Abstract
The disruption of cerebral cellular energy metabolism represents the initial phase in the pathogenesis of neonatal hypoxic-ischemic encephalopathy (HIE). This study aimed to investigate the significance of glycemic variability (GV) and lactate (LAC) metabolic levels for early assessment of HIE. A retrospective study was conducted on asphyxiated neonates admitted to our hospital from January 2018 to January 2024. Neonates ultimately diagnosed with HIE were categorized into the HIE group, while those excluded from the HIE diagnosis were allocated to the control group. GV was assessed using the difference between maximum and minimum (max-min), standard deviation (SD), and coefficient of variation (CV). Lactate clearance rate (LCR) was used as an indicator of lactate metabolism. We found that GLU CV and LCR were independent risk factors for brain injury following asphyxia. The combination of GLU CV and LCR demonstrated a sensitivity of 84.2% and specificity of 78.6% in predicting HIE, and achieved a sensitivity of 90.0% and specificity of 61.1% in predicting moderate-severe HIE. Early monitoring of GV and LAC levels can serve as valuable indicators for predicting neonatal HIE and assessing disease severity.
Similar content being viewed by others
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) is a perinatal brain injury resulting from hypoxia and ischemia affecting fetal or neonatal brain tissue due to various perinatal factors. This condition represents a prevalent form of neonatal central nervous system injury, with severe cases leading to varying degrees of long-term sequelae and, in extreme instances, neonatal mortality1,2,3,4.
The pathogenesis of HIE encompasses a multitude of intricate pathophysiological processes, with the disruption of cerebral cellular energy metabolism being the predominant factor contributing to post-hypoxic-ischemic brain injury5,6. During hypoxia-ischemia, aerobic metabolism is compromised, leading to an increase in anaerobic metabolism, elevated lactate production, and subsequent tissue acidosis7. Stress factors such as hypoxia and acidosis can also induce abnormal fluctuations in glucose levels8characterized by early stress-induced hyperglycemia followed by significant glycogen depletion at a later stage. Studies have demonstrated that stress-induced hyperglycemia is a significant risk factor for multi-organ damage9,10and neonatal hypoglycemia in the context of hypoxia can result in more severe brain injury11. Consequently, monitoring only hypoglycemia or hyperglycemia does not adequately capture the impact of glucose fluctuations on brain injury following asphyxia. In recent years, with the progressive refinement of glucose management protocols, it has become increasingly evident that controlling the magnitude of GV is more critical than managing glucose levels at isolated time points12. Elevated levels of GV and LAC have been found to be associated with brain injury in adults, as well as increased mortality in critically ill patients13,14,15,16. Consequently, it is reasonable to hypothesize that monitoring GV and LAC levels may offer a valuable reference for the early prediction and severity assessment of neonatal HIE. The aim of this study was to investigate the significance of GV and LAC metabolic levels in the early evaluation of HIE, thereby providing a reference for clinical management.
Materials and methods
Patient selection
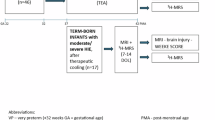
A retrospective study was conducted on 136 full-term neonates diagnosed with asphyxia who were admitted to the Neonatal Intensive Care Unit (NICU) of the Affiliated Hospital of Yangzhou University between January 2018 and January 2024. To establish the diagnosis and severity of encephalopathy, a neurological examination was performed within 6 h of birth according to the National Institute of Child Health and Human Development (NICHD) classification for modified Sarnat staging17. Simultaneously, the data from brain computed tomography (CT) or magnetic resonance imaging (MRI) as well as amplitude-integrated electroencephalogram (aEEG) were retrospectively analyzed and utilized for reference. The brain CT and MRI imaging results, as well as the aEEG findings, were evaluated by radiologists and electroencephalographers who were blinded to the study details. In accordance with the established inclusion and exclusion criteria, a total of 38 asphyxiated neonates diagnosed with HIE were categorized into the HIE group, while 56 neonates not diagnosed with HIE were allocated to the control group (Fig. 1). The HIE group was further subdivided into mild, moderate, and severe subgroups based on clinical grading. Neonates with moderate and severe HIE received therapeutic hypothermia (TH) initiated within the first 6 h of life and maintained for a duration of 72 h. This study adhered to medical ethical standards and received approval from the Institutional Review Board (IRB) of the Affiliated Hospital of Yangzhou University (No. 2022-YKL3-06-006). All methods were performed in compliance with the relevant guidelines and regulations established by the hospital’s IRB. Due to the retrospective nature of the study, the IRB of the Affiliated Hospital of Yangzhou University waived the need of obtaining informed consent. The inclusion criteria were as follows: gestational age of 37 to 42 weeks, birth weight ≥ 1800 g, admission to the NICU within 0.5 h postpartum, no prior exposure to medications that could influence blood glucose and LAC levels such as glucocorticoids, catecholamines, and maternal absence of gestational diabetes. Exclusion criteria encompassed conditions such as congenital genetic metabolic diseases, intrauterine infection, and congenital malformations.
