Abstract
This work investigates the design and application of a Mo-doped hydrolyzed ZIF-8 (h-ZIF-8) composite on a ZnS substrate for the photodegradation of malachite green (MG) dye using an H2O2-assisted advanced oxidation process (AOP) under sunlight irradiation. The innovative h-ZIF-8 photocatalyst, synthesized via a simple aqueous reflux process, is more stable than standard methanol-synthesized ZIF-8, which generally disintegrates under extended water exposure. The partly hydrolyzed ZIF-8 structure, with hydroxyl groups substituting for the cleaved Zn-N bonds, allows for improved photocatalytic activity. Using Response Surface Methodology (RSM) and Central Composite Design (CCD), the best conditions for 99.14% MG degradation are as follows: 0.05 mM H2O2, 6.5 mg catalyst, 66.5 ppm MG, and 52.9 min of sunlight exposure. The experimental findings closely match RSM predictions, with 98.95% degradation achieved after 50 min. An all-solid-state Z-scheme electron transfer channel using Mo nanoparticles allows for efficient charge separation between the ZnS and h-ZIF-8 interfaces, which reduces electron-hole recombination and increases photocatalytic performance. High-resolution liquid chromatography-mass spectrometry (HR-LCMS) detects intermediate phases of MG breakdown and provides insight into the degradation route. The catalyst is resilient across several water matrices (tap, lake, and wetland water) and various coexisting inorganic (cations and anions) and organic substances, and successfully degrades many dye pollutants, including crystal violet, rhodamine B, brilliant green, Victoria blue, methylene blue, and Congo red, with over 80% efficiency. The catalyst remains 76% efficient after six cycles, demonstrating its reusability. This study demonstrates substantial progress in stabilizing ZIF-8 for solar energy conversion and water treatment applications, highlighting the potential of hydrolyzed ZIF-8 in sustainable environmental remediation.
Similar content being viewed by others
Introduction
First reported in 1995, Metal-organic frameworks (MOFs), constructed of a metal node/metal cluster and organic linkers, are considered a new class of porous materials with remarkable properties such as high porosity, wide functionality, tunable structure, surface area, and pore size, and broad applicability1. Owing to these properties, MOFs exhibit potential applications in a wide range of fields, including environmental, energy, biomedical, optoelectronics, fuel generation, and energy storage. Among them, the utilization of MOFs in aqueous applications such as wastewater treatment, water splitting, and solar-fuel generation has recently experienced a significant increase. MOFs as photocatalysts for water remediation have particularly received much research interest with the anticipation that the tunable nature of the characteristic MOF properties leads to a sustainable and novel solution to the existing challenges in wastewater remediation scenarios2. MOFs can be broadly categorized into a variety of groups, including isoreticular MOFs, Zeolitic imidazolate framework (ZIF), porous coordination network (PCN), Materials Institute Lavoisier (MIL), porous coordination polymers (PCP), and University of Oslo (UiO) MOFs. Among them, ZIF-8, a prominent member of the ZIF family, is a widely studied MOF in various applications, including photocatalysis.
ZIF-8 is a member of the zeolitic imidazole frameworks, a subclass of MOFs, which is formed via the self-assembly of tetrahedrally coordinated Zn2+ ions linked to the N-atoms of deprotonated 2-methylimidazolate anions to create a unique, highly porous sodalite (SOD) topology, isomorphic with zeolites3. Extensive studies have been steered over the years in applying ZIF-8 in varying fields, such as a membrane for gas separation, desalination, and liquid separation, and as a photocatalyst and adsorbent for metal ions and organic pollutants removal from an aqueous medium. ZIF-8s are commonly synthesized using a variety of techniques, such as in-situ precipitation, solvothermal, sonochemical, microwave-assisted, and electrochemical synthetic routes using different solvents (methanol, ammonia (aq), N, N-dimethylformamide (DMF)) at various temperature conditions varying from room temperature to up to 200 °C. Additionally, some pioneering research has been carried out to synthesize ZIF-8 in the presence of additives like sodium formate, n-butylamine, triethylamine, and polyamine, with a strategy to optimize and control the size and morphology of the ZIF-8 crystals. However, organic solvents and additives are often toxic and expensive. Moreover, organic solvents like DMF trapped inside the ZIF-8 pores are difficult to remove. Therefore, considerable work has lately focused on developing green techniques without requiring chemical solvents to synthesize ZIF-8.
Water-based fabrication of ZIF-8 at room temperature can pave the path for a more ecological and green synthesis approach. However, this method still faces significant hurdles in producing high-crystallinity pure-phase ZIF-8. Several recent studies have reported the instability of ZIF-8 in an aqueous solution. Initially, Liu et al.4 observed that water treatment at 80 °C with a low ZIF-8/water mass ratio resulted in the structural collapse of ZIF-8 and a phase change to ZnO.
Later, Zhang et al.5 found the irreversible disintegration of ZIF-8 in water at room temperature, creating an unidentified compound accompanied by reduced porosity.
While numerous studies have been conducted on the post-synthetic stability of ZIF-8 for water-based applications, a few studies have also focused on the various polymorphic structures of ZIF-8 created due to the partial collapse or hydrolysis of the ZIF framework. Polymorphism is, in fact, one of the effective strategies for engineering the pore structures of MOF materials. A large portion of prior research into ZIF-8 has concentrated on developing stable or water-resistant frameworks to tackle instability difficulties; however, the potential advantages and possible application domains of hydrolyzed MOFs have been mostly overlooked6. It is, therefore, crucially important to identify the possible performances of hydrolyzed MOFs for application purposes. Very recently, Jeong et al.7 discovered that the hydrolyzed-MOF-derived materials exhibited enhanced electrochemical performance as an electrode material for lithium storage. Similarly, Cui et al.8 reported a dramatic improvement in photocatalytic hydrogen evolution over hydrolyzed MOF-5, a zinc-based MOF. Therefore, it is desirable to examine the functions of hydrolyzed MOFs in photocatalytic reactions in an aqueous solution.
Herein, hydrolyzed ZIF-8 (h-Z8) was chosen as a representative to evaluate the possibilities of photocatalytic applications of h-Z8. The hydrolyzed ZIF-8-derived Mo@h-ZIF-8/ZnS (MZ@h-Z8) photocatalyst was fabricated via a facile reflux synthesis approach. Impressively, the molybdenum-doped h-ZIF-8 exhibited a fused crystallite topology integrated with the ZnS particles, resulting in the formation of a compact all-solid-state Z-scheme heterojunction interface that markedly reduces the recombination rate of the photogenerated charge carriers. As a result, the as-synthesized MZ@h-Z8 catalyst exhibited excellent photocatalytic activity towards the degradation of a wide range of dye molecules including malachite green, crystal violet, rhodamine B, brilliant green, Victoria blue, methylene blue, and Congo red under solar light irradiation via an H2O2-assisted advanced oxidation process (AOP), demonstrating the great potential of hydrolyzed ZIF-8 in water-based applications. In this research work, Malachite green (MG) was chosen as a model contaminant for a detailed study on the process optimization using RSM, degradation mechanism, and the effect of pH, different inorganic and organic co-existing ions, and various water matrices on the catalyst’s photoactivity. Moreover, all the photodegradation trials were conducted under natural sunlight, which is a cost-effective alternative to conventional UV and visible light sources such as halogen lamps, xenon lamps, and LED bulbs. Finally, a plausible degradation pathway and mechanism were determined using the XPS-VB analysis, radical scavenging experiments, and LC-MS analysis. In conclusion, this recent research gives fresh insights into developing highly efficient hydrolyzed MOF-based photocatalysts and simultaneous solar light harvesting for aqueous environmental remediation.
Experimental
Materials
Zinc Nitrate [Zn(NO3)2.6H2O], (SRL, 99%), sodium sulfide flakes [Na2S.9H2O] (RANKEM, 98%), Ammonium heptamolybdate [(NH4)6Mo7O24.4H2O] (SRL, 99%), Cetyltrimethylammonium bromide [CTAB] ( SRL, 99%), 2-Methylimidazole [Hmim, C4H6N2] (TCI, > 97%), Malachite green [MG] (SRL), hydrogen peroxide [H2O2] (Merck, 30% v/v), Ethanol (Sigma Aldrich, 99.9%). All the reagents were of AR grade and were used without further purification.
Synthesis of ZnS
The ZnS was fabricated using zinc nitrate and sodium sulfide as Zn and S sources by employing CTAB as a stabilizing and capping agent. Firstly, 1 g of Zn(NO3)2.6H2O was dissolved in 40 mL of distilled water, and 0.02 g of CTAB was added to this solution and magnetically stirred for 1 h at room temperature. Separately, 0.806 g of Na2S.9H2O was dissolved in a minimum quantity of distilled water and was added to the above solution dropwise under continuous stirring. The mixture was then allowed to stir for another 3 h. The resultant greyish-white precipitate was then separated by centrifugation and washed thoroughly with distilled water and ethanol several times. The prepared ZnS sample was dried at 50 °C overnight.
