Abstract
This study investigated the effects of zearalenone (ZEN) and nanozeolite (NZ) on arugula (Eruca sativa) under deficit irrigation in a controlled greenhouse environment. Using a split-plot design, two irrigation levels (100% and 70% field capacity: FC) were applied, along with ZEN (60 and 120 ppb), NZ (1.5 and 3 ppm), and a control with no additives. Both ZEN and NZ significantly enhanced arugula’s drought tolerance by improving photosynthetic efficiency, water use efficiency (WUE), plant water status, growth, proline accumulation, and antioxidant activity. Notably, NZ at 3 ppm was most effective, increasing net photosynthesis by 2.84-fold, WUE by 2-fold, and reducing transpiration by 4.33-fold and electrolyte leakage by 1.19-fold under 70% FC compared to the control. NZ at this concentration also improved chlorophyll fluorescence, raising Fv/Fm by 52% (up to 0.8) and lowering non-photochemical quenching (NPQ) to 0.33, indicating enhanced photosystem II efficiency and reduced photoprotective demand. ZEN at 120 ppb also improved drought resilience by increasing Fv/Fm, reducing NPQ, enhancing chlorophyll content, maintaining higher relative water content and osmotic potential, increasing leaf area and shoot fresh weight, and boosting proline accumulation, though its effects were generally less pronounced than those of NZ. These findings suggest ZEN and NZ as promising amendments for improving arugula’s resilience to water stress in controlled environments, with potential applications in water-scarce agricultural systems pending field validation.
Similar content being viewed by others
Introduction
Arugula (Eruca sativa), a leafy vegetable in the Brassicaceae family, is valued for its nutrient-rich profile, including flavonoids and glucosinolates, which provide antioxidant and health benefits1,2,3. However, its productivity is increasingly challenged by drought stress, a major environmental constraint reducing crop yield through impaired photosynthesis, water relations, and oxidative damage4,5. Drought-tolerant arugula genotypes exhibit higher proline accumulation and antioxidant enzyme activity compared to sensitive ones6. Plants adapt to drought via stomatal closure, enhanced root water uptake, and accumulation of protective compounds like proline7,8,9. Drought-induced reactive oxygen species (ROS) further limit photosynthetic capacity, necessitating innovative strategies to enhance resilience10.
Zearalenone (ZEN), a metabolite from Fusarium graminearum, acts as a plant growth regulator, enhancing root development and drought tolerance11,12,13. Synthesized via a polyketide pathway, ZEN regulates fungal sexual development and promotes haploid embryo formation, respiration, and CO₂ absorption in plants14,15,16. Zearalenone supplementation (0.2 mL L⁻¹) enhances microspore embryogenesis and green plant regeneration in winter wheat12. In legumes like peas and lupines, ZEN mitigates drought stress, increases antioxidant levels (e.g., β-carotene, tocopherols) in seeds, and boosts root mass for improved nutrient and water uptake without accumulating in seeds, ensuring agricultural safety16,17. Zearalenone’s ability to enhance antioxidant defenses and root growth makes it a promising tool for drought resilience17. Recent agricultural trends highlight innovative methods like nano-fertilizers (NFs) to manage water scarcity and reduce chemical fertilizer use18. NFs enhance nutrient absorption and utilization under stress, offering eco-friendly and cost-effective solutions for sustainable agriculture19,20. Water retention agents like zeolite, bentonite, and perlite, especially in nano form, alleviate drought stress21. Nanozeolites (NZ) reduce reactive oxygen species (ROS), boost antioxidant activity, and enhance enzyme function during drought22,23,24. Nanozeolite’s high surface area, mesoporous structure, and nutrient-loading capacity enable slow nutrient release, improving fertilizer efficiency and ecosystem health25. With high cation exchange capacity (CEC), NZ enhances membrane stability, water relations, and photosynthetic protection, promoting plant resilience and phytohormone biosynthesis under drought26,27,28,29. Nanozeolite’s capacity to improve soil water retention and nutrient availability positions it as a sustainable amendment for water-limited environments28.
Global water scarcity and climate change necessitate resilient crops to address food security30. This study hypothesizes that ZEN and NZ enhance arugula’s drought tolerance by improving photosynthetic efficiency, water relations, and antioxidant defenses under regulated deficit irrigation (RDI). By evaluating these amendments in a greenhouse setting, this research addresses a critical gap in developing sustainable strategies for drought resilience in nutrient-rich leafy vegetables, offering novel insights into ZEN and NZ as synergistic tools for water-limited environments.
Results
Chlorophyll fluorescence
All chlorophyll fluorescence parameters (F₀, Fm, Fv, Fv/Fm, non-photochemical quenching (NPQ)) were significantly affected by both irrigation regime and chemical treatments (Fig. 1a–e). Under deficit irrigation (70% FC), F₀ increased (up to 407.4 in the control), while Fv and Fv/Fm decreased (lowest Fv/Fm: 0.5 in the control), indicating greater stress. The highest Fv and Fv/Fm values (Fv/Fm up to 0.8) were observed in NZ (3 ppm) and ZEN (120 ppb) under 100% FC, while the lowest values were consistently found in the control under 70% FC. Fm followed a similar pattern, with NZ (3 ppm) under 100% FC showing the highest values. The highest NPQ was observed in the control under 70% FC (0.49), while the lowest NPQ values were recorded for ZEN 120 ppb (0.32) and NZ 3 ppm (0.33) under 100% FC.
Effects of zearalenone (ZEN) and nanozeolite (NZ) on the minimum fluorescence: F0 (a), maximum fluorescence: Fm (b), variable fluorescence: Fv (c), maximum efficiency of photosystem II: Fv/Fm (d), None Photochemical Quenching: NPQ (e) under regulated deficit irrigation at 100% FC and 70% FC. Values are means of three replicates ± SE.
Gas exchange parameters
Gas exchange was strongly influenced by both irrigation and treatments (Fig. 2a–e). Stomatal conductance (gs) and net photosynthesis (An) were highest in NZ (1.5 and 3 ppm) under 100% FC (gs: 0.80 mmol m⁻² s⁻¹; An: 23.4 µmol CO₂ m⁻² s⁻¹) and lowest in the control under 70% FC (gs: 0.38 mmol m⁻² s⁻¹; An: 4.1 µmol CO₂ m⁻² s⁻¹). Transpiration (E) was lowest in NZ treatments under 100% FC, while WUE peaked in NZ (3 ppm) under 100% FC and was lowest in the control and ZEN treatments under both irrigation regimes. Intercellular CO₂ concentration was also higher in NZ (3 ppm) under 100% FC and lowest in the control under 70% FC.
