Abstract
Epidemiological association between psoriasis and T2DM suggests shared pathophysiology that are to be explored. Microarray expression profiles for psoriasis and T2DM were obtained from the Gene Expression Omnibus (GEO) database. The “limma” package in R software was used to screen the differentially expressed genes (DEG). GO and KEGG enrichment analysis were further conducted to explore the functions of co-DEGs. By intesecting genes of the key disease-related modules from WGCNA with co-DEGs, candidate co-driver genes were identified and their PPI network was constructed. Hub genes with good diagnostic potential were obtained by ROC analysis and their expression was further compared in validation datasets as well as clinical samples. The crucial co-driver genes, identified by a consistently differential expression pattern, were further subjected to a series of analyses, including Gene Set Enrichment Analysis (GSEA), immune cell infiltration analysis, gene-chemistry networks analysis, gene-transcription factors (TF) network analysis, and gene-miRNA regulatory network analysis. In our study, 71 co-DEGs were identifed from psoriasis and T2DM training datasets. KEGG analysis revealed enrichment of pathways including toll-like receptor signaling pathway, cytokine-cytokine receptor interactions, chemokine signaling pathway, NOD-like receptor signaling pathway and cytosolic DNA-sensing pathway. By intersecting the critical WCGNA modules with co-DEGs, 33 candidate co-driver genes were obtained. 11 of them showed interactions with others on PPI network and 7 revealed good diagnostic value with AUC > 0.7 by ROC analysis. 4 genes, namely BEX5, EPHX2, GPRASP1, and RBP4 were finally identified as crucial co-driver genes with a consistent differential expression pattern in both training and validation datasets as well as validation experiments using clinical samples. GSEA analysis revealed that these crucial co-driver genes were involved in cytokine receptor interaction, proteasome, ribosome, apoptosis and so on. Immune cell infiltration and correlation analyses highlighted their roles in the immune microenvironment. Lastly, these genes targeted 76 skin and metabolic diseases and 135 chemicals were predicted to exert an modulatory effect of their expression. 13 TFs and 79 miRNAs were identified to modulate their expression. The integrated bioinformatics analysis conducted in our study identified co-DEGs and enriched immune-inflammatory pathways, providing novel insights into the pathogenesis underlying the comorbidity of psoriasis and T2DM. The crucial co-driver genes warrants further experimental validation and exploration to unveal the common pathophysiology.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by sustained hyperglycemia resulting from impairments in insulin secretion, action, or both. Type 2 diabetes mellitus (T2DM) constitutes approximately 90% of all diabetes cases. The diagnosis of T2DM is established when a patient fulfills one or more of the following criteria: a glycated hemoglobin (HbA1C) level of 6.5% or higher, a fasting blood glucose level of 126 mg/dL or higher, or a 2-h postprandial glucose level of 200 mg/dL or higher1. The pathogenesis of T2DM involves a complex interplay of genetic, metabolic, and environmental factors2.
Psoriasis is one of the most prevalent inflammatory skin diseases, characterized by a chronic, relapsing–remitting course, with prevalence rates ranging from 0.1 to 8% across different geographical regions. It affects over 125 million people globally3. Plaque psoriasis, the most common form, presents as round, erythematous, dry, itchy patches of skin covered with scales. The pathogenesis of psoriasis involves the activation of inflammatory pathways in both innate and adaptive immune cells leading to excessive proliferation and dysfunctional differentiation of keratinocytes (KCs), acanthosis, neovascularization, and immune cells infiltration4. The etiology of psoriasis is multifaceted, involving a variety of intrinsic and extrinsic factors. Over the past several decades, accumulating evidence has highlighted a correlation between DM and psoriasis, suggesting that individuals with psoriasis are at a higher risk of developing DM, while DM is considered an intrinsic risk factor for the exacerbation of psoriasis5,6,7,8. In 2019, Mamizadeh et al.9 conducted a systematic review and meta-analysis of 38 eligible studies, which included 922,870 cases and 12,808,071 controls. Their research indicated an estimated odds ratio (OR) of 1.69 for the development of DM in patients with psoriasis (95% Confidence Interval [CI] 1.51–1.89; P < 0.001). Moreover, several studies have identified a positive correlation between the severity of psoriasis and the incidence of T2DM10,11,12. This implies that psoriasis and T2DM may share certain common pathophysiological mechanisms.
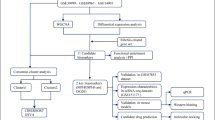
With the advancement of bioinformatics technology, it has become feasible to rigorously analyze potential associations between comorbidities, and such analyses have been extensively conducted. The present study aims to identify crucial genes and key pathways shared between psoriasis and T2DM utilizing robust analytic tools including limma, WGCNA, PPI, ROC, GSEA, ssGSEA and integrating sequencing data from multiple publicly available microarray datasets. Validation of candidate genes was further conducted using clinical samples. The identification of key co-driver genes and shared pathways associated with the comorbidity of psoriasis and T2DM is novel and may inform future translational studies.
Materials and methods
Data source and study design
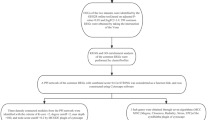
Expression profiling data of arrays of psoriasis and T2DM were downloaded from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo)13 The accession numbers for the datasets are GSE13355, GSE14905, GSE76894, and GSE41762. GSE13355 includes skin samples from 58 psoriasis patients and 64 normal controls while GSE76894 contains pancreatic islet samples from 19 T2DM patients and 84 normal controls. These datasets were analyzed as the training set of this study. GSE14905 comprises skin samples from 33 psoriasis patients and 21 normal controls, and GSE41762 includes pancreatic islet samples from 20 T2DM patients and 57 normal controls. The latter two datasets were utilized as the validation set in this study. Figure 1 illustrates the workflow of this study.
Identification of differentially expressed genes in psoriasis and T2DM
The ‘limma’ R package was used to identify differentially expressed genes (DEGs) in the datasets GSE13355 and GSE76894 under the cutoff criteria of |Log2FC|≥ 0.5 and adjusted p-value < 0.05. Volcano plots and heatmaps were utilized to visualize the DEGs, while Venn diagrams were employed for the screening of co-expressed differentially expressed genes (co-DEGs) across the two datasets.
