Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) has become the most prevalent chronic liver disease globally, imposing significant economic burdens. In this study, we analyzed serum indicators—including ALT, AST, De-Ritis ratio, fasting blood glucose (FBG), lipid profiles, and blood pressure—among 162,699 Chinese individuals from Ping An Healthcare Diagnostics Center, stratified by gender, age, and weight. Comparative analysis revealed significant differences in these biomarkers between MASLD and Normal groups, enabling the identification of markers associated with MASLD subhealth. The overall MASLD prevalence was 36.57% (23.96% in men and 12.61% in women). Notable discrepancies were observed for ALT, De-Ritis ratio, triglycerides, and HDL-C across all groups, with AST, FBG, blood pressure, TC, and LDL-C differing significantly in most groups. Our findings highlight the association of some critical metabolic and hepatic biomarkers with MASLD, underscoring the imperative need for enhanced monitoring of metabolic health indicators. This approach may facilitate the identification of individuals at risk for MASLD in the Chinese population, paving the way for timely interventions.
Similar content being viewed by others
Introduction
Fatty liver disease is characterized by the excessive accumulation of fat in liver cells due to various causes and is primarily classified into alcoholic fatty liver disease and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). MASLD has emerged as the most prevalent liver disease in clinical practice and is a leading cause of chronic liver injury worldwide, presenting a spectrum of conditions ranging from simple steatosis to nonalcoholic steatohepatitis, cirrhosis, and even hepatocellular carcinoma1.
The increasing prevalence of MASLD poses a significant economic burden globally. Epidemiological studies indicate that the global prevalence of MASLD is approximately 30–32% in the general population2, with rates of 27.4% in Asia3,4 and exceeding 33% in China5,6. Due to the often-asymptomatic nature of the disease, the actual number of affected individuals is likely underestimated, with around 20% of patients presenting with irreversible advanced fibrosis or hepatocellular carcinoma before diagnosis7. Currently, MASLD can be managed through conservative and surgical interventions, but there are no specific pharmacological treatments approved for this condition. While several drugs are under investigation, pioglitazone and vitamin E are currently recommended in guidelines for select patients8. Lifestyle modifications, including dietary changes, weight loss, and management of underlying metabolic syndrome, are the primary therapeutic approaches, although they often prove challenging to maintain8,9.
Several indicators contribute to the development of MASLD, including obesity, age, gender, Type 2 diabetes mellitus (T2DM), dyslipidemia, and metabolic syndrome. Gender, age, and body mass index (BMI) significantly influence MASLD incidence. Research indicates a higher prevalence of MASLD among males compared to females, attributed to lifestyle differences, hormonal variations, and metabolic responses to lipids10,11. Age also plays a crucial role influencing the incidence of MASLD, which peaks between 30 and 70 years. The rising prevalence of MASLD in younger populations can likely be attributed to unhealthy dietary patterns and lifestyle choices, such as late nights, lack of exercise, and high-fat diets, which can adversely affect liver function12,13,14. The rising incidence of MASLD parallels the obesity epidemic, with prevalence rates in obese individuals surpassing those in the general population15,16,17,18. A high BMI correlates with an increased prevalence of MASLD, and some patients with MASLD may present with normal BMI in population screening or health check programs, highlighting the strong association between MASLD, obesity, and metabolic syndrome19. T2DM and obesity are major independent indicators for MASLD20,21. Epidemiological studies indicate that MASLD patients have a two-fold increased risk of developing T2DM, and vice versa, with this relationship largely mediated by insulin resistance (IR)22. T2DM patients are at a heightened risk for both the presence and severity of MASLD23,24.
Research emphasizes that preventing the onset and progression of MASLD should be a primary objective moving forward, with indentification of key indicators being paramount25,26. Serum levels of liver function enzymes (ALT, AST) and the De-Ritis ratio are closely associated with liver function status27,28. FBG is the standard diagnostic criterion for T2DM and reflects islet β-cell function, generally indicating basal insulin secretion29. As such, FBG is the most commonly used indicator for T2DM diagnosis. Therefore, this study focuses on serum liver function enzymes and FBG levels within the Chinese population, categorizing collected data to minimize interference from gender, age, and weight, to analyze whether these indicators associated with MASLD. The findings aim to provide valuable insights into the associations with MASLD, supporting further research into its prevention and treatment.
Results
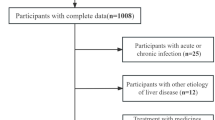
We collected data from a total of 162,699 individuals who underwent health check-ups from July 2022 to June 2023. Among these, 59,502 individuals were diagnosed with MASLD, resulting in a prevalence of 36.57%. This included 38,984 males (23.96%) and 20,513 females (12.61%) diagnosed with MASLD (Fig. 1; Table 1).
