Abstract
Legumes, being richer in protein, dietary fiber, vitamins, and minerals, offer a promising ingredient for enhancing the nutritional quality of baked products. This study examined the effect of germinated chickpea powder (GCPP) at different supplements of 10%, 20%, 30%, and 40% combined with turmeric rhizome powder (TRP) and carrot powder (CP), as substitutes for extracted wheat flour in biscuit formulation. The study prepared GCPP, added it to TRP and CP as substitutes for 72% extraction wheat flour (WF) in biscuit formulation, highlighting their nutraceutical properties. The composition of the biscuits was altered according to the specified ratios: Blends of 10%, 20%, 30%, and 40% (w/w) of GCPP/WF, with fixed additions of 5% of CP and 1% of TRP for all samples except the control (100% WF). A farinograph was used to record the dough’s rheological characteristics, and biscuits’ instrumental quality and nutritional value were recorded with assessment of sensory qualities by nutritionists from the Agricultural Research Center. Farinograph analysis shows significant increases (P ≤ 0.05) in water absorption, dough stability, dough development time, and softening degree. Based on physicochemical analysis, higher GCPP concentrations in the flour blends led to higher levels of protein, ash, fiber, and crude fiber contents, but with lower carbohydrates and caloric content. In addition, when GCPP, CP, and TRP were added to flour blends and biscuits samples, a significant increase (P ≤ 0.05) in the essential amino acid profile and biological value was revealed. The biscuits produced had a lower diameter and a lower spread ratio with increased hardness compared to purely wheat flour ones. Moreover, the total scoring of sensory evaluation was decreased. GCPF can be used as a WF substitute in bread goods that contain higher nutritional values and bioactive substances but have lower sensory appeal.
Similar content being viewed by others
Introduction
With about 800 genera and over 20,000 species, the Fabaceae family, also referred to as “legumes” or “pulses,” is one of the largest plant groups. It encompasses a broad variety of plants, including grain, pasture, and agroforestry species, and includes dicotyledonous crops, which can be either annual or perennial. In addition to being an important and sustainable source of protein, dietary fiber, and phytochemicals, legumes have a variety of functions in the fields of medicine, economics, biodiversity, and environmental stability1.
Legumes are used as nutritional supplements or as partial replacements for wheat flour in baked goods because of their chemical composition2 Due to their dietary fiber content, research has shown that pulses have several health benefits, such as a lower glycemic index that is advantageous for diabetic patients, increased feelings of fullness, and possible protection against cancer and cardiovascular diseases3,4 Legumes are typically eaten as a cooked meal or as toasted dry seeds for snacking5.
Numerous studies have also demonstrated the advantages of replacing meat with plant-based foods high in protein for the environment, animal welfare, and human health, especially in low-income nations. The consumption of plant-based proteins from legumes is increasing on a global basis. They are healthier and more sustainable than animal-based proteins6.
Chickpea (Cicer arietinum L.) is the third most important legume globally, following soybean and common bean, based on total production. It is primarily cultivated in warm climates, particularly in countries such as India, Pakistan, Iran, Ethiopia, and the Mediterranean region7 The crop mainly comes in two types: kabuli and desi. Kabuli chickpeas are known for their large, smooth, beige seeds, whereas desi chickpeas feature smaller, rough-textured seeds with a darker outer coat8 (Fig. 1).
Chickpeas: Classes, types, and varieties9.
Different chickpea cultivars possess unique physicochemical, nutritional, and functional properties. Brown chickpeas are ideal for vegan burgers, bread, and meat extenders, while beige chickpeas are better suited for other food products due to their enhanced functional properties. Both desi and kabuli varieties are rich in starch, protein, carbohydrates, lipids, fiber, flavonoids, and essential micronutrients such as zinc, iron, and manganese, and vitamin B12. These nutrients support the potential of chickpeas in managing chronic conditions such as diabetes and hyperlipidemia10.
Additionally, processing methods such as germination, cooking, or toasting can further improve protein digestibility11 The current trend is towards a diverse array of resilient and tolerant plants that thrive in challenging conditions, so they can offer superior nutritional benefits compared to conventional pulses. Kotsiou et al.12. demonstrated that incorporating chickpea flour improved the quality of bakery items such as bread. Utilizing the nutritional potential of chickpeas to create nutrient-rich cookies shows promise in promoting improved health and nutrition13.
