Abstract
During early pregnancy in mice, leukemia inhibitory factor (LIF) regulates embryo implantation by activating the JAK/STAT3 signaling pathway. The STAT3 pathway has been recognized to play a critical role in embryo implantation; however, it remains unclear whether STAT3 activation alone is sufficient to induce implantation. In this study, we investigated the effects of RO8191, a potential STAT3 activator, on embryo implantation through a series of studies with different mouse models. We found that RO8191 can induce embryo implantation and decidual reaction by activating STAT3, but not STAT1, signaling in both epithelial and stromal compartments in delayed implantation models. Furthermore, RO8191 was able to rescue implantation and establish pregnancy even in uterine epithelial-specific Lifr conditional knockout (cKO) mice, which exhibit infertility due to implantation failure. In contrast, in uterine epithelial-specific Stat3 or Gp130 conditional knockout (cKO) mice, which also show embryo implantation failure, RO8191 induces only a partial decidual response. These results suggest that STAT3, Gp130 and LIFR each play distinct roles in embryo implantation and development. Although the detailed mechanisms underlying RO8191’s action remain to be elucidated, our findings provide insights supporting its potential application in treating recurrent implantation failure.
Similar content being viewed by others
Introduction
Embryo implantation is a crucial stage in early mammalian gestation. The blastocyst hatches, attaches, adheres, and invades the receptive uterus in humans and mice1. Failure of embryo implantation is a significant challenge in the field of infertility treatment. Previous studies have shown that the success rate of implantation per embryo hovers around 25% in human populations with normal fertility, according to both natural cycles and assisted reproductive technology indications1. Although the exact molecular mechanisms of blastocyst implantation are not fully understood, existing research has revealed that successful implantation depends on the synchronization of endometrial receptivity with blastocyst activation2. For example, insufficient endometrial receptivity can lead to recurrent implantation failure (RIF) in clinical pregnancies3,4. Endometrial receptivity is controlled by the hormones 17β-estradiol (E2) and progesterone (P4)5, which are primarily synthesized by the ovaries.
In mice, embryo implantation occurs on the fourth day of pregnancy (D4) (D1=the day the vaginal plug is found). This process is triggered by the transient elevation of E26,7. E2 can stimulate the release of leukemia inhibitory factor (LIF), a cytokine that belongs to the interleukin (IL)-6 family cytokines (including oncostatin M, IL-11, IL-27, ciliary neurotrophic factor [CNTF], and cardiotrophin-1 [CT-1])8. It is widely accepted that LIF is expressed in the uterine glandular epithelium (GE) during the pre-implantation phase9 and is considered crucial for implantation10. The LIF interacts with the LIF receptor (LIFR) on the luminal epithelium and forms a heterodimer with the glycoprotein 130 (Gp130)11. This complex heterodimer activates the JAK/STAT3 signaling pathway, leading to the phosphorylation of Janus kinase and subsequent phosphorylation of STAT312,13. Following phosphorylation, p-STAT3 translocates to the nucleus, where it mediates the expression of a variety of genes in the cell11.
Infertility due to failure of embryo adhesion has been observed in Lif-deficient mice14. The importance of LIF for embryo implantation has been further demonstrated by the finding that intraperitoneal administration of a LIF antagonist15 or anti-LIF antibody16 in the C57BL/6J (B6) mouse strain prevented embryo implantation, leading to infertility. The importance of LIF in embryo implantation was also demonstrated in the delayed implantation (DI) mouse model. The DI mouse model provides a valuable experimental system for investigating molecular mechanisms underlying embryo implantation17. This model achieves implantation arrest through ovariectomy (OVX) performed prior to the E2 surge on D4, with delayed implantation maintained via continuous P4 supplantation such as medroxyprogesterone acetate. Intriguingly, injection of recombinant LIF or recombinant CT-1 can induce embryo implantation by activating STAT3 signaling in the uterine epithelium in DI mice (ICR or B6 strain)18. In contrast, CNTF, which belongs to the same IL-6 cytokine family as LIF and CT-1, neither induced embryo implantation nor activated STAT3 phosphorylation in DI mice. Mice with a conditional knockout of Stat3 in the uterine epithelium exhibit infertility due to implantation failure19. Similarly, mice with a conditional knockout of either Lifr20,21 or Gp13022 in the uterine epithelium are also infertile due to failure of blastocyst implantation. These findings suggest that the epithelial-originated LIFR/Gp130-JAK/STAT3 signaling pathway is essential for successful embryo implantation in mice.
Successful embryo implantation can be achieved through activation of STAT3, but to date, no pharmacological agents have been used to activate the STAT3 pathway and induce implantation in mice. The purpose of the present study was to determine whether the pharmacological agents could induce embryo implantation by activating the JAK/STAT3 signaling pathway. We found that RO8191, an interferon-α receptor 2 agonist23, is a useful STAT3 activator that can induce embryo implantation.