Functional enrichment analysis of co-DEGs
The co-DEGs identified were subjected to enrichment analysis including Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway using the R package clusterProfiler. The GO terms are classified into three independent, hierarchical ontologies: Biological Process (BP), Molecular Function (MF), and Cellular Component (CC). Statistical significance for all enrichment results was set at p < 0.05.
Weighted gene correlation network analysis (WGCNA)
The “WGCNA” package in R was employed to establish a gene coexpression network and facilitate the identification of disease-related gene modules. All genes from the training dataset of individual disease were used to construct an input matrix and deviated or apparently abnormal gene was removed. The second step was to calculate Pearson’s correlation matrices for each paired gene and use the average linkage technique and a weighted correlation coefficient to generate a weighted adjacency matrix. Next, the adjacency of the genes was computed utilizing the optimal “soft” thresholding power (β), and it was converted into a topological overlap matrix (TOM). Average linkage hierarchical clustering was applied to classify modules based on TOM. According to the standard of the ‘Dynamic Hybrid’ cut algorithmin using the R package dynamicTreeCut, each module was set to contain no fewer than 100 genes of GSE13355 and 20 genes of GSE76894. Similar modules were subsequently merged based on the threshold of 0.3. Finally, Pearson’s method was used to calculate the correlation between the merged modules and disease occurrence, and the modules with the strongest positive and negative correlations with the disease were selected as core modules.
The intersecting genes between the psoriasis-related core module and the T2DM-related core module were further analyzed by intersecting with co-DEGs identified previously. This analysis resulted in the identification of potential co-driver genes for cormobidity of psoriasis and T2DM.
Construction of protein–protein interaction (PPI) networks
Potential co-driver genes (n = 33) were imported into the STRING database (https://string-db.org/) to predict functional protein associations and interactions. The resulting PPI network was filtered for high-confidence interactions (minimum required interaction score > 0.4)14. Unconnected nodes were removed to identify hub co-driver genes within the network.
Receiver operating characteristic (ROC) curves analysis
To evaluate the diagnostic specificity and sensitivity of the hub co-driver genes for psoriasis and T2DM, we conducted ROC curve analysis using the R package pROC (v1.18.0)15. The area under the curve (AUC) with 95% confidence intervals (CI) was calculated separately for each disease to quantify diagnostic performance. Greater AUC values indicate stronger diagnostic performance.
Expression of hub common driver genes in validation datasets and clinical samples
We selected genes with AUC greater than 0.7 and analyzed their expression in the validation datasets for psoriasis (GSE14905) and T2DM (GSE41762)16. Genes that exhibited differential expression between the control group and patients were identified as crucial co-driver genes for this study if they demonstrated consistent changes across both the training and validation sets. To further verify the reliability of our in silico findings, we collected whole blood samples from 8 healthy controls (HC), 8 psoriasis patients, 13 T2DM patients, and 4 psoriatic patients with T2DM. We analyzed the mRNA expression levels of BEX5, EPHX2, GPRASP1, and RBP4 using qPCR (Comprehensive details are available in Supplementary material). This study received approval from the Ethics Committee of Yunnan Hospital of Traditional Chinese Medicine (Approval No: YYLW-2025-008) and complied with the Declaration of Helsinki (1996) as well as relevant Chinese clinical research regulations. All participants were provided with written informed consent after a detailed verbal and written disclosure of the purpose, procedures, potential benefits, and risks of the study.
Gene set enrichment analysis (GSEA) analysis of the crucial co-driver genes
To elucidate biological functions and signaling pathways of the crucial co-driver genes, we conducted GSEA on psoriasis and T2DM training datasets using the R package GSEA (v4.0.3)17. Analyses were performed separately for each disease with an adjusted p-value threshold of < 0.05. Enriched KEGG pathways common to both datasets were identified through intersection analysis. Within these shared pathways, core gene sets were characterized. The enrichment results were rank-ordered by normalized enrichment score, with pathways meeting p < 0.01 considered statistically significant.
Immune cell infiltration analysis and its correlation with the crucial co-driver genes
To elucidate the differences in the immune microenvironment between psoriasis and T2DM, we conducted an immune infiltration analysis utilizing single-sample gene set enrichment analysis (ssGSEA) through the GSVA R package. A predefined gene set comprising 782 markers for 28 immune cell types18enabled the quantification of immune cell infiltration levels. Comparisons of immune cell infiltration scores between the disease and control groups were visualized using ggplot2boxplots, with inter-group significance assessed by t-test or Wilcoxon rank-sum test (p < 0.05). Finally, the expression matrix of crucial co-driver genes were correlated with immune cell abundance and statistically significant associations (Pearson correlation, p < 0.05) were illustrated as a correlogram using ggplot2.
Disease/chemistry and TF/miRNA prediction of the crucial co-driver genes
The Comparative Toxicogenomics Database (CTD) was used to assess gene-disease in order to predict novel associations19. Gene-chemistry networks were employed to identify potential drugs capable of modulating the expression of the crucial co-driver genes, namely BEX5, EPHX2, GPRASP1, RBP4. Cytoscape software (http://www.cytoscape.org, latest version 3.9.1) was used for visualizing disease-gene and chemistry-gene networks20. To gain further insights into the regulatory mechanism of these genes, transcription factors (TFs) and microRNAs (miRNAs) associated with these genes were predicted using miRNet (https://www.mirna.ca/) based on RegNetwork database21,22. We utilized cytoscape software to construct topological networks for TFs-gene and miRNA-gene interactions.
Results
Identification of co-DEGs in psoriasis and T2DM
The differential expression analysis of the GSE13355 dataset identified 1,227 up-regulated and 1,128 down-regulated genes associated with psoriasis, as depicted in the volcano plot and heatmaps (Fig. 2A,B). The top 5 most differentially expressed gene are annotated in the volcano plot. In a similar manner, the GSE76894 dataset, which includes patients with T2DM, revealed 157 up-regulated and 424 down-regulated genes (Fig. 2C,D). Finally, a total of 71 overlapping DEGs, listed in Supplementary Table S1, were identified comprising 26 up-regulated and 45 down-regulated genes, as shown in the venn diagram (Fig. 2E) Additionally, a total of 29 GO functional entries were enriched by GO analysis (Fig. 2F). The KEGG pathway analysis highlighted several enriched pathways, including cytokine-cytokine receptor interaction, chemokine signaling pathway, Toll-like receptor signaling pathway, NOD-like receptor signaling pathway and cytosolic DNA-sensing pathway (Fig. 2G).
