Abstract
Hypogammaglobulinemia (HGG) is a common complication of liver transplantation (LT). However, the impact of underlying liver disease severity on post-LT immunoglobulin dynamics remains unclear. We aimed to evaluate the differences in serum immunoglobulin G (IgG) levels based on the pre-transplantation model for end-stage liver disease (MELD) scores. We collected data from patients who underwent LT between July 2016 and December 2022 at a tertiary hospital. Propensity score matching was performed between the low and high MELD groups and IgG dynamics were evaluated. The significance of peri-LT HGG on clinical outcomes was also evaluated. In a matched population (1:1 propensity score matching), the median serum IgG levels decreased significantly from 1,606.5 mg/dL pre-LT to 1,011.6 mg/dL 1-month post-LT. IgG levels before and after transplantation (1 month, 6 months, and 1 year) did not differ significantly between the groups. Overall, 36.0% of patients developed HGG within 1 year after transplantation. Multivariable Cox regression analysis showed that pre-LT HGG was independently associated with mortality. In conclusion, HGG is frequent complication in peri-LT period and pre-LT HGG is significantly associated with mortality. No significant difference in HGG according to the MELD score was observed.
Similar content being viewed by others
Introduction
Infectious complications are common after liver transplantation (LT) and increase the risk of morbidity and mortality. LT recipients are at a higher risk of developing bacterial infections (incidence, 33–68%) than other transplant recipients, owing to the complexity of the surgical procedure1,2. Additionally, cytomegalovirus and fungal infections occur at rates of 22–29% and 2–42%, respectively3. Infectious complications following LT are associated with a high mortality rate of 30–50%4. The risk of infectious complications increases with high Model for End-stage Liver Disease (MELD) scores, surgery duration, re-transplantation, surgical complications, and renal replacement therapy5.
Hypogammaglobulinemia (HGG) is common after solid organ transplantation (SOT) and associated with an increased risk of post-LT infectious complications6. HGG can result from various medications, including corticosteroids, mycophenolate, and anti-CD20 agents, which are commonly used before and after transplantation7. HGG occurs in 47% of LT recipients within the first week after transplantation and is associated with the development of infectious complications, sepsis, and post-LT mortality8,9.
In patients with chronic liver disease, immunoglobulin G (IgG) levels are affected by the underlying liver disease10,11. In advanced chronic liver disease, IgG levels may be elevated because of increased intrahepatic inflammation and reduced liver function in clearing antigens and endotoxins from the gastrointestinal tract10,11,12. However, the impact of liver disease severity on IgG dynamics before and after LT remains unclear. It is also uncertain whether IgG levels influence post-LT survival. Furthermore, the role of HGG in this relationship is unknown, though it may be especially relevant for recipients with high MELD scores, who face a higher risk of infection.
Therefore, in this study, we aimed to analyze the differences in IgG dynamics and the occurrence of HGG according to the MELD score in LT recipients and determine the differences in patient outcomes.
Methods
Study design and population
In this retrospective study, we analyzed prospectively collected data from 597 adult patients who underwent LT between July 2016 and December 2022 at Severance Hospital, Seoul, Korea. Clinical information and blood samples were obtained prospectively after informed consent was provided. Patients with cholangiocarcinoma (n = 22), combined organ transplantation (n = 9), re-transplantation (n = 9), and those who died within 2 days post-LT (n = 2) were excluded, leaving a final cohort of 555 eligible patients.
Data acquisition and definitions
Data on the following baseline characteristics were prospectively collected: demographic characteristics, body mass index (BMI), comorbidities, underlying liver disease, hepatocellular carcinoma (HCC), MELD score13, Child–Turcotte–Pugh (CTP) score14, ascites, esophageal varix, and encephalopathy. Transplantation-related variables collected included donor age, sex, and BMI; ABO incompatibility; desensitization method; donor type; and the Milan criteria. During surgery, cold ischemic time, operative time, number of packed red blood cell (RBC) transfusions, simultaneous splenectomy, and the graft-to-recipient weight ratio (GRWR) were recorded. The GRWR was calculated using the formula: GRWR = (graft weight [g]/recipient weight [g]) × 100. Cold ischemic time was defined as the period from graft inflow clamping in the donor to reperfusion in the recipient7.
