sphaeromyxidae). Syst. Parasitol. 92, 81–99 (2015).
Eiras, J. C., Cruz, C. & Saraiva, A. Synopsis of the species of Ceratomyxa Thélohan, 1892 (Cnidaria, Myxosporea, Ceratomyxidae) described between 2007 and 2017. Syst. Parasitol. 95, 427–446 (2018).
Matsche, M. A., Yurakhno, V., Zhang, J. & Sato, H. Synopsis of the species of the genus Zschokkella Auerbach, 1910 (Myxozoa: Bivalvulida: Myxidiidae). Syst. Parasitol. 98, 25–55 (2021).
Barman, G. D., Chanda, S., Panigrahi, A. K. & Eiras, J. C. Synopsis of tailed Myxobolidae (Cnidaria, Myxozoa, Myxosporea) infecting Indian fishes. Folia Parasitol. 69, 1–5 (2022).
Eiras, J. C., Barman, G. D., Chanda, S. & Panigrahi, A. K. An update of the species of Myxosporea (Cnidaria, Myxozoa) described from Indian fish. J. Parasit. Dis. 47, 12–36 (2023).
Rangel, L. F., Rocha, S. & Santos, M. J. Synopsis of the species of Ortholinea Shulman, 1962 (Cnidaria: Myxosporea: Ortholineidae). Syst. Parasitol. 101, 37 (2024).
Yang, L. et al. Phylogenetic placements of the barbin genera Discherodontus, Chagunius, and Hypselobarbus in the subfamily Cyprininae (Teleostei: Cypriniformes) and their relationships with other barbins. Zootaxa 3586, 26–40 (2012).
Kottelat, M. The fishes of the inland waters of Southeast Asia: A catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bull. Zool. 27, 1–663 (2013).
Zheng, L. P., Yang, J. X. & Chen, X. Y. Molecular phylogeny and systematics of the Barbinae (Teleostei: Cyprinidae) in China inferred from mitochondrial DNA sequences. Biochem. Syst. Ecol. 68, 250–259 (2016).
Froese, R. & Pauly, D. Fishbase, Genus Barbonymus. http://www.fishbase.org/Nomenclature/ScientificNameSearchList.php? (2018).
Kottelat, M. Fishes of Laos (WHT Publications, 2001).
Cheng, P., Baran, E. & Touch, B. T. Synthesis of all published information on java barb Barbonymus gonionotus (trey chhpin) based on Fishbase 2004. Worldfish Center and Inland Fisheries Research and Development Institute, Cambodia (2004).
Batubara, A. S. et al. Morphometric variations of the Genus Barbonymus (Pisces, Cyprinidae) harvested from Aceh Waters, Indonesia. Fish. Aquat. Life 26, 231–237 (2018).
Garcia, L. M. B. Species composition and length-weight relationship of fishes in the Candaba Wetland on Luzon Island, Philippines. J. Appl. Ichthyol. 26, 946–948 (2020).
Kenthao, A. & Jearranaiprepame, P. Ecomorphological diversification of some barbs and carps (Cyprininae, Cyprinidae) in the Lower Mekong Basin of Thailand. Zoology 143, 125830 (2020).
Mansour, O., Idris, M., Noor, N. M. & Das, S. K. Growth performance of tinfoil barb (Barbonymus schwanenfeldii) fry feeding with different protein content diets. AACL Bioflux. 10, 475–479 (2017).
Zakaria, M. H. et al. Embryonic and larval development of lemon fin barb hybrid (♂ Hypsibarbus wetmorei x♀ Barbonymus gonionotus. J. Environ. Biol. 39, 732–740 (2018).
Székely, C., Shaharom-Harrison, F., Cech, G., Mohamed, K. & Molnár, K. Myxozoan pathogens of Malaysian fishes cultured in ponds and net-cages. Dis. Aquat. Org. 83, 49–57 (2009).
Ky, H. & Te, B. Q. Parasites on Freshwater fish in Viet Nam (Science and Technics Publishing House, 2007).