Identification of shared DEGs. Heatmap of DEGs from psoriasis datasets (A) and T2DM datasets (C), red means up-regulated, blue means down-regulated. Sample means sample group; Volcano plots showing DEGs in psoriasis datasets (B) and T2DM datasets (D). Red/green dots represent up-/down-regulated genes; (E) Venn diagram for intersecting genes between psoriasis and T2DM. (F) GO functional enrichment analysis of co-DEGs; (G) The enriched KEGG pathways of co-DEGs.
WGCNA identifies key modules associated with psoriasis and T2DM
We performed WGCNA on the psoriasis dataset GSE13355 and the T2DM dataset GSE76894 to explore the correlations between clinical traits and genes. The samples clustered well without any outliers; therefore, no samples were excluded (Fig. 3A,B). The optimal soft threshold value, determined by R2 = 0.85, was 7 for the psoriasis dataset and 13 for the T2DM dataset (Fig. 3C,D). A total of 26 modules were generated in the psoriasis dataset using dynamic tree cutting, which were subsequently merged into 10 modules with a cut line set to 0.3. Similarly, 20 modules were generated in the T2DM dataset, which were then merged into 13 modules (Fig. 3E,F). Finally, we calculated the correlations between the modules and clinical traits. Among the 10 modules in the psoriasis dataset, the MEblue module, which contains 7854 genes, exhibited the highest correlation with disease onset (Cor = 0.95, P = 1e−62 < 0.001) (Fig. 3G). In the T2DM dataset, the MEblack module, containing 3,098 genes, showed the highest correlation with T2DM occurrence (Cor = − 0.36, P = 2e−04 < 0.05) (Fig. 3H).
Weighted gene co-expression network analysis (WGCNA) of psoriasis cohort and T2DM cohort. Clustering of module eigengens in psoriasis (A) and T2DM (B) dataset. Determination of the soft threshold value in psoriasis (C) and T2DM (D) dataset. The result from dynamic tree cutting in the psoriasis (E) and T2DM (F) cohort; Gene dendrograms were obtained by average linkage hierarchical clustering. The colored row underneath the dendrogram shows the module assignment determined by the dynamic tree cut method. Heatmap of the correlation analysis of module eigengenes with clinical phenotypes in psoriasis (G) and T2DM (H). Red color represents positive correlation and blue color represents negative correlation.
Identification of the crucial co-driver genes for psoriasis and T2DM
After intersecting the co-DEGs with critical module genes derived from WGCNA, we identifed 33 potential co-driver genes (Fig. 4A) which are listed in Supplementary Table S2. Subsequently, we constructed PPI networks for these genes (Fig. 4B) and excluded those without interactions, leading to the identification of 11 hub co-driver genes. The ROC analysis for these genes in psoriasis and T2DM datasets are presented in Fig. 4C,D, respectively, with the AUC values of ROC analysis provided in Supplementary Table S3. A total of seven genes exhibited AUC values exceeding 0.7 in both datasets. We subsequently compared the expression patterns of these genes between patients and controls in both the training and validation sets for psoriasis and T2DM, respectively (Fig. 5A–D). Four crucial co-driver genes (BEX5, EPHX2, GPRASP1, RBP4) were identified, demonstrating consistent changes across both the training and validation sets.
Identification of crucial co-driver genes in psoriasis and T2DM. (A) Venn diagram of co-driver genes extracted from co-DEGs and WGCNA key modules; Red = positive correlation and green = negative correlation; (B) Protein–protein interaction (PPI) network of co-driver genes. (C, D) ROC curve analyses of hub co-driver genes in psoriasis and T2DM.
Validation of the crucial co-driver genes in clinical samples
We conducted a Real-Time quantitative PCR (qPCR) assay to evaluate the crucial co-driver genes using whole-blood samples from healthy controls, psoriasis patients, T2DM patients and psoriasis patients with T2DM. The results indicated that, compared to healthy controls, the expression levels of BEX5, EPHX2, GPRASP1, and RBP4 were significantly decreased in both psoriasis and T2DM patients (P < 0.05). Notably, this reduction was even more pronounced in psoriasis patients with T2DM (P < 0.01) (see Fig. 5E–H).
Gene set enrichment analysis (GSEA) of the crucial co-driver genes
The GSEA was conducted on the four crucial co-driver genes in psoriasis (Fig. 6A, C, E, G) and T2DM datasets (Fig. 6B,D,F,H) to assess their relevant biological functions and signaling pathways. These genes are implicated in cytokine receptor interactions, proteasome, ribosome, cell cycle, and apoptosis, among others.
Immune cell infiltration analysis and its correlation with the crucial co-driver genes
Enrichment analyses of co-DEGs from psoriasis andT2DM datasets revealed a significant role for immune response regulation. We compared patterns of immune cell infiltration between patients and controls in the two training datasets using ssGSEA. The enrichment scores indicated significant differences in 26 types of immune cells between the control group and the psoriatic patient group (P < 0.05) (Fig. 7A). In the psoriasis training set, BEX5 and GPRASP1 had the highest correlation with CD56dim natural killer cells (r = − 0.77 and − 0.85, respectively). EPHX2 showed the strongest correlation with memory B cells (r = − 0.61), while RBP4 had the highest correlation with activated CD4+ T cells (r = − 0.62) (Fig. 7C). In the T2DM dataset, the enrichment scores of 9 different immune cells showed significant differences between control and T2DM patient group (P < 0.05) (Fig. 7B). The four key comorbid genes—BEX5, EPHX2, GPRASP1, and RBP4—exhibited the strongest correlation with neutrophils, with correlation coefficients of r = -0.66, -0.59, -0.75, and -0.59, respectively (Fig. 7D). Comprehensive details are available in Supplementary Tables S4–7.
Correlation of the crucial co-driver genes and immune cell infiltration in psoriasis and T2DM. Differences in the immune cell enrichment scores between psoriasis and Control (A), T2DM and control (B). (C, D) Association between BEX5, EPHX2, GPRASP1, RBP4 and immune cell infiltration in psoriasis and T2DM (Orange = positive correlation, blue = negative correlation, and the number = the correlation coefficient);
Disease/chemistry and TF/miRNA prediction of the crucial co-driver genes
The crucial co-driver genes were analyzed for their association with gene-related skin and metabolic diseases in the CTD database, using an Inference Score greater than 15. A total of 76 diseases were identified and illustrated in Fig. 8A. Additionally, 135 related chemicals were predicted to exert a modulatory effect on the four crucial co-driver genes (Fig. 8B). Furthermore, the shared gene-TF network and gene-miRNA regulatory network identified 13 TFs and 79 miRNAs using the miRNet database, which may influence the expression of these four genes (Fig. 8C). Details are provided in Supplementary Table S8.