Carrot (Daucus carota L.) is the most important crop of the Apiaceae family. It is a root vegetable that can be found all over the world. Originally utilized for medicinal purposes, carrots were later employed as food. Carrots with purple and yellow flesh were the first to be cultivated. Orange carrots became more popular in Central Europe around the 15th and 16th centuries. As more people became aware of orange carrots’ high provitamin A concentration, their popularity quickly increased14.
Carrot roots are a valuable source of essential vitamins and bioactive compounds that contribute to their nutritional quality. They are particularly rich in vitamin A (β-carotene), which plays a critical role in vision and increases immune system functions, and vitamin E, which aids cell signaling and membrane stability. Additionally, carrots provide B vitamins like thiamine, riboflavin, cobalamin, and pyridoxine, which are crucial for metabolism and brain health. Among organic acids, ascorbic acid (vitamin C) stands out for its antioxidant properties and roles in blood pressure regulation, iron absorption, and immune support15,16.
Carrots also contain benzoic and hydroxycinnamic acids, which possess antibacterial, anti-mutagenic, and anti-inflammatory benefits, and gallic acid, which inhibits mutagenesis17 Consequently, carrots can be incorporated as a functional ingredient in various products to enhance their biological and nutritional value18.
Turmeric, scientifically known as Curcuma longa, this golden spice is a perennial herb with rhizomes that has been employed in traditional medicine to address, prevent, and manage various health conditions, including cancer, diabetes, arthritis, diarrhea, inflammation, psoriasis, hepatobiliary disorders, and gastric and peptic ulcers19 In South Asia, it’s also used in beauty rituals and as an anti-inflammatory and antibacterial agent20.
Turmeric contains polyphenols, alkaloids, diterpenes, sesquiterpenes, triterpenoids, and sterols, according to chemical analysis. The most researched ingredient in turmeric is curcumin, which makes up 2–5% of the plant. Turmeric’s antibacterial, antimutagenic, anticancer, insecticidal, larvicidal, and radioprotective qualities have all been shown in laboratory experiments. Additionally, research on animals has demonstrated that turmeric is useful in reducing inflammation, atherosclerosis, cancer, diabetes, depression, and neurological illnesses21.
This innovation of using alternatives such as legume flours not only addresses the growing consumer demand for healthier snack options but also contributes to better digestive health, improved satiety, and supports public health initiatives focused on combating nutrient deficiencies.
So, the aim of the study is To study the effect of replacing refined wheat flour with 10%, 20%, 30%, and 40% germinated chickpea powder combined with turmeric and carrot powders on the functional properties, dough characteristics, quality, sensory attributes, bioactive compounds, and nutritional value of biscuits.
Materials and methods
Collection of raw materials
The chickpea was acquired from the Agriculture Research Center in Giza. Wheat flour (72% extraction) was supplied by the North Cairo Flour Mills Company. Other ingredients, including turmeric rhizome powder (Curcuma longa L), carrot (Daucus carota L.), sugar (sucrose), fresh eggs, baking powder, vanilla, skimmed milk, and butter, were purchased from local markets. All ingredients were sourced from Egypt.
Preparation of germinated chickpea flour
Approximately 2 kg of kabuli chickpeas underwent manual inspection to eliminate any impurities. Then to initiate germination, they were cleaned with distilled water, immersed for 12 h, positioned between two sheets of filter paper, and kept in a dark place at 30 °C for 48 h. The sprouted chickpeas were then dried for eight hours at 60 °C in a drying oven. A laboratory cutter mill was then used to grind the dry chickpeas into a fine powder, which was then filtered via a 60 μm mesh screen. The final powder was gathered and kept at 4 °C in an airtight container.
Preparation of carrot powder
Carrot powder was obtained by the method described by Marvin22 The carrot fruits were washed in portable water, peeled, sliced into 5–6 mm thickness; the sliced carrots were blanched for 3 min in hot water containing sodium metabisulfite to prevent discoloration. Then they were immediately cooled by exposure to air and dried in a cabinet drier at 50 °C for 12 h. The dried fruit was ground to a fine powder (model HL3294/C Phillips) and sieved with a 0.150 μm sieve and was packaged in a black polythene bag.
Determination of chemical composition
The physico-chemical characteristics of both raw and processed legumes were evaluated using AACC23 techniques. These analyses included measurements of protein concentration, fat content, dietary fiber, available carbohydrates, and mineral composition.