HGG was defined as a measured IgG level of < 700 mg/dL7. The presence of a portosystemic shunt (PSS) was estimated based on the presence of esophageal varix and hepatic encephalopathy15. The degree of PSS was classified according to the severity of each variable. Outcome variables included patient mortality, infection-related mortality, graft failure, bloodstream infection (BSI), cytomegalovirus (CMV) infection, and invasive pulmonary aspergillosis (IPA). Graft failure was defined as the need for re-transplantation or patient death. BSI was defined according to the National Healthcare Safety Network Patient Safety Manual16. CMV infection was defined by a positive CMV nucleic acid amplification test in conjunction with the initiation of antiviral therapy; cases with pre-transplant CMV viremia were excluded from this analysis. IPA was defined as either proven or probable17. All outcomes were measured from the date of LT.
Blood samples were prospectively collected on the day of LT, immediately before surgery, and at 1, 6, and 12 months post-LT. Serum from these samples was used to measure IgG levels.
Immunosuppressive regimens
All patients received basiliximab as an induction immunosuppressant on the day of transplantation and on postoperative day 4. ABO-incompatible LT recipients received rituximab according to a desensitization protocol. Maintenance immunosuppressants included calcineurin inhibitors, steroids, and mycophenolate mofetil, or, less commonly, a double regimen of tacrolimus and steroids. In patients with renal insufficiency or HCC, mycophenolate was replaced by everolimus 4 weeks post-transplantation.
Immunoglobulin G measurements
A quantitative enzyme-linked immunosorbent assay (ELISA) (88-50550, Invitrogen, Carlsbad, CA, USA) was used to measure IgG levels in prospectively collected blood samples. Serum samples were stored at −70 °C and thawed at 4 °C before use. The capture antibody was diluted in phosphate-buffered saline (PBS) coating buffer and applied to a 96-well plate, incubated overnight at 4 °C. The plate was washed twice with PBS containing 0.05% Tween 20 (PBS-T), then blocked with buffer for 2 h at room temperature, followed by washing with PBS-T. Serum samples were diluted 1:500,000 with Assay Buffer A (a 1:20 dilution of Assay Buffer A concentrate) and incubated for 2 h. The plate was washed four times, and horseradish peroxidase-conjugated anti-human IgG monoclonal antibody was added. After 1 h, the wells were washed four times, and tetramethylbenzidine substrate solution was added. After color development, a stop solution was added to terminate the reaction. Absorbance was measured at 450 nm using a microplate reader. All procedures followed the manufacturer’s protocol. IgG levels measured using ELISA were calibrated against laboratory measurements.
MELD group categorization and propensity score matching
Patients with pre-LT MELD scores of < 30 and ≥ 30 were categorized into low and high MELD groups, respectively. Propensity score matching was performed to address potential biases and confounding factors. Propensity scores were estimated using logistic regression, with the high MELD group as the dependent variable and relevant covariates, such as age, sex, transplantation year, HCC, CTP score, ABO incompatibility, and Milan criteria, as independent variables. The nearest-neighbor technique was used for matching.
Statistical analysis
A two-tailed independent t-test or Mann–Whitney U test was used for continuous variables to compare clinical characteristics and outcomes. The Shapiro–Wilk test determined normality, and non-parametric tests were applied if the data were not normally distributed. Continuous variables are presented as mean ± standard deviation if normally distributed or as median (interquartile range) if not. Pearson’s chi-square test or Fisher’s exact test was used for categorical variables. The Wilcoxon signed-rank test compared changes in serum IgG levels within the same group across multiple time points. Survival analysis was performed using the log-rank test to compare mortality between groups.