Synthesis of hydrolyzed ZIF-8 (h-Z8)
Typically, 1 g Zn(NO3)2.6H2O and 2.4 g hmim were dissolved separately in 50 mL distilled water. The Zn solution was then slowly added to the ligand solution under stirring to obtain a cloudy solution. The reaction mixture was then transferred to a round-bottom flask and refluxed at 90 °C for 12 h under continuous magnetic stirring. The resultant white precipitate of h-Z8 was collected by centrifugation, washed, and dried at 50 °C overnight.
Synthesis of Mo@h-ZIF-8/ZnS
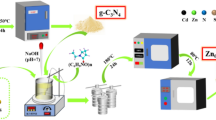
The Mo@h-Z8/ZnS composite was synthesized using a simple reflux condensation method, following a previous report with some modifications. Firstly, 200 mg of the previously prepared ZnS was dispersed in 50 mL of distilled water. To this, a 30 mL aqueous solution of 1 g Zn(NO3)2.6H2O and 3 g (NH4)6Mo7O24.4H2O was added and stirred vigorously for 1 h. Finally 30 mL of aqueous solution containing 2.4 g of the hmim ligand was added to the above mixture dropwise, and the resultant solution was refluxed at 90 °C for 12 h. After cooling naturally, the resultant off-white precipitate was collected and washed several times with distilled water and ethanol and dried at 50 °C for 24 h. The as-synthesized photocatalyst was labeled as Mo@h-ZIF-8/ZnS (MZ@h-Z8) (Fig. 1).
Material characterization
The powder X-ray diffraction (XRD) studies of the as-synthesized MZ@h-Z8 photocatalyst were performed on a Phillips X’PERT’3 pro (radiation source: Cu Kα; 2θ = 5–65° ) powder XRD diffractometer. The Fourier-transform spectroscopy (Nicolet IS50 USA) was used to evaluate the surface functional groups of the MZ@h-Z8 photocatalyst. The FEI-Quanta FEG 200 F field emission scanning electron microscope (SEM) and JEM-2100 PlusElectron high-resolution transmission electron microscope (HRTEM) investigated the morphology, elemental composition, lattice fringes, and mapping of the prepared catalyst. The optical properties and band gap of the catalyst were measured using the Agilent technologies CARY 5000 UV-vis diffuse reflectance spectrometer (UV-DRS) and F-4600 FL photoluminescence spectrometer. The X-ray photoelectron spectroscopy (XPS) of the sample was recorded on the Thermo Scientific NEXSA surface analyzer equipped with a monochromated Al kα (1486.6 eV) X-ray source. The high-resolution liquid chromatography-mass spectroscopy (HR-LCMS) data were measured on the Xevo XS QTof (Waters ACQUITY UHPLC) mass spectrometer. The absorbance studies of the degradation process were carried out on a GENESYS 10 S UV-vis spectrometer with a scan speed of 2 nm/s.
Experimental design
The influence of various reaction parameters, H2O2 dosage (A), catalyst dosage (B), dye concentration (C), and reaction time (D), on the photocatalytic performance of MZ@h-Z8 towards the degradation of MG dye was investigated using response surface methodology (RSM) with a central composite design (CCD). The experimental design was created using Design Expert Software (DOE), version 13 (https://www.statease.com/software/design-expert/). The interaction between the different parameters was studied based on the RSM, which combines both mathematical and statistical approaches9. The experimental data were adjusted to optimize photodegradation efficiency to a maximum value. The interaction between a single parameter and multiple parameters and their association with the photodegradation rate of MG dye was examined using the set of reaction conditions provided by the RSM software. An experimental design consisting of a total of 30 experimental runs (16 factor points, 8 axial points, and 6 center points) was conducted based on the following empirical model equation (Eq. 1):
Here, N is the total number of experimental runs, and k is the number of interacting parameters.
The relation between the photodegradation efficiency and the influence of various reaction parameters was predicted based on the quadratic equation (Eq. 2) given by the software as follows10:
In this equation, Xi represents the coded value of the ith parameter, while β0, βi, and βij correspond to the zero, first, and second-order coefficients, respectively.
The accuracy of the developed simulation and the statistical significance of the constant regression coefficients were assessed using analysis of variance (ANOVA). ANOVA was also employed to investigate the individual, interaction, and quadratic impacts of the process variables on the efficiency of MG degradation using MZ@h-Z8 photocatalyst. Furthermore, the coefficient of determination (R²) was compared with the adjusted R² value to assess the stability and validity of the predicted model.
Experimental photocatalytic evaluation
The photodegradation experiments using the MZ@h-Z8 photocatalyst were conducted in 100 mL beakers containing 50 mL of the MG dye solutions. All the photodegradation analyses were carried out under sunlight irradiation during the month of June 2024 (24°45’26.9” N; 92°47’32.7” E) between the time 11 am – 1 pm. The photocatalytic breakdown of the MG dye was initiated by employing the predetermined optimized values of H2O2 amount, catalyst dosage, dye concentration, and sunlight irradiation time. Typically, 0.5 mM H2O2 and 6.5 mg of the MZ@h-Z8 catalyst were added to 50 mL of 66.5 ppm dye solution. The reaction mixture was held in the dark under continuous stirring for 30 min to attain an adsorption-desorption equilibrium before irradiating with sunlight for 53 min. The progress of the degradation was recorded by measuring the absorbance of MG every 10 min using a UV-vis spectrometer.
The degradation efficiency (η) can be calculated using Eq. 3.
The pseudo-first-order reaction rate constant (k) under the optimum reaction conditions can be obtained from the slope of the ln(Co/C) vs. time graph according to Eq. 4.
where C and Co are the concentration of the MG solution at reaction time, t = t, and t = 0, respectively.
Although the current photodegradation studies focus on environmental water remediation, the experiments were conducted using deionized water. Therefore, to acquire a greater insight into the working of the prepared photocatalyst in real-life circumstances, the influence of different natural samples of water on the photocatalytic activity was also analyzed. Moreover, the MZ@h-Z8 catalyst was employed to degrade other organic dye pollutants such as Crystal Violet (CV), Rose Bengal (RB), Brilliant Green (BG), Victoria Blue (VB), Methylene Blue (MB), and Congo Red (CR). The contaminant concentrations were all maintained at 66.5 ppm with a catalyst dosage of 6.5 mg. A neutral pH (pH = 6.85) was maintained throughout all the experimental studies.
Materials characterization
Crystallographic studies
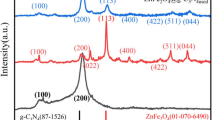
Figure 2a, depicts the findings of an X-ray diffraction (XRD) analysis performed to assess the crystal structure of the synthesized pristine ZnS, hydrolyzed ZIF-8, and MZ@h-Z8 composite. The XRD pattern of the fabricated composite confirms the presence of the h-ZIF-8 and ZnS crystalline phases and the successful formation of the Mo@h-ZIF-8/ZnS. The pristine ZnS showed characteristic peaks at 2θ values of 28.96°, 48.08°, 56.84°, and 77.88°, which correspond to the (111), (220), (311), and (331) crystalline planes, respectively. These planes can be attributed to the closed-packed cubic Sphalerite crystal system of ZnS with cell parameters a = b = c = 5.345 Å, matching with the JCPDS file no: 80 − 002011. The broadened nature of the XRD peaks is due to the nanocrystalline nature of the ZnS samples. However, a blue shift in the three peak positions was detected at 2θ values of 28.53°, 47.95°, 56.58°, and 77.23°, which can be linked with the development of the Mo-doped h-ZIF-8 on the ZnS surface. The h-ZIF-8 sample exhibited its characteristic peaks at 2θ values of 10.43°, 14.04°, 16.81°, 20.77°, 23.10°, 24.62°, 26.40°, 27.27°, 28.13°, 28.56°, 30.01°, 34.38°, and 35.33°, which were corroborated with a previous study12. The hydrolysis of the ZIF-8 occurs as a result of the partial cleavage of the Zn-N on one side of the tetrahedrally linked framework. Partial hydrolysis splits a coordination bond to the centroid Zn, allowing a hydroxyl to coordinate with Zn and a proton to coordinate with nitrogen in a hmim ligand. This coordination bond cleavage and formation results in an overall increase in the oxygen content in the hydrolyzed sample, as evident from the EDAX analysis discussed in the following section. Since ZIF-8 is composed of tetrahedrally coordinated Zn(II) and hmim linkers, (Zn(hmim)2), complete hydrolysis of ZIF-8 would result in a mixture of ZnO and Zn(OH)2. The absence of any distinct ZnO and Zn(OH)2 in the hydrolyzed ZIF-8 and composite sample further confirmed the partial hydrolysis of the ZIF-8 structure. The crystallographic pattern of the MZ@h-Z8 composite was analogous to the pristine hydrolyzed species, except for changes in some peak intensities. This suggests the highly dispersed in-situ incorporation of the Mo6+ entities within the pores of the h-ZIF-8/ZnS framework13. As reported previously, Mo incorporation may not be detected by XRD since it may be below the detection limit14. However, the occurrence of some new peaks around 7.47°, 12.64°, 18.13°, and 25.22° can be attributed to the pure phase ZIF-8, indicating incomplete hydrolysis of the zeolitic framework15. The crystallite size of the hydrolyzed ZIF-8 sample and the composite was estimated using Scherrer’s equation, as shown in Eqs. 5,
Here, D represents the crystallite size of the particles, k is a dimensionless shape factor (= 0.89), is the wavelength of X-ray radiation (1.54060 Å), β represents the peak broadening at full width at half maximum (fwhm), and θ is Bragg’s diffraction angle. The average particle size of pristine h-ZIF-8 and MZ@h-Z8 was calculated to be 43.726 nm and 48.073 nm, respectively. Besides, both the pristine h-ZIF-8 and the MZ@h-Z8 exhibited a high crystallinity of 75.58% and 85.85%, respectively, as calculated through the integration method using Eq. 6.