General data
Demographic and clinical information were collected for all enrolled infants, including gender, gestational age, birth weight, mode of delivery, and perinatal medical history.
Arterial lactate data collection
Arterial blood gas analysis was conducted to measure the arterial LAC levels at admission (LACTP0) and 6 h thereafter (LACTP6). The LCR was subsequently calculated using the formula: LCR = [(LACTP0 - LACTP6) / LACTP0] × 100%18.
Glucose monitoring
All included infants underwent glucose monitoring at 3-hour intervals throughout the first day of life. If the glucose levels fell below 2.6 mmol/L or exceeded 8.3 mmol/L, the monitoring frequency was increased to every 30 min to 1 h, and appropriate interventions were initiated. Specifically, when the blood glucose level was below 2.6 mmol/L, an intravenous bolus of 10% glucose (2 ml/kg) was administered, followed by an increase in the glucose infusion rate. When the blood glucose level exceeded 8.3 mmol/L, the glucose infusion rate was decreased. Notably, none of the infants developed hyperglycemia requiring insulin therapy. Data from glucose monitoring within the first 24 h post-admission were collected. The following metrics were calculated: average glucose (GLU ave), the difference between the maximum and minimum of glucose values (GLU max-min), standard deviation of glucose (GLU SD), and coefficient of variation of glucose (GLU CV). GLU max-min, GLU SD, and GLU CV were utilized as indicators of GV19.
Statistical analysis
All statistical analyses were performed using SPSS software (version 26.0, IBM Corporation, Armonk, NY, USA). For continuous variables, the Student’s t-test was used for parametric testing and the Mann-Whitney U-test was used for nonparametric testing. Analysis of variance (ANOVA) was employed to conduct comparisons among multiple groups. For categorical variables, the chi-square test was used. Univariate analyses were performed to identify possible risk factors that might be associated with HIE individually. Multicollinearity was tested among all factors identified as possibly associated with HIE (P < 0.05). In the multivariate analysis, the variables were further entered into the logistic regression model to determine independent predictors of HIE. The performance of factors identified as significantly related to HIE development from the regression analysis was assessed by receiver operating characteristic (ROC) curve analysis and estimation of the corresponding AUC.
Results
Demographic and clinical data of the control and HIE groups
A total of 136 asphyxiated neonates were admitted during the research window, of whom 94 met the inclusion criteria and had comprehensive clinical data. Among these neonates, 38 infants diagnosed with HIE were assigned to the HIE group, while 56 infants who were not diagnosed with HIE were allocated to the control group (Fig. 1). There were no significant differences in the demographic and general clinical characteristics between the two groups (Table 1).
Arterial lactate and the development of HIE
According to the inclusion criteria, all newborns were admitted to NICU within 0.5 h after birth. Consequently, the timing of LACTP0 corresponded to within 0.5 h post-birth, while LACTP6 corresponded to approximately 6 h post-birth. TH was initiated 4.6 ± 1.1 h post-birth, thereby minimizing its influence on LACTP6 measurements. There was no significant difference in the LACTP0 levels between the two groups (P > 0.05). However, 6 h after admission, the LACTP6 levels were significantly higher, and the LCR levels were significantly lower in the HIE group compared to the control group (P < 0.05) (Table 2).
Glycemic variability and the development of HIE
There was no significant difference in the GLU ave levels between the two groups (P > 0.05). In comparison to the control group, the HIE group exhibited significantly higher values for GLU max-min, GLU SD, and GLU CV (P < 0.05) (Table 3).
Relationship between GV and LAC metabolic levels
In the HIE group, GLU max-min, GLU SD, and GLU CV exhibited significant positive correlations with LACTP6 and significant negative correlations with LCR (p < 0.05). In contrast, GV showed no significant correlation with lactate in the control group (p > 0.05) (Fig. 2).
