Abstract
The return of large carnivores to human-dominated landscapes complicates predator–prey dynamics. While predator and anthropogenic effects are well-documented in intact systems, their interplay in fragmented landscapes remains understudied. We examined whether apex predators still regulate prey and mesopredators in the large mammal community of the Golan Heights—a mosaic of nature reserves, farmland and military zones—where wolves (Canis lupus), golden jackals (Canis aureus) and wild boar (Sus scrofa) are culled to mitigate agricultural losses and human-wildlife conflict. Using 60 camera traps and high-resolution culling data, we quantified predator–prey and intraguild relationships, identifying land protection thresholds at which they shifted. We found that endangered mountain gazelles (Gazella gazella) were most active in protected areas (top 50% of sites) with higher wolf activity and consistently avoided jackals. Species-specific culling increased jackal activity but decreased boar activity in nonprotected areas (lower 65% and 62% of sites, respectively), outweighing the suppressive effects of wolves. While jackal culling modestly benefited gazelles in protected areas, the positive association between wolves and gazelles was sevenfold stronger. These findings suggest that apex predators may maintain their ecological roles in fragmented landscapes up to a threshold of human disturbance, beyond which top-down regulation weakens and ecosystem function deteriorates.
Similar content being viewed by others
Introduction
Large carnivores have undergone widespread declines globally due to various human pressures, including habitat loss, hunting and conflict with humans1. However, recent improvements in conservation policies and legislative protection have facilitated their recovery in some human-dominated landscapes2,3. These landscapes—characterized by extensive land modification, habitat fragmentation and agriculture—now cover approximately 75% of global terrestrial areas4. Large carnivores (i.e., > 15 kg) function as apex predators in food webs, exerting far-reaching ecological impacts through both direct predation and indirect effects on prey and competitor behavior5,6,7. Two key mechanisms underpinning these effects are (1) “landscape of fear”, in which prey and mesopredators alter their spatial and temporal activity patterns in response to perceived predation risk8,9,10, and (2) “mesopredator release”, where the loss or suppression of apex predators allows mid-sized carnivores (< 15 kg) to proliferate, often leading to increased predation on their smaller prey11,12. Together, these processes form the foundation of apex predator theory (used hereafter), which describes how top predators structure ecosystems through both lethal and non-lethal effects. While these dynamics are well-documented in relatively undisturbed landscapes6,13, their applicability to human-modified systems remains less explored14,15. As large carnivores recolonize increasingly altered habitats, research is needed to determine whether they retain key ecological functions or if human pressures disrupt their regulatory roles in these complex environments14,16. This study examines how an apex predator interacts with both prey and mesopredators in a human-dominated landscape, identifying the thresholds at which its functional role is maintained or diminished.
Apex predators are expected to regulate predator–prey dynamics by controlling populations of large herbivores such as elk (Cervus canadensis), white-tailed deer (Odocoileus virginianus) or wild boar (Sus scrofa) which can overexploit native vegetation5,17. Additionally, by exerting top-down pressure on intermediate-sized carnivores, apex predators are expected to control mesopredator populations that would otherwise impose strong predation pressure on smaller prey species9,18,19. However, humanized habitats often favor highly adaptable mesopredators, particularly those capable of exploiting anthropogenic resources such as garbage, crop fields, or domestic animal waste. This resource plasticity enables mesopredators such as foxes (Vulpes sp.), raccoons (Procyon sp.) and feral cats (Felis catus silvestris) to thrive and spread even in the presence of larger carnivores11,18. A striking example is the current rapid expansion of the golden jackal (Canis aureus) across Europe, which coincides with continental gray wolf (Canis lupus) recovery20; yet, the ecological consequences of this expansion and the extent to which wolf recovery may regulate it remain largely understudied. While some studies from human-dominated landscapes suggest apex predators suppress both herbivores and mesopredators, human disturbance can often confound these trophic dynamics and obscure efforts to quantify them21,22. In ecosystems already strained by human pressures, understanding the capacity of apex predators to fulfill their ecological roles is essential to inform conservation and management strategies23.