Materials and methods
Animals
This study was approved by the Ethics Review Board for Animal Experiments at Nagoya University (approval number: A240034-002) and the Ethical Committee for Vertebrate Experiments at Azabu University (approval number: 230327-9). All experiments were conducted in accord with the relevant guidelines and regulations, including the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
ICR (Japan SLC, Shizuoka, Japan), C57BL/6J (Jackson Laboratory Japan, Kanagawa, Japan), and the following mouse strains, all aged over seven weeks, were used in the experiments: LtfiCre/+ mouse < Ltftm1(icre)Tdku/J, JAX: 026030 >24, Stat3flox/flox (Stat3f/f) < Stat3tm2Aki>25, Gp130flox/flox (Gp130f/f) mouse < Il6sttm1Wme>26, and Lifrflox/flox (Lifrf/f) < Lifrtm1c(EUCOMM)Hmgu>. To obtain the Lifrflox/flox mice (tm1c), Lifrtm1a(EUCOMM)Hmgu mice purchased from the European Mouse Mutant Archive (EMMA) (strain ID: EM:06941), harboring a knockout first allele, were crossed with FLPe transgenic mice < Tg(CAG-flpe)36Ito> (RBRC01834)27 FRT-LacZ-neo-FRT cassette was removed and loxP-flanked exon 4 was left. Stat3f/f, Gp130f/f and Lifrf/f mice were crossed with LtfiCre/+ mouse to generate uterine epithelial-specific gene deficient (conditional knockout, cKO) (Stat3 cKO, Gp130 cKO or Lifr cKO) mice as previously described22, respectively. The primers used for genotyping were; 5′-GTTTCCTCCTTCTGGGCTCC-3′, 5′-TTTAGTGCCCAGCTTCCCAG-3′ and 5′-CCTGTTGTTCAGCTTGCACC-3′ for LtfiCre; 5′-CCTGAAGACCAAGTTCATCTGTGTGAC-3′, 5′-CACACAAGCCATCAAACTCTGGTCTCC-3′ and 5′-GATTTGAGTCAGGGATCCTTATCTTCG-3′ for Stat3flox; 5′-GGCTTTTCCTCTGGTTCTTG-3′ and 5′-CAGGAACATTAGGCCAGATG-3′ for Gp130flox; 5′-TGAGAGCACGGAAGCTCTTT-3′ and 5′-ACTGCCCGACAAGGTTTTTA-3′ for Lifrflox.
All the mouse strains, except ICR, were maintained in a C57BL/6 strain background and housed in the barrier facility at Azabu University. ICR mice were housed in the barrier facility at Nagoya University and Azabu University. The mice were fed ad libitum under controlled light-dark cycles (12 h of light followed by 12 h of dark) at 22 ± 3 °C. The first day of pregnancy (D1) was determined as the morning when a vaginal plug was observed in the female that had been mated with fertile wildtype males on the previous evening.
RO8191 treatment in DI mice
The experimental procedure is shown in Fig. 1A. To induce delayed implantation (DI) model mice, plug-positive ICR females were ovariectomized between 1300 and 1530 h on D3 under sevoflurane (193-17791, FujiFilm Wako Pure Chemicals, Osaka, Japan) anesthesia, and siliconized medroxyprogesterone acetate (MPA, 100 µl/head; Pfizer Inc, New York, NY, USA) was injected subcutaneously in the ventral region. The RO8191 (T22142, TargetMol, Boston, MA, USA, or SML1200, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sesame oil (196-15385, FujiFilm Wako Pure Chemicals, or S3547, Sigma-Aldrich), and a single intraperitoneal (i.p.) injection of RO8191 (400 µg/head) or sesame oil was performed at 1300 h on D7. E2 (E2758, Sigma-Aldrich, 25 ng/head) was used to obtain control samples. These female mice were euthanized in a carbon dioxide chamber, followed by dissection at 1300 h on D10 to count the number of implantation sites. The saline solution was used to flush the uterine horns to check for the presence of embryos if the uteri did not show the implantation sites. DI mice were excluded from the statistical analysis if they had no implantation sites and no blastocyst was recovered. ICR females injected with sesame oil were used as a control. The implantation rates of two groups (oil and RO8191) were defined as the ratio of the number of mice with implantation sites to the total number of mice after being injected with oil or RO8191. Uterine tissues were collected at 6 and 24 h after treatment with either sesame oil or RO8191. Samples from 6 h were used for immunohistochemistry, and those from 24 h for Western blot analysis.
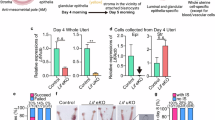
RO8191 induces embryo implantation in delayed implantation (DI) model mice. (A) The experimental procedure for the artificial DI mouse. Ovariectomy (OVX) and subcutaneous administration of siliconized medroxyprogesterone acetate (MPA) were performed on D3. A single injection of oil or RO8191 was performed on D7. Females were euthanized and dissected on D10. (B) Representative images of the gross uterine morphology. Blastocysts were recovered from oil-treated mice (white arrowheads). Implantation sites (yellow arrowheads on right panel) were visible in DI mice after RO8191 treatment. Scale bars: 1 cm for uterine images and 200 μm for blastocysts. (C, D) Implantation rate (C) and the number of implantation sites (D) in the oil and RO8191-treated DI mice. *Significantly different (p < 0.0001). (E) Representative histological image of uterine cross-sections of implantation sites on D10 after RO8191 treatment in DI mice. Right panels are higher magnification of left panels indicated by yellow line. Lower panels indicated abnormal embryo development and decidual reaction. Scale bars: 100 μm. dec: decidua; em: embryo.