Logistic regression analysis of risk factors for the development of HIE
Elevated GLU CV and reduced LCR were identified as independent risk factors associated with HIE, with an OR (95% CI) of 4.752 (1.249–8.667) and 4.149 (1.378–7.382), respectively (Fig. 3A).
ROC curve analysis of risk factors for the development of HIE
The ROC curve analysis showed that in predicting HIE, the combined detection of GLU CV and LCR yielded the highest AUC of 0.883, and the Youden index was the largest when the cut-off value was 0.71, with a sensitivity and specificity of 84.2% and 78.6%, respectively (Fig. 3B).
General clinical data of different clinical grading groups for HIE
The HIE group was further subdivided into mild (n = 20), moderate (n = 12), and severe (n = 6) subgroups based on clinical grading. In order to identify moderate and severe HIE and initiate brain protection strategies as soon as possible, moderate and severe HIE were combined as a moderate-severe HIE group for analysis. There were no significant differences in the demographic and general data across all clinical grading groups (P > 0.05), with the exception of the 5-minute Apgar score and the proportion of patients undergoing brain MRI scanning (p < 0.05) (Table 4).
Arterial lactate and clinical severity of HIE
There were no significant differences in the LACTP0 levels among the groups (P > 0.05). However, at 6 h post-admission, the LACTP6 levels increased, while LCR decreased as the severity of HIE worsened (P < 0.05) (Table 5).
Glycemic variability and clinical severity of HIE
There was no significant difference in the GLU ave levels among the groups (P > 0.05). However, GLU max-min, GLU SD, and GLU CV showed an increasing trend with the severity of HIE, and these differences were statistically significant (P < 0.05) (Table 6).
Logistic regression analysis of risk factors for the severity of HIE
In multiple regression analysis, GLU CV and LCR were identified as significant risk factors for moderate-severe HIE, with an OR (95% CI) of 3.718 (0.001–7.322) and 1.434 (1.001–1.945), respectively (Fig. 4A).
ROC curve analysis of risk factors for the severity of HIE
The ROC curve analysis showed that in predicting moderate-severe HIE, the combined detection of GLU CV and LCR yielded the highest AUC of 0.736, and the Youden index was the largest when the cutoff value was 0.51, with a sensitivity and specificity of 90.0% and 61.1%, respectively (Fig. 4B).
Discussion
In this study, we have demonstrated a significant association between elevated GV levels and reduced LCR levels and the development and severity of brain injury in neonates with HIE following asphyxia. Furthermore, our findings suggest that the combined detection of GV and LCR holds potential predictive value for the early identification and evaluation of HIE.
The impairment of energy metabolism in cerebral cells following hypoxic-ischemia represents the initial stage in the progression of HIE. The accumulation of LAC in tissues during anaerobic metabolism, along with fluctuations in glucose levels under stress conditions, is closely associated with brain injury. Consequently, maintaining homeostasis and stabilizing glucose levels are crucial for mitigating brain injury after hypoxic-ischemia5,6,7. Research has demonstrated that the metabolic interplay between glucose and LAC is disrupted following HIE, leading to disorders in LAC and glucose metabolism20. Both hyperglycemia and hypoglycemia are prevalent among asphyxiated neonates21. While hypoglycemia is a significant risk factor for hypoxic-ischemic brain injury22hyperglycemia and erratic glucose fluctuations also impair cerebral cell energy metabolism, contributing to neurological damage23,24. Therefore, this retrospective study aims to predict the risk of HIE and evaluate its severity by monitoring changes in LAC and glucose levels.
GV represents the magnitude of glucose fluctuation within a given time period, reflecting an unstable condition where glucose levels oscillate between maximum and minimum values. Research has demonstrated that maintaining long-term stable glucose levels can decrease the incidence of brain injury and mortality risk25,26. Currently, GV has gained widespread application in adult endocrinology research; however, its utility in predicting neonatal HIE remains unreported. In this study, we found that the levels of GLU max-min, GLU SD, and GLU CV in the HIE group were significantly higher than those in the non-HIE group (P < 0.05). It is hypothesized that asphyxia-induced elevation in stress hormone secretion stimulated increased glycogenolysis and gluconeogenesis, leading to stress hyperglycemia. If hypoxia remains uncorrected, anaerobic metabolism intensifies, resulting in glycogen depletion and subsequent hypoglycemia. The significant fluctuations in glucose caused by this neuroendocrine response are closely associated with the development of hypoxic-ischemic brain injury19. Further comparative analysis of GV indexes among neonates in different clinical grading groups revealed that GLU max-min, GLU SD, and GLU CV progressively increased with the severity of HIE (P < 0.05). This observation is consistent with previous studies27,28which have demonstrated that significant fluctuations in glucose levels, particularly extreme highs and lows, can lead to abnormal cerebral blood flow and neuronal stress damage, thereby supporting our findings.