Some statistically significant differences in serum monitoring indicators between individuals of different genders, ages, and BMI groups in both MASLD and corresponding normal groups to identify key indictors for MASLD (Table 2). All corresponding ANOVA P-values and Pearson’s correlation results are displayed in (Table 3). ROC evaluations showed that ALT and TG achieved AUC of 0.775 and 0.782, respectively, indicating good discriminatory capacity between normal and MASLD (Table 4; Fig. 6). Strikingly, SBP yielded an AUC of 0.979, reflecting excellent sensitivity and specificity, and highlighting its potential utility for early clinical identification of MASLD. Multivariable logistic regression further revealed that ALT, AST, FBG, and TG were independently associated with MASLD risk. Specifically, elevated ALT and TG levels conferred higher odds of disease, while increases in AST and FBG likewise signaled greater susceptibility (Table 5). Together, these findings emphasize the clinical relevance of metabolic and hepatic biomarkers for the identification of individuals at risk for MASLD (Tables 4 and 5; Fig. 6).
ALT
In males, ALT levels showed significant differences across all MASLD groups compared to their corresponding normal groups in the normal BMI, overweight, and obesity categories at different age stratifications. The average ALT values for all normal groups (Mean ± SD) were below 20 U/L, significantly lower than the maximum normal reference value of 40 U/L. Nearly half of the MASLD groups had ALT values below 40 U/L (Fig. 2). In females, ALT levels exhibited similar patterns, with average values for all normal groups also below 20 U/L. ALT values for all MASLD groups were below 35 U/L, within the normal range (Fig. 2). ALT levels were higher in MASLD groups than in corresponding normal groups across most age and BMI strata (Fig. 2). Among the 44,253 MASLD participants tested for ALT, 18,509 males and 13,330 females had normal values, accounting for a substantial 71.95% (Table 2).
Comparative analysis of ALT data between normal and MASLD groups of the normal BMI, overweight, obesity at different age stratifications. Data are represented as Mean ± SD. Comparison ALT between MASLD and its corresponding normal groups of the normal BMI, overweight, obesity. *p < 0.05; **p < 0.01; ***p < 0.001.
AST
In males, AST levels showed significant differences across major MASLD groups and their corresponding normal groups. The average AST values for all normal and MASLD groups were below 36 U/L, remaining under the maximum normal reference value of 40 U/L (Fig. 3). In females, AST levels demonstrated significant differences in most MASLD groups and their normal counterparts, similar to males. The average AST values for all normal and MASLD groups were below 30 U/L, well below the maximum normal reference value (Fig. 3). Higher mean AST levels were observed in MASLD groups compared to corresponding normal groups (Fig. 3). Among the 44,351 MASLD participants tested for AST, 25,476 males and 14,420 females had normal values, accounting for a total of 89.96% (Table 2).
Comparative analysis of AST data between normal and MASLD groups of the normal BMI, overweight, obesity at different age stratifications. Data are represented as Mean ± SD. Comparison AST between MASLD and its corresponding normal groups of the normal BMI, overweight, obesity. *p < 0.05; **p < 0.01; ***p < 0.001.
De-Ritis ratio
In males, the De-Ritis ratio showed significant differences across all MASLD groups and their corresponding normal groups in the normal BMI, overweight, and obesity categories at various age stratifications. The AST/ALT average values for most normal groups (Mean ± SD) ranged from 0.8 to 1.3, while the AST/ALT average values for major MASLD groups ranged from 0.65 to 1.0 (Fig. 4). In females, the AST/ALT ratios also showed significant differences across all groups, mirroring the male results. The average AST/ALT values for most normal groups ranged from 1.1 to 1.5, while the average values for all MASLD groups fell between 0.8 and 1.25, remaining within the normal range (Fig. 4). Lower De-Ritis ratios were generally observed in MASLD groups relative to their corresponding normal groups (Fig. 4). Among the 44,252 MASLD participants tested for the De-Ritis ratio, 12,111 males and 10,823 females had normal values, comprising 51.83% of the total (Table 2).
Comparative analysis of De-Ritis ratio (AST/ALT) data between normal and MASLD groups of the normal BMI, overweight, obesity at different age stratifications. Data are represented as Mean ± SD. Comparison AST/ALT between MASLD and its corresponding normal groups of the normal BMI, overweight, obesity. *p < 0.05; **p < 0.01; ***p < 0.001.
FBG
In males, FBG levels showed significant differences in most MASLD groups compared to their corresponding normal groups, except for the elderly group aged > 65 years. The average FBG values for most normal and MASLD groups ranged from 4.9 to 6.1 mmol/L, falling within the normal range (Fig. 5). In females, FBG levels exhibited significant differences in major MASLD groups compared to normal groups. The average FBG values for all normal groups were between 4.9 and 6.1 mmol/L, while the average values for all MASLD groups ranged from 4.9 to 6.8 mmol/L, nearing normal values (Fig. 5). Among the 13,075 MASLD participants tested for FBG, 6,585 males and 3,605 females had normal ALT values, accounting for a total of 77.93% (Table 2).
Comparative analysis of FBG data between normal and MASLD groups of the normal BMI, overweight, obesity at different age stratifications. Data are represented as Mean ± SD. Comparison FBG between MASLD and its corresponding normal groups of the normal BMI, overweight, obesity. *p < 0.05; **p < 0.01; ***p < 0.001.