RO8191 treatment in conditional knockout mice
The experimental procedure is shown in Fig. 3A. Plug-positive females of Stat3, Gp130 or Lifr cKO were administered with a single i.p. injection of RO8191 (400 µg/head; dissolved in sesame oil) or sesame oil at between 1330 and 1700 h on D4. Plug-positive females of Stat3f/f, Gp130f/f or Lifr f/f were injected with sesame oil as a pregnant control group. The number of implantation sites was counted macroscopically on D7. If there was no implantation site, the uterine horns were flushed with saline solution to retrieve the embryos and determine the success of mating. Mice were excluded from the statistical analysis if they had no implantation sites and no blastocyst was recovered. Images were saved in PNG format at a resolution of 806 × 1441 pixels. ImageJ software (version 1.54f; National Institutes of Health, Bethesda, MD, USA) was utilized to measure the areas of uterine bulges. The scale was set using the ‘Set Scale’ function based on a 1 cm scale bar. The bulges were selected using ‘Oval selection’. The measurements were recorded in square centimeters (cm²). The size of each bulge was measured in the control and cKOs (Stat3, Gp130, and Lifr) uteri on D7. Statistical analysis was conducted using GraphPad Prism (version 10.0; GraphPad Software), with results expressed as mean ± standard error of the mean (SEM). One-way ANOVA followed by Tukey’s multiple comparison test was used to assess group differences, with significance set at p < 0.05. In this study, we defined the bulges in the cKOs uterus as the putative implantation site.
To assess the effect of RO8191 on fertility in Lifr cKO, a single i.p. injection of RO8191 was administrated at 1600 h at D4 of pregnancy. For immunochemical analysis of uteri from Gp130 cKO and Lifr cKO, uterine tissues were collected 6 h after administration of RO8191 or sesame oil at 1000 h on D4.
Isolation of LE and ST
After the injection of RO8191 or sesame oil for 24 h, DI mice were sacrificed by inhalation of carbon dioxide, and the uterine horns were collected. For the sesame oil injection group, each uterine horn was rinsed with 0.5 mL saline solution to recover blastocysts, while the uteri without embryos were excluded from sampling and discarded. The uteri were washed three times briefly with saline solution on ice. All uterine horns were opened longitudinally and cut into 2 mm pieces. The uterine pieces were collected in a tube and kept at -80 °C overnight and incubated with RNAlater stabilization solution (AM7020, Thermo Fisher Scientific, Waltham, MA, USA) at -80 °C for 1 h. The luminal epithelium (LE) and stroma (ST) were separately isolated from the uterine pieces using forceps on the inner surface of the endometrium and collected by gravity sedimentation, washed in RNA stabilization solution, and stored at -80 °C prior to protein extraction.
Cell culture and IFN-γ treatment
A549 cells (JCRB0076) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS; HyClone, Cytiva, Marlborough, MA, USA), 2 mM l-glutamine (Sigma-Aldrich), 0.14% sodium hydrogen carbonate (NaHCO3; Sigma-Aldrich), and 100 U/mL penicillin-0.1 g/mL streptomycin (Meiji, Tokyo, Japan) at 37 °C in a 5% CO2 atmosphere. A549 cells at 80–90% confluence were treated with 10 IU/ml IFN-γ (Kyowa Pharmaceutical Industry, Osaka, Japan) or an equivalent volume of sterile water for 1 h.
Protein extraction and Western blot analysis
Total protein was isolated using RIPA lysis buffer containing a 1% protease inhibitor cocktail (25955-24; Nacalai tesque, Kyoto, Japan). Liver tissue was obtained from nonpregnant ICR mice. The A549 cells were washed three times with phosphate-buffered saline (PBS) before collection. Samples were lysed on ice for 5 min and centrifuged at 15,000 rpm for 30 min at 4 °C. Protein concentrations were determined using the Qubit™ protein assay kit (2411539, Thermo Fisher Scientific). Equal amounts of protein from LE (25 µg), ST (25 µg), liver (10 µg) and A549 cells (10 µg) were separated on an 8% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred onto nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk (Megmilk Snow Brand, Tokyo, Japan) in TTBS (Tris-buffered saline with 0.01% detergent) for 30 min at room temperature (RT). Membranes were incubated overnight at 4 °C with the following primary antibodies in diluted in TTBS containing 5% bovine serum albumin (010-25783, FujiFilm Wako Pure Chemicals): STAT3 (79D7, #4904, 1:2,000), p-STAT3 (Tyr705, D3A7, #9145, 1:2,000), STAT1 (D1K9Y, #14994, 1:1,000), or p-STAT1 (Tyr701, 58D6, #9167, 1:1,000) (all from Cell Signaling Technology, Danvers, MA, USA). After six 5-minute washes in TTBS, membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (PI-1000-1; Vector Labs; Newark, CA, USA) for 1 h at RT, followed by another six 5-minute washes with TTBS. Membranes immersed in Western BLoT Ultra Sensitive HRP Substrate (#T7104A; Takara, Shiga, Japan) were placed in the Lumicube (Liponics, Tokyo, Japan). The aperture was adjusted to f/2.8, ISO to 12,800, and the exposure time to 5–30 s. Molecular weights were determined using ImageJ software (version 1.54f; National Institutes of Health).
Histological analysis
Uterine tissues were fixed in 4% paraformaldehyde (PFA) solution in PBS (pH 7.4) using standard histological techniques28,29. The tissues were then dehydrated through a graded ethanol series, cleared with xylene, and embedded in paraffin. Transverse sections (5 μm thickness) were cut and stained with the routine hematoxylin and eosin (H&E). Micrographs were captured using a BZ-X700 microscopy (Keyence, Osaka, Japan).