Preparation of biscuits
Samples of biscuits were made following the standard protocol for semi-hard sweet biscuits utilized by Bisco Misr Company in Cairo, Egypt, as detailed in Fig. 2. Based on the method described by Mohamed et al.24 this procedure incorporated certain modifications.
The biscuit manufacturing process employed the conventional creaming technique outlined by Chinma et al.25 A Kenwood mixer (HM 430) was used to combine fat and sugar until the mixture achieved a fluffy consistency. Eggs and milk were then added while continuing to mix. The dough was formed by incorporating baking powder, ground nutmeg, composite flour, carrot powder, turmeric rhizome powder, and salt into the mixture. Once the dough was removed from the bowl, it was kneaded on a flat surface to achieve a consistent texture. It was then rolled out into thin sheets using a rolling pin and cut into the preferred shapes with a cutter. The shaped pieces were arranged on a greased baking tray and baked at 180 °C for 17 min (as presented in Fig. 3). A control sample consisting of biscuits made exclusively with wheat flour was also prepared. Blends of 10%, 20%, 30%, and 40% (w/w) of GCPP/WF, with fixed additions of 5% and 1% of CP and TRP respectively, were used for all samples except the control (100% WF).
Evaluation of biscuit characteristics
Rheological properties of dough
Farinograph measurements were used to evaluate how adding WF, GCPP, CP, and TRP affected the dough’s mixing properties. The rheological characteristics of flour samples were assessed using a Brabender farinograph (mixing bowl 300 g, Brabender OHG, Duisburg, Germany HZ50), based on the AACC22 procedure 54-21.02. Water absorption, dough development time, dough stability time, softening degree, and arrival time were among the Farinograph parameters that were determined by the analysis.
Proximate composition of biscuits
The proximate composition of biscuits provides essential information about their nutritional quality, including ashes, protein, fat, fiber, and carbohydrates. Understanding these components is important for assessing the overall quality, stability, and health impact of biscuits26.
Amino acid profile of biscuit samples
Amino acids of all samples were estimated according to the method suggested by AOAC27 The calorimetric method previously published by Miller28 was used to assess the tryptophan content.
Using the formula given by Alsmeyer et al.29the computed protein efficiency ratio (C-PER) was calculated as follows: −0.4687 + 0.454 (leucine)−0.105 (tyrosine).
The biological value (BV) was calculated using the formula BV = 49.9 + 10.53 C-PER, as explained by Farag et al.30.
Physical characteristics of biscuits
The diameter (D) and thickness (T) of biscuits were measured for groups of 10 biscuits, following the procedure mentioned by AACC31 Then, the spread ratio was calculated as the ratio of diameter (D) to thickness (T). In addition, Texture profile analysis was done by A Brookfield CT3 Texture Analyzer No. M08-372-C0113 (version 2.1, 1000-gram unit). The hardness of samples was auto- recorded using TA-CT-PRO software. Following the method described by AACC22, samples underwent two compression cycles at 40% deformation with a trigger load of 5 N and a test speed of 2 mm/s. All procedures were done under ambient conditions.
Sensory evaluation of the end product of biscuits
The sensory characteristics of biscuits produced were evaluated by a panel of ten staff members from the Bread and Pastry Research Department, Agricultural Research Center, Giza. The evaluation followed the scoring scheme outlined by the AACC30, assessing the following attributes: crust color (20 points), taste (20 points), odor (20 points), general appearance (20 points), and texture (20 points), with a total possible score of 100.
Statistical analysis of the data collected
The SPSS 26.0 program32 was used to analyze the data from the experiments, which were recorded as means ± standard deviations (SD) of triplicate measurements. Analysis of variance (ANOVA) was used to compare the means between groups, and Duncan’s multiple range test was then performed to perform pairwise comparisons between the group means. The significance level was determined at a p-value of ≤ 0.05.
Results and discussion
Chemical composition of raw materials (on a dry weight basis)
Table 1 shows the results of the proximate analysis of WF, GCPP, CP, and TRP. Crude protein and ether extract contents were highest in GCPP (26.0% and 7.5%, respectively). Ash content was highest in CP (6.50%), while crude fiber content was highest in TRP (10.40%). Moreover, the available carbohydrate content and caloric value were highest in WF, at 85.10% and 414.95 kcal/100 g, respectively. These findings are in line with Saeed et al.33.