Changes in LAC levels serve as sensitive indicators of the degree of tissue and cellular hypoxia. Research has confirmed that neonates experiencing hypoxic-ischemic events exhibit increased anaerobic metabolism, which can lead to hyperlactatemia and accumulation of LAC in the brain. This process is closely associated with the onset and progression of HIE29. We observed that at 6 h post-admission, the LAC levels in the HIE group were significantly higher compared to the control group, whereas the LCR levels were notably lower. The LAC and LCR levels at 6 h post-admission were correlated with disease severity (P < 0.05), which was consistent with prior research29.
In the metabolic processes of neonatal HIE, glucose and LAC exhibit interactive relationships that collectively influence cerebral cellular energy metabolism20. This study further investigated the correlation between GV and LAC indices. The results demonstrated that no significant correlation was observed between GV and the lactate indices in the control group (p > 0.05). In contrast, within the HIE group, GLU max-min, GLU SD, and GLU CV were positively correlated with the LAC levels and negatively correlated with LCR (p < 0.05). Although the association within the HIE group was not very pronounced, potentially due to the low proportion of neonates with severe HIE, we hypothesize that this association may become stronger as the proportion of neonates with increasing HIE severity rises. It is hypothesized that following hypoxic-ischemic injury, neurons are unable to secure a stable energy supply due to erratic fluctuations in glucose levels, and LAC can serve as an alternative energy source, which has been termed “alternative brain fuel“30. Consequently, there exists a compensatory relationship between LAC and glucose in the metabolic alterations observed in HIE.
Therefore, GV and LAC levels are intricately associated with brain injury following hypoxic-ischemic events. It is crucial to maintain the homeostasis of glucose and LAC metabolism in the clinical management of asphyxiated neonates to mitigate the risk of brain injury. Multivariate logistic regression analysis revealed that increased GLU CV (OR: 4.752, 95% CI: 1.249–8.667) and decreased LCR (OR: 4.149, 95% CI: 1.378–7.382) were independent risk factors for HIE following asphyxia. Additionally, elevated GLU CV (OR: 3.718, 95% CI: 0.001–7.322) and reduced LCR (OR: 1.434, 95% CI: 1.001–1.945) were associated with an increased risk of moderate-severe HIE. These findings suggest that higher GV and lower LCR levels may play partial roles in the development and progression of HIE. However, GLU CV for moderate-severe HIE exhibit imprecision (owing to wide 95% CI), potentially influenced by the proportion of HIE severity. Consequently, these findings should be interpreted with clinical prudence and in conjunction with neuroimaging and neuroelectrophysiological data. Further analysis of the ROC curve demonstrated that the combination of GLU CV and LCR achieved the highest diagnostic efficiency for both the occurrence and severity of HIE. Specifically, the AUC for predicting HIE was 0.883, with a sensitivity of 84.2% and a specificity of 78.6%. For moderate-severe HIE, the AUC was 0.736, with a sensitivity of 90.0% and a specificity of 61.1%.
Our study has some limitations. First it is a single center study, with a relatively small sample size that precluded more in-depth analyses. Second, given that blood glucose monitoring was performed intermittently rather than continuously, the available blood glucose data fail to comprehensively capture the trends of blood glucose fluctuations following asphyxia. Furthermore, owing to the scarcity of blood glucose data within the first six hours postpartum, it was not feasible to analyze the significance of GV during the TH therapeutic intervention window for clinical decision-making purposes. Finally, this study did not evaluate the long-term neurodevelopmental outcomes in infants with HIE.
Conclusions
Our study indicates that elevated GV and reduced LCR are indicative of cellular energy depletion and impaired homeostasis in neonatal HIE. The concurrent evaluation of GV and LCR may serve as a valuable tool for the early prediction and assessment of HIE severity. Prompt monitoring of glucose fluctuations and LAC metabolism could offer novel insights for the prevention and management of brain injury following hypoxic-ischemic events.