Immunohistochemistry
Immunohistochemistry (IHC) was performed as previously described with minor modifications22. Briefly, the paraffin sections (5 μm) were deparaffinized, hydrated, and used for antigen retrieval by auto-claving in 10 mM sodium citrate buffer (pH 6.0) for 5 min. The sections were further incubated in 3% hydrogen peroxide diluted with methanol for 15 min. After blocking, the slides were incubated with the following primary antibodies overnight at 4 °C: p-STAT3 (Tyr705, D3A7, #9145, 1:200, Cell Signaling Technology) or alkaline phosphatase (ALPL; AF2910, 1:100, R&D Systems, Minneapolis, MN, USA). The same slides were followed by incubation with biotin-conjugated secondary antibody (Anti-Rabbit: 711-065-152 or Anti-Goat: 705-065-147; 1:500, Jackson Immuno Research, West Grove, PA, USA) and peroxidase-conjugated streptavidin (016-030-084, 1:500, Jackson Immuno Research). Signals were detected by 3,3’-diaminobenzene tetrahydrochloride (DAB; 040-27001, FujiFilm Wako Pure Chemicals) in 50 mM Tris buffer (pH 7.6). After counter-staining with hematoxylin, the sections were dehydrated and mounted with Pathomount (164-28492, FujiFilm Wako Pure Chemicals).
Statistical analysis
The parameter data including the implantation sites and implantation rate in the DI and cKOs mice were expressed as mean ± SEM or mean. Statistical analysis was performed by using independent Student’s t test at the GraphPad Prism (version 10.0; GraphPad Software). P values less than 0.05 were considered statistically significant.
Results
RO8191 promotes blastocysts implantation in DI mice
RO8191 has been reported to bind to the interferon-α receptor 2 (INFAR2) and promote phosphorylation of STAT323. To validate the promotion activity of RO8191 for implantation in mice, the DI mouse was established by ovariectomy and subcutaneous injections of MPA on D3, leading to embryos remaining unattached to the luminal epithelium and stated in the uterine lumen. Subsequently, RO8191 or oil was injected into the DI mice on D7 (Fig. 1A). No implantation site was detected in the oil-injected mice (n = 5), and hatched blastocysts were recovered after washing the uterus lumen on D10 (Fig. 1B). On the other hand, distinct implantation sites were observed in approximately 80% (15/19) of the RO8191-injected DI mice on D10 (Fig. 1B-D). In the samples without distinct implantation sites after RO8191 injection (4/19), hatched blastocysts were recovered as like oil-injected mice. The number of average implantation sites was 6.7 ± 1.0 in RO8191 group (Fig. 1D). We confirmed the growing embryo in the implantation sites in RO8191-treated mice by the histological observation on D10 (Fig. 1E, upper panels), although some part of implantation sites showed abnormal embryo development and decidual reaction (Fig. 1E, lower panels). These findings indicates that RO8191 can initiate blastocyst implantation in DI mice.
Phosphorylation of STAT3 but not STAT1 by RO8191
To evaluate whether RO8191 initiates implantation by regulating the JAK/STAT3 signaling pathway, the LE cells was collected from DI mice 24 h after RO8191 injection. STAT3 expression was comparable between the oil- and RO8191-injected mice by Western blot (WB) analysis (Fig. 2A). Phosphorylated-STAT3 (p-STAT3) was detectable in the LE after the intraperitoneal injection of RO8191 in DI mice. High expression levels of both STAT3 and p-STAT3 were observed in the liver tissue from nonpregnant ICR mice as a control. Since RO8191 mimics IFN by binding IFNAR2 to induce IFN-stimulated gene (ISG) expression23, STAT1 and p-STAT1 levels were also examined. WB analysis showed that STAT1 was expressed in both LE and ST cells from oil- and RO8191-injected mice, whereas p-STAT1 was undetectable in these groups (Fig. 2B). A549 cells treated with IFN-γ were loaded as a control (Fig. 2B). To further verify whether administration of RO8191 activates STAT3 signaling in the endometrium, immunohistochemical staining was conducted for p-STAT3 on uterine sections collected 6 h after RO8191 treatment in DI mice. P-STAT3 was detected not only in the nuclei of the uterine epithelium including the glandular epithelium, but also uterine stroma after RO8191 treatment (Fig. 2C). E2-treated uterus was used as a control (Fig. 2C). These results showed that RO8191 induces embryo implantation via activating STAT3 signaling pathway in DI mice.
RO8191 activates STAT3, but not STAT1, in the endometrium. (A) Western blot analysis of STAT3 and phospho-STAT3 (p-STAT3) proteins after RO8191 treatment. The predicted band sizes are 89 kDa (STAT3α), 69 kDa (STAT3β), and 66 kDa (p-STAT3). (B) Western blot analysis of STAT1 and p-STAT1 after RO8191 treatment. A549 cells treated with IFN-γ were loaded as a control. The predicted band sizes are 91 kDa (STAT1α and p-STAT1α), 84 kDa (STAT1β and p-STAT1β). Blot images were obtained from one gel, and dividing lines indicate their separate origins. LE and ST protein lysates were pooled from at least three different individuals. (C) Representative immunohistochemical images of p-STAT3 expression in the uterus after RO8191 treatment. E2-treated uterus is shown as a control. Specimens were prepared at least three different individuals (two individuals in the case of E2). Scale bar: 100 μm. le: luminal epithelium; st: stroma.