FTIR analysis
Figure 2b compares the Fourier transform infrared (FTIR) spectra of h-ZIF-8, pristine ZnS, and MZ@h-Z8, employed to study the chemical functional groups of the samples. The ZnS spectrum exhibits two prominent peaks at 636 and 1004 cm− 1, corresponding to the Zn-S stretching vibration and the resonance interaction between the S2− vibrational modes16. Additionally, the peaks at 1119 cm− 1 can be attributed to the asymmetric vibration of the Zn-S bonds. The other broad peaks around 1626 and 3290 cm− 1 arise due to the H2O bending and O-H stretching vibrations from the adsorbed water molecules on the ZnS surface. The existence of weak transmittance peaks for the ZnS indicates weak van der Waals interactions between the inserted ZnS nanoparticles and the molybdenum-doped h-ZIF-8. The FTIR spectrum of the hydrolyzed ZIF-8 exhibited distinct bands at 686, 755, 993, 1144, 1307, 1423, 1455, 1571, and 3280 cm− 1. The peaks around 3280 and 2918 cm− 1 can be ascribed to the N-H and C-H stretching vibration in the imidazolate ring. The transmittance band at 1571 cm− 1 can be attributed to the C = N stretching vibrations, while the peaks at 1455 and 1423 cm− 1 correspond to the vibrational stretching modes of the imidazole ring15. Similarly, the bands at 1307 and 1144 cm− 1 are attributed to the bending modes of vibrations of the imidazole ring. The C-N and C-H bending vibrational modes of the imidazole framework can be confirmed by the peaks positioned at 993 and 755 cm− 1, respectively17. Additionally, the peak at 686 cm− 1 can be accredited to the out-of-plane bending vibration of the imidazolate moiety18. As observed in Fig. 2b, the FTIR data of the hydrolyzed sample is closely comparable to previous literature on pristine ZIF-8. This finding indicates the retention of most of the chemical structure of ZIF-8 even in the hydrolyzed product. Besides, the broadening of the peak from 2600 to 3500 cm− 1 compared to a relatively smooth baseline observed for pristine pure phase ZIF-8 can be related to the intermolecular H-bonding between the -OH group and the re-protonated -NH group on imidazolate ring of the hydrolyzed species.
Optical properties
Analyzing the light absorption capacity and its optical properties is crucial in understanding the catalytic activity of a photocatalyst. Henceforth, UV-DRS, Tauc plot, PL analysis, and XPS-VB studies were employed to assess the bandgap, light absorption capacity, band positions, and the recombination rate of the photogenerated charge carriers in the MZ@h-Z8 catalyst. To draw a comparative analysis, the light-harvesting capacity of the pristine ZnS, h-ZIF-8, and the MZ@h-Z8 was measured using the UV-DRS absorption plot as shown in Fig. 2c. Additionally, the band gaps of the materials were determined, as depicted in Fig. 2d, by extrapolating the absorption edge of the Tauc plots of (αhν) vs. energy, derived from Eq. 7.
Here, α is the absorption coefficient, hν is the energy of incident light, β is an energy constant, and Eg represents the optical energy band gap of the samples.
The UV-DRS plot (Fig. 2c) shows that the hydrolyzed ZIF-8 has a very low range of absorbance, primarily in the UV region below 200 nm. On the other hand, ZnS and MZ@h-Z8 exhibit similar absorbance patterns with maximum light absorbance ranging from 250 nm to 350 nm, beyond which the intensity decreases and remains constant up to 700 nm, at which point the composite photocatalyst, MZ@h-Z8, shows a slight rising bump in the absorbance spectra. Further, the calculated band gaps of the pristine ZnS, h-ZIF-8, and the composite MZ@h-Z8 obtained from the Tauc plots (Fig. 2d) were found to be 3.38 eV, 5.00 eV, and 3.44 eV, respectively. The synthesized MZ@h-Z8 showed improved optical properties as the gap of h-ZIF-8 drastically decreased from 5 eV to 3.44 eV, showing the formation of a desirable heterojunction with enhanced photocatalytic activity.
In addition to a suitable band gap and light-absorption capacity, prolonged recombination rate and interfacial transfer of the photogenerated e−-h+ pairs in the heterojunction are crucial in designing an effective photocatalyst. Accordingly, photoluminescence (PL) spectroscopy was employed to gain insight into the charge recombination rate of the photogenerated charge carriers in the prepared photocatalysts. Figure 2e depicts the PL spectra of pure ZnS, h-ZIF-8, and MZ@h-Z8 excited at 272 nm. All the samples exhibited similar spectra, with the highest intensity corresponding to ZnS and the lowest intensity to that of the composite. Despite possessing higher light absorption capacity, ZnS has a higher charge recombination rate, leading to lower photoactivity. The decreased peak intensity in the fabricated composite suggested the successful formation of a tight interface between the ZnS and h-ZIF-8, resulting in a proper electron transfer channel and reduced recombination rate. This inference further validates the formation of a suitable composite photocatalyst with improved catalytic activity.
Chemical state analysis
The elemental composition and the surface chemical state analysis of the MZ@h-Z8 photocatalyst were investigated using X-ray photoelectron spectroscopy (XPS). The characteristic peaks at 284.7, 531.38, 399.08, 1021.5, 232.14, and 161.64 eV in the survey spectrum (Fig. 3a) illustrate the co-existence of C, O, N, Zn, Mo, and S in the prepared photocatalyst. Furthermore, the atomic concentration of each element is 54.82, 7.93, 16.12, 10.23, 2.29, and 8.61% for C, O, N, Zn, Mo, and S, respectively. The short scan spectrum of Zn 2p (Fig. 3b) shows two symmetric peaks located at 1021.49 eV and 1044.52 eV with a binding energy difference of 23.03 eV and can be related to the Zn 2p3/2 and Zn 2p1/2 spin-orbital splitting of Zn2+ state in the metal sulfide as well as in the hydrolyzed ZIF-8 species19. In the 3d high-resolution spectrum of Mo (Fig. 3c), a doublet could be identified at 232.05 eV and 235.21 eV, corresponding to the Mo6+ state of molybdenum20. Reports suggest that Mo6+ usually crystallizes as orthorhombic MoO3 and incorporates into the voids of the ZIF-8 matrix instead of forming a complex with the imidazolate ligands13. This observation further validates the absence of characteristic Mo peaks in the XRD spectrum. By analyzing the C 1s spectrum (Fig. 3d), two peaks were deconvoluted and connected to the C-C/C = C/C-H bonds of the imidazole ring at 248.88 eV and C-N bonds at 285.83 eV21,22. Similarly, the short scan XPS spectrum of O 1s can be deconvoluted into two peaks located at 530.65 eV and 531.50 eV, as shown in Fig. 3e. The two peaks can be majorly attributed to the metal-oxygen bonding of the MoO3 moiety and the Zn-OH bonding, thereby confirming the partial hydrolysis of the ZIF-8 framework23,24. Figure 3f depicts the deconvoluted spectrum of N 1s, with three subpeaks at 397.89 eV, 398.97 eV, and 400.12 eV. The peak at 397.89 eV can be assigned to the Zn-N bonding in the h-ZIF-8 framework25. Moreover, the peaks at 398.97 eV and 400.12 eV are related to the pyridinic-N and pyrrolic-N of the imidazole ring, respectively26. The high-resolution spectrum of S 2p (Fig. 3g) shows two peaks at 161.16 eV and 162.38 eV, corresponding to the S 2p3/2 and S 2p1/2 core levels of S The peak positions confirm the existence of the S2− phase of the Zn-S bonding in the synthesized sample27.