Disease/chemistry and TF/miRNA prediction of the crucial co-driver genes. (A) CTD-predicted disease-gene interactions (Key comorbid genes are shown in red and related diseases in green); (B) Gene-chemistry network (The diagnostic markers are shown in red and the related compounds in yellow.); (C) TF/miRNA-mRNA network(Key comorbid genes are shown in red, transcription factors in yellow and miRNA in green).
Discussion
T2DM is recognized as one of the most well-established comorbidities of psoriasis. Many studies have established that psoriasis acts as an independent risk factor for insulin resistance23,24,25. Moreover, diabetes markedly reduces therapeutic response rates, as indicated by both PASI75 and PASI50 endpoints, in psoriatic patients treatmed with either methotrexate or cyclosporine-based regimens26. Nevertheless, research on the shared pathophysiology between psoriasis and T2DM remains limited27,28. In this study,we identified 71 overlapping DEGs from the psoriasis and T2DM datasets. GO and KEGG analysis revealed enrichment in the toll-like receptor signaling pathway, cytokine-cytokine receptor interactions, chemokine signaling pathway, NOD-like receptor (NLR) signaling pathway and cytosolic DNA-sensing pathway, suggesting that these genes play a crucial role in the regulation of immune responses.
Emerging evidence indicates that dendritic cells, Th17 cells, and keratinocytes constitute a pathogenic triad in psoriasis. Tumor necrosis factor-alpha (TNF-α) and interleukin-23 (IL-23) produced by dendritic cells promote the differentiation of Th17 cells, which in turn produce key psoriatic cytokines, including IL-17, interferon-gamma (IFN-γ), and IL-22. The activity of these cytokines results in skin inflammation and further actives keratinocytes. Additionally, other cells and signaling pathways are implicated in the pathogenesis of psoriasis, including Th9 cells, Th22 cells, CD8+ cytotoxic T cells, neutrophils, γδ T cells, as well as the cytokines and chemokines they secrete4. Similarly, insulin resistance affects immune cells such as T cells, B cells, macrophages, and neutrophils, leading to an imbalance between pro-inflammatory and anti-inflammatory responses. Elevated levels of pro-inflammatory cytokines (e.g., TNF-α, IL-6) and adipokines (e.g., leptin, resistin) exacerbate insulin resistance, thereby promoting a vicious cycle of metabolic and immune dysregulation. This interplay contributes to the chronic low-grade inflammation underlying the pathogenesis of T2DM, further impairing insulin signaling and glucose metabolism29. Therefore, the interactions between cytokines and their receptors, along with the enriched chemokine signaling pathways derived from co-DEGs, represent vital mechanisms in the co-morbidity of T2DM and psoriasis.
At the first line of defense, the skin is continuously exposed to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Toll-like receptors (TLRs), expressed in a cell type-specific manner by various skin cells, play a fundamental role in detecting invading pathogens or damage and initiating the innate immune responses necessary for pathogen clearance and tissue repair. However, aberrant activation of TLRs canl exaggerate T cell-mediated autoimmune activation and trigger various intracellular pathways, resulting in the production of proinflammatory cytokines, chemokines,and the expression of co-stimulatory molecules that contribute to the development of psoriasis30.
In particular, TLRs including TLR1, TLR2, TLR4, TLR5, TLR7/8 and TLR9, along with TLR pathway-associated proteins, such as IRAK, TRAF, and SOCS, have been found dysregulated in psoriasis31. TLR-mediated activation of keratinocytes, plasmacytoid dendritic cells (pDCs), and/or conventional dendritic cells (cDCs) initiates early innate immune events that link to T cell activation and the development of autoimmunity in psoriasis32. The tripartite interaction between gut microbiota, the host immune system, and metabolism is a major factor in the pathophysiology of T2DM33,34,35. As the critical role of intestinal immunity and intestinal barrier function in the development of T2DM is increasingly acknowledged, intestinal epithelial cells (IECs) has emerged as vital modulators of obesity and glucose homeostasis through their effect on lipopolysaccharide (LPS) signaling. Metabolic endotoxemia, characterized by increased LPS levels in the blood, occurs when intestinal barrier function is compromised. Mohan et al.36 reported that TLR2 and TLR4 expression, along with their ligands LPS, and functional activation are elevated in monocytes from recently diagnosed T2DM patients, contributing to a proinflammatory state. Lu et al.37demonstrated that intestinal TLR4 regulatse the interaction between host and microbiota, thereby affecting metabolic syndrome. The knockout of TLR4 specifically in IECs led to increased body weight and impaired glucose metabolism. The metabolic effects of IEC-specific TLR4 knockout could be reversed by antibiotic treatment, suggesting that TLR4 expression in IECs play a central role in protective host-microbe interactions. Furthermore, TLR4 has been implicated in beta cell failure during diet-induced obesity in mice, as high-fat diet (HFD)-fed TLR4 knockout mice exhibit preserved insulin secretory function, lower inflammatory markers,and no macrophage infiltration in pancreatic islets38. Additionally, TLR activation through ligands such as increased free fatty acids and lipid derivatives from adipocytes and the skeletal muscles has been reported to mediate insulin resistance39.
The NLRs, a superfamily of multidomain-containing proteins, serve as modulators of innate immunity by detecting cellular stress and microbial infection. They play critical roles controlling inflammasome activation, antigen presentation, transcriptional regulation and cell death. Mari et al.40analyzed the transcriptomes of normal, lesional and non-lesional psoriatic epidermis, revealing that the NLR signaling genes NOD2, PYCARD, CARD6 and IFI16 are upregulated in psoriatic epidermis. All components necessary for the active inflammasome are present in the keratinocytes of psoriatic skin, underscoring the significance of NLR signaling and inflammasome activation in psoriasis. A study by Lai et al.41reported elevated GSDMD expression and increased levels of pro-pyroptosis mediators in the lesional skin tissues of psoriasis patients. GSDMD knockdown in the IMQ-induced psoriasis-like mouse model suppressed pyroptosis and alleviated skin lesion severity and inflammatory cell infiltration by inhibiting the NLR pathway, an effect that could be reversed by NLR pathway activator treatment. Furthermore, Abhijit et al.42reported that monocytes from diabetes patients exhibited increased expression of NOD1 and NOD2, correlating with poor glycemic control and insulin resistance. High glucose levels activated NOD expression and enhanced the actions of NOD ligands. Additionally, obesity fosters conditions that favour the activation of pro-inflammatory responses mediated by TLR4, NOD1 and NLRP3, including contributions from microbiota-derived factors43.