The effects of RO8191 on cKOs mice
Since the epithelial-derived LIF-LIFR/Gp130-JAK/STAT3 signaling pathway is critical for establishing embryo implantation in mice, RO8191 was administered to Stat3 cKO19, Gp130 cKO22 and Lifr cKO20,21,30 mice to examine whether their implantation failure phenotype can be restored (Fig. 3A). As a pregnant control, Stat3f/f, Gp130f/f and Lifrf/f mice were injected with the same dose of sesame oil (Fig. 3A; Table 1).
RO8191 induces decidual reaction in mice with genetic implantation failure. (A) The experimental procedure for the genetically modified mice. For control, a single oil injection was performed on D4. For cKOs, a single injection of oil or RO8191 was performed on D4. Females were euthanized and dissected on D7. (B–D) Uterine morphology of cKOs mice after the injection of oil or RO8191. The white arrowheads indicate the presumptive implantation sites, including uterine swelling. Scale bars: 1 cm. (E) The implantation rate in cKOs mice after oil or RO8191 administration. (F) The number of implantation sites in cKOs mice after the injection of oil or RO8191. (G) The size of implantation sites in cKOs mice after oil or RO8191 administration. *Significantly different (p < 0.05).
In the Stat3f/f (n = 5), Gp130f/f (n = 3), and Lifrf/f (n = 4) mice after oil injection on D4, implantation sites were lined along with the uteri on D7 and no defect of embryo implantation was found in each mouse strain (Fig. 3B-D; Table 1). The average number of implantation sites was 5.6, 9.7, and 8.3 in Stat3f/f, Gp130f/f and Lifrf/f mice, respectively (Table 1). Surprisingly, implantation sites, including small uterine swellings, were observed in Stat3 cKO (2/4; 2 of 4 mice), Gp130 cKO (4/5; 4 of 5 mice), and Lifr cKO (3/3; 3 of 3 mice) mice injected with RO8191 (Fig. 3B-F). The average number of estimated implantation sites was 2.5, 4.4, and 8.7 in Stat3, Gp130, and Lifr cKO mice, respectively (Fig. 3F). Administration of sesame oil in cKOs mice did not induce implantation (Fig. 3B-F). The size of the decidual swelling can serve as an indicator of the extent of decidualization in the uterus. To quantify these differences, we measured the size of decidual swellings at implantation sites in cKOs mouse strains. The size of the implantation sites varied among the different cKOs mouse strains. The average area of implantation sites was 0.077 cm², 0.061 cm², 0.039 cm², and 0.121 cm² in control, Stat3, Gp130, and Lifr cKO mice, respectively (Fig. 3G). The areas of the bulges in Stat3 cKO and Gp130 cKO were notably smaller compared to the control, whereas those in Lifr cKO was larger (p < 0.05). The bulges areas in Stat3 cKO mice were markedly larger than those in Gp130 cKO mice (Fig. 3G; p < 0.05).
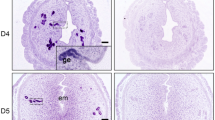
Histological analysis revealed that uterine sections of all cKOs mice exhibited the changes in stromal cells (Fig. 4A). In both the control and Lifr cKO uterus, post-implantation embryos were located at the antimesometrial side of the crypt, surrounded by the decidualized stomal cells (Fig. 4Aa, d). Degenerated embryos were observed in the uterus of Stat3 and Gp130 cKO mice (Fig. 4Ab, c). In the Stat3 cKO uterus, LE was broken and shed, and the development of blood vessels was observed at implantation sites (Fig. 4Ab’). Histological analysis of Gp130 cKO mice uterus showed that the leukocyte infiltration and embryo death had occurred (Fig. 4Ac’). We further examined the expression of alkaline phosphatase (ALPL), whose activity serves as a marker of decidualization, by IHC. The Lifr cKO uterus showed ALPL expression pattern similar to that of the control (Fig. 4B). In contrast, weaker expression was observed in the Stat3 cKO, whereas stronger but disorganized expression was seen in the Gp130 cKO (Fig. 4B). No ALPL expression was detected in non-implanted uteri, including the oil-treated Lifr cKO uterus (Fig. 4B).
Effect of RO8191 on the STAT3 signaling pathway in knockout mice. (A) Histological characterization of uterine cross-sections from presumed implantation sites on D7. Images a’-d’ are higher magnifications of images a-d, with further magnification in the yellow frames in b’ and c’. Scale bars: 100 μm. dec: decidua; le: luminal epithelium; em: embryo; bv: blood vessels; hem: hemorrhage; li: leukocyte infiltration. (B) Representative images of alkaline phosphatase (ALPL) expression. Scale bar: 100 μm. em: embryo; le: luminal epithelium; st: stroma. (C) The experimental procedure for maintaining pregnancy in Lifr cKO mice. A single injection of RO8191 was performed on D4, and females underwent cesarean section (CS) on D20-22. A single injection of RO8191 was sufficient to establish pregnancy in Lifr cKO mice. Representative images show an Lifr cKO female on D15 and the offspring obtained by CS. (D) Representative images of p-STAT3 in Gp130 cKO and Lifr cKO uterus 6 h after RO8191 treatment (D4 1600 h). Scale bar: 100 μm. le: luminal epithelium; st: stroma.