Data availability
The original rearch data presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
Gale, C., Statnikov, Y., Jawad, S., Uthaya, S. N. & Modi, N. Neonatal brain injuries in england: population-based incidence derived from routinely recorded clinical data held in the National neonatal research database. Arch. Dis. Child. Fetal Neonatal Ed. 103(4), F301–F306. https://doi.org/10.1136/archdischild-2017-313707 (2018).
Bruns, N. et al. Short-term outcomes of asphyxiated neonates depending on requirement for transfer in the first 24 h of life. Resuscitation 202(0), 110309. https://doi.org/10.1016/j.resuscitation.2024.110309 (2024).
Glass, H. C. et al. Predictors of death or severe impairment in neonates with Hypoxic-Ischemic encephalopathy. JAMA Netw. Open. 7(12), e2449188. https://doi.org/10.1001/jamanetworkopen.2024.49188 (2024).
Bonifacio, S. L., Liu, J., Lee, H. C., Hintz, S. R. & Profit, J. Trends in HIE and use of hypothermia in California: opportunities for improvement. Pediatrics 154(3), e2023063032. https://doi.org/10.1542/peds.2023-063032 (2024).
Yang, M., Wang, K., Liu, B., Shen, Y. & Liu, G. Hypoxic-Ischemic encephalopathy: pathogenesis and promising therapies. Mol. Neurobiol. 62(2), 2105–2122. https://doi.org/10.1007/s12035-024-04398-9 (2025).
Greco, P. et al. Pathophysiology of hypoxic-ischemic encephalopathy: a review of the past and a view on the future. Acta Neurol. Belg. 120(2), 277–288. https://doi.org/10.1007/s13760-020-01308-3 (2020).
Olofsson, P. Umbilical cord pH, blood gases, and lactate at birth: normal values, interpretation, and clinical utility. Am. J. Obstet. Gynecol. 228(0), S1222–S1240. https://doi.org/10.1016/j.ajog.2022.07.001 (2023).
Liu, C. H. et al. Effect of birth asphyxia on neonatal blood glucose during the early postnatal life: A multi-center study in Hubei province, China. Pediatr. Neonatol. 64(5), 562–569. https://doi.org/10.1016/j.pedneo.2021.11.016 (2023).
Sperry, J. L. et al. Early hyperglycemia predicts multiple organ failure and mortality but not infection. J. Trauma. 63(3), 487–493. https://doi.org/10.1097/TA.0b013e31812e51fc (2007).
Garcia Whitlock, A. E. et al. Loss of FOXO transcription factors in the liver mitigates stress-induced hyperglycemia. Mol. Metab. 51(0), 101246. https://doi.org/10.1016/j.molmet.2021.101246 (2021).
Mietzsch, U. et al. Early glycemic state and outcomes of neonates with hypoxic-Ischemic encephalopathy. Pediatrics 152(4), e2022060965. https://doi.org/10.1542/peds.2022-060965 (2023).
See, K. C. Glycemic targets in critically ill adults: A mini-review. World J. Diabetes. 12(10), 1719–1730. https://doi.org/10.4239/wjd.v12.i10.1719 (2021).
Qi, L. et al. Association of glycemic variability and prognosis in patients with traumatic brain injury: A retrospective study from the MIMIC-IV database. Diabetes Res. Clin. Pract. 217(0), 111869. https://doi.org/10.1016/j.diabres.2024.111869 (2024).
Bagshaw, S. M., Egi, M., George, C. & Bellomo, R. Early blood glucose control and mortality in critically ill patients in Australia. Crit. Care Med. 37(2), 463–470. https://doi.org/10.1097/CCM.0b013e318194b097 (2009).
Schurr, A. Lactate: A major and crucial player in normal functioning of the brain and in brain pathological conditions. J. Physiol. 586(11), 2665–2666. https://doi.org/10.1113/jphysiol.2008.155416 (2008).
Andersson, M. et al. Prehospital lactate analysis in suspected sepsis improves detection of patients with increased mortality risk: an observational study. Crit. Care. 29(1), 38. https://doi.org/10.1186/s13054-024-05225-2 (2025).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705. https://doi.org/10.1001/archneur.1976.00500100030012 (1976).
Abdelaziz, T. A., Karam, N. A., Ismail, W. I., Askary, N. M. A. & Baz, E. G. Lactate dynamics in paediatric patients with severe sepsis: insights from a prospective cohort study. BMC Pediatr. 24(1), 345. https://doi.org/10.1186/s12887-024-04809-9 (2024).