Blood pressure
In males, SBP and DBP levels exhibited significant differences in most MASLD groups compared to normal groups, particularly for the youngerly group aged from 18 to 29 years (Figure S1-S2 and Table 2). Specifically, the proportion of male MASLD patients with normal SBP values was as low as 27.96%, approximately 7% higher than that observed in female MASLD patient groups. In females, SBP and DBP levels showed significant differences in most MASLD groups compared to their corresponding normal groups, except for the elderly group aged > 65 years. The average SBP values in all MASLD groups ranged from 110 to 150 mmHg, with some patients exceeding normal limits, while the average DBP values remained within the standard clinical range (Table 2).
Lipid-related profiles
In females, TC and TG levels displayed significant differences across all MASLD groups compared to their corresponding normal groups in the normal BMI, overweight, and obesity categories at different age stratifications (Figure S3-S4). In males, TG also showed significant differences across all groups, mirroring the females’ results. And the average TG values for both normal and MASLD males’ groups exceeded 1.7 mmol/L, surpassing the standard clinical reference threshold (Table 2). Moreover, we observed an intriguing finding. Across various age subgroups, TC did not exhibit statistically significant differences between MASLD and normal groups across obesity categories, whereas TG levels demonstrated significant statistical differences within the corresponding age strata of these two groups (Figure S4).
Among the 43,524 MASLD participants tested for LDL-C, 14,090 males and 7,776 females had normal values, accounting for a total of 50.06% and 50.56% respectively (Figure S5-S6 and Table 2). Although the average LDL-C values in all normal and MASLD groups range from 2.5 to 3.2mmol/L, significant statistical differences were observed in most age and BMI comparisons, with the exception of the male obesity group (Figure S5). Additionally, compared to LDL-C, HDL-C exhibited more pronounced and comprehensive statistical differences between MASLD and normal groups, particularly in various BMI categories among female subjects. Lower HDL-C were generally observed in MASLD groups (below 1.4 mmol/L) relative to their corresponding normal groups (below 1.5 mmol/L).
Discussion
The liver plays a crucial role in fat metabolism within the human body. Excessive fat intake, combined with diminished liver function, can result in significant fat accumulation in the liver, leading to conditions such as MASLD and liver fibrosis. If left untreated, these conditions can progress to liver failure30,31. Therefore, early diagnosis of fatty liver is essential, along with effective measures to reverse the condition and inhibit liver fibrosis and cirrhosis. However, the asymptomatic nature and insidious onset of MASLD pose challenges for timely clinical diagnosis32.
Liver biopsy is the “gold standard” for diagnosing MASLD, but its invasiveness and associated discomfort limit clinical use33. In contrast, ultrasound examination is a non-invasive, cost-effective, and widely used method in clinical practice for assessing MASLD. However, its diagnostic accuracy may be limited by equipment quality and operator expertise, leading to potential misclassification34gained popularity in MASLD diagnosis due to advancements in technology and research. These markers can gauge liver damage effectively, offering low trauma and high detection efficiency35,36. Therefore, identifying reliable serum biomarkers for MASLD is crucial for exploring their potential role in risk stratification.
Liver function enzymes, including ALT and AST, serve as biomarkers for liver injury. Elevated levels of these enzymes indicate hepatocellular, with ALT being more specific for hepatic injury than AST36. Behnaz Amernia et al. suggested that the aspartate aminotransferase to platelet ratio index (APRI) and the De-Ritis ratio could serve as alternatives to FibroScan for assessing hepatic fibrosis in MASLD patients, highlighting their utility in clinical practice37. Additionally, an elevated De-Ritis ratio serves as a simple, accessible marker associated with increased risk of MASLD, cardiovascular disease, all-cause mortality, and diabetes27.
MASLD is a highly prevalent condition associated with metabolic syndrome, often characterized by mild elevations in AST/ALT levels22,38. In chronic liver diseases, these enzymes are routinely used to reflect the degree of hepatic injury and disease progression28. Recent research indicates that the interpretation of elevated AST and ALT levels should be informed by clinical presentation and the degree of elevation, with normal ALT values being < 29–33 U/L in males and 19–25 U/L in females36. The present study also observed this pattern in a large population-based Chinese cohort8. Our research findings align with this conclusion.
AST, ALT, and the De-Ritis ratio reflect disease severity in various chronic liver diseases, including MASLD39 alcohol-related liver disease (ALD)40,41 hepatitis C, and autoimmune liver disease42. In MASLD, ALT levels tend to be higher than AST, consistent with prior studies. The AST/ALT ratio aids in determining the etiology of abnormal liver tests. Generally, conditions like chronic viral hepatitis and MASLD usually show ALT > AST, while AST > ALT may occur in patients with cirrhosis such as ALD or ischemic hepatitis, with approximately 90% of patients of ALD patients having AST > ALT and over 70% exhibiting a De-Ritis ratio > 236. Elevated liver enzyme levels are associated with increased mortality due to hepatocellular damage and systemic disorders, while low transaminase levels may indicate higher mortality linked to liver senescence, affecting both individuals with and without MASLD43.