The cGAS-STING pathway was discovered as an important DNA-sensing machinery that detects cytosolic DNA and triggers an immune response44. Yu et al.45explored the role of STING in inflammation associated with psoriasis, demonstrating that STING acts as a self-DNA sensor in macrophages and KCs of psoriatic skin, synergistically contributing to the inflammation that exacerbates psoriasis. Sun et al.46reported the upregulation of cGAS-STING signaling in psoriatic lesions by analyzing samples from clinical patients and IMQ-treated mice. Utilizing a conditional Sting-knockout transgenic mouse model, they further elucidated the impact of cGAS-STING signaling in DCs on the activation of IL-17- and IFN-γ-producing T cells in psoriatic inflammation. Additionally, the cGAS-STING signaling pathway has been shown to play a crucial role in the development and progression of diabetes through its regulation of immune responses, inflammation, insulin resistance, and β-cell dysfunction47,48.
By integrating the WCGNA modules and co-DEGs, 33 candidate co-driver genes were obtained and subjected to PPI network and ROC curve analysis. 7 genes demonstrated strong diagnostic performance with AUC greater than 0.7 in both datasets. Upon comparing the expression pattern of these genes in both training and validation datasets of psoriasis and T2DM cohorts, 4 genes, namely BEX5, EPHX2, GPRASP1, and RBP4, exhibited consistent downregulation, which were further confirmed from qPCR ananlysis in whole blood samples from clinical patients.
Members of the Bex (Brain expressed X-linked) gene family are among the most commonly identified genes in subtractive hybridization screens aimed at discovering ventral mesencephalic genes during the development stage of ventral mesencephalic dopamine neurons. Bex5 is present in humans and monkeys, but not rodents. This gene is localized to the X chromosome and exhibits 56% identity with human Bex3 (pHGR74, NADE). Bex5 proteins have been characterized as cytoplasmic proteins that are widely expressed and degraded by the proteasome49. To date, there has been no published research on the role of Bex5 in either psoriasis or T2DM, indicating a need for further investigation.The EPHX2 gene is located on chromosome 8 and encodes a highly conserved protein comprising 555 amino acids. The EPHX2 protein, also known as soluble epoxide hydrolase (sEH), is predominantly found in the cytoplasm50 and exhibits a broad expression pattern51. While EPHX2 plays a crucial role in the metabolism of xenobiotic epoxides, its primary physiological function involves the metabolism of epoxides derived from fatty acids. Among the most well-known substrates of EPHX2 are epoxyeicosatrienoic acids (EETs), which are derived from arachidonic acid (AA) and are subsequently metabolized into dihydroxyeicosatrienoic acids (DHETs)52. EETs have been demonstrated to exhibit beneficial effects across various conditions53. In contrast, epoxidized products of linoleic acid (LA), known as epoxyoctadecenoic acids (EpOMEs), are cytotoxic and impair mitochondrial respiration by uncoupling oxidative phosphorylation54. DiHOMEs, which possess greater cytotoxicity, are produced from EpOMEs catalyzed by EPHX255. Additionally, EPHX2 is involved in the metabolism of epoxides into their corresponding 1,2-diols, such as dihydroxyeicosatrienoic acids (DiHETEs) and dihydroxydocosahexaenoic acids (DiHDPEs), which are derived from eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These compounds play a crucial role in regulating blood pressure, inflammation, and metabolic disorders related to obesity56. EPHX2, with its broad expression and diverse substrates, has been implicated in numerous pathophysiological processes and has been extensively investigated as a potential therapeutic target. Notably, interventions aimed at increasing circulating EETs have been shown to enhance insulin sensitivity and prevent diabetes in animal models57. A randomized, double-blind, placebo-controlled crossover study evaluated the effect of a sEH inhibitor GSK2256294 in patients with obesity and prediabetes. The study revealed that administration of GSK2256294 for 7 days effectively inhibited sEH activity in plasma, muscle, and adipose tissue; however, it did not result in improvements in insulin sensitivity or blood pressure58. Functional variants of EPHX2 encode enzymes with altered hydrolase activity, specifically increased activity due to the Lys55Arg variant and decreased activity due to the Arg287Gln variant59. Ramirez et al.60 reported no relationship between the Lys55Arg genotype and insulin sensitivity or secretion in their study. In contrast, the EPHX2 287Gln variant was associated with higher insulin sensitivity index. Furthermore, plasma EETs were found to correlate with insulin sensitivity index and were decreased in individuals with metabolic syndrome. Conversely, a study conducted in a Japanese population reported an association between the EPHX2 rs751141 (Arg287Gln) variant and insulin resistance in patients with T2DM61. Additionally, the missense variant rs57699806 in EPHX2 was linked to an increased risk of gestational diabetes mellitus (GDM) in a Chinese population62. High-glucose-induced oxidative stress has been shown to suppress sEH levels in a hepatocarcinoma cell line (Hep3B)63 and sEH levels were found decreased in streptozotocin-induced diabetic mice in vivo64. Therefore, further investigation is required to elucidate the role of EPHX2 activity and function in the pathogenesis of diabetes. Another study examined the effects of inhibiting sEH on skin homeostasis and identified a protective role for sEH in the skin. Global deletion of the sEH (sEH−/− mice) resulted in thicker differentiated spinous and corneocyte layers compared to wild-type mice, demonstrating a hyperkeratosis phenotype that was replicated in wild-type mice treated with a sEH inhibitor. sEH deletion made the skin more susceptible to inflammation, as evidenced by the development of thicker imiquimod-induced psoriasis plaques in sEH−/− mice compared to the control group, along with increased infiltration and activation of neutrophils in response to mechanical stress65. To sum up, EPHX2 may play a vital role in the comorbidity of psoriasis and T2DM, and further exploration is needed to unveil its mechanism of action.