As regard mineral and vitamin composition of WF, GCPP, CP, and TRP, it was found that Potassium (K), phosphorus (P), magnesium (Mg), iron (Fe), and manganese (Mn) were highest in TRP, whereas calcium (Ca), sodium (Na), and zinc (Zn) were most abundant in CP. Additionally, CP exhibited the highest levels of ascorbic acid and β-carotene, at 45.5 mg and 120 µg, respectively.
Rheological characteristics of dough
The farinograph parameteres of WF and its blends with GCPP, CP and TRP are presented in Table 2.
It was observed that the water absorption of WF gradually increased as the level of substitution with GCPP increased. The higher fiber levels of GCPP, CP, and TRP than WF may be the cause of the dough’s increased water absorption. These results are consistent with Abd El-Moniem and Yaseen34who revealed that increasing the amount of fiber sources added to WF resulted in the produced dough absorbing more water. This may be due to fiber’s high water hydration capacity35.
The dough development time is the interval between adding water and when the dough reaches its maximum torque. The water hydrates the components of the flour during this mixing period. The current farinograph results revealed that adding GCPP, CP, and TRP lengthened the time needed for dough to develop; this could be because the plant sources delayed the hydration and development of gluten (because fibers and starches from plants “steal” water, so gluten needs more time to develop properly).
In addition, it was observed that the stability time of the control dough was 9.0 min, and that the addition of GCPP, CP, and TRP dough increased this time to approximately 10, 11.5, 12.5, and 13.0 min for B10%, B20%, B30%, and B40%, respectively. This stability time is a crucial indicator of dough strength based on the quantity and quality of dough gluten.
Proximate composition of biscuits
Table 3 shows that the replacement of WF with GCPP, CP, and TRP, with different supplemented ratios of B10%, B20%, B30%, and B40%, significantly improved the nutritional composition of the biscuits. Protein content increased from 9.97% in the control to a range from 10.21 to 13.77%, while fat content rose from 12.26% to 13.45–14.69%. Similarly, ash content increased significantly from 0.55% to 1.16–2.39%, and crude fiber content improved from 0.36% to 1.29–3.19%. In contrast, carbohydrate content decreased from 76.68% in the control to 65.96% in B40%. Although energy content was significantly higher in the control (467.52 kcal), it slightly decreased to a range of 467.21–460.57 kcal with the incorporation of GCPP, CP, and TRP.
These findings agree with Saeed et al.32who revealed that the increased contents of protein, crude fiber, ash, and fat enhance the nutritional profile of food products. Although there are numerous health benefits associated with consuming fiber, such as improved metabolic parameters, microbiota composition, and the synthesis of beneficial metabolites, Western countries continue to have low fiber intake. According to Kumar et al.36 and Sreerama et al.37 suggested that the food industry should focus on enhancing food products by increasing their fiber content, which presents an opportunity for food reformulation.
Amino acid profile of biscuits
The amino acid analysis presented in Table 4 revealed that biscuits supplemented with GCPP, CP, and TRP (B10%, B20%, B30%, B40%) had significantly (p ≤ 0.05) higher essential amino acid content compared to the control (WF-only biscuits), while the control sample contained higher levels of non-essential amino acids. The table also shows that the C-PER and BV were higher in biscuits prepared from GCPP, CP, and TRP-WF biscuits at different supplemented ratios than in the control sample. Our results are coordinate with Šramková et al.38 who reported that wheat protein is notably low in tryptophan, threonine, and lysine, which limits its overall nutritional value.
Physical characteristics of biscuits
The dimensional characteristics of prepared biscuits with different levels of GCPP, CP, and TRP are represented in Table 5. The values of diameter and spread ratio decreased with the increased concentration of GCPP, CP, and TRP in biscuit samples.
The diameter of biscuits prepared with GCPP, CP, and TRP ranged from 58.50 to 53.33 mm, while their spread ratio varied from 6.16 to 4.19. In comparison, the control biscuits had a larger diameter of 60.50 mm and a higher spread ratio of 7.12. This finding might be due to the increased hydrophilic sites and water-soluble protein in GCPP, which compete for the limited free water in the dough, thereby increasing its viscosity39 Moreover, the germination process leads to partial enzymatic degradation of starch, resulting in higher dextrin content in GCPP. This enhances the water absorption capacity of the dough and further reduces biscuit spread and diameter40. Similar results were also observed in biscuits made with roasted and germinated black gram flour41 and germinated lupine flour42.