Leaf water status
Significant differences were observed in all measured indices of leaf water status, including relative water content (RWC), water potential, osmotic potential, and turgor potential (Fig. 3a–d). RWC was highest in NZ (3 ppm) under 100% FC (93.3%) and lowest in the control under 70% FC (49.3%). Both NZ and ZEN at higher concentrations maintained RWC above 60% under deficit irrigation, indicating improved tissue hydration (Fig. 3a). Water potential was highest in ZEN (120 ppb, -0.97 bar) and NZ (3 ppm, -0.5 bar) under 100% FC, and lowest in the control under 70% FC (-8.6 bar), showing severe water stress in the control (Fig. 3b). Osmotic potential varied significantly: the most negative value (-9.2 bar) was in the control under 70% FC, while the highest was in ZEN (120 ppb) + 100% FC (-2.0 bar), followed by NZ (3 ppm) under 100% FC (-2.3 bar). NZ and ZEN, especially at higher concentrations, improved osmotic adjustment and cellular hydration (Fig. 3c). Turgor potential was highest in NZ (3 ppm) under 70% FC (3.80 bar), not significantly different from ZEN (60 ppb, 3.43 bar) and ZEN (120 ppb, 3.07 bar) under the same regime. The lowest turgor (0.6 bar) was in the control under 70% FC, indicating loss of cell turgidity. Some treatments under 100% FC (ZEN 120 ppb and NZ 1.5 ppm) also showed relatively low turgor, possibly due to rapid cell expansion (Fig. 3d).
Electrolyte leakage
Electrolyte leakage was highest in the control under both irrigation regimes (42.3–48.1%) and lowest in NZ (3 ppm) under both conditions, as well as in NZ (1.5 ppm) and ZEN (120 ppb) under 100% FC (Fig. 4a).
Leaf area, aerial and root fresh weight
Leaf area and aerial fresh weight were greater under 100% FC, with the largest values in ZEN (120 ppb) and NZ (3 ppm) under 100% FC. The smallest values were in the control and ZEN (60 ppb) under 70% FC (Fig. 4b, c). Root fresh weight was 53% higher under 70% FC, with all treatments outperforming the control in deficit irrigation (Fig. 4d).
Chlorophyll index
Chemical treatments significantly increased the chlorophyll index, while irrigation levels and their interaction had no significant effect (Fig. 5a–b), with the highest value in ZEN (120 ppb, 34.7), followed by NZ (1.5 and 3 ppm). The lowest index was in the control (25.5).
Effects of zearalenone (ZEN) and nanozeolite (NZ) on chlorophyll index (a), proline (c), superoxide dismutase activity (e), and simple effects of regulated deficit irrigation at 100% FC and 70% FC on chlorophyll index (b), proline (d), superoxide dismutase activity (f). Values are means of three replicates ± SE.
Proline content
Both deficit irrigation and chemical treatments increased leaf proline content (Fig. 5c–d). The highest proline (0.66 µmol g⁻¹ FW) was in NZ (3 ppm), not significantly different from NZ (1.5 ppm), and the lowest in the control (0.35 µmol g⁻¹ FW).
Superoxide dismutase (SOD) activity
SOD activity was significantly higher under 70% FC (42.1 U mg⁻¹ FW) than 100% FC (24.5 U mg⁻¹ FW). All treatments except ZEN (60 ppb) and the control showed the highest SOD activity (Fig. 5e–f).
Discussion
Responses of chlorophyll fluorescence to NZ and ZEN under RDI
Water scarcity, a pressing global challenge, significantly impairs agricultural productivity by disrupting plant water status, photosynthesis, and cellular homeostasis31,32. Drought stress reduces the F₀ and Fm, lowering the Fv/Fm ratio, which indicates impaired photochemical efficiency of photosystem II (PSII) and decreased plant productivity under water-limited conditions33,34,35. The Fv/Fm ratio serves as a key indicator of the maximum quantum efficiency of PSII, providing insights into photosynthetic performance36. Under drought stress, plants unable to dissipate excess solar energy via fluorescence transfer this energy to oxygen, generating reactive oxygen species (ROS) that damage the D1 protein in the PSII reaction center, potentially leading to membrane destruction and chlorophyll oxidation21.
In this study, NZ and ZEN treatments, particularly at higher concentrations, significantly enhanced chlorophyll fluorescence parameters (Fv, Fm, Fv/Fm) under both normal and RDI at 70% FC (Fig. 1b–d). These improvements were more pronounced under normal irrigation compared to RDI, likely due to greater water availability supporting photosynthetic processes. Nanozeolite (3 ppm) increased Fv/Fm by 52% under 70% FC, consistent with Mahmoud et al.21who reported a 30% increase in Fv/Fm in coriander (Coriandrum sativum L.) treated with zeolite nanoparticles under drought stress. This amplified response in arugula may reflect its high metabolic demand as a leafy vegetable. Similarly, ZEN treatment increased Fv/Fm to 0.8, comparable to the 20–30% enhancement observed with 24-epibrassinolide in wheat under heat stress37suggesting a shared mechanism of stabilizing PSII under abiotic stress15. Additionally, NPQ was significantly lower in treated plants (0.33–0.34 for ZEN and NZ vs. 0.49 in controls at 70% FC), indicating reduced photoprotective demand and improved photosynthetic efficiency under drought stress (Fig. 1e).
Nanozeolite enhances soil water-holding capacity and nutrient availability due to its high cation-exchange capacity and mesoporous structure, directly improving soil properties under RDI38,39. By reducing water loss through evaporation, NZ mitigates water stress, enabling plant to improve chlorophyll fluorescence parameters and tolerance to deficit irrigation. Research has shown that nutrient deficiencies can lead to decreased photosynthetic efficiency, as indicated by lower chlorophyll fluorescence values40,41. A study demonstrated that applying zeolite nanoparticles significantly decreased leaf chlorophyll degradation and improved parameters such as Fv/Fm and overall photosynthetic efficiency in plants experiencing drought stress through their high cation exchange capacity, which improves nutrient availability for chlorophyll stability, mesoporous structure for soil water retention to support stomatal conductance, and adsorption of reactive oxygen species to minimize oxidative damage21,42.