Székely, C., Shaharom-Harrison, F., Cech, G., Ostoros, G. & Molnár, K. Myxozoan infections in fishes of the Tasik Kenyir water reservoir, Terengganu, Malaysia. Dis. Aquat. Org. 83, 37–48 (2009).
Suhaimi, N. S., Székely, C., Cech, G., Sellyei, B. & Borkhanuddin, M. H. New freshwater Ceratomyxa species, Ceratomyxa schwanefeldii n. sp. (Myxozoa: Ceratomyxidae) from the gall bladder of tinfoil barb, Barbonymus schwanefeldii (Cyprinidae, Cypriniformes) in Malaysia. Parasitol. Int. 108, 103073 (2025).
Lom, J. & Dyková, I. Myxozoan genera: Definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol. 53, 1–36 (2006).
Suhaimi, N. S., Sellyei, B., Udvari, Z., Székely, C. & Cech, G. Characterization of four novel actinospore types of fish parasitic myxozoans and the occurrence of Branchiodrilus hortensis and Ophidonais serpentina from fish farms of Hungary. Int. J. Parasitol. Parasites. Wildl. 25, 100994 (2024).
Suhaimi, N. S., Sellyei, B., Cech, G., Székely, C. & Borkhanuddin, M. H. First record and description of actinospore stages (raabeia, triactinomyxon, and aurantiactinomyxon types) of fish parasitic myxozoans from Malaysia. Int. J. Parasitol. Parasit. Wildl. 24, 100964 (2024).
Liu, X. H. et al. Morphological and molecular characterisation of Myxobolus pronini n. sp. (Myxozoa: Myxobolidae) from the abdominal cavity and visceral serous membranes of the gibel carp Carassius auratus gibelio (Bloch) in Russia and China. Parasit. Vectors 9, 1–11 (2016).
Úngari, L. P. et al. Description of a new species of myxobolid parasite, Henneguya pindaibensis n. sp. (Cnidaria: Myxosporea), infecting the gills of Boulengerella cuvieri (Spix and Agassiz, 1829) from Brazil. Parasitol. Int. 83, 102319 (2021).
Barta, J. R. et al. Phylogenetic relationships among eight Eimeria species infecting domestic fowl were inferred using complete small subunit ribosomal DNA sequences. J. Parasitol. 83, 262–271 (1997).
Hallett, S. L. & Diamant, A. Ultrastructure and small subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the coast of Queensland. Australia. Dis. Aquat. Org. 46, 197–212 (2001).
Whipps, C. M., Adlard, R. D., Bryant, M. S. & Kent, M. L. Two unusual myxozoans, Kudoa quadricornis n. sp. (Multivalvulida) from the muscle of godspotted trevally (Carangoides fulvoguttatus) and Kudoa permulticapsula n. sp. (Multivalvulida) from the muscle of Spanish mackerel (Scomberomorus commersoni) from the Great Barrier Reef, Australia. J. Parasitol. 89, 168–173 (2003).
Fiala, I. The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. Int. J. Parasitol. 36, 1521–1534 (2006).
Køie, M., Karlsbakk, E. & Nylund, A. The marine herring myxozoan Ceratomyxa auerbachi (Myxozoa: ceratomyxidae) uses Chone infundibuliformis (Annelida: polychaeta: Sabellidae) as invertebrate host. Folia. Parasitol. 55, 100–104 (2008).
Diamant, A., Whipps, C. M. & Kent, M. L. A new species of Sphaeromyxa (Myxosporea: Sphaeromyxina: Sphaeromyxidae) in devil firefish, Pterois miles (Scorpaenidae), from the northern Red Sea: Morphology, ultrastructure, and phylogeny. J. Parasitol. 90, 1434–1442 (2004).
Hillis, D. M. & Dixon, M. T. Ribosomal DNA: Molecular evolution and phylogenetic inference. Q. Rev. Biol. 66, 411–453 (1991).
Kearse, M. et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. Mega X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Ronquist, F. et al. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across large model space. Syst. Biol. 61, 539–542 (2012).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 9, 772 (2012).