Catalyst morphology and elemental mapping
The structural morphology, elemental mapping, and microstructures of the synthesized photocatalyst, MZ@h-Z8, were established using scanning electron microscopy (SEM) and Transmission electron microscopy (TEM).
As depicted in Fig. 4(a), the SEM image of the pristine ZnS revealed irregular crystals forming an agglomerated structure. On the other hand, the Mo@ZIF-8 structure displayed in Fig. 4(b) shows aggregated sheet-like structures in the micrometer range. However, on forming composites of the Mo@ZIF-8 with the ZnS crystals, the SEM images (Fig. 4(c-d)) showed well-defined but irregularly shaped spherical and polyhedral beads condensed and stacked together to form a continuous sheath-like structure with a rugged surface. The SEM micrographs closely resembled the structures of hydrolyzed ZIF-8 obtained from the product prepared under Zn: hmim: H2O molar ratio of 1:2:156 in a previous study12. The average size of the photocatalyst particles varied widely, falling in the range of 60–360 nm, with an average size of ∼186 nm (Fig. 4e). The vast difference between the crystallite size obtained from Scherrer’s equation and the particle size calculated from the quantitative histogram equipped with the Lorentzian function, can be attributed to the agglomeration and condensation of the ZIF-8 particles in the hydrolyzed structure. The arrangements of the catalyst particles, as observed from the SEM images, result in the formation of a moderately porous structure with a number of channels on the catalyst surface. Consequently, it can be assumed that the adsorption of the dye molecules during the degradation process on the catalyst surface gets enhanced, increasing the photocatalytic activity of the MZ@h-Z8. The elemental composition of the synthesized catalyst was further evaluated using EDAX analysis, as shown in Fig. 4f. The results confirm the co-existence of carbon, oxygen, zinc, molybdenum, nitrogen, and sulfur with an atomic percentage of 57.05, 8.48, 8.98, 1.84, and 18.20%, respectively. The absence of any residual elements ensures the purity of the sample.
The TEM studies and elemental mapping further allowed the study of the microstructures and elemental arrangement in the MZ@h-Z8 crystals. The TEM images (Fig. 5(a-b)) revealed a definite grain boundary of h-ZIF-8 encapsulating the ZnS crystals exhibiting a core-shell-like structure. As observed in Fig. 5b, the ZnS particles form an agglomerated mass in the center of the core-shell structure. Moreover, Fig. 5c shows the lattice fringes of 0.308 nm, corresponding to the (111) distinct plane of ZnS. The lattice plane also corroborated with the highest intensity peak observed from the XRD analysis at 28.53°. A further look at the catalyst surface (Fig. 5d) shows spherical particles deposited on the MZ@h-Z8 surface, which may be attributed to the MoO3 particles entrapped in the catalyst framework. The interplanar distance of 0.321 nm on the deposited particles corresponds to the (021) lattice plane of orthorhombic MoO3 (Fig. 5e). The SAED image (Fig. 5f) of the MZ@h-Z8 photocatalyst reveals diffused patterns arising due to the presence of both crystalline and amorphous phases arranged in a core-shell-like structure28,29. The diffraction pattern of the ZnS core corresponding to the (220), (311), and (331) lattice planes could be identified in the SAED pattern. The d-spacing, calculated from the diffraction patterns observed in the SAED image, was in accordance with the lattice spacing of cubic ZnS from the JCPDS database. The absence of any lattice planes of the h-ZIF-8 in the SAED image was likely because the h-ZIF-8 acquires an amorphous state in the shell structure. The HRTEM images (Fig. 5c) also clearly show the crystalline ZnS core encircled by the potentially amorphous-like nature of the h-ZIF-8 shell. Furthermore, the elemental mapping (Fig. 5g) of the MZ@h-Z8 composite depicts that Zn and S mostly occupy the core positions while the C, N, and O are distributed throughout the mapping images. This further confirms that the ZnS primarily forms the core of the photocatalyst, while the Mo-doped h-ZIF-8 framework completely encapsulates the ZnS core. The Mo mapping showing particles scattered randomly over the map indicates the insertion of the MoO3 particles at random positions in the h-ZIF-8 framework. The elemental mapping analysis confirms the existence of all the elements and their distribution in the synthesized photocatalyst.
Optimization of the reaction parameters using RSM
Response surface methodology (RSM) was applied to analyze the influence of various factors on the degradation rate of MG dye. A central composite design was employed to establish the interaction between the reaction parameters to obtain the maximum degradation efficiency of MG in aqueous solutions. The CCD generated a set of 30 experimental runs, depicting the four variables, H2O2 dosage (A), photocatalyst dosage (B), initial dye concentration (C), and reaction time (D), along with the predicted and actual responses (degradation efficiency) as shown in Table 1. The resulting equation predicting MG degradation is as follows (Eq. 8):
The above equation represents the combined interaction and influence of reaction parameters (variables) on the degradation efficiency (%) of MG dye (response). A negative sign indicates antagonist interaction, while a positive sign indicates the synergistic effect of the parameter on the degradation efficiency.
The significance of the above-predicted model was examined using ANOVA results, as shown in Table 2. The significance of the regression model can be evaluated using the Fisher test (F value), the probability of error (P value), and the sum of squares, with P values required to be less than 0.05 to indicate statistical significance. An F value of 72.36, a P value below 0.0001, and a high sum of squares of 390.97 demonstrate the relevance of the chosen quadratic model. In this case, A, B, D, A², B², C², D² are significant model terms influencing the degradation efficiency. The reproducibility of the results and the validity of the model are commonly evaluated using the percentage coefficient of variance (CV). Ideally, the CV should range from 0.5 to 10%. In this case, a low CV of 0.6656% indicates that the proposed quadratic model demonstrates good reproducibility and a strong correlation between the actual and predicted degradation efficiency. Additionally, high values of correlation coefficients (R2 = 0.995, Adj R2 = 0.990, and Pred R2 = 0.961) indicated that the equation is well-fitted30. The adequate precision of 26.081 for the MG dye degradation demonstrates a favorable response, signifying an adequate signal. A ratio exceeding 4 is preferred, as it suggests that the model is capable of achieving the expected performance31.
The diagnostic plots were used to examine the quality of the predicted model. The linear fit plot of externally studentized residuals and normal probability percent is shown in Fig. 6a. The figure shows a normal linear distribution, which supports the model’s outcomes. The alignment of the data points with the regression line signifies a strong correlation between the actual and predicted values, indicating that the model accurately predicts the response to changes in the independent variables. The plot of predicted degradation efficiency versus externally studentized residuals is shown in Fig. 6b. The model was validated as the predicted values remained independent of the actual values. Furthermore, the residuals on the plot were randomly distributed within a ± 4 range, suggesting the model’s ability to provide accurate predictions. The plot of externally studentized residuals against the run number is shown in Fig. 6c. The independent residuals showed no distinct pattern and were randomly scattered around the center line within a range of ± 4, indicating the model’s accuracy and precision without any data discrepancies. The plot of actual versus predicted degradation efficiency (Fig. 6d) shows a close correlation, indicating the model’s applicability to the empirical data. The Box-Cox plot (Fig. 6e) for power transformations indicated a lambda value of 1, suggesting that no transformation is necessary for the response concerning the degradation of MG. The Cook’s distance plot in Fig. 6f offered further statistical validation. Of the 30 experimental runs, 28 had Cook’s distance values below 0.4, with most runs close to zero, suggesting a significant influence on the model’s predictive capability32.