Zearalenone significantly enhances plant growth and development by altering the status and functionality of the photosynthetic apparatus, as evidenced by increased Fv/Fm ratios, reduced NPQ, and enhanced chlorophyll content and net photosynthetic15. Similarly, in our study, ZEN-treated arugula exhibited higher Fv/Fm values, reduced NPQ, and increased chlorophyll content, confirming the positive effect of ZEN on the photosynthetic apparatus under both optimal and deficit irrigation. Lower NPQ in treated arugula reflects improved CO₂ assimilation and reduced energy dissipation as heat, consistent with enhanced photosynthetic efficiency, in agreement with previous findings36,37. ZEN’s role in enhancing root hydraulic conductivity and antioxidant defenses further minimized PSII damage, reducing the need for NPQ activation16. This aligns with observations in drought-stressed plants, where high NPQ indicates increased photoprotective demand, whereas drought-tolerant plants maintain lower NPQ due to improved photosynthetic performance33,34,35.
Responses of photosynthetic parameters to NZ and ZEN under RDI
In our study, ZEN and NZ significantly influenced various photosynthetic parameters, including stomatal conductance (gs), transpiration rate (E), net photosynthetic rate (An), intercellular CO2 concentration (Ci), and water use efficiency (WUE) under both irrigation conditions (Fig. 2a-e). Zeolites are known for their high cation-exchange capacity and ability to retain substantial amounts of water, which helps maintain soil moisture levels, especially during drought conditions. This property is crucial for plant health and photosynthesis, as improved water availability facilitates better gs and enhances An by ensuring that plants have sufficient water to support these processes43,44. Additionally, zeolites can adsorb CO2 molecules, increasing the concentration of CO2 available near the stomata44,45,46. This enhancement allows for greater carbon fixation during photosynthesis, thereby improving overall efficiency. The increased intercellular CO2 concentration promotes higher rates of photosynthesis, as plants can utilize more carbon for energy production44. Elevated CO₂ concentrations near the stomata generally trigger partial stomatal closure, thereby minimizing water loss without compromising carbon assimilation or photosynthetic efficiency47,48,49. The inverse relationship between gs and transpiration rate E under deficit irrigation with NZ (3 ppm) and ZEN (120 ppb) treatments likely stems from NZ’s enhanced soil water retention and ZEN’s improved root hydraulic conductivity, enabling sustained gs (0.80 mmol m⁻² s⁻¹) for CO₂ uptake while reducing E by 433% through optimized stomatal regulation21,28,50. Both ZEN concentrations (60 and 120 ppb) under 70% FC enhanced net photosynthesis (An) compared to the control, likely due to improved root hydraulic conductivity and elevated proline and SOD activity, which sustained higher gs and Ci while mitigating drought-induced oxidative stress14,16,28. A study focusing on the impact of N-zeolite on coriander plants under drought stress reported significant enhancements in photosynthetic parameters compared to untreated control plants21. In another study involving soybean and wheat plants, ZEN was shown to increase net photosynthesis and transpiration rates, which are closely related to stomatal function. Specifically, soybean plants exhibited an average increase of approximately 13.6% in An during early vegetative growth stage and final phases, while wheat demonstrated a less pronounced response but still an increase of over 24% near maturity15.
Responses of water status to NZ and ZEN under RDI
The findings indicated a notable interaction between the treatments applied and the levels of irrigation concerning the water status of the leaves (Fig. 3a-d). Research demonstrates that RDI significantly enhances WUE and minimizes water waste, a critical factor in horticultural production amid water scarcity challenges. RDI promotes water conservation while ensuring economic viability in water-limited conditions by optimizing the balance between water use, crop yield, and product quality51,52. Under drought conditions, NZ mitigates water stress by improving WUE and maintaining higher RWC in plants. Nanozeolite enhances soil structure and moisture retention, playing a pivotal role in reducing water stress under RDI53. By sustaining soil hydration, NZ supports plant water relations, minimizing the physiological impact of water deficits and bolstering arugula’s drought resilience. This is achieved through improved soil moisture retention and reduced evaporation losses, which maintain higher turgor pressure during periods of limited water availability53,54. Similar improvements in water use efficiency under drought stress have also been reported in other crops using biological amendments, such as mycorrhizal inoculation in hemp (Cannabis sativa L.)55. Zeolite enhances the soil’s ability to retain water; studies have shown that its addition can significantly increase water content in sandy soils by up to 60% at specific matric potentials21. This increase in soil moisture directly affects the availability of water to plants, improving overall water potential42. The porous structure of zeolite reduces evaporation rates from the soil surface, further contributing to moisture retention. This is particularly important in light-textured soils, where zeolite can decrease the rate of water discharge and enhance hydraulic parameters such as saturation and residual moisture56. In saline soils, osmotic pressure can hinder plant growth by affecting turgor pressure57,58. Zeolite has been shown to improve the osmotic potential of soil by facilitating the removal of harmful ions like sodium and boron from irrigation water, helping maintain a favorable osmotic environment for plants59. The high cation exchange capacity (CEC) of zeolite enables it to act as a chemical sieve, selectively retaining beneficial nutrients while blocking harmful ions. This selective absorption enhances nutrient availability and contributes to better osmotic conditions for plant roots56. By increasing soil moisture and reducing osmotic stress, zeolite application helps maintain turgor pressure in plant cells. Turgor pressure is crucial for cell expansion and overall plant health, as it supports structural integrity and influences physiological processes such as photosynthesis59. A crucial aspect of a plant’s tolerance to water deficit is the preservation of cell membrane integrity and stability under such conditions. The cell membrane is essential for regulating the movement of nutrients, water, and other vital molecules in and out of the cell, as well as for maintaining cellular homeostasis2,60. A common approach to evaluate the permeability and integrity of plant cell membranes under stress is through measuring electrolyte leakage. In this study, when applied to water-stressed arugula plants using RDI, treatments with ZEN and NZ significantly decreased electrolyte leakage compared to control plants (Fig. 4a). These results align with findings from Dziurka et al.16which demonstrated that foliar application of ZEN on pea and lupine at the onset of drought effectively reduced electrolyte leakage and alleviated drought stress impacts. Similarly, treating strawberry plants with nanozeolite particles resulted in a notable reduction in electrolyte loss; untreated plants exhibited significantly higher relative electrolyte leakage than those treated with NZ, suggesting that NZ offers enhanced protection for plant membranes22. Both natural and synthetic zeolites have been shown to mitigate the negative effects of soil abiotic stresses, supporting the notion that they can protect cell membranes from oxidative damage61.