Even when apex predators successfully recolonize human-dominated areas, they face a wide range of novel anthropogenic pressures24. Habitat fragmentation, human activity in wild areas, depletion of wild prey substituted by domestic animals and human-wildlife conflict can all affect predator distribution and behavior patterns, as well as the way they interact with species at lower trophic levels25,26. Among these pressures, culling management is a widespread tool for reducing livestock depredation27. While culling has shown some success with solitary carnivores such as American black bears (Ursus americanus) and Eurasian lynx (Lynx lynx)28,29, social species often exhibit compensatory behaviors like increased reproduction or dispersal, making long-term control difficult30,31,32. Furthermore, heavy culling can threaten population viability when thresholds are exceeded33. Beyond direct interventions, extensive land-use changes such as agricultural expansion and urban development reduce habitat availability and disrupt the spatiotemporal patterns of interspecific interactions22,26. Taken together, these overlapping pressures challenge the capacity of apex predators to maintain their ecological roles in human-modified landscapes.
As human-induced pressures intensify, natural trophic interactions within ecosystems can break down, disrupting or collapsing ecological balance34,35. An ecosystem out of balance reveals a critical breaking point, i.e., a threshold beyond which ecosystems lose their capacity for natural regulation36,37. Notably, this breaking point does not necessarily correspond to the extinction of apex predators but marks the boundary at which they cease to realize their ecological roles, even in seemingly suitable habitats38. Where apex predators have become functionally extinct, humans may replace apex predators as the dominant regulatory force, directly and indirectly controlling prey and mesopredator populations24. For example, overhunting of prey species can reduce predator numbers and weaken top-down control39, while anthropogenic food subsidies, such as livestock carcasses, garbage dumps and crop surpluses, can eliminate natural bottom-up regulation such that mesopredator or prey populations inflate beyond the regulatory capacity of natural top-down control40,41. In some cases, apex predators themselves may become reliant on these human-provided resources, further weakening their role in structuring ecological communities12,42,43. While these dynamics highlight the complexity of predator–prey interactions in anthropogenic landscapes, the thresholds at which apex predators lose their regulatory function remain poorly understood, particularly in fragmented or actively managed landscapes. A key challenge is disentangling natural trophic interactions from anthropogenic influences affecting species’ spatiotemporal patterns at the community level32,44. As landscapes become increasingly fragmented and shared among humans and wildlife, quantifying breaking points of predator–prey interactions is crucial for understanding how factors such as land use, resource availability and human activity shape the capacity of apex predators to maintain ecological balance in diverse multi-use landscapes16,45.
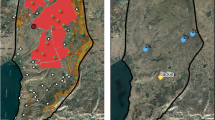
The Golan Heights in the Middle East provides an ideal system for testing apex predator function in a human-dominated mosaic landscape, as it hosts high biodiversity in a matrix of diverse land uses, including agriculture, recreation and military activity (Fig. 1A). Over the past half-century, wildlife protection laws and increased cattle and crop availability have allowed wildlife in this region to recover substantially, resulting in some of the highest global densities of grey wolves, golden jackals and wild boar46,47,48; it is also a last stronghold of the endangered mountain gazelle (Gazella gazella)49,50. Although all wild species are legally protected, wolf, jackal and wild boar populations are actively managed through culling programs to mitigate livestock depredation and crop damage51 (Fig. 1B). This unique context—characterized by apex predator recovery, high mesopredator densities and human intervention—offers a rare opportunity to examine how predators retain their ecological roles in a fragmented landscape. By leveraging fine-scale culling data across multiple trophic levels, we can assess community-level responses to both natural and anthropogenic pressures, identifying the thresholds at which human activity disrupts apex predator function.
Maps of the Golan Heights showing (A) land cover (base colors) with semi-transparent land-use polygons overlaid and (B) the spatial arrangement of sampling sites used in the study, categorized as high culling, low culling, nature reserve, or minefield. High/low culling zones were delineated from ten years of spatially explicit wolf and jackal culling records to depict relative removal intensity (see Methods); exact culling locations are not shown for confidentiality. Nature reserves and minefields represent no-culling areas, with recreation also absent from the latter. Maps created by the authors in QGIS version 3.40 (http://qgis.org)52.