The table also displays the texture of biscuit samples to measure the hardness level, i.e., breakability, which was increased with the level of GCPP, CP, and TRP replacement. The hardness value of B10%, B 20%, B 30%, and B 40% was observed as 18.90, 20.30, 22.19, and 25.33, respectively, with GCPP 40% showing the highest value of hardness (25.33). This can be explained by the lower level of gluten content due to fat replacement with CP and GCPP, causing a weak gluten network, which affected the hardness of biscuits43 and due to the higher fiber contents of GCPP, CP, and TRP44. Similar findings were reported by Saeed et al.45 when black gram flour was used as a fat replacer in biscuits.
Sensory analysis of biscuits
Table 6 depicts the effect of the incorporation of GCPP, CP, and TRP substitutes on the sensory properties of biscuit samples. The observations revealed that the biscuits prepared by substituting GCPP 10% such as control biscuits concerning color, taste, appearance, texture, and overall acceptability, while increasing the concentration of GCPP above 10% resulted in a significant decrease (P < 0.05) in those sensory scores.
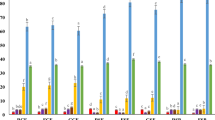
Color is a critical quality attribute in baked products, as it not only influences consumer appeal but also serves as an indicator of the formulation and processing conditions. In the current study, higher concentrations of GCPP 40% resulted in the darkest color of biscuit samples, which might be possible due to the increased rate of Millard reaction and caramelization process during baking, because of the high sugar and protein content Color was illustrated in Fig. 4 measured by colorimeter that works by measuring the light reflected or transmitted by the biscuit at different wavelengths46.
Colorimetric Analysis of Biscuit Composition Ingredients and Final Products. Each measurement was taken using a portable colorimeter to evaluate L (lightness), a (red/green axis), and b (yellow/blue axis) values, providing quantitative assessment of ingredient and product color. A: Turmeric rhizomes powder, showing high b value indicating intense yellow pigmentation. B: Germinated chickpea powder, with the highest L value indicating lightness.C: Carrot powder, with moderate a (redness) and b (yellowness) values, contributing to the orange tone. D: Wheat flour, showing low a and b values and high lightness (L), as the base flour.E: Control biscuit (100% wheat flour), exhibiting a pale color profile with high L and low and high b values. F: Biscuit sample B10 (10% composite flour), with slightly reduced L and increased a and b value, indicating yellow color development. G: Biscuit sample B20 (20% composite flour), showing further reduction in L and increased a and b values, indicating enhanced color intensity. H: Biscuit sample B30 (30% composite flour), displaying reduced L and elevated a and more elevated b values, contributing to a deeper orange-brown tone. I: Biscuit sample B40 (40% composite flour), showing the lowest L, indicating the darkest and most intense coloration among all formulations.
Biscuit samples prepared from GCPP 40% showed the lowest texture score of 15.80; the texture was affected by the level of fat and increased concentration of starch and non-starch compounds, i.e., fibers and proteins, and their resulting interactions with each other, which developed firm texture47,48. The color and appearance of the final product of different biscuits were illustrated in Fig. 5.
Images of the Final Biscuit Product vs. the control. Control biscuit:100% wheat flour. B10: 10% GCPP, 84% wheat flour, 5% carrot powder (CP), and 1% turmeric powder (TRP), B20 : 20% GCPP, 74% wheat flour, 5% CP, and 1% TRP, B30: 30% GCPP, 64% wheat flour, 5% CP, and 1% TRP, B40: 40% GCPP, 54% wheat flour, 5% CP, and 1% TRP.
Conclusion
This study’s findings revealed that biscuits prepared from different proportions of germinated chickpea powder combined with carrot powder and turmeric rhizome powder, compared with biscuits made from wheat flour only, had a dough with higher water absorption, longer development, and stability time. The produced biscuits had higher crude protein, fat content, and crude fiber, while having a lower carbohydrate and caloric content. These biscuits were also rich in essential amino acids, with a higher computed protein efficiency ratio and biological value. But the overall sensory characteristics of these biscuits had lower scores comparable to those made with wheat flour.