Responses of growth parameters to NZ and ZEN under RDI
In our research, the application of ZEN and NZ treatments significantly enhanced various growth metrics in arugula, including leaf area, aerial and root fresh weights (Fig. 4b-d). This finding aligns with existing research that indicates zeolites improve soil physicochemical properties, which in turn promotes plant growth. Specifically, zeolites increase CEC, water retention, and nutrient availability in the root zone, benefiting crops like wheat, maize, and tomatoes62,63. The results from our study are consistent with findings from Rahmani et al.21 where zeolite application to Nigella sativa resulted in a notable increase in plant height—approximately 3.2 mm for each ton of zeolite applied per hectare. Furthermore, the number of branches per plant improved with zeolite treatment, indicating enhanced overall vegetative growth. This suggests that zeolite not only supports root development but also contributes to above-ground biomass accumulation. Zeolites have been shown to elevate soil moisture levels and hydraulic conductivity, which is particularly advantageous under limited irrigation conditions64. Our findings corroborate this assertion, as the incorporation of zeolites into soil has been associated with improved growth indicators such as increased plant height, dry weight, and root development. For example, studies involving tomato seedlings demonstrated enhanced growth when zeolite was mixed into growth substrates65. Additionally, zeolites may positively influence photosynthesis by adsorbing CO2 and lowering leaf temperatures—factors that can further enhance plant growth under specific conditions44.
Responses of some antioxidants to NZ and ZEN under RDI
The accumulation of proline is a well-documented physiological response in various plant species experiencing drought stress. This amino acid is essential for plant adaptation to water scarcity10. In our experiment, plants subjected to RDI irrigation showed a proline concentration that was 46% higher compared to those under normal irrigation (Fig. 5c). The enzymes pyrroline-5-carboxylate synthase (P5CS) and proline dehydrogenase (PRODH) play crucial roles in regulating proline accumulation through their reciprocal activities66. This experiment demonstrated that treatments significantly increased proline content in arugula compared to the control group, particularly in plants treated with NZ (Fig. 5d). Moreover, soil amendments with zeolites have been shown to alleviate various abiotic stresses, including water and salt stress, by enhancing soil properties and nutrient availability. This alleviation can activate the plant’s natural defense mechanisms, including the accumulation of proline, which is recognized as a stress-responsive metabolite67. Zeolites function as chelating agents and soil conditioners, slowing the release of nutrients from fertilizers to ensure consistent nutrient availability while improving soil water retention and aeration by preserving beneficial nutrients in the root zone61,68. Additionally, zeolites can minimize the uptake of potentially toxic elements by plants, which might otherwise induce stress and lead to increased proline accumulation as a response. By mitigating these toxic effects, zeolites indirectly support plants in maintaining higher proline levels, thereby enhancing their ability to manage stress60. Cataldo et al.69 supported this hypothesis by demonstrating that zeolites enhance nutrient availability to plants. This improved nutrient uptake can enhance overall metabolic efficiency in plants, including the biosynthetic pathways responsible for proline accumulation under stress conditions.
Superoxide dismutase plays a vital role in the plant’s antioxidant defense system by neutralizing superoxide radicals and alleviating oxidative stress. In this study, SOD activity under RDI was found to be 71% greater than under normal irrigation conditions (Fig. 5e). Moreover, some experiments have indicated that drought treatment can lead to a remarkable 418% increase in SOD activity in one drought-resistant barley strain and a 59% increase in another resistant strain by the end of the 12th day of observation70. Results showed that all treatments applied, with the exception of the ZEN 60 ppb and control groups, demonstrated significantly elevated levels of SOD activity (Fig. 5f). Zearalenone supports arugula’s tolerance to RDI by enhancing biochemical defenses, such as proline accumulation and SOD activity, which mitigate oxidative stress. Additionally, its promotion of root development indirectly improves soil properties, aiding water and nutrient acquisition under water-limited conditions. This indicates that these treatments effectively enhance the plant’s antioxidant defense mechanisms, likely by triggering stress responses that promote SOD production. For example, a pot experiment with Chinese cabbage (Brassica rapa subsp. Pekinensis) revealed that both nanozeolite and conventional zeolite treatments increased antioxidant enzyme activities in the plants’ shoots and roots71.
While this study provides comprehensive insights into the physiological and biochemical responses of arugula to water stress under ZEN and NZ treatments, several limitations must be acknowledged. The absence of water productivity measurements (yield per unit water applied) limits our understanding of the relationship between water use and yield outcomes, despite the observed 200% increase in WUE with NZ (3 ppm) under 70% FC. Additionally, the controlled greenhouse conditions minimized environmental variability, which may not reflect field conditions with fluctuating temperatures, soil heterogeneity, or biotic stresses. The short experimental duration captured acute responses but did not assess long-term impacts on yield stability or soil health. The lack of molecular data, such as transcriptomic or proteomic profiles, restricts our understanding of the genetic and signaling pathways underlying ZEN and NZ’s effects. Future research should include field trials to validate scalability, long-term studies to assess sustained benefits, and omics approaches to elucidate molecular mechanisms. Exploring combined ZEN and NZ applications could also uncover synergistic effects, while assessing NZ’s impact on soil microbial communities could clarify its environmental sustainability.
Conclusion
NZ (3 ppm) was the most effective amendment, significantly enhancing photosynthetic efficiency (Fv/Fm 52%, An 284%), WUE (200%), and growth under 70% FC, while reducing transpiration and electrolyte leakage. ZEN (120 ppb) also improved chlorophyll content (35%), proline (37%), and SOD activity (74%), though less effectively than NZ. These findings highlight NZ and ZEN as promising tools for enhancing arugula’s drought resilience in controlled settings, with potential to reduce irrigation needs in water-scarce regions. Field trials are needed to validate scalability and assess long-term impacts on yield and soil health, while ZEN’s safety must be confirmed to ensure consumer and environmental safety.