Rambaut. A. FigTree V. 1.4.4: Tree Figure Drawing Tool. http://tree.bio.ed.ac.uk/software/figtree/ (2018).
Chinh, N. N. et al. Synopsis of myxosporean species (Cnidaria: Myxozoa) parasitizing fishes from Vietnam. Syst. Parasitol. 100, 325–344 (2023).
Molnár, K., Cech, G. & Székely, C. Histological and molecular studies of species of Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea) in the gills of Abramis, Blicca and Vimba spp.(Cyprinidae), with the redescription of M. macrocapsularis Reuss, 1906 and M. bliccae Donec & Tozyyakova, 1984 S. yst. Parasitol. 79, 109–121 (2011).
Donec, Z. S. & Shulman, S. S. Cnidosporidia. In Key to the Parasites of Freshwater Fishes of the USSR (ed Bauer, O. N.) Vol. 1, 88–251 (1984).
Fomena, A., Lekeufack Folefack, G. B. & Tang, C. II. New species of Myxobolus (Myxosporea: Myxobolidae) parasites of fresh water fishes in Cameroon (Central Africa). J. Biol. Sci. 7, 1171–1178 (2007).
Wang, M. M., Zhang, J. Y. & Zhao, Y. J. Morphological description and molecular identification of Myxobolus dajiangensis n. sp. (Myxozoa: Myxobolidae) from the gill of Cyprinus carpio in southwest China. PeerJ 10, e13023 (2022).
McAllister, C. T., Cloutman, D. G., Leis, E. M., Camus, A. C. & Robison, H. W. A new Myxobolus (Cnidaria: Myxosporea: Myxobolidae) from the gills of the southern striped shiner, Luxilus chrysocephalus isolepis (Cypriniformes: Leuciscidae), from southwestern Arkansas, USA. Syst. Parasitol. 100, 215–229 (2023).
Zhang, B., Guo, Q., Tu, X. & Gu, Z. Identification of Myxobolus distalisensis n. sp. (Cnidaria: Myxozoa) infecting yellow catfish Tachysurus fulvidraco (Richardson), with a supplement description of M. voremkhai (Akhmerov, 1960) Landsberg and Lom, 1991. Syst. Parasitol. 100, 473–485 (2023).
Cech, G., Molnár, K. & Székely, C. Molecular genetic studies on morphologically indistinguishable Myxobolus spp. infecting cyprinid fishes, with the description of three new species, M. alvarezae sp. nov., M. sitjae sp. nov. and M. eirasianus sp. nov. Acta. Parasitol. 57, 354–366 (2012).
Zhang, X. Y., Yao, X., Zhou, F., Yang, C. Z. & Liu, Y. Identification of Thelohanellus pseudonikolskii n. sp. and Myxobolus koi Kudo, 1920 from goldfish Carassius auratus. Aquac. Rep. 24, 101167 (2022).
Molnár, K., Eszterbauer, E., Székely, C., Dán, Á. & Harrach, B. Morphological and molecular biological studies on intramuscular Myxobolus spp. of cyprinid fish. J. Fish. Dis. 25, 643–652 (2002).
Molnár, K., Marton, S., Székely, C. & Eszterbauer, E. Differentiation of Myxobolus spp. (Myxozoa: Myxobolidae) infecting roach (Rutilus rutilus) in Hungary. Parasitol. Res. 107, 1137–1150 (2010).
Székely, C. et al. Myxozoan infections of the three Indian major carps in fish ponds around Meerut, UP, India, with descriptions of three new species, Myxobolus basuhaldari sp. n., M. kalavatiae sp. n. and M. meerutensis sp. n., and the redescription of M. catlae and M. bhadrensis. Parasitol. Res. 114, 1301–1311 (2015).
Eiras, J. C., Molnár, K. & Lu, Y. Synopsis of the species of Myxobolus bütschli, 1882 (Myxozoa: Myxosporea: Myxobolidae). Syst. Parasitol. 61, 1–46 (2005).