Fuentes, B. et al. Glucemie variability: prognostic impact on acute ischemic stroke and the impact of corrective treatment for huperalycemia. The GLIAS-III translational study. J. Trans. Med. 18(1), 414. https://doi.org/10.1186/s12967-020-02586-4 (2020).
Galderisi, A. et al. Glucose-to-lactate ratio and neurodevelopment in infants with hypoxic-ischemic encephalopathy: an observational study. Eur. J. Pediatr. 182(2), 837–844. https://doi.org/10.1007/s00431-022-04694-3 (2023).
Basu, S. K. et al. Hypoglycaemia and hyperglycaemia are associated with unfavourable outcome in infants with hypoxic ischaemic encephalopathy: a post hoc analysis of the CoolCap study. Arch. Dis. Child. Fetal Neonatal Ed. 101(2), F149–155. https://doi.org/10.1136/archdischild-2015-308733 (2016).
Basu, S. K., Salemi, J. L., Gunn, A. J. & Kaiser, J. R. Hyperglycaemia in infants with hypoxic-ischaemic encephalopathy is associated with improved outcomes after therapeutic hypothermia: a post hoc analysis of the CoolCap study. Arch. Dis. Child. Fetal Neonatal Ed. 102(4), F299–F306. https://doi.org/10.1136/archdischild-2016-311385 (2017).
Gao, X. et al. Hyperglycemia aggravates the cerebral ischemia injury via protein O-GlcNAcylation. J. Alzheimers Dis. 94(2), 651–668. https://doi.org/10.3233/JAD-230264 (2023).
Ali, M. A. M. et al. Glucose instability and outcomes of neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia. Brain Dev. 46(8), 262–267. https://doi.org/10.1016/j.braindev.2024.05.003 (2024).
Kramer, A. H., Roberts, D. J. & Zygun, D. A. Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit. Care. 16(5), R203. https://doi.org/10.1186/cc11812 (2012).
Panayi, A. C. et al. Effect of Liberal glucose control on critically ill patients: a systematic review and meta-analysis. BMC Endocr. Disord. 25(1), 36. https://doi.org/10.1186/s12902-025-01864-w (2025).
Lei, H. & Gruetter, R. Metabolic and perfusion responses to acute hypoglycemia in the rat cortex: A non-invasive magnetic resonance approach. J. Neurochem. 154(1), 71–83. https://doi.org/10.1111/jnc.15028 (2020).
Yang, W. et al. NLRP3 inhibitor alleviates glycemic variability-induced cognitive impairment in aged rats with type 2 diabetes mellitus. Mol. Cell. Endocrinol. 595(0), 112406. https://doi.org/10.1016/j.mce.2024.112406 (2025).
Wu, T. W. et al. Cerebral lactate concentration in neonatal hypoxic-ischemic encephalopathy: in relation to time, characteristic of injury, and serum lactate concentration. Front. Neurol. 9(0), 293. https://doi.org/10.3389/fneur.2018.00293 (2018).
Harris, D. L., Weston, P. J. & Harding, J. E. Alternative cerebral fuels in the first five days in healthy term infants: the glucose in well babies (GLOW) study. J. Pediatr. 231(0), 81–86e2. https://doi.org/10.1016/j.jpeds.2020.12.063 (2021).
Acknowledgements
We are grateful to the participants and the staff of the NICU for their support and assistance in this study.
Funding
This study was funded by Maternal and Child Health Research Project of Jiangsu Province (F202071) and Maternal and Child Health Outstanding Talent Project of Jiangsu Province (SWBFY 2021-9).
Author information
Authors and Affiliations
Contributions
LZ and CW contributed to the conception and design of the study and wrote the manuscript. QJ and LJ contributed to the design of the study and reviewed the manuscript. FW, HL and LJ corrected the draft. QJ and HL completed the statistical analysis. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics statement
This study adhered to medical ethical standards and received approval from the Institutional Review Board (IRB) of the Affiliated Hospital of Yangzhou University (No. 2022-YKL3-06-006). Due to the retrospective nature of the study, the IRB of the Affiliated Hospital of Yangzhou University waived the need of obtaining informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Wang, C., Jia, Q. et al. The significance of glycemic variability in conjunction with lactate metabolism levels in the clinical assessment of neonatal hypoxic-ischemic encephalopathy. Sci Rep 15, 29072 (2025). https://doi.org/10.1038/s41598-025-14871-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14871-7