Effect of interacting variables on the degradation efficiency
The 3D surface graphs were used to evaluate the influence of different individual reaction parameters on the photodegradation of MG. The surface plots show the relationship between two factors that affect degradation efficiency while keeping the other factors constant. Figure 7a shows the effect of catalyst loading and H2O2 dosage on degradation efficiency. Both factors significantly affected the degradation efficiency. The degradation of MG increases when catalyst dosage increases to an optimum value and starts to decline above that. The rise in the degradation efficiency was due to the presence of more exposed active sites for the interaction of dye molecules and increased production of reactive oxygen species. A decline in the degradation of MG dye at higher catalyst loading resulted from the increased opacity of the solution caused by an excess amount of the photocatalyst, which hinder the path of photons from reaching the surface of the photocatalyst. The increasing H2O2 dosage increases the rate of MG degradation due to the production of hydroxyl radicals, which facilitates the degradation of MG dye. However, higher H2O2 dosages negatively impacted the degradation efficiency due to the self-quenching of hydroxyl radicals, which ultimately decreased the amount of reactive hydroxyl radicals in the solution. Figure 7b shows the effect of dye concentration and H2O2 dosage on degradation efficiency. A minor increase in the degradation of MG dye was observed initially with increasing dye concentration, but the degradation decreased at higher concentrations. An increased dye concentration increased the opacity of the solution, which hindered the penetrating power of photons in the solution. Additionally, an increased dye concentration would require an increased amount of photocatalyst, which would increase the opacity of the solution, thereby decreasing the degradation efficiency. The effect of H2O2 dosage and reaction time on the degradation efficiency is shown in Fig. 7c. It is clearly visible that the photodegradation of MG dye increases with increasing reaction time and almost becomes constant above 55 min. Figure 7d shows the effect of initial dye concentration and catalyst dosage on degradation efficiency. Increasing dye concentration decreased the degradation efficiency, while the photocatalyst loading increased the degradation of MG to only an optimum value. The effect of varying catalyst loading and initial dye concentration with reaction time is shown in Fig. 7(e-f). Both factors significantly affected the degradation of MG dye up to an optimum value and then negatively affected the degradation efficiency at higher concentrations. Optimizing these parameters is crucial for enhancing degradation efficiency while minimizing operational time and costs. Consequently, all four parameters were optimized to achieve maximum MG dye degradation efficiency.
The RSM-CCD model predicted the optimum values of reaction parameters as H2O2 dosage = 0.05 mM, catalyst dosage = 6.5 mg, dye concentration = 66.5 ppm, and time = 52.9 min, predicting a maximum efficiency of 99.14%, with a desirability value of 1.00. Therefore, to appraise the effectiveness of the predicted model, the parameters were rounded off as in run number 3, and the actual degradation efficiency of 98.95% was achieved within 50 min of light irradiation, which is very close to the predicted value (99.14%). Thus, the prepared MZ@h-Z8 photocatalyst is an effective material for degrading MG dye from an aqueous solution. The optimum reaction conditions with the desirability factor are shown in Fig. 8. The results confirm that the RSM is an effective approach for optimizing reaction parameters.
Photocatalytic analysis
The photocatalytic experiment for the degradation of MG dye was carried out using the predetermined optimized reaction conditions (0.05 mM H2O2, 6.5 mg photocatalyst, 50 mL of 66.5 ppm MG solution, and 53 min of sunlight irradiation time) obtained from the RSM analysis. Figure 9a shows the degradation profile of MG with irradiation time. The MG degradation rapidly increased, reaching 72.71% within 20 min and gradually increasing to 98.9% by 50 min. The degradation kinetics were established using the Langmuir-Hinshelwood (L-H) model and were found to follow the pseudo-first-order kinetics with a rate constant of 0.0874 min− 1 (Fig. 9b).
To enhance the understanding of the photocatalytic efficacy of the synthesized heterojunction photocatalyst, the degradation of MG was also assessed using pristine ZnS, h-ZIF-8, and Mo@h-ZIF-8, and under varying reaction conditions, as displayed in Fig. 9(c-d). All the degradation experiments were monitored under 50 min of sunlight irradiation. No change in the MG concentration in a dark condition suggests that the decolorization of the MG dye occurs primarily by the photodegradation process rather than the physicochemical adsorption of the dye on the MZ@h-Z8 surface. However, the dye exhibited some photolysis with a degradation of about 12.4% in 50 min. Photolysis refers to the degradation of the dye in the absence of any catalyst under light irradiation. The degradation by photolysis is primarily driven by the generation of reactive oxygen species (ROS) by photosensitization of the MG dye under light illumination. On the other hand, pristine ZnS could degrade up to 41.78% MG in the presence of the external oxidant, H2O2. Interestingly, over 63% and 72% breakdown of MG could be achieved when the degradation experiments were carried out using pristine hydrolyzed ZIF-8 and Mo@h-ZIF-8 in the presence of H2O2. The results imply that both the pristine hydrolyzed h-ZIF-8 and the Mo-doped h-ZIF-8 are particularly effective photocatalysts operating through an advanced oxidation process (AOP). Moreover, the degradation efficiency rapidly rose to over 98.9% with the MZ@h-Z8 heterojunction. This rapid increment in the degradation efficiency using the MZ@h-Z8 composite can be attributed to the formation of a tight heterojunction between the ZnS nanostructure and the Mo-doped h-ZIF-8 framework, resulting in reduced bandgap and efficient charge separation and transfer channels in the synthesized composite.
The optimized catalyst was also employed to study the degradation of the 66.5 ppm MG dye in solutions containing various inorganic ions (Na+, Mg2+, Ca2+, Al3+, Cl−, CO32−, SO4−, and NO3−), organic contaminants (Sodium dodecyl sulfate (SDS), acetone, humic acid, and urea) as well as in diverse water samples, such as tap, lake water, and wetland water. The water samples were collected from different locations in Assam, India.
Effect of various cations
The photocatalytic activity of the MZ@h-Z8 for MG degradation was tested in the presence of inorganic cations such as Na+, Mg2+, Ca2+, and Al3+ at a constant concentration of 0.01 M. Figure 10a depicts a drastic drop in the degradation efficiency to 17.5% in the presence of Al3+ ions. Presumably, Al3+ with the smallest size, immensely absorbs on the catalyst surface, thereby blocking the active sites for effective dye degradation. On the other hand, Na+, Mg2+, and Ca2+ had a negligible effect on the photodegradation of MG.
Effect of various anions
Previous reports have shown a marked effect of inorganic anions on the photodegradation of various organic pollutants in an aqueous medium. To test the photoactivity of the MZ@h-Z8 under such an environment, the extent of degradation of MG was analyzed in the presence of 0.01 M Cl−, CO32−, SO4−, and NO3− (Fig. 10b). While a negligible effect was observed for Cl− and CO32− ions, some hindrance in the degradation efficiencies was observed in the presence of SO4− and NO3−. Such a reduction in the degradation efficiencies can be attributed to the radical trapping and quenching of reactive h+ and •OH species by the SO4− and NO3− ions6.
Effect of various organic compounds
The photocatalytic activity of the MZ@h-Z8 was also tested in the presence of different organic compounds such as SDS, acetone, humic acid (HA), and urea (Fig. 10c). These compounds are widely used organic chemicals of industrial importance and are commonly found in industrial wastewater. While a negligible effect on the degradation efficacy was observed in the presence of acetone, the efficiency was reduced to 56.4%, 62.3%, and 72.9% in the presence of SDS, humic acid, and urea, respectively. SDS, urea, and HA compete for the ROS to undergo simultaneous degradation alongside the dye molecules, thereby reducing the overall efficiency of the effective MG degradation33.
Figure 10d depicts the degradation efficiency of the MZ@h-Z8 photocatalyst towards MG degradation in different water matrices. Maximum degradation of 96.7, 95.1, and 92.7% was observed for tap water, lake water, and wetland water, respectively. The minor deviation in the degradation efficiency results from the presence of various dissolved oxygen, organic matter, and salts in the different water samples, which may either hinder the light absorption capacity of the catalyst by increasing the solution turbidity or may compete for photogenerated ROS with the MG molecules to undergo self-degradation.
Furthermore, the MZ@h-Z8 catalyst showed 98.5, 66.9, 96.2, 88.7, 76.9, 81.5% photodegradation of CV, RB, BG, VB, MB, and CR, as shown in Fig. 10e. The results suggest that the synthesized catalyst exhibits excellent catalytic activity towards various classes of dyes, especially triarylmethane dyes, and can be considered a potential catalyst for the removal of dye pollutants from an aqueous medium. To gain a more in-depth knowledge of the photocatalytic activity of the prepared MZ@h-Z8 catalyst in a real-life scenario, a 10 ppm solution of all 7 dyes was prepared and mixed together (total volume 70 mL), and the degradation efficacy of the catalyst was monitored for the dye mixture (Fig. 10f). Interestingly, around 46% efficiency was observed at a 50 min time interval using 1 mL H2O2 and 12 mg catalyst. However, on extending the light irradiation time to 180 min, over 75% degradation of all the dyes was observed simultaneously, indicating a remarkable performance of the prepared catalysts for the remediation of dye-polluted water.
Effect of pH and pHZPC of the photocatalyst
The pH of the reaction mixture plays a crucial role in the catalytic activity of a photocatalyst by affecting the surface charge of the catalyst. The point of zero charge (pHZPC) of the MZ@h-Z8 was determined to be 5.4 from the zeta potential vs. pH plot, as depicted in Fig. 11a. If the pH of the solution is below the pHZPC, the surface charge on the catalyst will be predominantly positively charged, while at higher pH than the pHZPC, the surface attains a negative charge, facilitating the interaction with positively charged molecules.