Medications and lifestyle interventions significantly influence AST and ALT levels. For instance, semaglutide has been shown to alleviate symptoms in Metabolic Dysfunction-Associated Steatohepatitis (MASH) patients, leading to significant reductions in ALT and AST values, as confirmed by liver biopsy, with a reported 17-unit decrease compared to the placebo group in a randomized trial44. Similarly, Matthew J et al. reported that liraglutide improved AST and ALT levels over 12 weeks in a double-blind study45,46 while pioglitazone yielded significant enhancements in liver histology and reductions in liver biomarkers in patients with prediabetes or T2DM and MASLD47. Additionally, aerobic exercise has been shown to significantly improve various health indicators in MASLD patients, including ALT, AST, SBP, DBP, TG, TC, LDL-C, HDL-C, and BMI. High-intensity interval training particularly improves ALT levels, while resistance training significantly reduces AST and TG levels48.
T2DM is an independent risk factor for MASLD, and resolving hepatic steatosis may help prevent the onset of T2DM22,49. In the global burden study of MASLD, T2DM prevalence in MASLD patients is 22.5% − 43.6%, and up to 80% of MASLD patients may develop new-onset T2DM50. In addition, Suwała et al. reported that (pre)diabetes is associated with a 36% higher risk of developing MASLD regardless of the diagnostic criterion adopted51. These findings underscore the close association between T2DM and MASLD and highlight the importance of considering this relationship in clinical practice. FBG not only serves as a marker for diabetes but may also be a significant biomarker for MASLD29,52. Recent studies have emphasized the role of metabolic and hepatic biomarkers in assessing the risk and progression of MASLD. Huang et al. developed a metabolome-derived score for predicting MASH and liver disease mortality using machine learning53. The score, based on clinical parameters and serum metabolites, accurately identified MASH in Chinese and Finnish individuals and outperformed existing fibrosis scores in predicting mortality. In addition, Gao et al. reported that the triglyceride-glucose body mass index (TyG-BMI) was the most valuable indicator of all-cause and cardiovascular mortality in patients with MASLD54. The study revealed a U-shaped relationship between the TyG-BMI and mortality, with an inflection point at 270.49, indicating that both high and low values of TyG-BMI were associated with increased mortality risk. Furthermore, Zhang et al. found that elevated TyG-related indices are associated with increased mortality in MASLD patients with prediabetes or diabetes, highlighting the importance of metabolic and hepatic biomarkers such as ALT, AST, FBG, TG, SBP, and DBP in risk assessment and treatment management55.
The observed biomarker patterns in this large-scale cohort can be understood in the context of MASLD’s core pathophysiology. Chronic hyperinsulinemia and systemic IR promote increased lipolysis in adipose tissue, leading to elevated free fatty acid flux to the liver. This excess substrate, together with IR-driven upregulation of hepatic de novo lipogenesis and impaired suppression of gluconeogenesis, results in progressive triglyceride accumulation within hepatocytes56. As hepatic fat storage capacity approaches the individual’s personal fat threshold, lipids begin to “spill over” into ectopic depots, generating toxic lipid intermediates such as ceramides and diacylglycerols57. These lipotoxic species impair mitochondrial function, activate inflammatory pathways, and induce hepatocellular injury—manifesting clinically as subtle increases in ALT and changes in the De-Ritis ratio even before overt enzyme elevation58. Concomitantly, IR-associated dyslipidemia, reflected by higher triglycerides and lower HDL-C in our MASLD participants, indicates impaired clearance of atherogenic remnant particles58. The small but consistent increases in fasting glucose, alongside elevated systolic blood pressure, further reflect systemic metabolic stress and endothelial dysfunction accompanying hepatic steatosis. Together, these findings support the concept that MASLD is a systemic metabolic disorder with hepatic and extrahepatic manifestations, and they highlight the potential value of composite risk markers—such as TG/HDL-C ratio, TyG index, or waist-to-height ratio (WHtR)—that integrate these interrelated processes for earlier detection and risk stratification.
Furthermore, we found that Pearson correlation coefficients are very small but significant due to the large sample size in (Table 3). It is worth noting that statistical significance does not always equate to clinical significance. In chronic liver diseases like MASLD, small effects can still have substantial clinical implications. For example, slight elevations in ALT or AST can indicate underlying hepatic dysfunction, particularly when associated with other metabolic risk factors. Furthermore, we observed the majority of MASLD patients in our study presented with biomarker levels within normal ranges, suggesting a potential patterns of subclinical progression. Our findings highlight the necessity for a more nuanced approach in clinical evaluations, emphasizing that even modest changes in biomarkers—despite being statistically small—can signify increased risk for more severe liver conditions. These observations may advocate for enhanced monitoring and early intervention strategies in at-risk populations. In conclusion, while the Pearson correlation coefficients may be small, they indicate clinically relevant associations that must not be overlooked in the management of MASLD.