Molnár, K., Eszterbauer, E., Marton, S., Székely, C. & Eiras, J. C. Comparison of the Myxobolus fauna of common barbel from Hungary and Iberian barbel from Portugal. Dis. Aquat. Org. 100, 231–248 (2012).
Qadri, S. S. On a new myxosporidian, Thelohaenellus boggoti n. sp., from Indian fresh water fish Labeo boggot. Arch. Protistenkd. 106, 218–222 (1962).
Singh, R. & Kaur, H. Two new and one already known species of the genus Thelohanellus (Myxozoa: Myxosporea: Bivalvulida) parasitizing fresh water fishes in wetlands of Punjab, India. Biologia 70, 85–93 (2015).
Chen, Q. L., Ma, C. L. & Fauna, S. Myxozoa: Myxosporea (Science Press, 1998).
Molnár, K. & Eszterbauer, E. Specificity of infection sites in vertebrate hosts. In Myxozoan Evolution, Ecology and Development 295–313 (2015).
Eiras, J. C., Zhang, J. & Molnár, K. Synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea, Myxobolidae) described between 2005 and 2013. Syst. Parasitol. 88, 11–36 (2014).
Carriero, M. M., Adriano, E. A., Márcia, R. M., Silva, C. P. S. & Maia, A. A. M. Molecular phylogeny of the Myxobolus and Henneguya genera with several new South American species. PLoS ONE 8, e73713 (2013).
Shin, S. P. et al. The phylogenetic study on Thelohanellus species (Myxosporea) in relation to host specificity and infection site tropism. Mol. Phylogenet. Evol. 72, 31–34 (2014).
Ahmad, I. & Kaur, H. Prevalence, site and tissue preference of myxozoan parasites infecting gills of cultured fingerlings of Indian major carps in District Fatehgarh Sahib, Punjab (India). J. Parasit. Dis. 42, 559–569 (2018).
Eszterbauer, E. Genetic relationship among gill-infecting Myxobolus species (Myxosporea) of cyprinids: molecular evidence of importance of tissue-specificity. Dis. Aquat. Org. 58, 35–40 (2004).
Kaur, H. et al. Species diversity of the genus Thelohanellus Kudo, 1933 (Myxozoa: Bivalvulida) parasitizing fishes in Indian subcontinent. J. Parasit. Dis. 41, 305–312 (2016).
Borkhanuddin, M. H. et al. Description of myxosporeans (Cnidaria: Myxozoa) infecting the popular food fish Notopterus notopterus (Pisces: Notopteridae) in Malaysia and India. Food Waterborne Parasitol. 20, e00092 (2020).
Lukphet, J. Parasites of Pla Soi Nok Khao Osteochilus hasselti (Cuvier & Valenciennes) in Srinakarin reservior, Changwat Kanchanaburi (1997).
Thumvittayakul, W., U-taynapun, K. & Wongsawad, C. Myxozoa parasites from some freshwater fishes in Chiang Mai Province (2018).
Acknowledgements
We thank the staff members at the Biodiversity Laboratory, Faculty of Science and Marine Environment, Universiti Malaysia Terengganu, for their assistance during the investigation. We also thank Ms. Györgyi Pataki for the histological slides.
Funding
Open access funding provided by HUN-REN Veterinary Medical Research Institute. This work was supported by the Stipendium Hungaricum Program.
Author information
Authors and Affiliations
Contributions
N.S.S., C.S. and B.S. conceived and designed the study; N.S.S. collected the samples and analyzed the data; C.S. and M.H.B. provided sampling resources and permits; G.C. assisted in molecular analyses; N.S.S writing original draft; K.M., G.C., B.S., C.S., review and editing. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors consent to publish the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suhaimi, N.S., Székely, C., Molnár, K. et al. Biodiversity and five novel species of myxozoan parasites in Barbonymus spp. (Cyprinidae, Cypriniformes) from Malaysia. Sci Rep 15, 29134 (2025). https://doi.org/10.1038/s41598-025-14254-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14254-y