The cationic dyes readily interact with the negatively charged catalyst surface via electrostatic interactions at pH 7 (pH > 5.4) to effectively break down with over 85% − 99% degradation efficiencies. Although the anionic dyes were less readily degraded with 81.5% efficiency for CR and 66.9% for RB, sufficient degradation was observed using the MZ@h-Z8 catalyst even at pH 7. Possible degradation mechanisms for the anionic dyes could thus be attributed to non-electrostatic interactions, such as π-π stacking interactions or H-bonding; photocatalytic oxidation pathways (via reactive oxygen species), and photosensitization.
As the degradation efficiency was tested in an acidic and alkaline environment (Fig. 11b), a slight drop in the degradation of the cationic dyes was observed, probably because the catalyst surface attains a positive charge at a low pH. Interestingly, a steeper drop in the efficiencies was observed at pH 10, which could be attributed to the excess−OH ions quenching the reactive •OH radicals. MG and CV were instantly hydrolyzed in the alkaline medium in the presence of H2O2, thereby limiting their study above pH 7. In the case of the anionic dyes, there was a rise in the efficiencies at pH 4 as the catalyst surface attained a positive charge, increasing its interaction with the anionic dyes. However, as the pH was further raised above 7, a sharp drop in the degradation was observed. This could be attributed to the increased−OH ions and a predominant negatively charged catalyst.
Photocatalytic reaction mechanism and degradation pathway
It is widely established that AOP-assisted photodegradation processes are directly influenced by the light absorption capacity, reactive oxygen species (ROS) generation, and effective charge separation and transfer between the catalyst interface. A set of radical trapping experiments were performed to explore the mechanism of the photocatalytic process and evaluate the ROS involved in the degradation process. Benzoic acid, ascorbic acid, potassium persulfate (K2S2O8), and disodium EDTA (Na2-EDTA) were used to trap hydroxyl radicals (•OH), superoxide radicals (•O2−), holes (h+), and electrons (e−) produced in the reaction mixture during the photodegradation process. For this purpose, 0.01 M of the scavenging agents were typically added to the photocatalytic reaction system containing the set quantities of dye solution, H2O2, and catalyst dosage. As shown in Fig. 12(a-b), the degradation of MG dramatically falls to 17.1% and 43.5% with the addition of benzoic acid and ascorbic acid, respectively, indicating the maximum contribution of •OH and •O2− radicals in the breakdown of MG dye. Additionally, K2S2O8 and Na2-EDTA scavenge the electrons and holes in the reaction system, thereby preventing the production of ROS and decreasing the overall degradation efficiency to 65.8% and 58.9%, respectively. Thus, the extent of influence of the active species on the photodegradation of MG follows the order: •OH > •O2− > h+ > e−.
Meanwhile, the conduction band (CB) and valence band (VB) positions of the h-ZIF-8 and the ZnS also play a vital role in understanding the charge transfer pathway in the reaction mechanism. The electronic valence band position of h-Z8 and pristine ZnS was determined from the XPS-VB analysis as shown in Fig. 12(c-d). Here, the EVB was found to be 2.31 and 1.30 eV for h-Z8 and ZnS, respectively. The band gap (Eg) of 3.38 eV and 5 eV for ZnS and h-ZIF-8 was previously determined from the (αhν)2 vs. hν plot derived from the UV-DRS analysis (Fig. 2d). The ECB of both the components was thereby calculated using the equation EVB = ECB + Eg, and were found to be -2.69 eV and − 2.08 eV, respectively.
Based on the band positions and the radical scavenging experiments, plausible all-solid-state electron transfer pathways and degradation mechanisms for the breakdown of MG dye were proposed, as illustrated in Fig. 13.
As light illuminates the MZ@h-Z8 photocatalyst, electrons are simultaneously excited from the VB of the ZnS and h-ZIF-8 and accumulate in their conduction bands. Subsequently, the photoexcited electrons on the CB of h-ZIF-8 transfer through the Mo NPs and recombine with the holes left behind in the VB of ZnS. Here, the Mo NPs function as an electron mediator, facilitating the development of an all-solid-state Z-scheme heterojunction with rapid interfacial electron transfer. Furthermore, this pathway effectively separates the photogenerated electrons and holes, slowing down the charge recombination rate, as evident from the PL analysis. Thus, incorporating ZnS and Mo NPs in the h-ZIF framework acts as a channel for promoting charge transfer by modifying photoelectron movement, leading to increased charge separation and enhanced activity.
As the VB position of h-ZIF-8 (2.31 V vs. NHE) is higher than the standard potential of H2O/•OH (2.8 V vs. NHE), the H2O molecules easily interact with the h+ on the h-ZIF-8 to yield highly reactive •OH radicals. Additionally, the external oxidant H2O2 undergoes photolysis to produce ample •OH in the reaction mixture, which directly influences the breakdown of the MG dye. Similarly, the accumulated photoelectrons in the CB of ZnS and h-Z8 have a much more negative band potential of -2.69 and − 2.08 V (vs. NHE) than the standard reduction potential of O2/•O2− (-0.33 V vs. NHE)33. Consequently, the electrons in the CB of both h-Z8 and ZnS can reduce O2 to produce •O2− radicals. These ROS generated on the catalyst surface may attack the MG dye molecules, initiating a degradation process and breaking them into simpler molecules40. The reactions involved in the breakdown of MG using the MZ@h-Z8 catalyst under sunlight irradiation can be summarized as shown in Eqs. 9–21.
Furthermore, the bandgap of MZ@h-Z8 was found to be 3.44 eV, which is higher than that of conventional semiconductor photocatalysts. Consequently, the MZ@h-Z8 may not be stimulated by solar light to its fullest capacity, as ultraviolet light comprises just 4% of solar energy. Hence, alongside direct degradation, dye-sensitization processes may significantly contribute to the degradation of the MG dye. In the photosensitization process, the dye molecules capture the visible spectrum of the solar light to generate excited photoelectrons. The photoexcited electrons are then ejected from the LUMO of the MG dye into the conduction band of the photocatalyst, which further interacts with O2 to produce •O2− radicals. This process thus extends the light absorption capacity of the MZ@h-Z8 photocatalyst, boosting its catalytic activity towards the degradation of MG.
Following the degradation experiments, GC-MS was implemented to determine the photodegradation pathway and the degradation products of the MG dye under solar light irradiation, as depicted in Fig. 14(a-b). The MG was degraded extensively after 50 min of sunlight irradiation, catalyzed by the ROS generated from the MZ@h-Z8 catalyst. Some intermediates, such as N-(4-((4-aminophenyl)phenyl)methylene)cyclohexa-2,5-dien-1-ylidene)-N-methylmethanaminium (m/z = 301), N-(4-(hydroxy(4-(methylamino)phenyl)(phenyl)methylcyclohexa-2,5-dien-1-ylidene)-N-methylmethanaminium (m/z = 333), N-(4-benzoylcyclohexa-2,5-dien-1-ylidene)-N-methylmethanaminium (m/z = 226), 4-benzhydrylaniline (m/z = 259), N-(4-benzoylcyclohexa-2,5-dien-1-ylidene)methanaminium (m/z = 212), and (4-aminophenyl)(phenyl)methanone (m/z = 197) were detected from the GC-MS spectra of the degraded products. It was proposed that the eluted products occurred from the demethylation, hydroxylation, and oxidation of the triarylmethane structure of the MG dye41. Subsequent deamination, deformation, oxidation, and ring opening result in the formation of 4-(dimethylamino)benzaldehyde (m/z = 149), 4-(dimethylamino)benzoic acid (m/z = 165), 1,4-benzendicarboxylic acid (m/z = 166), penta-1,4-dien-3-aminium (m/z = 84), and buta-1,3-dien-2-amine (m/z = 69)42. Consequently, the intermediates undergo a sequence of reactions and cleavage of benzene rings, forming simpler and less toxic compounds. Finally, the smaller organic molecules may further break down and undergo mineralization to produce carbon dioxide and water.
To further confirm the extent of mineralization of the MG molecules, the residual total organic carbon (TOC) was analyzed for the degraded sample. The TOC values for the unreacted dye solution and the degraded solutions were analyzed to be 126.4 mg/L and 25.16 mg/L, giving a TOC removal efficiency of 80%. Such a high degree of mineralization depicts efficient breakdown of the dye molecules.