Given the pathophysiological associations described above, ALT, AST, the De-Ritis ratio, FBG, SBP, DBP, TG, TC, LDL-C and HDL-C are candidate biomarkers associated with MASLD, warranting further investigation for their potential in screening and risk evaluation. These indicators reflect key aspects of liver function, glucose metabolism, and lipid profiles, all of which are mechanistically implicated in the development and progression of MASLD. Their combined use allows for a more comprehensive evaluation of metabolic risk and disease burden, particularly in large-scale screening settings where non-invasive, accessible biomarkers are essential.
Additionally, it is imperative to consider an array of non-invasive markers of steatosis, such as the Fatty Liver Index (FLI)59 Hepatic Steatosis Index (HSI)60 Visceral Adiposity Index (VAI)61 and ZJU index62. FLI, which incorporates BMI, waist circumference, TG, and GGT, demonstrates an accuracy of 0.84 in detecting fatty liver59. HSI, by integrating BMI and the ALT/AST ratio, shows remarkable accuracy in assessing MASLD, with high sensitivity and specificity in the Korean population60. VAI not only reflects fat distribution but also its functional status, and is strongly associated with cardiometabolic risk61. The ZJU index, combining BMI, FBG, TG, and the ALT/AST ratio, is particularly suitable for the Chinese population62. Each of these markers has unique advantages and provides clinicians with a variety of options. Future research could further explore how to combine these markers to enhance the understanding of their associations with MASLD63. In summary, the integrated use of these non-invasive markers is expected to contribute significantly to more precise management and prompt intervention in MASLD.
Despite the large sample size and population-based design, several limitations warrant consideration. First, participants were recruited from health check-up centers, which may underrepresent lower socioeconomic or less health-conscious groups and thus limit generalizability. Second, anthropometric measures such as waist circumference were not collected, precluding assessment of central adiposity and comparison with indices like the WHtR, TG/HDL-C ratio, and TyG index. Third, our analysis was restricted to routine biochemical indicators without imaging or histological confirmation, limiting evaluation of MASLD severity. Fourth, while several biomarkers were identified, no predictive model was established. Future studies should integrate diverse data sources and advanced approaches such as machine learning. Fifth, the cross-sectional design prevents causal inference, and longitudinal follow-up is needed to delineate the natural history of MASLD.
Our findings revealed significant differences in ALT, De-Ritis ratio, TG and HDL-C across all groups, while AST, FBG, DBP, SBP, TC and LDL-C showed significant differences in most groups, except among the elderly. Notably, we observed that the majority of MASLD patients exhibited biomarker levels within normal reference ranges, indicating a potential pattern of subclinical progression. Among these markers, ALT and the De-Ritis ratio were more frequently elevated in MASLD compared to AST and FBG, indicating their potential clinical utility as critical indicators. These findings underscore the potential role of routine monitoring of these metabolic and hepatic biomarkers in population-level MASLD in China, supporting further research into their clinical utility.
Methods
Data collection source
Data were collected from the Ping An Healthcare Diagnostics Center between July 2022 and June 2023, primarily from 11 diagnostic centers across China, including Shanghai, Xi’an, Shenyang, Nanchang, Xiangyang, Wuhan, Chongqing, Xiamen, Guangzhou, Qingdao, and Hefei. Participants included healthy or sub-healthy individuals who had not undergone major surgery or been diagnosed with related diseases prior to their physical examinations. Specifically, individuals with chronic liver diseases (e.g., viral hepatitis, alcoholic liver disease), severe cardiovascular diseases, uncontrolled hypertension, Type 1 diabetes mellitus, severe kidney disease, malignancies, or those undergoing immunosuppressive therapies were excluded.
For experiments involving human participants, informed consent must have been obtained. For participants who were unable to provide informed consent themselves (e.g., minors or individuals with impaired decision-making capacity), informed consent was obtained from a parent and/or legally authorized representative prior to participation. All participants or their guardians signed forms agreeing to the use of their data for health management research. Our study does not involve the use of patients’ tissue samples. All data used in this research were anonymized, and no patient names or identifying features are included in the manuscript. And the study was reviewed and approved by the Ping An Healthcare Diagnostics Center.
The study was performed in accordance with the Declaration of Helsinki and all relevant national and international guidelines and regulations. The experimental protocol was reviewed and approved by the Ping An Healthcare Diagnostics Center. All methods were carried out in strict compliance with the approved protocol and the aforementioned guidelines.
Basic data collection
Participants provided personal information, including gender and age, when selecting special combined health check-up services. They were required to arrive for the check-up in a fasting state. Medical staff measured height and weight using the Dingheng Electronics DHM-200 Electronic Scale, with height measured in meters and weight measured in kilograms. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Participants were categorized into BMI classes based on both Chinese and World Health Organization (WHO) criteria. According to the Chinese classification, underweight is defined as a BMI < 18.5 kg/m2, normal BMI as 18.5 to 23.9 kg/m2, overweight as 24 to 27.9 kg/m2, and obesity as BMI ≥ 28 kg/m2. The WHO criteria define underweight as a BMI < 18.5 kg/m2, normal BMI as 18.5 to < 25 kg/m2, overweight as 25 to < 30 kg/m2, and obesity as BMI ≥ 30 kg/m264,65,66. A summary of participant comparisons regarding height and weight is presented in Table S1.