Recommendations
Considering the current findings, it is possible to produce high-quality baked goods using ingredients such as germinated chickpea powder, turmeric rhizome powder, and carrot powder. While the caloric difference between the control biscuit and the produced enriched biscuits is minimal, the enhanced nutritional and bioactive properties make these products beneficial as nutrient-dense snacks, especially for individuals at risk of malnutrition. Moreover, these biscuits may also serve as a healthy snack option for patients with obesity when consumed in a reasonable quantity. However, incorporating these functional ingredients may impact sensory attributes. Therefore, to improve overall acceptability, it is recommended to optimize their levels, including moisture-enhancing and flavor-masking agents, and adjust baking conditions to achieve desirable texture and color.
Data availability
Data is provided within the manuscript.
References
Ranamukhaarachchi, S. & Nanayakkara, D. Legumes: Cultivation, Uses, and Benefits.. IntechOpen. https://doi.org/10.5772/intechopen.1009148 (2025).
Duodu, K. G. & Minnaar, A. Legume composite flours and baked goods: Nutritional, functional, sensory, and phytochemical qualities. In Advances in Food and Nutrition Research. 61, 147–184 (2011).
Chillo, S., Laverse, J., Falcone, P. M., Protopapa, A. & Del Nobile, M. A. Influence of addition of buckwheat flour and durum wheat Bran on spaghetti quality. J. Cereal Sci. 47 (2), 144–152. https://doi.org/10.1016/j.jcs.2007.03.004 (2008).
Goni, I. & Valentin-Gamazo, C. Chickpea flour ingredient slows glycemic response to pasta in healthy volunteers. Food Chem. 81, 511–515 (2003).
Rachwa-Rosiak, D., Nebesny, E. & Budryn, G. Chickpeas composition, nutritional value, health benefits, application to bread and snacks: a review. Crit. Rev. Food Sci. Nutr. 55, 11371145 (2015).
Hayes, M., Mullen, A. M., Fenelon, M. & Tiwari, B. Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Food Secur. 6, 53. https://doi.org/10.3390/foods6070053 (2017).
FAOSTAT DATA. Agricultural production and production indices data: crop primary. http://faostat3.fao.org/home/E (2013).
Mohammadi, K. Nutritional composition of Iranian desi and Kabuli Chickpea (Cicer arietinum L.) cultivars in autumn sowing. Int. J. Agricultural Biosystems Eng. 9, 514–517. https://doi.org/10.5281/zenodo.1106401 (2015).
Canadian Grain Commission. Retrieved from https://www.grainscanada.gc.ca/en/grain-quality/official-grain-grading-guide/22-chickpeas/classes-types-varieties.html (1 August 2021).
Summo, C. et al. Data on the chemical composition, bioactive compounds, fatty acid composition, physico-chemical and functional properties of a global Chickpea collection. Data Brief. 27, 104612. https://doi.org/10.1016/j.dib.2019.104612 (2019).
Xu, Y. X., Thomas, M. & Bhardwaj, H. L. Chemical composition, functional properties and microstructural characteristics of three Kabuli Chickpea (Cicer arietinum L.) as affected by different cooking methods. Int. J. Food Sci. Technol. 49, 12151223 (2014).
Kotsiou, K., Sacharidis, D. D., Matsakidou, A., Boliaderis, C. G. & Lazaridou, A. Physicochemical and functional aspects of composite wheat-roasted chickpea flours in relation to dough rheology, bread quality and staling phenomena. Food Hydrocoll. 124, 107322 (2021).
Kaur, R. & Prasad, K. Technological, processing and nutritional aspects of Chickpea (Cicer arietinum) - a review. Trends Food Sci. Technol. 109, 448–463. https://doi.org/10.1016/j. tifs.2021.01.044 (2021).
Simon, P. W. Domestication, historical development and modern breeding of Carrot. Plant. Breed. Reviews. 19, 157–190 (2000).
Luby, C. H., Maeda, H. A. & Goldman, I. L. Genetic and phenological Var.ation of tocochromanol (Vitamin E) content in wild (Daucus Carota L. Var. Carota) and domesticated Carrot (D. Carota L. Var. sativa). Hortic. Res. 1, 15 (2014).