We tested whether Lifr cKO mice treated with RO8191 were able to carry pregnancies to term (Fig. 4C). Single RO8191 injection was performed at 1600 h on D4. All RO8191-treated Lifr cKO mice (n = 3) were pregnant and a total of 6 live pups out of 15 observed implantation sites were confirmed by cesarean section at term pregnancy (Fig. 4C; Table 2). We further examined STAT3 status after RO8191 injection in Gp130 cKO or Lifr cKO mice. P-STAT3 positive cells were detected in the stromal cells but not epithelial cells in RO8191 treatment in Gp130 cKO (Fig. 4D). On the other hand, p-STAT3 was observed not only in the stromal cells but also epithelial cells in RO8191 treatment in Lifr cKO (Fig. 4D).
Discussion
It is widely accepted that a transient elevation of E2 in the uterus can induce LIF secretion from the glandular epithelium at D4 of pregnancy in mice. LIF activates the JAK/STAT3 signaling pathway through LIFR/Gp130 and phosphorylation of STAT3 in the uterine epithelium, which is critical for embryo implantation14,31. Therefore, uterine epithelial cKO mice lacking Stat3, Gp130, or Lifr exhibit defects in STAT3 signaling, resulting in implantation failure19,20,21,22. Although the detailed mechanisms of downstream STAT3 signaling remain elusive, rescuing implantation failure through STAT3 activation offers considerable potential for future applications.
The purpose of this study was to test whether activation of STAT3 signaling by RO8191 can initiate embryo implantation in mice. RO8191 functions similarly to type I interferons (IFNs) as a ligand: it binds to the IFN-α receptor 2 (IFNAR2) homodimer, phosphorylates and activates the STAT protein family, including STAT1, STAT2, STAT3, STAT5, and STAT6, and induces IFN-inducible gene expression23, while type I IFNs phosphorylate STAT proteins by inducing heterodimerization of IFNAR1 and IFNAR2. The previous report demonstrated that RO8191 can elevate p-STAT3 levels in the immortalized goat endometrial epithelial cells and restore the expression of endometrial receptivity-related genes caused by the silencing of STAT332. This result highlights the potential of RO8191 as a pharmacological agent for STAT3 activator in animals.
In the present study, DI mice injected with RO8191 showed high implantation rates, a substantial number of implantation sites, succeed embryo development and decidualization (Fig. 1). The WB analysis and IHC results suggest that RO8191 can directly activate the STAT3 signaling pathway in epithelial and stromal cells (Fig. 2A, C). Previous research has shown that mouse LE cells treated with LIF in vitro exhibited a clear single band of p-STAT3 by WB analysis31 and DI mice injected with LIF also showed a high expression level of p-STAT318. Conversely, p-STAT1 was not detected in uterine LE and ST cells following RO8191 treatment, indicating that RO8191 does not activate the STAT1 signaling pathway in the uterus (Fig. 2B). We first considered the possibility that RO8191 may bind the IFNAR2, which is known to be expressed in the luminal epithelium33, and/or Gp130/LIFR, activating STAT3 and regulating gene expression, and eventually rescuing embryo implantation in DI mice. In DI mice, individual physiological differences may result in abnormal embryo development and decidualization at some implantation sites. It is also possible that RO8191 induced the expression of ISGs through STAT pathways other than STAT3, such as STAT2, STAT5, and STAT6, which could have contributed to the abnormal development observed at certain implantation sites.
In this study, the uterine epithelium cKOs (Stat3, Gp130, and Lifr) also exhibited a high implantation rate and a substantial number of presumed implantation sites after RO8191 treatment (Fig. 3). This indicated that RO8191 affect the embryo-uterine interactions even in the absence of STAT3, Gp130, and LIFR in the uterine epithelium. Importantly, histological observations and ALPL staining showed stromal cell decidual reaction in all cKOs uteri after RO8191 treatment (Fig. 4A, B). IHC results from DI mice, Gp130 cKOs and Lifr cKOs treated with RO8191 showed that RO8191 not only activated STAT3 in the epithelium but also directly activates the STAT3 pathway in uterine stromal cells. It has been reported that activation of the EGR1/WNT4 signaling pathway via LIF-STAT3 in the endometrial stroma is important in decidualization34. Our results in the present study may indicate that the decidual response during implantation is not solely dependent on the activation of the STAT3 pathway in LE cells.
An interesting finding was that only the Lifr cKO treated with RO8191 exhibited an adequate decidual reaction to support blastocyst development. The size of the Lifr cKO implantation site observed in D7 was larger than that of the controls. Previous study has reported that the loss of LIFR promotes the production of inflammatory cytokines35. Loss of LIFR may alter the uterine immune environment, leading to the activation or enhancement of compensatory immune-regulatory pathways. Therefore, in Lifr cKO mice, it is possible that a moderately inflammatory response, in combination with the STAT3 activation induced by RO8191, contributes to enhance signaling pathways that facilitate the proliferation of stromal cells during decidualization. To determine whether blastocyst development continues in Lifr cKO mice, we assessed pregnancy continuity after a single dose of RO8191 on D4 at 16:00. The results confirmed that embryos developed to term, although the number of implantation sites harboring embryos was reduced compared to that observed on D7. Significantly, we were able to restore the infertility phenotype in a mouse model of complete implantation failure by administering the drug. Further investigation of the effects of RO8191 is expected to elucidate the signals involved in embryo implantation. Also, further research into the timing and administration method of RO8191 would be required to maximize its effectiveness. Since epithelial STAT3 activation after RO8191 administration was observed in the Lifr cKO, but not in the Gp130 cKO (Fig. 4D), RO8191 may directly bind to Gp130.