Data for 99 individuals aged < 18 and 4,624 individuals with a BMI < 18.5 were excluded from further analysis due to their small numbers and low prevalence of MASLD (Table 1). To further investigate influencing indicators, we categorized the data based on gender, age, and BMI. Gender groups were divided into male and female cohorts, and data (including MASLD and normal groups) were compared across age intervals (< 18, 18–29, 30–39, 40–49, 50–65, > 65 years) for both genders67. Additionally, participants were grouped according to BMI: underweight, normal weight, overweight, and obese64,65,66.
Ultrasound examination of MASLD
Examining physicians and diagnostic doctors at the Ping An Healthcare Diagnostics Center adhered strictly to the guidelines outlined in the 2018 update for the prevention and treatment of MASLD regarding examination procedures and diagnosis (Fig. 1). Ultrasound examinations were conducted using three advanced ultrasound systems: the GE LOGIQ E9 Ultrasound System (General Electric Company, Chicago, Illinois, USA), the GE Vivid E80 Ultrasound System (General Electric Shanghai Medical Equipment Co., Ltd., Shanghai, China) and the Philips EPIO 7 Color Ultrasound Diagnostic System (Philips Healthcare, Amsterdam, Netherlands). Both devices provided high-resolution imaging capabilities essential for accurately assessing liver conditions.
Detection of serum biochemical indicators
Medical staff followed the operating procedures established by the Ping An Healthcare Diagnostics Center for blood collection, sample storage, and processing. Serum samples were analyzed according to the center’s protocols. Testing kits for ALT and AST, as well as glucose test kits, were provided by Mindray and Beckman. Biochemical analyses were conducted using the Beckman AU680 biochemical analyzer primarily in Shanghai, the Beckman AU5821 in Guangzhou, and the fully automated Mindray BS2000 biochemical luminescence analyzer in Xiamen and Shenyang. The standard units for ALT and AST were U/L, while FBG was measured in mmol/L.
We separated all data on ALT, AST, De-Ritis ratio, FBG, TG, TC, SBP, DBP, HDL-C and LDL-C into male and female groups, and analyzed their distributions within and between Normal and MASLD groups (Figs. 2, 3, 4 and 5, S1-S6). We also categorized the data into various age stratifications based on differing MASLD incidence rates. Furthermore, we analyzed serum monitoring indicator levels in MASLD groups and their corresponding normal groups for the normal BMI, overweight, and obesity groups. Finally, inspired by the study conducted by Abenavoli et al.58,68,69 we performed ROC curve evalutions and Logistic regression analyses to assess the ability of metabolic and hepatic biomarkers for distinguishing normal individuals from MASLD in the Chinese population (Tables 4 and 5; Fig. 6). In the multivariable Logistic Regression analysis, we excluded AST/ALT to avoid collinearity issues.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., San Diego, California, USA). Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were summarized as counts and percentages. The Kolmogorov-Smirnov test was applied solely to assess the normality of the data distributions. Given the large sample size, the Student’s t-test was used for comparisons between two groups, and one-way analysis of variance (ANOVA) was used for comparisons among more than two groups. In the figure legends, “n” represents the number of participants. Correlations were assessed using Pearson correlation tests. A two-sided p-value of < 0.05 was considered statistically significant.
Data availability
The data that support the findings of this study are available from the Ping An Healthcare Diagnostics Center, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request to the corresponding author (Shuqiong Huang, [huangsq@xmu.edu.cn]) and with permission of the Ping An Healthcare Diagnostics Center.
Abbreviations
- MASLD:
-
Metabolic dysfunction-associated steatotic liver disease
- FBG:
-
Fasting blood glucose
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- T2DM:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
References
Fang, J. et al. Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications. Front. Cell. Infect. Microbiol. 12, 997018 (2022).
Golabi, P., Isakov, V. & Younossi, Z. M. Nonalcoholic fatty liver disease: disease burden and disease awareness. Clin. Liver Dis. 27, 173–186 (2023).
Lonardo, A., Byrne, C. D., Caldwell, S. H., Cortez-Pinto, H. & Targher, G. Global epidemiology of nonalcoholic fatty liver disease: Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 1388–1389 (2016).
Wiest, R., Albillos, A., Trauner, M., Bajaj, J. S. & Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 67, 1084–1103 (2017).
Zhou, J. et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology 71, 1851–1864 (2020).
Wong, W. K. & Chan, W. K. Nonalcoholic fatty liver disease: A global perspective. Clin. Ther. 43, 473–499 (2021).
Leung, H. et al. Risk assessment with gut Microbiome and metabolite markers in NAFLD development. Sci. Transl Med. 14, eabk0855 (2022).
Pouwels, S. et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 22, 63 (2022).
Romero-Gómez, M., Zelber-Sagi, S. & Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 67, 829–846 (2017).