Materials and methods
Plant material and design experiment
This study investigated the effects of nanozeolite (NZ) and zearalenone (ZEN) treatments combined with regulated deficit irrigation (RDI) on the growth of arugula (Eruca sativa) in a greenhouse setting from October 2023 to January 2024. Conditions were maintained at 20–25 °C (day), ≥ 15 °C (night), 16 h light (4380 lx), and 60–70% relative humidity. A split-plot design was used with two irrigation regimes as main plots: (1) full irrigation (100% field capacity: FC), and (2) deficit irrigation (70% FC). Subplots included five treatments: (a) control (no additives), (b) ZEN at 60 ppb, (c) ZEN at 120 ppb, (d) NZ at 1.5 ppm, and (e) NZ at 3 ppm, with three replicates (30 experimental units total). Concentrations were selected based on prior studies 14,16,21 and preliminary trials confirming efficacy and safety for arugula.
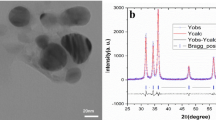
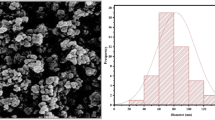
The growing medium comprised cocopeat, perlite, and peat moss (60:20:20). ZEN (Sigma-Aldrich, ≥ 98% purity) was dissolved in 1% ethanol to create a 1000 µg/L stock solution, stored at 4 °C, and diluted with deionized water to 60 and 120 µg/L. Arugula seeds were primed in these solutions for 12 h before sowing. NZ (clinoptilolite, < 100 nm, ZeoTech Inc.) was characterized for particle size (mean: 80 nm, surface area: 150 m²/g) and incorporated into the medium. Control plants received no additives.
Before sowing, Regulated deficit irrigation (RDI) was established by saturating control trays with water until free drainage ceased, then weighing them to determine field capacity (FC), measured as 36% gravimetric soil moisture content (w/w), with a permanent wilting point (PWP) of 25% (w/w), yielding an available water capacity (AWC) of 11% (w/w). The 70% FC treatment was defined as maintaining soil moisture at 70% of AWC (approximately 32.7% w/w), inducing mild drought stress above the PWP. Soil moisture was monitored daily using the gravimetric method: pots were weighed, and water loss was calculated relative to the initial weight at 100% or 70% FC. Irrigation was applied to restore target moisture levels, typically requiring 300 mL/pot every 24 h for 100% FC and 300 mL/pot every 72 h for 70% FC, with volumes and frequencies adjusted based on daily measurements to account for environmental variations (e.g., temperature, humidity). This ensured precise maintenance of soil moisture throughout the experiment, regardless of plant growth stage or external conditions.
The seeds used in our study were purchased from Rocalba S.A., a company based in Spain. On October 15, 2023, arugula F1 seeds were soaked in ZEN solutions (60 and 120 ppb) for seed priming and sown in trays containing the prepared medium amended with NZ (1.5 or 3 ppm) where applicable. Seed germination occurred within 7–10 days (October 15–22, 2023). On December 5, 2023, physiological and biochemical measurements, including chlorophyll fluorescence, photosynthetic gas exchange, plant water relations, electrolyte leakage, chlorophyll index, proline content, and superoxide dismutase (SOD) activity, were conducted on fully expanded leaves at the maximum growth stage. On December 24, 2023, leaf area, aerial fresh weight, and root fresh weight were measured by harvesting five plants per replicate. Plants were harvested on January 5, 2024, for the final evaluation of yield and component traits.
Chlorophyll fluorescence
Chlorophyll fluorescence parameters (F₀, Fm, Fv, Fv/Fm, NPQ) were measured on fully expanded leaves (n = 3 plants/replicate) after 25 min dark adaptation using a Junior-PAM fluorometer (Walz, Germany). Fv/Fm represents the maximum quantum yield of photosystem II (PSII), and NPQ indicates non-photochemical quenching36.
Photosynthetic gas exchange
Gas exchange (stomatal conductance: gs, transpiration rate: E, net photosynthetic rate: An, intercellular CO₂: Ci, water use efficiency: WUE) was measured (n = 3 plants/replicate) using a KR8700 system (Korea Tech Inc.) at 400 µmol mol⁻¹ CO₂, 1000 µmol m⁻² s⁻¹ photosynthetic photon flux density (PPFD), 1.0 kPa vapor pressure deficit (VPD), and 25 °C73.
Plant water relations
The evaluation of plant water relations was conducted by assessing the water status through the relative water content (RWC) of fully expanded leaves, following the methodology outlined by Silva et al.74. The formula used is RWC = [Wf−Wd]/[Wt− Wd]×100, where Wf, Wd, and Wt represent the fresh, dry, and turgid weights, respectively. Leaf water potential (ψw) was determined using a Scholander pressure chamber (Wescor C-52, Logan, UT, USA) as described by Scholander et al.75. Following ψw, leaves were immediately wrapped in aluminum foil, frozen by immersing in liquid nitrogen and stored at − 18 °C. Leaf osmotic potential (ψπ) was measured on sap pressed from the thawed tissue at 25 ± 1 °C with a vapor pressure osmometer (model 5100B, Wescor, Logan, UT, USA). Turgor potential (ψp) was calculated as the difference between the ψπ and ψw76.
Electrolyte leakage
The procedure involved measuring electrolyte leakage to evaluate the integrity of plant cell membranes77. Ten leaf sample segments were added to each test tube, along with 10 ml of deionized water, and the mixtures were shaken at room temperature for 24 h. The initial electric conductivity (EC1) of each solution was measured using an electrical conductivity meter (Apera EC20, Germany). Subsequently, the samples were placed in a water bath heated to 100 °C for 20 min. After cooling to room temperature, a second measurement of electric conductivity (EC2) was taken. The percentage of electrolyte leakage was calculated using the formula: (EC1/EC2) × 100.