Embryo development in the Stat3 and Gp130 cKO uterus could not be rescued by RO8191, as no post-implantation embryos were observed, although RO8191 exhibited low toxicity in cell culture23 and positive effects in Lifr cKO mice (Fig. 4A). In the Lifr cKO uterus, post-implantation embryos were located on the antimesometrial side of the crypt, and angiogenesis appeared to be preserved, similar to that in the control group. These observations suggest that RO8191 effectively induced embryo implantation, and the role of LIFR in post-implantation embryo development and angiogenesis might be limited. Despite exhibiting key features of embryo invasion, such as partial uterine epithelial breakdown, shedding, and blood vessel development (Fig. 4A)36, the Stat3 cKO uterus showed a weaker decidual reaction (Fig. 4B). In contrast, the Gp130 cKO uterus did not show these characteristics but exhibit strong alkaline phosphatase expression (Fig. 4B). This suggested that embryonic development was already initiated but terminated due to the aberrant decidual reaction in the Stat3 and Gp130 cKO uterus. In Stat3 cKO uterus, endometrial decidual response and angiogenesis may result from the direct activation of the STAT3 signaling pathway in the uterine stromal cells by RO8191. However, STAT3 in epithelial cells might play a critical role in endometrial decidualization to support embryo development, and its absence could lead to aberrant decidual response and subsequent pregnancy failure. This hypothesis is also supported by the finding that p-STAT3 was detected in the uterine epithelium following RO8191 administration in the Lifr cKO, but not in the Gp130 cKO (Fig. 4D). In the Gp130 cKO uterus, an excessive inflammatory response and embryo death were observed after RO8191 treatment (Fig. 4A). Decidualization is typically accompanied by leukocyte infiltration, with approximately 15% of uterine decidual cells being immune cells, primarily uterine NK cells, macrophages, and T lymphocytes37. However, abnormal leukocyte infiltration also can lead to the early embryo loss38. Previous research has shown that the absence of epithelial Gp130 reduces the expression of the progesterone receptor and ALOX15, a downstream target of the progesterone receptor22. This reduction leads to an excessive inflammatory response in Gp130 cKO mice at D422. These findings indicate that Gp130 deficiency leads to abnormal leukocyte infiltration, which may contribute to embryonic loss and disrupt the angiogenesis in early pregnancy. Although RO8191 was able to initiate the decidualization process by acting directly on stromal cells, it failed to alleviate the excessive inflammatory response in Gp130 cKO mice. The marked failure of decidualization in the Stat3 and Gp130 cKO uteri suggests that STAT3 and Gp130 play crucial but distinct roles in the RO8191-mediated signaling, which regulates decidualization and embryonic development.
In summary, our present study suggests that RO8191 can contribute to embryo implantation and decidualization by activating STAT3 signaling in the endometrium, potentially through binding the IFNAR2 and/or Gp130/LIFR. The epithelial STAT3 and Gp130 are indispensable for coordinating the embryonic development and the decidual response followed by RO8191-mediated embryo implantation. RO8191 also has the advantage of convenient oral administration in mice, which is patient-friendly for the future application23. Although the further analysis needs to elucidate the detailed mechanism of RO8191 on implantation, it is promising that RO8191 has potential for the treatment of implantation failure.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author, Eiichi Hondo, upon reasonable request.
References
Macklon, N. S., Geraedts, J. P. M. & Fauser, B. C. J. M. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum. Reprod. Update. 8, 333–343 (2002).
Giudice, L. C. Potential biochemical markers of uterine receptivity. Hum. Reprod. 14 (Suppl 2), 3–16 (1999).
Coughlan, C. et al. Recurrent implantation failure: definition and management. Reprod. Biomed. Online. 28, 14–38 (2014).
Kliman, H. J. & Frankfurter, D. Clinical approach to recurrent implantation failure: evidence-based evaluation of the endometrium. Fertil. Steril. 111, 618–628 (2019).
Wang, H. & Dey, S. K. Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7, 185–199 (2006).
Kojima, Y., Tam, O. H. & Tam, P. P. L. Timing of developmental events in the early mouse embryo. Semin. Cell Dev. Biol. 34, 65–75 (2014).
Yang, Z. M. et al. Leukemia inhibitory factor, LIF receptor, and gp130 in the mouse uterus during early pregnancy. Mol. Reprod. Dev. 42, 407–414 (1995).
Hu, W., Feng, Z., Teresky, A. K. & Levine, A. J. p53 regulates maternal reproduction through LIF. Nature 450, 721–724 (2007).
Song, H., Lim, H., Das, S. K., Paria, B. C. & Dey, S. K. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF- deficient mice. Mol. Endocrinol. 14, 1147–1161 (2000).
Robb, L., Dimitriadis, E., Li, R. & Salamonsen, L. A. Leukemia inhibitory factor and interleukin-11: cytokines with key roles in implantation. J. Reprod. Immunol. 57, 129–141 (2002).
Onishi, K. & Zandstra, P. W. LIF signaling in stem cells and development. Development 142, 2230–2236 (2015).
Salleh, N. Diverse Roles of Prostaglandins in Blastocyst Implantation. The Scientific World Journal e968141 (2014). (2014).
Pastuschek, J. et al. Stimulation of the JAK/STAT pathway by LIF and OSM in the human granulosa cell line COV434. J. Reprod. Immunol. 108, 48–55 (2015).
Stewart, C. L. et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79 (1992).
White, C. A. et al. Blocking LIF action in the uterus by using a pegylated antagonist prevents implantation: A nonhormonal contraceptive strategy. Proc. Natl. Acad. Sci. U S A. 104, 19357–19362 (2007).