We asked whether apex predator theory holds in a mosaic landscape where predator–prey dynamics are influenced by both natural and human-mediated factors. We analyzed the population-level responses of gazelles, wild boar, jackals and wolves to the combined pressures of predation, culling and land management. We hypothesized that: (H1) Wolves and jackals, abundant sympatric predators of mountain gazelles, would suppress gazelle activity, reflecting additive top-down control50,53; (H2) Consequently, areas with intense wolf and jackal culling would serve as conservation refuges for gazelles, given the known link between dense predator populations and increased fawn mortality50; (H3) Increased wolf activity would suppress jackal and wild boar activity, in line with the mesopredator release and landscape of fear theories10,54,55,56 and wolves’ known reliance on boar as prey in Mediterranean landscapes57. This effect would demonstrate wolves’ ecological role in regulating both mesopredators and prey; (H4) Lastly, anthropogenic pressures, such as culling and land use change, would either outweigh or interact with natural trophic interactions to shape the structure of the large mammal community, as observed in other fragmented landscapes where human activities often overwhelm natural ecological processes21,58.
To test these hypotheses, we applied integrated N-mixture and structural equation models to systematic camera trap data, incorporating high-resolution culling records and land-use layers to quantify lethal risk and human disturbance gradients for apex predators, mesopredators and prey. We used a priori directed acyclic graphs to visualize hypothesized relationships among species, natural predator–prey dynamics and human-mediated pressures (Fig. 2). Our findings reveal that even in a fragmented, human-dominated landscape, apex predators remain functionally important within remaining protected patches, amplifying the ecological benefits of these areas. We also found that the suppressive effect of mesopredator culling was amplified within refugia but reversed outside them, suggesting that apex predators’ regulatory control begins to deteriorate with increasing anthropogenic pressure.
A priori directed acyclic graph illustrating our hypotheses: (H1) wolves and jackals suppress gazelles; (H2) predator culling indirectly benefits gazelle conservation; (H3) wolves suppress overabundant agricultural pests (i.e., jackals and wild boar); and (H4) anthropogenic effects outweigh or interact with natural trophic interactions. Green lines indicate hypothesized positive relationships, while red lines indicate hypothesized negative relationships. Dashed lines represent indirect effects, and arrows denote the hypothesized direction of each relationship. Arrow width conveys conceptual weight: anthropogenic pressures (culling, land use) are drawn thicker than natural trophic links to reflect H4; widths are schematic and not scaled to effect sizes.
Results
We generated 23,000 independent detections of our four study species, comprising 1043 independent detections of wolves, 11,959 of jackals, 4937 of wild boar and 5061 of mountain gazelles (Table 1). Naive occupancy, or the proportion of sampling locations with detections, was high for all four species—95% for wolves, 100% for jackals, 97% for wild boar and 88% for gazelles. Jackals were the most abundant species, averaging 149.46 ± 67.54 SD sightings per two-week period per sampling location, followed by wild boar (129.1 ± 36.79 SD), gazelle (112.61 ± 45.10 SD) and lastly wolves (26.08 ± 8.03 SD).
Our top structural equation model (SEM) linked three species-specific GLMs for jackal, wild boar, and gazelle, with wolf activity modeled exogenously (Table 2, Fig. 3). This structure ranked above alternative SEMs that included a separate wolf GLM (see Supplementary Table S1 for full model rankings). Parallel N-mixture models produced consistent patterns but showed poorer fit and less stable p-values (Supplementary Table S2).
Final structural equation model (SEM) illustrating the interactive effects of culling, predators and land management on the large mammal community in the Golan Heights. Line width represents effect size, with solid lines denoting statistically significant effects (P < 0.05) and dashed lines representing non-significant effects included in the top SEM. Interactive effects are indicated by lines merging into grey circles, with corresponding graphs displaying these interactions in detail: (A) the interaction between culling and land protection for golden jackals; (B) the interaction between wolf activity and land protection for gazelles; and (C) the interaction between culling and land protection for wild boar. Dotted lines denote threshold values in land protection at which the direction or strength of culling or predator effects shifts: in panel B, the threshold is shown as a vertical line (0.114, 50th quantile), while in panels A and C, the threshold is represented by a horizontal line where the slope of the effect changes (jackals: 0.267, 65th quantile; wild boar: 0.196, 62nd quantile). These threshold values highlight how species responses depend on the surrounding degree of protection and human pressure.