Leaf area, aerial and root fresh weight
On the 50th day following the initiation of the RDI, five plants from each replicate were carefully removed from their pots, and their roots were thoroughly rinsed with tap water to remove any contaminants. The plants were then divided into two sections: aerial parts and roots. Each section’s weight was recorded using a precise balance.
Chlorophyll index
The chlorophyll index was measured using a SPAD-502 chlorophyll meter (Minolta Camera Co., Japan), while leaf area was assessed with a portable leaf area meter (Model MK2; Delta-T Devices, Cambridge, UK).
Proline content
The method for quantifying proline content, as described by Bates et al.78began with a 0.5 g leaf sample being homogenized in 10 mL of 3% (w/v) sulfosalicylic acid, followed by centrifugation at 5000 g for 10 min. The resulting supernatant (2 mL) was mixed with 2 mL of acid ninhydrin reagent and 2 mL of glacial acetic acid, and the mixture was heated in a water bath for one hour. After cooling, 4 mL of toluene was added, and the solution was stirred rapidly for 20 s. Free proline was then quantified spectrophotometrically at 520 nm, using toluene as a blank for calibration.
Superoxide dismutase (SOD)
For the extraction of superoxide dismutase (SOD), fresh leaf samples (0.5 g) were ground using a pre-chilled pestle and mortar in liquid nitrogen. The ground tissue was then extracted with 50 mM potassium phosphate buffer (pH 7.8), supplemented with 0.5 mM EDTA. The resulting homogenate was transferred to centrifuge tubes and centrifuged at 15,000 g for 15 min at 4 °C. The supernatant obtained was utilized for the enzyme activity assay. Total SOD activity was measured by assessing the inhibition of nitro blue tetrazolium (NBT) photochemical reduction, following the protocol established by Sairam et al.79. One unit of SOD activity was defined as the amount of enzyme needed to achieve 50% inhibition of NBT reduction, as determined by monitoring absorbance at 560 nm.
Statistical analysis
Data were analyzed using ANOVA in a split-plot design, with irrigation as the main plot and treatments as subplots (n = 3 replicates). Means were compared using LSD (P < 0.05) in SAS.
Data availability
Data is provided within the supplementary information files.
References
Nikzad, S., Mirmohammady Maibody, S. A. M., Ehtemam, M. H., Golkar, P. & Mohammadi, S. A. Response of seed yield and biochemical traits of Eruca sativa mill. To drought stress in a collection study. Sci. Rep. 13, 11157 (2023).
Yang, T., Samarakoon, U., Altland, J. & Ling, P. Photosynthesis, biomass production, nutritional quality, and flavor-related phytochemical properties of hydroponic-grown arugula (Eruca sativa Mill.)‘standard’under different electrical conductivities of nutrient solution. Agronomy 11, 1340 (2021).
Jaafar, N. S. & Jaafar, I. S. Eruca sativa linn.: pharmacognostical and Pharmacological properties and pharmaceutical preparations. Asian J. Pharm. Clin. Res. 12, 39–45 (2019).
Yoo, M. J. et al. Physiological and molecular modulations to drought stress in the brassica species. Int. J. Mol. Sci. 25, 3306 (2024).
Takahashi, F., Kuromori, T., Urano, K., Yamaguchi-Shinozaki, K. & Shinozaki, K. Drought stress responses and resistance in plants: from cellular responses to long-distance intercellular communication. Front. Plant. Sci. 11, 556972 (2020).
Lee, K., An, S. K., Ku, K. M. & Kim, J. The optimum substrate moisture level to enhance the growth and quality of arugula (Eruca sativa). Horticulturae 10, 483 (2024).
Pei, Y. Y., Lei, L., Fan, X. W. & Li, Y. Z. Effects of high air temperature, drought, and both combinations on maize: A case study. Plant Sci. 327, 111543 (2023).
Seleiman, M. F. et al. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10, 259 (2021).
Fila, G., Zeinalipour, N., Badeck, F. W., Delshad, M. & Ghashghaie, J. Application of water-saving treatments reveals different adaptation strategies in three Iranian melon genotypes. Sci. Hortic. 256, 108518 (2019).
Ghosh, U. K., Islam, M. N., Siddiqui, M. N., Cao, X. & Khan, M. A. R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant. Biol. 24, 227–239 (2022).
Biesaga-Kos´ cielniak, J. & Filek, M. Occurrence and physiology of Zearalenone as a new plant hormone. Sociology Org. Farming Clim. Change Soil. Science 419–435 (2010).
Biesaga-Kościelniak, J. Zearalenone as a new hypothetical regulator of plant growth and development. Monograph Inst. Plant. Physiology 10 (2001).
Biesaga-Kościelniak, J., Marcińska, I., Wędzony, M. & Kościelniak, J. Effect of Zearalenone treatment on the production of wheat haploids via the maize pollination system. Plant. Cell. Rep. 21, 1035–1039 (2003).
Weigt, D. et al. Effect of Zearalenone and hormone regulators on microspore embryogenesis in anther culture of wheat. Plants 8, 487 (2019).
Kościelniak, J., Biesaga-Kościelniak, J., Janeczko, A., Filek, W. & Kalaji, H. M. Can the Giberella Zeae toxin Zearalenone affect the photosynthetic productivity and increase yield formation in spring wheat and soybean plants? Photosynthetica 47, 586–594 (2009).
Dziurka, M., Maksymowicz, A., Ostrowska, A. & Biesaga-Kościelniak, J. The interaction effect of drought and exogenous application of Zearalenone on the physiological, biochemical parameters and yield of legumes. J Plant. Growth Regul 1–12 (2020).
Płażek, A. et al. Effects of Zearalenone and 24-epibrassinolide on the salt tolerance of selected monocotyledonous crop plants. J. Appl. Bot. Food Qual. 90, 280–287 (2017).
Marchiol, L. et al. Changes in physiological and agronomical parameters of barley (Hordeum vulgare) exposed to cerium and titanium dioxide nanoparticles. Int. J. Environ. Res. Public. Health. 13, 332 (2016).
Astaneh, N., Bazrafshan, F., Zare, M., Amiri, B. & Bahrani, A. Nano-fertilizer prevents environmental pollution and improves physiological traits of wheat grown under drought stress conditions. Scientia Agropecuaria. 12, 41–47 (2021).