Terakawa, J. et al. Embryo implantation is blocked by intraperitoneal injection with Anti-LIF antibody in mice. J. Reprod. Dev. 57, 700–707 (2011).
Lee, K. Y., Jeong, J. W., Tsai, S. Y., Lydon, J. P. & DeMayo, F. J. Mouse models of implantation. Trends Endocrinol. Metabolism. 18, 234–239 (2007).
Kobayashi, R. et al. The contribution of leukemia inhibitory factor (LIF) for embryo implantation differs among strains of mice. Immunobiology 219, 512–521 (2014).
Hiraoka, T. et al. Differential roles of uterine epithelial and stromal STAT3 coordinate uterine receptivity and embryo attachment. Sci. Rep. 10, 15523 (2020).
Fukui, Y. et al. Uterine epithelial LIF receptors contribute to implantation chamber formation in blastocyst attachment. Endocrinology 162, bqab169 (2021).
Terakawa, J. et al. LIFR-Mediated ERBB2 signaling is essential for successful embryo implantation in mice. Biomolecules 15, 698 (2025).
Namiki, T. et al. Uterine epithelial Gp130 orchestrates hormone response and epithelial remodeling for successful embryo attachment in mice. Sci. Rep. 13, 854 (2023).
Konishi, H. et al. An orally available, small-molecule interferon inhibits viral replication. Sci. Rep. 2, 259 (2012).
Daikoku, T. et al. Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology 155, 2718–2724 (2014).
Takeda, K. et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161, 4652–4660 (1998).
Betz, U. A. K. et al. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J. Exp. Med. 188, 1955–1965 (1998).
Kanki, H., Suzuki, H. & Itohara, S. High-efficiency CAG-FLPe deleter mice in C57BL/6J background. Exp. Anim. 55, 137–141 (2006).
Humason, G. L., Presnell, J. K. & Schreibman, M. P. Humason’s Animal Tissue Techniques (Johns Hopkins University,, 1997). Baltimore (Md.).
Suvarna, K. S., Layton, C. & Bancroft, J. D. Bancroft’s Theory and Practice of Histological Techniques (Elsevier, Place of publication not identified, 2019).
Cheng, J., Rosario, G., Cohen, T. V., Hu, J. & Stewart, C. L. Tissue-Specific ablation of the LIF receptor in the murine uterine epithelium results in implantation failure. Endocrinology 158, 1916–1928 (2017).
Cheng, J. G., Chen, J. R., Hernandez, L., Alvord, W. G. & Stewart, C. L. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proceedings of the National Academy of Sciences 98, 8680–8685 (2001).
Jia, Y. et al. LncRNA STAT3-AS regulates endometrial receptivity via the STAT3 signaling pathway. Theriogenology 216, 118–126 (2024).
Jang, H. et al. Characterization of interferon α and β receptor IFNAR1 and IFNAR2 expression and regulation in the uterine endometrium during the estrous cycle and pregnancy in pigs. Theriogenology 88, 166–173 (2017).
Liang, X. H. et al. Egr1 protein acts downstream of Estrogen-Leukemia inhibitory factor (LIF)-STAT3 pathway and plays a role during implantation through targeting Wnt4 *. J. Biol. Chem. 289, 23534–23545 (2014).
Kahn, L. M. et al. Roles for Leukemia-inhibitory factor receptor signaling in intestinal immunity. The J. Immunology 204, 158.5 (2020).
Whitby, S., Zhou, W. & Dimitriadis, E. Alterations in epithelial cell Polarity during endometrial receptivity: A systematic review. Front Endocrinol 11, (2020).
Erlebacher, A. Immunology of the Maternal-Fetal interface. Annu. Rev. Immunol. 31, 387–411 (2013).
Baines, M. G., Duclos, A. J., Antecka, E. & Haddad, E. K. Decidual infiltration and activation of macrophages leads to early embryo loss. Am. J. Reprod. Immunol. 37, 471–477 (1997).
Acknowledgements
We thank Dr. T. Daikoku (Kanazawa University) and Dr. S.K. Dey (Cincinnati Children’s Hospital Medical Center) for providing Ltf iCre mice, Dr. S. Akira (Osaka University) for providing Stat3flox mice, and Dr. W. Muller (University of Manchester) for providing Gp130flox mice. We also thank Medical Research Council (MRC) for providing Lifrtm1a(EUCOMM)Hmgu. FLPe transgenic mouse strain (RBRC01834) was provided by RIKEN BRC through the National BioResource Project of the MEXT/AMED, Japan. This work was supported by Grants-in-Aid for Scientific Research from the JSPS (23K23785 to E.H.). This work was partially supported by the Center for Human and Animal Symbiosis Science, Azabu University, and a research project grant awarded by the Azabu University Research Services Division.
Author information
Authors and Affiliations
Contributions
E.H. planned the experiments. E.H. J.S. J.T. S.O. S.T.and J.R. performed the experiments and made all figures. J.S. J.T. S.O. A.M. J.R. A.I. J.I. and E.H. analyzed the data. J.S. J.T. A.I. J.I. and E.H. wrote the manuscript. All authors agreed the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shu, J., Terakawa, J., Takikawa, S. et al. RO8191, a new compound for initiating embryo implantation in mice. Sci Rep 15, 32387 (2025). https://doi.org/10.1038/s41598-025-18471-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18471-3