Multivariable Cox proportional regression evaluated the effect of HGG on mortality, BSIs, and CMV infections. Variables with p < 0.05 from the univariable analysis were included in the multivariable analysis. Rituximab use was included as a covariate in the multivariable analysis, regardless of its significance in the univariable analysis, because of its potential effect on IgG levels. HGG after transplantation and rejection were treated as time-varying variables. Risk factors for the development of HGG before and 1 month after transplantation were analyzed using logistic regression. Differences were considered statistically significant at p < 0.05. All statistical analyses were conducted using R v.4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Among the 555 eligible patients, 444 were in the low MELD group and 111 in the high MELD group (Fig. 1). After propensity matching, 111 patients from each group were paired, with characteristics well balanced except for factors inherently different between low and high MELD patients, such as CTP score, ascites, encephalopathy, living-donor LT, and cold ischemic time.
The median patient age was 54 years (interquartile range [IQR]: 47–61 years), and 60.8% of the patients were male (Table 1). No significant differences in underlying liver diseases or comorbidities were found between the two groups. The MELD scores were 15.0 (IQR: 10.5–20.0) and 40.0 (33.0–40.0) in the low and high MELD groups, respectively.
Living-donor transplantation was more frequent in the low MELD group than in the high MELD group (97.3% vs. 36.9%; p < 0.001). The mean donor age was significantly higher in the high MELD group than in the low MELD group (35.5 ± 11.9 vs. 46.4 ± 16.1 years; p < 0.001). No significant differences were observed between the two groups regarding ABO incompatibility, desensitization medications, low GRWR, and the proportion of patients meeting the Milan criteria.
Mortality and infectious complication rates were significantly higher in the high MELD group compared to the low MELD group. The overall mortality rate was 9.0% and 29.7% in the low and high MELD groups, respectively. The median follow-up duration for the low and high MELD groups was 942.0 (IQR: 626.0-1616.5) and 655.0 (IQR: 174.0-1487.5) days, respectively (p = 0.002). Infectious complications, including 1-year BSI, CMV infection, and IPA, were significantly more frequent in the high MELD group compared to the low MELD group.
Dynamics of Immunoglobulin G levels
IgG levels were measured before and after transplantation to assess dynamics according to the MELD score. In the overall population, the median IgG level before transplantation was 1,606.5 mg/dL (IQR: 1,124.6–2,149.0; Table 2 and Figure S1). One month after transplantation, the median IgG level significantly decreased to 1,011.6 mg/dL (IQR: 733.4–1,387.1) (p < 0.001). At 6 months and 1 year, the median IgG levels were 1,070.5 mg/dL (IQR: 780.4–1,566.4) and 1,117.5 mg/dL (IQR: 799.6–1,403.1), respectively. These levels were higher than at 1 month, but the differences were not significant (p = 0.151 and 0.172, respectively).
Before transplantation, HGG occurred in 4.5% of LT recipients. One month after transplantation, the incidence of HGG increased to 21.4%. At 6 months and 1 year, the incidence rates of HGG were 16.3% and 14.5%, respectively. Overall, 36.0% of patients developed HGG within 1 year after transplantation.
We compared IgG levels between the low and high MELD groups before and after transplantation. Serum IgG levels were not significantly different between the two groups at any time point before transplantation or at 1 month, 6 months, and 1 year after transplantation (Figure S1). The incidence of HGG was also not significantly different between the groups.
We further analyzed the IgG dynamics and incidence of HGG in LT recipients who did not receive rituximab as induction therapy (Table S1). The median IgG levels decreased the least at 1 month post-LT and gradually increased thereafter, showing dynamics similar to the entire cohort. Within 1 year, 35.4% of patients developed HGG.
We also evaluated IgG dynamics according to the severity of PSS. Pre-LT IgG levels showed a increasing trend with higher PSS severity, but this was not statistically significant (Table S2 and Figure S2). Additionally, the occurrence of pre-LT hypergammaglobulinemia (IgG > 1600 mg/dL) also increased across no, mild, and severe PSS groups (43.2%, 52.7%, and 56.4%, respectively), without statistical significance (p = 0.287). After transplantation, no notable trends related to PSS severity were observed in IgG levels.