GPRASP1 is a member of the GPRASP(GPCR-associated sorting protein)/ARMCX (ARMadillo repeat-Containing proteins on the X chromosome) family, which comprises 10 proteins. It binds trafficking proteins to participate in post-endocytic sorting of G protein-coupled receptors (GPCRs)66. GPCR are one of the most abundant protein families encoded by the human genome and are involved in regulating numerous physiological functions67. Consequently, GPRASP1 can either silence signals by directing degradation receptors to lysosomes or amplify signals by promoting endosome formation68. Reports indicate that GPRASP1 may participate in vacuolar protein sorting and specific lysosomal degradation pathways, thereby controlling cellular responses to environmental signals 72. However, the direct involvement of GPRASP1 in diabetes or psoriasis has not yet been documented.
Retinol binding protein 4 (RBP4) is a member of the lipocalin family and serves as the primary transport protein of retinol (also known as vitamin A) in circulation69. Both diabetes and psoriasis have been reported to be potentially linked to alterations in vitamin A metabolism70,71. RBP4 has also been identified as an adipokine that influences insulin resistance, thereby contributing to an increased risk of diabetes72. Notably, RBP4-deficient mice exhibit a reduced propensity to develop insulin resistance73. Furthermore, transgenic overexpression of human RBP4 or the injection of recombinant human RBP4 into wild-type mice has been shown to induce glucose intolerance and insulin resistance. Mechanistically, the immune system—particularly antigen-presenting cells such as dendritic cells, macrophages, and CD4+ T cells—drives an inflammatory response within adipose tissue, mediating RBP4-induced insulin resistance74,75,76. Importantly, RBP4 levels are elevated in patients with psoriasis vulgaris, and systemic treatment can lower them77,78. In our analysis, we observed a decrease in RBP4 expression in the microarray profiles of both the training and validation datasets from skin lesions of psoriatic patients and islet tissue from patients with T2DM. Validation results of whole blood samples from patients also demonstrated a significant reduction in RBP4 RNA levels. The discrepancies between our findings and those of other studies may be attributed to variations in the quantity and types of detection samples, as well as the experimental techniques utilized. To clarify the changes of RBP4 related to the pathogenesis of psoriasis and T2DM, it is essential to utilize a larger clinical sample size with standardized detection techniques and sample types. Additionally, further exploration of potential mechanisms involved in disease onset is warranted.
In psoriasis, BEX5 and GPRASP1 exhibited the most pronounced negative correlations with CD56dim NK cells, which is consistent with emerging evidence of NK cell dysfunction in psoriatic inflammation79. The association between EPHX2 and memory B cells reinforces the role of B cell-mediated immunity in the progression of psoriasis, a pathway that is gaining increasing attention4,80,81.
Conclusion
In summary, our integrated bioinformatics analysis identified co-DEGs and enriched immune-inflammatory pathways, providing novel insights into the pathogenesis underlying the comorbidity of psoriasis and T2DM. Two crucial co-driver genes identified, namely BEX5 and GPRASP1, have not been previously reported in relation to either psoriasis or T2DM, warranting further exploration. However, it is important to note that this study is primarily computational. Although qPCR validation was conducted, enhancing the translational relevance of the identified crucial co-driver genes, the sample size was relatively small (n = 4–13 per group). Future studies with larger cohorts, as well as further investigations into the molecular mechanisms of the pathogenesis, are necessary to confirm these findings.
Data availability
The microarray datasets analyzed during the current study are available in the GEO repository (https://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE13355, GSE14905, GSE76894, and GSE41762. The detailed data supporting the validation of co-DEGs in clinical samples are provided within the Supplementary Information file.
References
C. American Diabetes Association Professional Practice, 2. Diagnosis and classification of diabetes: Standards of care in diabetes-2024. Diabetes Care 47, S20–S42 (2024).
Singh, A., Shadangi, S., Gupta, P. K. & Rana, S. Type 2 diabetes mellitus: A comprehensive review of pathophysiology, comorbidities, and emerging therapies. Compr. Physiol. 15, e70003 (2025).
Parisi, R. et al. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 369, m1590 (2020).
Sieminska, I., Pieniawska, M. & Grzywa, T. M. The immunology of psoriasis-current concepts in pathogenesis. Clin. Rev. Allergy Immunol. 66, 164–191 (2024).
Shapiro, J. et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: A case-control study. J. Am. Acad. Dermatol. 56, 629–634 (2007).
Brauchli, Y. B., Jick, S. S. & Meier, C. R. Psoriasis and the risk of incident diabetes mellitus: A population-based study. Br. J. Dermatol. 159, 1331–1337 (2008).
Solomon, D. H., Love, T. J., Canning, C. & Schneeweiss, S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann. Rheum. Dis. 69, 2114–2117 (2010).
Kamiya, K., Kishimoto, M., Sugai, J., Komine, M. & Ohtsuki, M. Risk factors for the development of psoriasis. Int. J. Mol. Sci. 20, 4347 (2019).
Mamizadeh, M., Tardeh, Z. & Azami, M. The association between psoriasis and diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 13, 1405–1412 (2019).
Armstrong, A. W., Harskamp, C. T. & Armstrong, E. J. Psoriasis and the risk of diabetes mellitus: A systematic review and meta-analysis. JAMA Dermatol. 149, 84–91 (2013).
Coto-Segura, P. et al. Psoriasis, psoriatic arthritis and type 2 diabetes mellitus: A systematic review and meta-analysis. Br. J. Dermatol. 169, 783–793 (2013).
Wan, M. T. et al. Psoriasis and the risk of diabetes: A prospective population-based cohort study. J. Am. Acad. Dermatol. 78, 315-322 e311 (2018).
Kang, J. et al. Identification of shared genes and pathways in periodontitis and type 2 diabetes by bioinformatics analysis. Front. Endocrinol. (Lausanne) 12, 724278 (2021).
Szklarczyk, D. et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447-452 (2015).
Robin, X. et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 12, 77 (2011).
Zhou, Y. et al. Bioinformatic analysis of the potential common pathogenic mechanisms for psoriasis and metabolic syndrome. Inflammation 46, 1381–1395 (2023).
Suarez-Farinas, M., Lowes, M. A., Zaba, L. C. & Krueger, J. G. Evaluation of the psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA). PLoS ONE 5, e10247 (2010).