DiStefano, J. K. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology 161 (2020).
Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 7, 851–861 (2022).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Targher, G., Byrne, C. D. & Tilg, H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and Pharmacological implications. Gut 69, 1691–1705 (2020).
Lei Ting, L. Y. Analysis of the detection rate of fatty liver detection rate and related risk factors in health examination personnel. Xinjiang Med. J. 53, 1458–1461 (2023).
Sun, M. & Sun, H. Recent prevalence and trends of obesity and metabolic dysfunction-associated steatotic liver disease (MASLD) among US adolescents: 1999 to 2020. Pediatr. Obes. 20, e70003 (2025).
Huang, C., Gao, Z., Huang, Z. & Xu, J. Nonlinear association between body roundness index and metabolic dysfunction associated steatotic liver disease in nondiabetic Japanese adults. Sci. Rep. 15, 15442 (2025).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism 92, 82–97 (2019).
Fazel, Y., Koenig, A. B., Sayiner, M., Goodman, Z. D. & Younossi, Z. M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 65, 1017–1025 (2016).
Fan, J. G., Kim, S. U. & Wong, V. W. New trends on obesity and NAFLD in Asia. J. Hepatol. 67, 862–873 (2017).
Younossi, Z. M. et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 69, 564–568 (2020).
Ferguson, D. & Finck, B. N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 17, 484–495 (2021).
Tanase, D. M. et al. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 3920196 (2020).
Caussy, C., Aubin, A. & Loomba, R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Curr. Diab Rep. 21, 15 (2021).
Zhang, H. et al. Global burden of metabolic diseases, 1990–2021. Metabolism 160, 155999 (2024).
Sheng, G. et al. Waist-to-height ratio and non-alcoholic fatty liver disease in adults. BMC Gastroenterol. 21, 239 (2021).
Araújo, A. R., Rosso, N., Bedogni, G., Tiribelli, C. & Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: what we need in the future. Liver Int. 38 (Suppl 1), 47–51 (2018).
Rief, P. et al. The AST/ALT (De-Ritis) ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Med. (Baltim). 95, e3843 (2016).
Liu, Y. et al. AST to ALT ratio and arterial stiffness in non-fatty liver Japanese population:a secondary analysis based on a cross-sectional study. Lipids Health Dis. 17, 275 (2018).
Stedman, M. et al. The experience of blood glucose monitoring in people with type 2 diabetes mellitus (T2DM). Endocrinol. Diabetes Metab. 5, e00302 (2022).
Bansal, S., Vachher, M., Arora, T., Kumar, B. & Burman, A. Visceral fat: A key mediator of NAFLD development and progression. Hum. Nutr. Metabolism. 33, 200210 (2023).
Ross, R., Soni, S. & Houle, S. A. Negative energy balance induced by exercise or diet: Effects on visceral adipose tissue and liver fat. Nutrients 12, (2020).
Aguilera-Méndez, A. [Nonalcoholic hepatic steatosis: a silent disease]. Rev. Med. Inst. Mex Seguro Soc. 56, 544–549 (2019).
Ahn, S. B. Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: current and future developments. Clin. Mol. Hepatol. 29, S150–s156 (2023).
Miles, D. A. et al. Pocket-Sized versus conventional ultrasound for detecting fatty infiltration of the liver. Dig. Dis. Sci. 65, 82–85 (2020).
Ramadan, A., Khalaf, A. R., Ray, E., Saad, Y. & A. & Serum Anandamide level as a potential indicator for nonalcoholic fatty liver disease severity. Eur. J. Gastroenterol. Hepatol. 33, e363–e367 (2021).
Kwo, P. Y., Cohen, S. M. & Lim, J. K. ACG clinical guideline: evaluation of abnormal liver chemistries. Am. J. Gastroenterol. 112, 18–35 (2017).
Amernia, B., Moosavy, S. H., Banookh, F. & Zoghi, G. FIB-4, APRI, and AST/ALT ratio compared to fibroscan for the assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease in Bandar abbas, Iran. BMC Gastroenterol. 21, 453 (2021).
Cotter, T. G. & Rinella, M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 158, 1851–1864 (2020).
Åberg, F. et al. A dynamic Aspartate-to-Alanine aminotransferase ratio provides valid predictions of incident severe liver disease. Hepatol. Commun. 5, 1021–1035 (2021).
Thiele, M. et al. Accuracy of the enhanced liver fibrosis test vs fibrotest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology 154, 1369–1379 (2018).
Whitfield, J. B. et al. Evaluation of laboratory tests for cirrhosis and for alcohol use, in the context of alcoholic cirrhosis. Alcohol 66, 1–7 (2018).
Botros, M. & Sikaris, K. A. The de Ritis ratio: the test of time. Clin. Biochem. Rev. 34, 117–130 (2013).
Grob, S. R., Suter, F., Katzke, V. & Rohrmann, S. The association between liver enzymes and mortality stratified by non-alcoholic fatty liver disease: An analysis of NHANES III. Nutrients 15, (2023).