Catalyst reusability
The stability and reusability of the MZ@h-Z8 catalyst were examined by employing the catalyst in six consecutive photodegradation cycles toward the degradation of MG under optimum reaction conditions. After each catalytic cycle, the used MZ@h-Z8 was collected from the reaction system by centrifugation and washed repeatedly with distilled water and ethanol to remove any residual organic molecules adsorbed on the catalyst surface and pores. Figure 15a depicts that the catalyst was reusable up to six catalytic cycles without significantly declining its photocatalytic activity. The slight decline in the degradation efficiency in each successive cycle may have resulted from the obstruction of the catalyst active sites by the intermediate molecules and a minor disruption in the Z-scheme heterojunction. Even after six cycles, the synthesized MZ@h-Z8 photocatalyst exhibited retention of ∼76% efficiency, depicting the excellent reusability of the photocatalyst. Additionally, the reused photocatalyst was subjected to XRD and SEM-EDAX analysis to test its stability and possible leaching of active sites of MZ@h-Z8 during its catalytic cycles. No notable change in the XRD pattern, with some minor changes in some peak intensities, indicated that the catalyst retained its crystalline structure after its reusability tests (Fig. 15b). Further, the SEM image confirms no change in the catalyst morphology (Fig. 15c). However, some changes in the elemental composition could be observed from the EDAX graph (Fig. 15d). The slight decline in the Mo and Zn concentrations and increase in the O concentration show some loss of active sites and adsorption of intermediate organic molecules on the catalyst surface.
The XPS spectra of the recycled catalyst are depicted in Fig. 16. Compared to the as-synthesized catalyst (Fig. 3), no obvious change was observed in the peak position and intensities of the framework atoms Zn and C, which suggested the basic overall framework of the MOF possessed good stability. However, certain changes in the peak position and intensity in the peak of O, N, and S were observed. The decrease in the intensity of Zn-OH/Mo-O-Mo bonding peak at 531.41 eV is probably due to the loss of Zn and Mo in the reused catalyst. Furthermore, an additional shoulder peak in the Mo spectrum at 226.08 eV, which could be attributed to either partial reduction of Mo6+ to M0 or partial sulfidation of Mo.
However, the overall high catalytic performance of the catalyst in the reusability cycles with minor structural or morphological changes indicated high stability of the hydrolyzed ZIF-8 structure without further hydrolysis or structural damage in the h-ZIF-8 framework. Thus, this study opens up a potentially novel scope for utilizing hydrolyzed MOFs in water-based applications under sunlight irradiation.
Comparison with other photocatalysts for MG removal
The photocatalytic activity of the MZ@h-Z8 composite was compared with different materials reported in the literature to draw a comparative analysis, particularly for MG degradation. Compared to several recently reported catalyst materials, superior removal of MG dye could be achieved using a small amount of the prepared MZ@h-Z8 catalyst (Table 3).
Conclusion
This work effectively shows that Mo-doped hydrolyzed ZIF-8 (h-ZIF-8) on a ZnS substrate may be used as an efficient, water-stable photocatalyst to degrade MG under sunlight in a hydrogen peroxide-assisted AOP. We overcome ZIF-8’s conventional water stability limits using an aqueous reflux technique, resulting in a redesigned structure with increased durability and reactivity. RSM-predicted optimal conditions revealed strong photocatalytic effectiveness, attaining close to 99% degradation in roughly 50 min, consistent with experimental results. Mechanistic research revealed an all-solid-state Z-scheme route in which Mo nanoparticles enhanced charge separation and decreased recombination at the ZnS and h-ZIF-8 interfaces. Our findings demonstrate the MZ@h-Z8 catalyst’s adaptability by proving its high degrading efficiency across various dye contaminants and environmental water samples, as well as in the presence of different inorganic and organic salts, while maintaining strong performance and reusability across several cycles. These findings emphasize the potential of hydrolyzed ZIF-8-based heterojunctions in solar-driven photocatalysis for long-term water treatment applications. Moreover, this technique presents a viable strategy for developing new photocatalysts with improved water stability and efficiency, opening the path for more widespread applications in solar energy conversion and environmental cleanup.
Data availability
“All data generated or analyzed during this study are included in this published article.”
References
Yaghi, O. M., Li, G. & Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nat. 1995. 378, 6558378. https://doi.org/10.1038/378703a0 (1995).
Bhuyan, A. & Ahmaruzzaman, M. Metal-organic frameworks: A new generation potential material for aqueous environmental remediation. Inorg. Chem. Commun. 140, 109436. https://doi.org/10.1016/J.INOCHE.2022.109436 (2022).
Ahmad, R., Khan, U. A., Iqbal, N. & Noor, T. Zeolitic imidazolate framework (ZIF)-derived porous carbon materials for supercapacitors: an overview. RSC Adv. 10, 43733–43750. https://doi.org/10.1039/D0RA08560J (2020).
Liu, X. et al. Improvement of hydrothermal stability of zeolitic imidazolate frameworks. Chem. Commun. 49, 9140–9142. https://doi.org/10.1039/C3CC45308A (2013).
Zhang, H., Liu, D., Yao, Y., Zhang, B. & Lin, Y. S. Stability of ZIF-8 membranes and crystalline powders in water at room temperature. J. Memb. Sci. 485, 103–111. https://doi.org/10.1016/J.MEMSCI.2015.03.023 (2015).
Bhuyan, A. & Ahmaruzzaman, M. Novel Zn(II)-imidazolate polymorph MOF loaded with Mo-QD clusters for solar light-driven H2O2-assisted synergistic photosensitized degradation of Gentian Violet. J. Alloys Compd. 950, 169747. https://doi.org/10.1016/J.JALLCOM.2023.169747 (2023).
Jeong, Y. C. et al. Function-regeneration of non-porous hydrolyzed-MOF-derived materials. Nano Res. 12, 1921–1930. https://doi.org/10.1007/S12274-019-2459-8/METRICS (2019).
Cui, L. et al. Dramatic enhancement of photocatalytic H2 evolution over hydrolyzed MOF-5 coupled Zn0.2Cd0.8S heterojunction. J. Colloid Interface Sci. 577, 233–241. https://doi.org/10.1016/J.JCIS.2020.05.023 (2020).
Gadore, V., Singh, A. K., Mishra, S. R. & Ahmaruzzaman, M. RSM approach for process optimization of the photodegradation of congo red by a novel NiCo2S4/chitosan photocatalyst. Sci. Rep. 14, 1–20. https://doi.org/10.1038/s41598-024-51618-2 (2024).
Soleimani, H., Sharafi, K., Amiri Parian, J., Jaafari, J. & Ebrahimzadeh, G. Acidic modification of natural stone for remazol black B dye adsorption from aqueous solution- central composite design (CCD) and response surface methodology (RSM). Heliyon 9, e14743. https://doi.org/10.1016/J.HELIYON.2023.E14743/ASSET/0635D925-8D08-460D-A68A-CADA63E092EB/MAIN.ASSETS/GR11.JPG (2023).
Sheshmani, S. & Mardali, M. Harnessing the synergistic potential of ZnS Nanoparticle-Interfacing Chitosan for enhanced photocatalytic degradation in aqueous media and textile wastewater. J. Polym. Environ. 1–23. https://doi.org/10.1007/S10924-024-03307-4/FIGURES/22 (2024).
Zhang, H., Zhao, M., Yang, Y. & Lin, Y. S. Hydrolysis and condensation of ZIF-8 in water. Microporous Mesoporous Mater. 288, 109568. https://doi.org/10.1016/J.MICROMESO.2019.109568 (2019).
Wang, T. et al. A highly efficient Ni–Mo bimetallic hydrogen evolution catalyst derived from a molybdate incorporated Ni-MOF. J. Mater. Chem. Mater. 6, 9228–9235. https://doi.org/10.1039/C8TA01325J (2018).
El-Sawaf, A. K. et al. A mesoporous mo and N Co-doped TiO 2 nanocomposite with enhanced photocatalytic efficiency. RSC Adv. 14, 3536–3547. https://doi.org/10.1039/D3RA07258D (2024).
Mishra, S. R., Verma, R., Gadore, V. & Ahmaruzzaman, M. Bioinspired fish scale-derived hydroxyapatite@ZIF-8 nanocomposite for enhanced removal of Rose bengal: theoretical RSM modeling and mechanistic insights. Biomass Convers. Biorefin. 1, 1–21. https://doi.org/10.1007/S13399-023-05202-9/TABLES/3 (2023).
Yadav, N., Yadav, G. & Ahmaruzzaman, M. Camellia sinensis leaf-assisted green synthesis of SO3H-functionalized zns/biochar nanocatalyst for highly selective solketal production and improved reusability in methylene blue dye adsorption. Renew. Energy. 224, 120176. https://doi.org/10.1016/J.RENENE.2024.120176 (2024).
Du, P. D., Hieu, N. T., Thien, T. V. & Ultrasound-Assisted Rapid ZIF-8 synthesis, porous ZnO Preparation by heating ZIF-8, and their photocatalytic activity. J. Nanomater. 2021, 9988998. https://doi.org/10.1155/2021/9988998 (2021).
Zhang, Y., Jia, Y. & Hou, L. Synthesis of zeolitic imidazolate framework-8 on polyester fiber for PM 2.5 removal. RSC Adv. 8, 31471–31477. https://doi.org/10.1039/C8RA06414H (2018).