Charoentong, P. et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 18, 248–262 (2017).
Chu, Y. et al. Identification of genes and key pathways underlying the pathophysiological association between nonalcoholic fatty liver disease and atrial fibrillation. BMC Med. Genomics 15, 150 (2022).
Wishart, D. S. et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082 (2018).
Liu, Z. P., Wu, C., Miao, H. & Wu, H. RegNetwork: An integrated database of transcriptional and post-transcriptional regulatory networks in human and mouse. Database (Oxford) 2015, bav095 (2015).
Zhang, Y. & Kiryu, H. Identification of oxidative stress-related genes differentially expressed in Alzheimer’s disease and construction of a hub gene-based diagnostic model. Sci. Rep. 13, 6817 (2023).
Karadag, A. S. et al. Is psoriasis a pre-atherosclerotic disease? Increased insulin resistance and impaired endothelial function in patients with psoriasis. Int. J. Dermatol. 49, 642–646 (2010).
Okan, G., Baki, A. M., Yorulmaz, E., Dogru-Abbasoglu, S. & Vural, P. Serum visfatin, fetuin-A, and pentraxin 3 levels in patients with psoriasis and their relation to disease severity. J. Clin. Lab. Anal. 30, 284–289 (2016).
Albareda, M. et al. Metabolic syndrome and its components in patients with psoriasis. Springerplus 3, 612 (2014).
Karpinska-Mirecka, A., Bartosinska, J. & Krasowska, D. The impact of hypertension, diabetes, lipid disorders, overweight/obesity and nicotine dependence on health-related quality of life and psoriasis severity in psoriatic patients receiving systemic conventional and biological treatment. Int. J. Environ. Res. Public Health 18, 13167 (2021).
Patrick, M. T. et al. Causal relationship and shared genetic loci between psoriasis and type 2 diabetes through trans-disease meta-analysis. J. Investig. Dermatol. 141, 1493–1502 (2021).
Wen, S. et al. Psoriasis exacerbates the state of insulin resistance in patients with Type 2 diabetes. Diabetes .Metab Syndr. Obes. 14, 2389–2397 (2021).
Berbudi, A., Khairani, S. & Tjahjadi, A. I. Interplay between insulin resistance and immune dysregulation in type 2 diabetes mellitus: Implications for therapeutic interventions. Immunotargets Ther. 14, 359–382 (2025).
Rahmani, F. & Rezaei, N. Therapeutic targeting of toll-like receptors: A review of toll-like receptors and their signaling pathways in psoriasis. Expert Rev. Clin. Immunol. 12, 1289–1298 (2016).
Chen, J. Q., Szodoray, P. & Zeher, M. Toll-like receptor pathways in autoimmune diseases. Clin. Rev. Allergy Immunol. 50, 1–17 (2016).
Sun, L., Liu, W. & Zhang, L. J. The role of toll-like receptors in skin host defense, psoriasis, and atopic dermatitis. J. Immunol. Res. 2019, 1824624 (2019).
Zhou, Z., Sun, B., Yu, D. & Zhu, C. Gut microbiota: An important player in type 2 diabetes mellitus. Front. Cell. Infect. Microbiol. 12, 834485 (2022).
Scheithauer, T. P. M. et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 11, 571731 (2020).
Riedel, S., Pheiffer, C., Johnson, R., Louw, J. & Muller, C. J. F. Intestinal barrier function and immune homeostasis are missing links in obesity and type 2 diabetes development. Front. Endocrinol. (Lausanne) 12, 833544 (2021).
Dasu, M. R., Devaraj, S., Park, S. & Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33, 861–868 (2010).
Lu, P. et al. Intestinal epithelial Toll-like receptor 4 prevents metabolic syndrome by regulating interactions between microbes and intestinal epithelial cells in mice. Mucosal Immunol. 11, 727–740 (2018).
Li, J. et al. TLR4 is required for the obesity-induced pancreatic beta cell dysfunction. Acta Biochim. Biophys. Sin. (Shanghai) 45, 1030–1038 (2013).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 116, 3015–3025 (2006).
Tervaniemi, M. H. et al. NOD-like receptor signaling and inflammasome-related pathways are highlighted in psoriatic epidermis. Sci. Rep. 6, 22745 (2016).
Lai, S. et al. Knockdown of GSDMD inhibits pyroptosis in psoriasis by blocking the NOD-like receptor signaling pathway. Int. Immunopharmacol. 147, 114036 (2025).
Shiny, A. et al. Convergence of innate immunity and insulin resistance as evidenced by increased nucleotide oligomerization domain (NOD) expression and signaling in monocytes from patients with type 2 diabetes. Cytokine 64, 564–570 (2013).
Rodrigues, E. L. R. et al. Microbiota and Nod-like receptors balance inflammation and metabolism during obesity and diabetes. Biomed. J. 46, 100610 (2023).
Yu, L. & Liu, P. Cytosolic DNA sensing by cGAS: Regulation, function, and human diseases. Signal Transduct. Target. Ther. 6, 170 (2021).
Yu, Y. et al. Cytosolic DNA-mediated STING-dependent inflammation contributes to the progression of psoriasis. J. Investig Dermatol. 142, 898–906 (2022).
Sun, X. et al. Targeting STING in dendritic cells alleviates psoriatic inflammation by suppressing IL-17A production. Cell. Mol. Immunol. 21, 738–751 (2024).
Mohammadi, S. & Khorasani, M. Implications of the cGAS-STING pathway in diabetes: Risk factors and therapeutic strategies. Int. J. Biol. Macromol. 278, 134210 (2024).
Hu, H. et al. cGAS-STING mediates cytoplasmic mitochondrial-DNA-induced inflammatory signal transduction during accelerated senescence of pancreatic beta-cells induced by metabolic stress. FASEB J. 36, e22266 (2022).
Alvarez, E., Zhou, W., Witta, S. E. & Freed, C. R. Characterization of the Bex gene family in humans, mice, and rats. Gene 357, 18–28 (2005).
Enayetallah, A. E., French, R. A., Barber, M. & Grant, D. F. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J. Histochem. Cytochem. 54, 329–335 (2006).
Gill, S. S. & Hammock, B. D. Distribution and properties of a mammalian soluble epoxide hydrase. Biochem. Pharmacol. 29, 389–395 (1980).