Yusuf, E., Tkacz, K., Turkiewicz, I. P., Wojdyło, A. & Nowicka, P. Analysis of chemical compounds’ content in different varieties of carrots, including qualification and quantification of sugars, organic acids, minerals, and bioactive compounds by UPLC. Eur. Food Res. Technol. 247, 3053–3062 (2021).
Mandrich, L., Esposito, A. V., Costa, S. & Caputo, E. Chemical composition, functional and anticancer properties of Carrot. Molecules 28(20). https://doi.org/10.3390/molecules28207161 (2023).
Ergun, M. Evaluating Carrot as a functional food. Middle East. J. Sci. 4, 113–119. https://doi.org/10.23884/mejs.2018.4.2.07 (2018).
Iweala, E. J. et al. Curcuma longa (Turmeric): ethnomedicinal uses, phytochemistry, Pharmacological activities and toxicity profiles—A review. Pharmacol. Research-Modern Chin. Med. 6, 100222 (2023).
Prasad, S. & Aggarwal, B. B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Benzie, I. F. F. & Wachtel-Galor S. eds. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; Ch. 13 https://www.ncbi.nlm.nih.gov/books/NBK92752/ (2011).
Jyotirmayee, B. & Mahalik, G. A review on selected Pharmacological activities of Curcuma longa L. Int. J. Food Prop. 25 (1), 1377–1398 (2022).
Marvin, S. Processing of dried carrots and Carrotpowder. Food Recipe Net. 14–20 (2009).
AACC. American Association of Cereal Chemists. Approved Methods of the A.A.C.C. Published by the American Association of Cereal Chemists, 10th Ed., St. Paul, MN. USA. (2000).
Mohamed, H. A., Elsoukkary, M. M., Doweidar, M. M. & Atia, A. A. Preparation, characterizations and health effects of functional biscuits containing iso flavones. Minufiya J. Agric. Res. 2 (29), 425–434 (2004).
Chinma, C. E. et al. Physicochemical and sensory properties and in-vitro digestibility of biscuits made from blends of tigernut (Cyperus esculentus) and pigeon pea (Cajanus cajan). Nigerian J. Nutritional Sci. 32, 55–62 (2011).
Manley, D. Manley’s Technology of Biscuits, Crackers and Cookies 4th edn. (Woodhead Publishing, 2011).
AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists. 18th edition, Washington DC, USA. (2005).
Miller, E. L. Determination of the Tryptophan content of feeding stuffs with particular reference to cereals. J. Sci. Food Agric. 18 (9), 381–387 (1967).
Alsmeyer, R. H., Cuningham, A. E. & Happich, M. L. Equations predict PER from amino acid analysis. Food Tech. 28 (7), 3440 (1974).
Farag, S. A., El-Shirbeeny, A. & Nassef, A. E. Physicochemical studies for Preparing quick-cooking rice by using gamma irradiation. Annals Agric. Sci. Moshtohor. 34, 641–652 (1996).
AACC. Approved Method of American Association of Cereal Chemists. Approved Methods the A.A.C.C. published by the American Association of Cereal Chemists 13th edn. (Inc, 2002).
(IBM Corp. IBM SPSS Statistics for Windows, Version 26.0. IBM Corp.) (2019).
Saeed, S. M. G. et al. Techno-functional, antioxidants, microstructural, and sensory characteristics of biscuits as affected by fat replacer using roasted and germinated Chickpea (Cicer arietinum L). Int. J. Food Prop. 26 (1), 2055–2077 (2023).
Abd El-Moniem, G. M. & Yaseen, A. A. High dietary fibre cookies from several sources of Bran or husk. Egypt. J. Food Sci. 21 (2), 157170 (1993).
Chen, H., Rubenthaler, G. L. & Schamus, E. C. Effect of Apple fiber and cellulose on the physical properties of wheat flour. J. Food Sci. 53 (1), 304–306 (1988).
Kumar, Y., Sharanagat, V. S., Singh, L. & Mani, S. Effect of germination and roasting on the proximate composition, total phenolics, and functional properties of black Chickpea (Cicer Arietinum). Legume Sci. 2 (1), e20. https://doi.org/10.1002/leg3.20 (2020).
Sreerama, Y. N., Sashikala, V. B., Pratape, V. M. & Singh, V. Nutrients and Antinutrients in Cowpea and Horse Gram Flours in Comparison to Chickpea Flour: Evaluation of Their FlourFunctionality. Food. Chem 131(2), 462–468. https://doi.org/10.1016/j.foodchem.2011.09.008 (2012).