Effect of HGG on clinical outcomes
Cox regression analysis was performed for mortality, BSIs, and CMV infections to determine the effect of HGG on clinical outcomes. The univariable analysis identified HGG before transplantation (HGGpre) as a significant risk factor for mortality (hazard ratio [HR] 4.06; 95% confidence interval [CI] 1.75–9.43; p = 0.001), whereas HGG at 1 month (HGG1mo) was not significantly associated with mortality (HR 0.90; 95% CI 0.37–2.18; p = 0.810; Table 3). Multivariable analysis was performed using a high MELD score, donor age, sex, BMI, living-donor transplantation, rituximab use, and number of RBC transfusions as confounders. After adjustment, HGGpre was significantly associated with mortality (adjusted HR [aHR] 2.82; 95% CI 1.10–7.20; p = 0.030).
In the univariable analysis, HGG1mo after transplantation was significantly associated with BSI (HR 2.19; 95% CI 1.08–4.45; p = 0.030), whereas HGGpre was not (HR 2.15; 95% CI 0.89–5.23; p = 0.090; Table 4). However, in the multivariable analysis, no significant association was observed between HGG1mo and BSI (aHR, 1.46; 95% CI 0.53–4.06; p = 0.463). Regarding CMV infection, HGGpre was significantly associated with increased risk in the univariable analysis (HR, 3.04; 95% CI, 1.15–8.06; p = 0.026; Table S3), whereas HGG1mo was not (HR, 1.31; 95% CI, 0.48–3.61; p = 0.602). HGGpre remained a significant risk factor for CMV infection in the multivariable analysis (HR, 2.96; 95% CI, 1.30–6.72; p = 0.009).
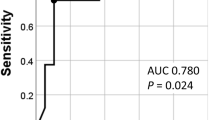
Kaplan–Meier curves also showed that HGGpre was associated with decreased survival compared to patients with no HGG (p = 0.002; Fig. 2). Further stratification was performed according to the HGGpre and MELD groups. The Kaplan–Meier curve showed a significant survival difference among the four groups (p < 0.001; Figure S3), with patients with HGGpre and a high MELD score having the worst prognosis, followed by patients with HGGpre and a low MELD score, no HGGpre and a high MELD score, and no HGGpre and a low MELD score.
Risk factors for HGG in LT recipients
The risk factors for HGG before and after LT were investigated using logistic regression. A history of diabetes was identified as a risk factor for HGGpre (odds ratio [OR] 4.73; 95% CI 1.27–22.47; p = 0.028; Table S4). Risk factors for HGG1mo were GRWR < 0.8 (OR 5.40; 95% CI 1.51–20.17; p = 0.009) and steroid pulse therapy within 1 month after LT (OR 3.67; 95% CI 1.67–8.02; p = 0.001).
Discussion
We investigated the differences in IgG dynamics according to MELD groups in LT recipients and the association between HGG and clinical outcomes. Overall, 36.0% of patients developed HGG within 1 year after transplantation. IgG levels and the proportion of patients with HGG before and after transplantation (1 month, 6 months, and 1 year) were not significantly different between the low and high MELD groups. HGG before transplantation was independently associated with mortality. Diabetes was significantly associated with HGGpre, whereas GRWR < 0.8 and steroid pulse therapy were associated with HGG1mo.
Overall, in a previous study, HGG occurred in 45% of SOT recipients in the first year of transplantation6. In LT, 14–47% of recipients developed HGG after transplantation, with rates varying depending on the time point after transplantation and definition of HGG8,9,18. Doron et al. measured IgG levels from 1 month after transplantation and found that 26% of recipients developed HGG within 1 year, with the definition of HGG being IgG < 560 mg/dL.9 Yoshizumi et al. measured IgG levels up to 1 week after transplantation and found that HGG, defined as IgG < 650 mg/dL, occurred in 47% of recipients8.