Imig, J. D. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 92, 101–130 (2012).
Imig, J. D. & Hammock, B. D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 8, 794–805 (2009).
Hildreth, K., Kodani, S. D., Hammock, B. D. & Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutr. Biochem. 86, 108484 (2020).
Moghaddam, M. F. et al. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat. Med. 3, 562–566 (1997).
Morisseau, C. & Hammock, B. D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 53, 37–58 (2013).
Ghoshal, K. et al. EET analog treatment improves insulin signaling in a genetic mouse model of insulin resistance. Diabetes 71, 83–92 (2021).
Luther, J. M. et al. GSK2256294 decreases sEH (soluble epoxide hydrolase) activity in plasma, muscle, and adipose and reduces F2-isoprostanes but does not alter insulin sensitivity in humans. Hypertension 78, 1092–1102 (2021).
Przybyla-Zawislak, B. D. et al. Polymorphisms in human soluble epoxide hydrolase. Mol. Pharmacol. 64, 482–490 (2003).
Ramirez, C. E. et al. Arg287Gln variant of EPHX2 and epoxyeicosatrienoic acids are associated with insulin sensitivity in humans. Prostaglandins Other Lipid Mediat. 113–115, 38–44 (2014).
Ohtoshi, K. et al. Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients. Biochem. Biophys. Res. Commun. 331, 347–350 (2005).
Lai, S. et al. Genetic variants in epoxyeicosatrienoic acid processing and degradation pathways are associated with gestational diabetes mellitus. Nutr. J. 22, 31 (2023).
Oguro, A., Fujita, N. & Imaoka, S. Regulation of soluble epoxide hydrolase (sEH) in mice with diabetes: High glucose suppresses sEH expression. Drug Metab. Pharmacokinet. 24, 438–445 (2009).
Oguro, A., Oida, S. & Imaoka, S. Down-regulation of EPHX2 gene transcription by Sp1 under high-glucose conditions. Biochem. J. 470, 281–291 (2015).
Naeem, Z. et al. Role of the soluble epoxide hydrolase in keratinocyte proliferation and sensitivity of skin to inflammatory stimuli. Biomed. Pharmacother. 171, 116127 (2024).
Kaeffer, J., Zeder-Lutz, G., Simonin, F. & Lecat, S. GPRASP/ARMCX protein family: Potential involvement in health and diseases revealed by their novel interacting partners. Curr. Top. Med. Chem. 21, 227–254 (2021).
Abu-Helo, A. & Simonin, F. Identification and biological significance of G protein-coupled receptor associated sorting proteins (GASPs). Pharmacol. Ther. 126, 244–250 (2010).
Du, J. et al. Comprehensive pan-cancer analysis of role of GPRASP1, associated with clinical outcomes, immune microenvironment, and immunotherapeutic efficiency in pancreatic cancer. Pathol. Res. Pract. 243, 154374 (2023).
Steinhoff, J. S., Lass, A. & Schupp, M. Biological functions of RBP4 and its relevance for human diseases. Front. Physiol. 12, 659977 (2021).
Zhang, Y., Wang, T., Hu, X. & Chen, G. Vitamin A and diabetes. J Med Food 24, 775–785 (2021).
Wang, H. M., Wu, C., Jiang, Y. Y., Wang, W. M. & Jin, H. Z. Retinol and vitamin A metabolites accumulate through RBP4 and STRA6 changes in a psoriasis murine model. Nutr. Metab. (Lond.) 17, 5 (2020).
Hu, C. et al. Effect of RBP4 gene variants on circulating RBP4 concentration and type 2 diabetes in a Chinese population. Diabet. Med. 25, 11–18 (2008).
Yang, Q. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 (2005).
Moraes-Vieira, P. M. et al. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell. Metab. 19, 512–526 (2014).
Moraes-Vieira, P. M. et al. Antigen presentation and T-cell activation are critical for RBP4-induced insulin resistance. Diabetes 65, 1317–1327 (2016).
Moraes-Vieira, P. M. et al. Retinol binding protein 4 primes the NLRP3 inflammasome by signaling through Toll-like receptors 2 and 4. Proc. Natl. Acad. Sci. U. S. A. 117, 31309–31318 (2020).
Gao, G., Cui, Y. & Cheng, H. Association between retinol binding protein-4 and psoriasis vulgaris: A systematic review and meta-analysis. Front. Med. (Lausanne) 10, 1208969 (2023).
Niu, R. et al. Pre-treatment plasma retinol binding protein 4 level and its change after treatments predict systemic treatment response in psoriasis patients. BMC Immunol. 25, 55 (2024).
Kucuksezer, U. C. et al. The role of natural killer cells in autoimmune diseases. Front. Immunol. 12, 622306 (2021).
Noor, A. A. M., Nor, A. & Redzwan, N. M. The immunological understanding on germinal center B cells in psoriasis. J. Cell. Physiol. 239, e31266 (2024).
Mizumaki, K., Horii, M., Kano, M., Komuro, A. & Matsushita, T. Suppression of IL-23-mediated psoriasis-like inflammation by regulatory B cells. Sci. Rep. 11, 2106 (2021).
Acknowledgements
We thank all consortium studies for publicly making the summary association statistics data available. We thank all the reviewers for their comments and contributions to this manuscript.
Funding
This work was supported by The Yunnan Provincial Science and Technology Department Science and Technology Programme Projects (202101AZ070001-168) and Joint Fund set up by Yunnan University of Chinese Medicine and its First Affiliated Hospital (XYLH202339).
Author information
Authors and Affiliations
Contributions
W-ZM and MJ: made major contributions to the conception and design of the study; the acquisition, analysis, interpretation of the data; and the writing and revision of the manuscript. Z-YW and HH: contributed to the data acquisition and analysis. QC and H-GL: made important contributions to the revision of the manuscript. W-JR contributed to the analysis of the data. Z-PL, CH and HW: have made contributions to the design, drafting, and revising of the articl. X-XY and Z-QM:put forward many important suggestions for the revision of this article, which greatly improved the text;Y-XS, LX and Y-JZ: made substantial contributions to the supervision and project administration of the study and the writing and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Z., Mu, J., Zhang, Y. et al. Bioinformatics analyses of comorbid mechanisms between psoriasis and type 2 diabetes mellitus. Sci Rep 15, 36772 (2025). https://doi.org/10.1038/s41598-025-20762-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20762-8