Zulfiqar, F., Navarro, M., Ashraf, M., Akram, N. A. & Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: advantages and limitations. Plant Sci. 289, 110270 (2019).
Mahmoud, A. W. M., Rashad, H. M., Esmail, S. E. A., Alsamadany, H. & Abdeldaym, E. A. Application of silicon, zinc, and zeolite nanoparticles—A tool to enhance drought stress tolerance in coriander plants for better growth performance and productivity. Plants 12, 2838 (2023).
Zeinalipour, N. & Saadati, S. Physiological and biochemical response of strawberry cv. diamond to nano zeolite soil application and cinnamic acid foliar application. Sci. Rep. 14, 28908 (2024).
Rahmani, R., Khalesro, S., Heidari, G. & Mokhatssi-Bidgoli, A. Vermicompost and zeolite improve yield, nutrient uptake, essential and fixed oil production, and composition of Nigella sativa L. Front. Sustain. Food Syst. 7, 1214691 (2023).
Mondal, M. et al. Zeolites enhance soil health, crop productivity and environmental safety. Agronomy 11, 448 (2021).
Singh, D., Sharma, A., Verma, S. K., Pandey, H. & Pandey, M. Impact of nanoparticles on plant physiology, nutrition, and toxicity: A short review. Next Nanatechnol. 6, 100081 (2024).
Priyanka, T. S., Kerketta, A., Topno, S. E., Mohiddin, S. G. & Tripathi, P. Effect of nano zeolite, nano micronutrients and biocapsules on plant growth, head yield and quality of broccoli (Brassica Oleracea var Italica). Int. J. Environ. Clim. Change. 12, 58–65 (2022).
Mahmoudi, M. The effect of nanozeolite on morphological and physiological properties of Rosa damascene. J. Med. Plants Biotechnol. 7, 19–35 (2022).
Rasheed, A. et al. The role of nanoparticles in plant biochemical, physiological, and molecular responses under drought stress: A review. Front. Plant. Sci. 13, 976179 (2022).
Chandrashekar, H. K. et al. Nanoparticle-mediated amelioration of drought stress in plants: a systematic review. 3 Biotech. 13, 336 (2023).
El Moll, A. Elsevier,. Water resources and climate change: regional, national and international perspective. in Sustainable and Circular Management of Resources and Waste Towards a Green Deal 309–336 (2023).
Farooq, M., Hussain, M., Wahid, A. & Siddique, K. H. M. Drought stress in plants: an overview. Plant responses to drought stress: From morphological to molecular features 1–33 (2012).
Zaib, M. et al. Innovative approaches utilizing Plant-Based techniques for soil conservation: an In-depth review. Int. Res. J. Educ. Technol. 5, 319–330 (2023).
Wang, B. et al. Physiological mechanism of drought-resistant rice coping with drought stress. J Plant. Growth Regul 1–14 (2022).
Yang, X. et al. Effects of drought stress at the booting stage on leaf physiological characteristics and yield of rice. Plants 13, 3464 (2024).
Kumar, V. et al. Combined supplementation of selenium and silica boosts growth and yield of rice (Oryza sativa L.) by stimulating photosynthetic efficiency and nutrient uptake. Physiology Mol. Biology Plants 1–21 (2025).
Maxwell, K. & Johnson, G. N. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 51, 659–668 (2000).
Jat, M., Ray, M., Ahmad, M. A. & Prakash, P. Unravelling the photosynthetic dynamics and fluorescence parameters under ameliorative effects of 24-epibrassinolide in wheat (Triticum aestivum L.) grown under heat stress regime. Sci. Rep. 14, 30745 (2024).
Kumar, S., Kaur, R., Kaushik, R., Kaur, S. & Vats, A. Exploring the potential of zeolites as a soil amendment: A review. ~ 237 ~ Int. J. Res. Agron. 8, 237–244 (2025).
Jakkula, V. S., Wani, S. P. & Zeolites Potential soil amendments for improving nutrient and water use efficiency and agriculture productivity. (2018).
Kalaji, H. M. et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth Res. 136, 329–343 (2018).
Smethurst, C. F., Garnett, T. & Shabala, S. Nutritional and chlorophyll fluorescence responses of Lucerne (Medicago sativa) to waterlogging and subsequent recovery. Plant. Soil. 270, 31–45 (2005).
Ibrahim, H. M. & Alghamdi, A. G. Effect of the particle size of clinoptilolite zeolite on water content and soil water storage in a loamy sand soil. Water (Basel). 13, 607 (2021).
Shamili, M., Dehghanpour, S. & Atrash, S. Zeolite alleviates defense responses in drought stressed Carrot (Daucus Carota L). J. Plant. Process. Function. 9, 27–36 (2020).
De Smedt, C., Steppe, K. & Spanoghe, P. Beneficial effects of zeolites on plant photosynthesis. Adv. Mater. Sci. 2, 1–11 (2017).
Zheng, J. et al. Zeolite enhances phosphorus accumulation, translocation, and partitioning in rice under alternate wetting and drying. Field Crops Res. 286, 108632 (2022).
De Smedt, C., Someus, E. & Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag Sci. 71, 1355–1367 (2015).
Flexas, J. et al. Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant. Cell. Environ. 39, 965–982 (2016).
Xu, Z., Jiang, Y., Jia, B. & Zhou, G. Elevated-CO2 response of stomata and its dependence on environmental factors. Front. Plant. Sci. 7, 657 (2016).
Field, C. B., Jackson, R. B. & Mooney, H. A. Stomatal responses to increased CO2: implications from the plant to the global scale. Plant. Cell. Environ. 18, 1214–1225 (1995).
Rahmani, R., Khalesro, S., Heidari, G. & Mokhtassi-Bidgoli, A. Vermicompost and zeolite improve yield, nutrient uptake, essential and fixed oil production, and composition of Nigella sativa L. Front. Sustain. Food Syst. 7, 1214691 (2023).
Chen, Y., Zhang, J. H., Chen, M. X., Zhu, F. Y. & Song, T. Optimizing water conservation and utilization with a regulated deficit irrigation strategy in Woody crops: A review. Agric. Water Manag. 289, 108523 (2023).