Interspecies effects
Interspecific paths in our top SEM revealed that wolf activity had a strong and statistically significant positive interaction with nature reserves on gazelle activity (β = 1.13, p = 0.0067), but small non-significant negative effects on both jackals (β = -0.15, p = 0.368) and boar (β = -0.10, p = 0.48). Gazelles were most active in areas within nature reserves where wolf activity was higher (Fig. 3B). Jackals demonstrated a significant positive correlation with boar activity patterns (β = 0.30, p = 0.032), a relationship that was not part of our original causal hypothesis (Fig. 1). Jackals, however, showed a consistent negative relationship with gazelle activity across all land uses (β = -0.26, p = 0.035), representing the only significant predator–prey relationship that remained independent of land-use context. Indirect or cascading effects, calculated by multiplying path coefficients59, revealed that the interactive effect of jackal culling cascaded through the jackal-gazelle relationship to indirectly affect gazelles based on land use (-0.629 × -0.26 = 0.164). This suggests that jackal culling had a small indirect effect on gazelle activity that was dependent on land use—positive in more protected areas and negative in less protected areas. In contrast with jackals, our top SEMs did not indicate any effect of boar activity on gazelles.
Culling and land use effects
Culling and land use were the strongest drivers of both jackals and wild boar activity, with significant interactive effects observed for both species (Fig. 3A, C), consistent with land protection functioning as an inverse subsidy gradient. For jackals, culling reduced their activity in low-subsidy natural areas but increased their activity in high-subsidy developed areas (β = -0.629, p = 0.015). In contrast, the interaction had an opposite effect on wild boar activity (β = 0.484, p = 0.012), where culling reduced boar activity in less natural areas but had minimal impact in protected areas, where culling was rarely applied (Fig. 2C, green slope with large confidence interval in protected areas). Shipley’s d-separation test revealed no significant relationships between the culling of wolves, jackals, or boars and gazelle activity, indicating that culling predators and boars had no direct benefit on gazelles.
Threshold-dependent species responses
Interactive effects on species’ activity were marked by distinct threshold values, where the level of one variable determined if the effect of another variable was absent, present, or reversed. For gazelles, the positive relationship with wolf activity was detected when land protection exceeded a value of 0.114 (Fig. 3B). This value corresponds to 11.4% protected land within a 7-km radius, proximity-weighted to emphasize nearby areas over distant ones (see Methods; Shamoon et al.60 for exponential decay approach). Though low in absolute terms, this represents the 50th quantile of the land protection gradient, meaning that half of the sampled sites were above this threshold and half were below. Similar context-dependent thresholds emerged for the effects of jackal and wild boar culling. For jackals, the shift occurred at a land protection value of 0.267 (65th quantile), with culling increasing jackal activity below this threshold but reducing it above (Fig. 3A). Wild boar showed the opposite pattern, with culling reducing wild boar activity below a land protection threshold of 0.196 (62nd quantile), but increasing activity in more protected areas (Fig. 3C).
Discussion
It has been argued that both the decline and recovery of apex predators may have far-reaching ecological impacts34,55,56,61. In human-dominated landscapes, wildlife must adapt to anthropogenic pressures such as wildlife management, land use change and human-wildlife conflict, complicating trophic dynamics and making them more challenging to interpret7,14. We tested the applicability of apex predator theory to the fragmented, mosaic landscape of the Golan Heights by analyzing fine-scale species-specific culling and activity data. This approach allowed us to assess the population-level responses of the large mammal community to intersecting pressures of predation, culling and land use, and to gain insights into community-level interactions within a multi-predator, multi-prey system. Our findings indicate that protected areas facilitated natural interactions between species, with a strong positive association between apex predator activity and endangered ungulate activity in protected areas. However, contrary to expectations of additive top-down control (H1), only jackals—not wolves—suppressed gazelle activity. Importantly, gazelles appeared to benefit more from high apex predator activity than from culling efforts aimed at reducing overabundant mesopredators (H2). We also found that land use and culling interacted to drive mesopredator activity patterns more strongly than apex predator activity, especially outside protected areas (H3). Taken together, these results suggest that apex predators can maintain their ecological roles even in highly fragmented systems, though only up to a breaking point of anthropogenic pressure. Beyond this threshold, however, particularly in intensively managed agricultural areas and regions with high levels of culling, trophic dynamics became disrupted (H4). Our results highlight the importance of maintaining protected areas and regulating anthropogenic food resources to enable apex predators to deliver the top-down ecosystem services essential for functional predator–prey relationships and ecosystem balance.