IgG levels start to decrease during surgery and reach their nadir 1 week after transplantation19. The IgG levels increase during the following 1 month and remain within the normal range thereafter. Therefore, the incidence of HGG may vary depending on the timing of IgG level measurement relative to transplantation, with the highest likelihood occurring within 1 week after transplantation. In this study, blood samples were collected 1 month after transplantation, and IgG levels were measured. The incidence of HGG was 21.4% and 36.0% at 1 month and over the entire period, respectively, which is lower than the 46% reported by a previous study that measured HGG within 1 week after transplantation6. These differences may be attributed to the recovery of IgG levels by 1 month after transplantation.
Several hypotheses have been proposed to explain the decrease in IgG levels after LT. Possible mechanisms include sequestration of immunoglobulins by the donor liver and the effects of immunosuppressive drugs19. After reperfusion of the transplanted graft, liver sinusoidal cells become activated and may contribute to the clearance of immunoglobulin complexes20. High-dose corticosteroids used during transplantation can rapidly decrease serum IgG concentrations21, and immunosuppressive agents, including calcineurin inhibitors, can decrease B-cell function in response to changes in T-cell function19,22. In addition, the removal of the diseased liver, which causes hypergammaglobulinemia, may also contribute to a long-term decrease in immunoglobulin levels12.
HGG after SOT is a known risk factor for poor clinical outcomes, especially for severe bacterial infections. In kidney transplant recipients, post-LT HGG significantly increased the risk of infectious complications, including BSIs, acute graft pyelonephritis, graft-related viral infections, and subsequent hospitalization23,24. In LT, studies have also shown that the risk of death and infectious complications was significantly higher in patients with HGG8,9. However, in contrast to the results of previous studies, peri-transplantation HGG was not identified as an independent risk factor for BSI. In previous studies, severe HGG, defined as a decrease below 400 mg/dL, further increased the risk of infectious complications6. However, in the present study, only a small number of patients developed severe HGG 1 month after transplantation (n = 9, 4.4%), and these cases could not be analyzed further. Therefore, further studies are needed to investigate the association between severe HGG and infectious complications in larger populations.
A high MELD score is a significant risk factor for infectious complications after LT. Previous studies have shown that an increase in the MELD score is significantly associated with infectious complications, including BSIs25. The frequency of BSIs after LT ranges from 8 to 28% and varies between studies depending on the pre-LT liver disease status and donor type26,27. In this study, the incidence of BSIs was 37.8% and 26.1% in the high and low MELD groups, respectively. We also hypothesized that the high MELD group might have a higher incidence of HGG, which could contribute to the higher incidence of infectious complications. However, no difference in the incidence of HGG was found between the two groups during the pre- and post-LT periods. This suggests that a MELD score ≥ 30 is not directly associated with HGG and that perioperative HGG is influenced by multiple factors.
Pre-transplant HGG was identified as a significant risk factor for all-cause mortality, further stratifying the risk of death beyond the MELD score categories. This study found diabetes mellitus to be a predisposing condition associated with pre-LT HGG. Given the mixed results in previous studies on IgG levels in diabetes28,29 and the small sample size of the present study, further research is needed to clarify this finding. Apart from one patient who underwent ABO-incompatible living-donor LT and received rituximab with six plasmapheresis sessions before LT, potential explanations for pre-LT HGG include a protein-losing condition7. Although the retrospective design limits confirmation, these patients may have experienced decreased IgG levels due to excessive protein loss, outweighing increased immunoglobulin synthesis from plasma cell activation secondary to liver disease. This protein-losing state may have been driven by heightened mucosal permeability due to inflammation, infection, or intestinal lymphangiectasias from severe portal hypertension and cirrhosis7. Future studies are needed to determine if prophylactic IgG replacement benefits pre-LT HGG.
This study is the first to demonstrate an association between a GRWR of < 0.8 and HGG 1 month after LT. One possible mechanism involves IgG loss through ascites, lymphatic drainage, or the intestinal mucosa due to prolonged high portal pressure, which might be more pronounced during the regeneration of small liver grafts—a common aspect of small-for-size syndrome in low-GRWR grafts30. HGG after the early post-LT period should be interpreted in the context of recipients’ nutritional status, infectious conditions, use of immunosuppressants, or treatment for acute rejection, such as steroid pulse therapy, which is a known risk factor for secondary HGG7 and was also identified in this study. Evidence of the association between low GRWR and the risk of severe infection after LT is limited, and the relationship between insufficient graft volume and post-LT HGG requires further investigation.