Yang, B. et al. Regulated deficit irrigation: an effective way to solve the shortage of agricultural water for horticulture. Stress Biology. 2, 28 (2022).
Al-Busaidi, A., Yamamoto, T. & Tanigawa, T. Effect of zeolite and irrigation frequency on barley using Sub-Surface dripper in hot environment. Journal King Abdulaziz University: Meteorol. Environ. & Arid Land. Agric. Sciences 22, (2011).
Ghodsi, M. H., Esfahani, M., Tehrani, M. M. & Aalami, A. Effect of fertilizer management and the application of zeolite on agronomic traits and grain yield of maize (Zea Mays L.) hybrids under deficit irrigation conditions. Iran. Agricultural Res. 39, 87–98 (2020).
Bahador, M., Tadayon, M. R., Soureshjani, K., Ghaffari, H. & H. & Radiation and water use efficiencies of mycorrhizal inoculated hemp under water-Deficit stress. J. Soil. Sci. Plant. Nutr. 23, 2202–2214 (2023).
Zahedi, H., Shirani-Rad, A. H. & Tohidi-Moghadam, H. R. Zeolite and selenium application and their effects on production and physiological attributes of Canola cultivars under water stress. Agrociencia 46, 489–497 (2012).
Huang, G. et al. Adaptation to low nitrogen and salt stresses in the desert Poplar by effective regulation of nitrogen assimilation and ion balance. Plant Physiol. Biochem. 193, 14–24 (2022).
Acosta-Motos, J. R. et al. Plant responses to salt stress: adaptive mechanisms. Agron. 2017. 7, 18 (2017).
Núñez-Gómez, D. et al. Evaluation of agricultural Soil-Improving zeolite for improving irrigation water quality. Appl. Sci. 14, 418 (2024).
Cadar, O. et al. Zeolites reduce the transfer of potentially toxic elements from soil to leafy vegetables. Materials 15, 5657 (2022).
Javaid, A., Munir, N., Abideen, Z., Siddiqui, Z. S. & Yong, J. W. H. The role of natural and synthetic zeolites as soil amendments for mitigating the harmful impacts of abiotic stresses to improve agricultural resilience. Plant Stress 100627 (2024).
Szatanik-Kloc, A., Szerement, J., Adamczuk, A. & Józefaciuk, G. Effect of low zeolite doses on plants and soil physicochemical properties. Materials 14, 2617 (2021).
Méndez Argüello, B. et al. Water holding capacity of substrates containing zeolite and its effect on growth, biomass production and chlorophyll content of solanum lycopersicum mill. Nova Scientia. 10, 45–60 (2018).
Taghdisi Heydarian, S. Z., Khorassani, R. & Emami, H. The effect of zeolite, manure and vermicompost on growth and micronutrients uptake by corn. Water Soil. 32, 763–778 (2018).
Domenico, P. Particle films: chabazitic zeolites with added microorganisms in the protection and growth of tomato plants (Lycopersicon esculentum L). GSC Adv. Res. Reviews. 4, 1–8 (2020).
Liang, X., Zhang, L., Natarajan, S. K. & Becker, D. F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 19, 998–1011 (2013).
Shahbaz, A. K. et al. Effects of Biochar and zeolite soil amendments with foliar proline spray on nickel immobilization, nutritional quality and nickel concentrations in wheat. Ecotoxicol. Environ. Saf. 173, 182–191 (2019).
Kalita, B., Bora, S. S. & Gogoi, B. Zeolite: a soil conditioner. Int. J. Curr. Microbiol. Appl. Sci. 9, 1184–1206 (2020).
Cataldo, E. et al. Application of zeolites in agriculture and other potential uses: A review. Agronomy 11, 1547 (2021).
Acar, O., Türkan, I. & Özdemir, F. Superoxide dismutase and peroxidase activities in drought sensitive and resistant barley (Hordeum vulgare L.) varieties. Acta Physiol. Plant. 23, 351–356 (2001).
Qin, Y. L. et al. Effect of nano zeolite on growth, activity of antioxidant enzyme, and chemical fractions and concentration of cd in Chinese cabbage. Huan Jing Ke Xue. 38, 1189–1200 (2017).
Tominaga, J., Shimada, H. & Kawamitsu, Y. Direct measurement of intercellular CO2 concentration in a gas-exchange system resolves overestimation using the standard method. J. Exp. Bot. 69, 1981–1991 (2018).
Guo, R., Sun, S. & Liu, B. Difference in leaf water use efficiency/photosynthetic nitrogen use efficiency of Bt-cotton and its conventional peer. Sci. Rep. 6, 33539 (2016).
Silva, M. A., Sharma, V., Jifon, J. L. & Silva, J. A. G. da. Assessment of chlorophyll and leaf relative water content as indicators of drought tolerance on sugarcane initial growth. (2010).
Scholander, P. F., Bradstreet, E. D., Hemmingsen, E. A. & Hammel, H. T. Sap pressure in vascular plants: negative hydrostatic pressure can be measured in plants. Sci. (1979). 148, 339–346 (1965).
García-Sánchez, F. & Syvertsen, J. P. Salinity tolerance of Cleopatra Mandarin and Carrizo Citrange citrus rootstock seedlings is affected by CO2 enrichment during growth. J. Am. Soc. Hortic. Sci. 131, 24–31 (2006).
Hatsugai, N. & Katagiri, F. Quantification of Plant Cell Death by Electrolyte Leakage Assay. Bio-protocol, 8 (5), e2758. Preprint at (2018).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant. Soil. 39, 205–207 (1973).
Sairam, R. K., Rao, K. V. & Srivastava, G. C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 163, 1037–1046 (2002).
Author information
Authors and Affiliations
Contributions
N.Z. Supervision, designed the research, carried out the experiment. F.G. Methodology, Data curation. S.S. Formal analysis, Writing – review & editing. M.J.A. Supervision. M.S. Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
We confirm that all the experimental research and field studies on plants (either cultivated or wild), including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation. All of the material is owned by the authors and/or no permissions are required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeinalipour, N., Galehdari, F., Saadati, S. et al. Zearalenone and nanozeolite improve Arugula (Eruca sativa) drought tolerance by enhancing photosynthesis and water relations. Sci Rep 15, 28770 (2025). https://doi.org/10.1038/s41598-025-14765-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14765-8