Šramková, Z., Gregová, E. & Šturdík, E. Chemical composition and nutritional quality of wheat grain. Acta Chim. Slovaca. 2 (1), 115–138 (2009).
Suriya, M., Rajput, R., Reddy, C. K., Haripriya, S. & Bashir, M. Functional and Physicochemical Characteristics of Cookies Prepared from Amorphophallus Paeoniifolius Flour. J. Food Sci. Tech 54(7), 156–2165. https://doi.org/10.1007/s13197-017-2656-y (2017).
Bolarinwa, I., Lim, P. & Muhammad, K. Quality ofGluten-Free cookies from germinated brown rice flour. Food Resh. 3 (3), 199–207. https://doi.org/10.26656/fr.2017.3(3).228 (2019).
Patel, M. & Rao, G. V. Effect of untreated, roasted and germinated black gram (Phaseolus mungo) flours on the Physico-Chemical and biscuit (Cookie) making characteristics of soft wheat flour. J. Cereal Sci. 22 (3), 285–291. https://doi.org/10.1006/jcrs.1995.0065 (1995).
Obeidat, B. A., Abdul-Hussain, S. S. & Al Omari, D. Z. Effect of addition of germinated lupin flour on the physiochemical and organoleptic properties of cookies. J. Food Process. Preser 37(5), 637–643 (2013).
Saeed, S. M. G. et al. Evaluation of the potential of Lotus root (Nelumbo nucifera) flour as a fat mimetic in biscuits with improved functional and nutritional properties. CYTA J. Food. 18 (1), 624–634. https://doi.org/10.1080/19476337.2020.1812727 (2020a).
Saeed, S. M. G., Ayesha, R., Ali, S. A., Ali, R. & Ahmed, R. Lotus Root (Nelumbo Nucifera Gaertn) Flour a Novel Ingredient for the Formulation of Traditional Unleavened Flatbread: Rheological, Physical and Nutritional Characteristics, and Sensory Attributes. J. Food Process Preser. https://doi.org/10.1111/jfpp.16078 (2021).
Saeed, S. M. G. et al. Utilization of Vigna Mungo Flour as Fat Mimetic in Biscuits: Its Impact on Antioxidant Profile, Polyphenolic Content, Storage Stability, and Quality Attributes. Legume. Sci. https://doi.org/10.1002/leg3.58. (2020).
Pathare, P. B., Opara, U. L. & Al-Said, F. A. J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 6, 36–60. https://doi.org/10.1007/s11947-012-0867-9 (2013).
Saeed, S. M. G., Ali, S. A., Faheem, K., Ali, R. & Giuffrè, A. M. The Impact of Innovative Plant Sources (Cordia Myxa L. Fruit (Assyrian Plum) and Phoenix Dactylifera L. Biowaste (Date Pit)) on the Physicochemical, Microstructural, Nutritional, and Sensorial Properties of Gluten-Free Biscuits. Micro struct. Nutr. Sens. Properties Gluten-Free Biscuits. Foods 11(15), 2346 https://doi.org/10.3390/foods11152346 (2022).
Ali, S. A. et al. Functionalization of pre-gelatinized Urad bean fermented by Saccharomyces cerevisiae MK-157 as a fat replacer and its impact on physico-chemical, micromorphology, nutritional and sensory characteristics of biscuits. Arab. J. Chem. 16 (9), 105029. https://doi.org/10.1016/j.arabjc.2023.10502 (2023).
Acknowledgements
The authors are thankful to the Deanship of Graduate Studies and Scientific Research at University of Bisha for supporting this work through the Fast-Track Research Support Program.
Author information
Authors and Affiliations
Contributions
MAA, GSE, AMH, and RAS”: contributed equally to this article as follows conception, design of the work, analysis, method and statistical analysis, interpretation of data, have drafted the work and substantively revised it. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Ethics approval and consent to participate
This study did not involve the recruitment of human or animal subjects.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahmed, M.A., El-Hadidy, G.S., Hamouda, A.M. et al. Preparation of high nutritional value biscuits from germinated chickpea, carrot, and turmeric rhizomes powder. Sci Rep 15, 34496 (2025). https://doi.org/10.1038/s41598-025-14810-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14810-6