This study had several limitations. First, being a single-center study conducted in Korea, its generalizability is limited. Second, propensity score matching was adequate to analyze differences in IgG dynamics based on MELD score, but may have reduced the sample size and limited the generalizability of secondary analyses. Additionally, post-LT serum IgG levels were measured 1 month after transplantation, which limited the identification of IgG decreases within the first month. The prevalence of HGG may be underestimated, as the lowest IgG levels typically occur within 1 month post-transplant19. Pre-LT IgG levels were measured from blood samples collected just before the transplant operation, potentially influenced by pre-LT plasmapheresis or rituximab administration in ABO-incompatible living-donor LT cases.
In conclusion, HGG occurred in 36% of LT recipients within the first year after transplantation and was associated with poor outcomes, including mortality and severe infection. No significant difference in HGG based on the MELD score was observed. However, pre-LT HGG further stratified the risk of death beyond the MELD score, warranting further study. Diabetes was significantly associated with HGGpre, whereas insufficient graft volume and steroid pulse therapy were significantly associated with HGG1mo. These findings highlight the need for early identification and targeted management of LT recipients at higher risk of HGG, particularly those with diabetes or small grafts. Further studies are needed to monitor HGG development in these high-risk recipients and determine whether additional treatment improves prognosis.
Data availability
The datasets used for the current study are available from the corresponding author upon reasonable request.
References
Kim, S. I. Bacterial infection after liver transplantation. World J. Gastroenterol. 20, 6211–6220 (2014).
Patel, R. & Paya, C. V. Infections in solid-organ transplant recipients. Clin. Microbiol. Rev. 10, 86–124 (1997).
Kim, Y. J. et al. Infectious complications in living-donor liver transplant recipients: a 9-year single-center experience. Transpl. Infect. Dis. 10, 316–324 (2008).
Snydman, D. R. Infection in solid organ transplantation. Transpl. Infect. Dis. 1, 21–28 (1999).
Hernandez Mdel, P., Martin, P. & Simkins, J. Infectious complications after liver transplantation. Gastroenterol. Hepatol. (N Y). 11, 741–753 (2015).
Florescu, D. F., Kalil, A. C., Qiu, F., Schmidt, C. M. & Sandkovsky, U. What is the impact of hypogammaglobulinemia on the rate of infections and survival in solid organ transplantation? A meta-analysis. Am. J. Transpl. 13, 2601–2610 (2013).
Otani, I. M. et al. Practical guidance for the diagnosis and management of secondary hypogammaglobulinemia: A work group report of the AAAAI primary immunodeficiency and altered immune response committees. J. Allergy Clin. Immunol. 149, 1525–1560 (2022).
Yoshizumi, T. et al. Decreased Immunoglobulin G levels after living-donor liver transplantation is a risk factor for bacterial infection and sepsis. Transpl. Infect. Dis. 16, 225–231 (2014).
Doron, S., Ruthazer, R., Werner, B. G., Rabson, A. & Snydman, D. R. Hypogammaglobulinemia in liver transplant recipients: incidence, timing, risk factors, and outcomes. Transplantation 81, 697–703 (2006).
Fallatah, H. I. & Akbar, H. O. Elevated serum Immunoglobulin G levels in patients with chronic liver disease in comparison to patients with autoimmune hepatitis. Libyan J. Med 5, 4857 (2010).
Simbrunner, B. et al. Dysregulated biomarkers of innate and adaptive immunity predict infections and disease progression in cirrhosis. JHEP Rep. 5, 100712 (2023).
Liu, W. T. et al. The injured liver induces hyperimmunoglobulinemia by failing to dispose of antigens and endotoxins in the portal system. PLoS One. 10, e0122739 (2015).
Kamath, P. S. & Kim, W. R. The model for end-stage liver disease (MELD). Hepatology 45, 797–805 (2007).
Child, C. G. & Turcotte, J. G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1, 1–85 (1964).