Loomba, R. et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 8, 511–522 (2023).
Armstrong, M. J. et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 64, 399–408 (2016).
Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387, 679–690 (2016).
Lian, J. & Fu, J. Pioglitazone for NAFLD patients with prediabetes or type 2 diabetes mellitus: A Meta-Analysis. Front. Endocrinol. (Lausanne). 12, 615409 (2021).
Xiong, Y., Peng, Q., Cao, C., Xu, Z. & Zhang, B. Effect of different exercise methods on non-alcoholic fatty liver disease: A meta-analysis and meta-regression. Int J. Environ. Res. Public. Health 18 (2021).
Arab, J. P., Arrese, M. & Trauner, M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu. Rev. Pathol. 13, 321–350 (2018).
Miao, L., Targher, G., Byrne, C. D., Cao, Y. Y. & Zheng, M. H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metabolism. 35, 697–707 (2024).
Suwała, S. & Junik, R. Assessment of the liver steatosis and fibrosis risk in metabolic syndrome and its individual components, considering the varying definitions used in clinical practice throughout time: A retrospective Cross-Sectional study. Biomedicines 12, 1739 (2024).
Niu, S. et al. Semaglutide ameliorates metabolism and hepatic outcomes in an NAFLD mouse model. Front. Endocrinol. (Lausanne). 13, 1046130 (2022).
Huang, Q. et al. A metabolome-derived score predicts metabolic dysfunction-associated steatohepatitis and mortality from liver disease. J. Hepatol. 82, 781–793 (2025).
Gao, X. et al. The association between different insulin resistance surrogates and all-cause mortality and cardiovascular mortality in patients with metabolic dysfunction-associated steatotic liver disease. Cardiovasc. Diabetol. 24, 200 (2025).
Zhang, Y., Wu, J., Li, T., Qu, Y. & Wang, Y. Association of triglyceride-glucose related indices with mortality among individuals with MASLD combined with prediabetes or diabetes. Cardiovasc. Diabetol. 24, 52 (2025).
Simancas-Racines, D. et al. Nutritional strategies for battling Obesity-Linked liver disease: the role of medical nutritional therapy in metabolic Dysfunction-Associated steatotic liver disease (MASLD) management. Curr. Obes. Rep. 14, 7 (2025).
Tantu, M. T. et al. Pathophysiology, noninvasive diagnostics and emerging personalized treatments for metabolic associated liver diseases. Npj Gut Liver. 2, 18 (2025).
Tauil, R. B. et al. Metabolic-Associated fatty liver disease: the influence of oxidative stress, inflammation, mitochondrial dysfunctions, and the role of polyphenols. Pharmaceuticals 17, 1354 (2024).
Bedogni, G. et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33 (2006).
Lee, J. H. et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 42, 503–508 (2010).
Amato, M. C. et al. Visceral adiposity index. Diabetes Care. 33, 920–922 (2010).
Wang, J. et al. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci. Rep. 5, 16494 (2015).
Han, A. L. & Lee, H. K. Comparison of the diagnostic performance of steatosis indices for discrimination of CT-Diagnosed metabolic Dysfunction-Associated fatty liver disease. Metabolites 12, 664 (2022).
Chen, K. et al. Prevalence of obesity and associated complications in china: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. https://doi.org/10.1111/dom.15238 (2023).
Younes, R. & Bugianesi, E. NASH in lean individuals. Semin Liver Dis. 39, 86–95 (2019).
Zhu, X. P. et al. Fatty liver is a sensitive early warning for hypertension and its complication in the Chinese population. Clin. Exp. Hypertens. 44, 306–312 (2022).
Fresneda, S. et al. Sex and age differences in the association of fatty liver index-defined non-alcoholic fatty liver disease with cardiometabolic risk factors: a cross-sectional study. Biol. Sex. Differ. 13, 64 (2022).
Abenavoli, L. et al. Use of metabolic scores and lipid ratios to predict metabolic dysfunction-associated steatotic liver disease onset in patients with inflammatory bowel diseases. JCM 14, 2973 (2025).
Scarlata, G. G. M. & Abenavoli, L. Gut microbiota: the pathogenetic Bridge between inflammatory bowel disease and metabolic-associated steatotic liver disease. Expert Rev. Gastroenterol. Hepatol. 19, 85–88 (2025).
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Natural Science Foundation of Xiamen, China (3502Z20227026) and Student Innovation and Entrepreneurship training program of Xiamen University (2024Y1022, 2025X632).
Author information
Authors and Affiliations
Contributions
HC, ZC contributed equally to this work. HC, ZC and XB wrote the manuscript. SH, JL, YW and LH collected health examination data. HC, XB grouped and analyzed data. ZL, DL reviewed and edited this paper. KX revised the manuscript. SH and KX conceived, designed, and implemented this paper. All of the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, H., Chen, Z., Bai, X. et al. Metabolic and hepatic biomarkers associated with MASLD in the Chinese population. Sci Rep 15, 31593 (2025). https://doi.org/10.1038/s41598-025-17219-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17219-3