Gadore, V., Ranjan Mishra, S. & Ahmaruzzaman, M. SnS2 modified carbonate-intercalated Ni-Zn-Fe trimetallic LDH as a novel photocatalyst for persulfate activation: influence of operational parameters, co-existing ions and organic compounds. Chem. Eng. J. 475, 146157. https://doi.org/10.1016/J.CEJ.2023.146157 (2023).
Li, Q. et al. Self-assembled mo doped Ni-MOF nanosheets based electrode material for high performance battery-supercapacitor hybrid device. Int. J. Hydrogen Energy. 45, 20820–20831. https://doi.org/10.1016/J.IJHYDENE.2020.05.143 (2020).
Mao, L., Wang, F. & Mao, J. Polar mesoporous zinc sulfide nanosheets encapsulated in reduced graphene oxide three-dimensional foams for sulfur host. Sci. Rep. 2020. 10 (10), 1. https://doi.org/10.1038/s41598-020-62037-4 (2020).
Wang, Y. et al. In situ growth of ZIF-8 nanoparticles on Chitosan to form the hybrid nanocomposites for high-efficiency removal of congo red. Int. J. Biol. Macromol. 137, 77–86. https://doi.org/10.1016/J.IJBIOMAC.2019.06.195 (2019).
Cao, L. et al. Controllable growth of Cu–Bi co-doped ZnO nanospheres on cotton fabrics and a study on their photocatalytic performance in visible light. RSC Adv. 11, 29416–29425. https://doi.org/10.1039/D1RA05317E (2021).
Zhou, K. et al. Ultrathin MoO3 nanocrystalsself-assembled on graphene nanosheets via oxygen bonding as supercapacitor electrodes of high capacitance and long cycle life. Nano Energy. 12, 510–520. https://doi.org/10.1016/J.NANOEN.2015.01.017 (2015).
Chafiq, M., Chaouiki, A., Suhartono, T. & Ko, Y. G. Albumin protein encapsulation into a ZIF-8 framework with Co-LDH-based hierarchical architectures for robust catalytic reduction. J. Mater. Chem. Mater. 11, 23984–23998. https://doi.org/10.1039/D3TA03623E (2023).
Patil, R. et al. An improved hummers method derived graphene oxide wrapped ZIF-8 polyhedron derived porous heterostructure for symmetric supercapacitor performance. RSC Sustain. 2, 233–238. https://doi.org/10.1039/D3SU00327B (2024).
Mishra, S. R., Panigrahi, B., Gadore, V., Sarkar, N. & Ahmaruzzaman, M. Enhanced photocatalytic performance of CuS/O,N-CNT composite for solar-driven organic contaminant degradation. Sci. Rep. 2024. 14, 1. https://doi.org/10.1038/s41598-024-68770-4 (2024).
Sanaee, M. R. & Bertran, E. Synthesis of carbon encapsulated Mono- and Multi-Iron nanoparticles. J. Nanomater. 2015 (450183). https://doi.org/10.1155/2015/450183 (2015).
Begum, K., Bhuyan, A. & Ahmaruzzaman, M. Ingenious development of bandgap engineered MIL-101(Fe) core-shell supported on SnO2/ZnO heterojunction and its photocatalytic activity towards Azo dye degradation: process optimization and mechanistic insight. J. Mol. Struct. 1321, 140135. https://doi.org/10.1016/J.MOLSTRUC.2024.140135 (2025).
Tripathi, P., Tiwari, S., Sonwani, R. K. & Singh, R. S. A step towards enhancing the efficiency of biofilm mediated degradation of brilliant green dye in packed bed bioreactor: statistical and toxicity analysis. Process Saf. Environ. Prot. 170, 1228–1239 (2023).
Hosseini-Nasab, S. J., Saber-Tehrani, M., Haghgoo, M. & Aberoomand-Azar, P. Control of porous properties of ambient dried sodium silicate-based aerogels using response surface methodology. J. Thermoplast. Compos. Mater. 0892705719876316. (2019).
Jawad, A. H., Hameed, B. H. & Abdulhameed, A. S. Synthesis of biohybrid magnetic chitosan-polyvinyl alcohol/mgo nanocomposite blend for remazol brilliant blue R dye adsorption: solo and collective parametric optimization. Polym. Bull. 80, 4927–4947. https://doi.org/10.1007/S00289-022-04294-Z/FIGURES/9 (2023).
Anindita Bhuyan, M. & Ahmaruzzaman Ternary 3D/2D/3D direct dual Z-scheme MOF-on-MOF-derived α-Fe2O3/g-C3N4/Fe-MOF photocatalyst for boosted sunlight-driven removal of metronidazole: effect of co-existing ions, mechanistic insights, and water matrices. Environ. Sci. Nano. https://doi.org/10.1039/D4EN00610K (2024).
Snigdha, A. et al. Facile green synthesis of SnO2:Zn@g-C3N4 heterojunction nanocomposite using Murraya paniculata leaves extract for effective photocatalytic degradation of organic dye malachite green by sunlight illumination. J. Mol. Struct. 1308, 138013. https://doi.org/10.1016/J.MOLSTRUC.2024.138013 (2024).
Karimi Anjiraki, M. et al. Magnetically recyclable PrFeO3/g-C3N4 nano-photocatalyst with Z-scheme structure: synthesis and characterization and its application for enhanced degradation of malachite green as contaminated water under sunlight. Inorg. Chem. Commun. 170, 113184. https://doi.org/10.1016/J.INOCHE.2024.113184 (2024).
Shyagathur, S. C. et al. Enhanced degradation of dyes using a novel CuS/g-C3N4/rGO ternary composite catalyst: synthesis, characterization, and mechanistic insights. Mater. Chem. Phys. 327, 129877. https://doi.org/10.1016/J.MATCHEMPHYS.2024.129877 (2024).
Yuan, N. et al. MIL-53(Fe)/Sn3O4 nanosheet photocatalysts for Visible-Light degradation of toxic organics in wastewater. ACS Appl. Nano Mater. 6, 9159–9174. https://doi.org/10.1021/ACSANM.3C00400/SUPPL_FILE/AN3C00400_SI_001.PDF (2023).
Christopher, J. J., Lydia, I. S., Sathiyan, A., Princy, J. & Merlin Fabrication of Cu3Mo2O9 doped MWCNT nanocomposites as efficient photocatalyst for malachite green dye degradation. Opt. Mater. (Amst). 156, 115935. https://doi.org/10.1016/J.OPTMAT.2024.115935 (2024).
Boucherdoud, A. et al. Experimental exploration and DFT analysis of the kinetics and mechanism of malachite green photodegradation catalyzed by polyaniline-copper oxide nanocomposite. J. Mol. Model. 30, 1–15. https://doi.org/10.1007/S00894-024-06039-3/FIGURES/14 (2024).
Bhuyan, A. Md. Ahmaruzzaman, Ultrasonic-assisted synthesis of highly efficient and robust metal oxide QDs immobilized-MOF-5/Ni-Co-LDH photocatalyst for sunlight-mediated degradation of multiple toxic dyes. J. Alloys Compd. 172781. https://doi.org/10.1016/J.JALLCOM.2023.172781 (2023).
Amigun, A. T., Adekola, F. A., Tijani, J. O. & Mustapha, S. Photocatalytic degradation of malachite green dye using nitrogen/sodium/iron-TiO2 nanocatalysts. Results Chem. 4, 100480. https://doi.org/10.1016/J.RECHEM.2022.100480 (2022).
Diao, Z., Li, M., Zeng, F., Song, L. & Qiu, R. Degradation pathway of malachite green in a novel dual-tank photoelectrochemical catalytic reactor. J. Hazard. Mater. 260, 585–592. https://doi.org/10.1016/J.JHAZMAT.2013.05.037 (2013).
Acknowledgements
The authors would like to acknowledge CIF NIT Silchar, SAIF IIT Madras, SAIF IIT Bombay, IAAST SAIF Guwahati, CNMS Bangalore, PR Testing Delhi, and SAIF IIT Patna for the analytical facilities. Anindita Bhuyan, thanks CSIR, New Delhi, for awarding the SRF-Direct Fellowship, 2024 (ID: 35110438).
Author information
Authors and Affiliations
Contributions
“A.B. and M. A. wrote the main manuscript text, commented, revised and corrected the whole manuscript and S. R. M. and V.G. edited, commented and revised the manuscript. All authors reviewed the manuscript.”
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bhuyan, A., Mishra, S.R., Gadore, V. et al. Hydrolyzed MOF based Mo@h-ZIF-8/ZnS composite for enhanced photodegradation of dye pollutants using a combined experimental and RSM approach. Sci Rep 15, 30181 (2025). https://doi.org/10.1038/s41598-025-16201-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16201-3