Ke, Q. et al. Prevalence, clinical characteristics, and outcomes of spontaneous portosystemic shunt in patients with hepatitis B-related cirrhosis: A multicenter study from China. Dig. Liver Dis. 55, 1382–1390 (2023).
CDC Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associated Bloodstream Infection). National Healthcare Safety Network (NHSN) Patient Safety Component Manual, 4 – 1. (2024).
Donnelly, J. P. et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 71, 1367–1376 (2020).
Gregorek, H. et al. Long-term monitoring of Epstein-Barr virus DNA load and humoral parameter abnormalities in pediatric liver transplant recipients before development of malignancy. Pediatr. Transpl. 14, 629–635 (2010).
González-Quintela, A. et al. Time-course changes of serum Immunoglobulins (IgA, igg, IgM) after liver transplantation for alcoholic cirrhosis. Transpl. Immunol. 11, 73–77 (2003).
Carles, J. et al. Preservation of human liver grafts in UW solution. Ultrastructural evidence for endothelial and Kupffer cell activation during cold ischemia and after ischemia-reperfusion. Liver 14, 50–56 (1994).
Posey, W. C., Nelson, H. S., Branch, B. & Pearlman, D. S. The effects of acute corticosteroid therapy for asthma on serum Immunoglobulin levels. J. Allergy Clin. Immunol. 62, 340–348 (1978).
Lavríková, P. et al. Tacrolimus has immunosuppressive effects on heavy/light chain pairs and free light chains in patients after heart transplantation: A relationship with infection. Transpl. Immunol. 50, 43–47 (2018).
Fernández-Ruiz, M. et al. Monitoring of Immunoglobulin levels identifies kidney transplant recipients at high risk of infection. Am. J. Transpl. 12, 2763–2773 (2012).
Jo, E. A. et al. The time-dependent changes in serum Immunoglobulin after kidney transplantation and its association with infection. Front. Immunol. 15, 1374535 (2024).
He, Q. et al. Risk factors of bloodstream infections in recipients after liver transplantation: a meta-analysis. Infection 47, 77–85 (2019).
Lee, I. K. et al. Risk factors and crucial prognostic indicators of mortality in liver transplant recipients with bloodstream infections: A comprehensives study of 1049 consecutive liver transplants over an 11-year period. J. Microbiol. Immunol. Infect. 57, 771–781 (2024).
Kim, H. K. et al. Epidemiology and clinical features of post-transplant bloodstream infection: an analysis of 222 consecutive liver transplant recipients. Infect. Chemother. 45, 315–324 (2013).
Ardawi, M. S., Nasrat, H. A. & Bahnassy, A. A. Serum Immunoglobulin concentrations in diabetic patients. Diabet. Med. 11, 384–387 (1994).
Guo, X. et al. Serum levels of Immunoglobulins in an adult population and their relationship with type 2 diabetes. Diabetes Res. Clin. Pract. 115, 76–82 (2016).
Masuda, Y. et al. Small-for-size syndrome in liver transplantation: definition, pathophysiology and management. Hepatobiliary Pancreat. Dis. Int. 19, 334–341 (2020).
Acknowledgements
None.
Funding
This research was funded by the Severance Hospital Research Fund for Clinical Excellence (SHRC) (Grant No. C-2024-0025). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: Lee Y, Min E-K, Lee JG, and Jeong SJ. Data curation: Lee Y, Min E-K, Joo DJ, Kim MS, Kim D-G, and Lee JG. Formal analysis and visualization: Lee Y. Investigation: Kim JI. Writing of the original draft: Lee Y and Min E-K. Funding acquisition: Jeong SJ. All the authors contributed to the methodology, review, and editing of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and Istanbul and approved by the Severance Hospital Institutional Review Board (Approval No. #4-2016-0323). Informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, Y., Min, EK., Kim, J.I. et al. Immunoglobulin G dynamics and outcomes of hypogammaglobulinemia in liver transplant recipients. Sci Rep 15, 31043 (2025). https://doi.org/10.1038/s41598-025-16543-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16543-y