Apex predators and prey relationships
Depending on community structure, apex predators can regulate prey populations by directly suppressing their abundance and activity17, while also shielding prey from other predators through interference competition19,55. We initially hypothesized that both wolves and jackals would suppress gazelle activity; however, our findings revealed contrasting responses to these two sympatric predators. Gazelle activity peaked in protected areas with high wolf activity but dropped significantly in areas with high jackal activity, regardless of land use. The negative effect of jackals on gazelles, recorded in other fragmented landscapes62, likely reflects the inflated jackal density of the Golan Heights31. In contrast, the positive interaction of wolves and land protection suggests that gazelles benefit most in protected areas where wolves are present—more so than in protected areas without wolves. This pattern aligns with theoretical models predicting that apex predators can buffer prey from mesopredators when mesopredator densities remain below a threshold and when the apex predator exerts a stronger top-down effect on mesopredators than on prey12,53. Since apex predators typically occur at lower densities than mesopredators, their presence may reduce overall predation pressure on prey when conditions allow for territorial stability and reduced human disturbance1,54,63. In our system, this dynamic appears most evident in protected areas, where wolves maintain stable territories and gazelles face less pressure from jackals, their more abundant predator. This may help explain why prey tolerate—or even select for—areas with higher apex predator activity45,55. The positive association between wolves and gazelles in protected areas highlights the nuance of contemporary predator–prey dynamics in increasingly fragmented ecosystems with overabundant mesopredator populations. At the same time, while we quantify top-down relationships using contemporaneous activity indices, bidirectional processes (e.g., wolves tracking gazelles) undoubtedly operate alongside them. Nevertheless, livestock biomass in the Golan exceeds gazelle biomass by roughly three orders of magnitude (∼103 × ; see Supplementary Note S1), making large grazers and other anthropogenic resources the dominant energetic attractors for wolves in most contexts.
In contrast to gazelles, wild boar showed only a small, non-significant response to wolves, despite being a principal wolf prey in the region46 and occurring at high density in our survey. This weak predator–prey signal fits the profile of hyperabundant, subsidy-driven generalists that rely heavily on human-provided resources40,41. Unlike gazelles, boar also face lower predation risk from jackals, which helps explain why their space use was shaped more by resource distribution and human-induced risk than by predator activity (expanded below in Weak mesopredator suppression, strong bottom-up resource subsidies).
Refuges for prey depend more on protection than predator removal
While predator culls are generally employed to mitigate livestock losses, they have also been used to aid in the conservation of endangered prey species. Studies in other systems have drawn varying conclusions regarding the effectiveness of predator removal in conserving prey species, especially when compared to mitigating other threats such as habitat loss, fragmentation, poaching, collisions and disease64,65. In wolf-caribou (Rangifer tarandus) dynamics, for example, some studies argue that predator control is ineffective because habitat loss poses a far greater challenge to caribou survival66, while others contend that predator control is necessary to prevent further declines of caribou subpopulations67. Prey responses can also be species-specific; for instance, coyote removal increased fawn survival in pronghorn (Antilocapra americana) but not in mule deer (Odocoileus hemionus) populations68. In Alaska, even large-scale predator removals (96% of bears and 75% of wolves) were less instrumental for moose calf survival than snow depth or temperature69. In our system, the positive relationship between wolves and gazelles in natural areas suggests that lethal wolf management would not create refuges for gazelles. While jackal culling provided some indirect benefits to gazelles, these were land use-dependent—limited to the same protected areas where wolf activity had a much stronger, nearly sevenfold, positive effect. This challenges the idea that predator culls necessarily serve as effective conservation tools for prey, particularly in fragmented landscapes where prey species face multiple other pressures. Instead, our findings highlight the greater importance of land protection, limiting anthropogenic food sources and maintaining apex predator populations in supporting endangered prey species.
Weak mesopredator suppression, strong bottom-up resource subsidies
While we expected wolves to suppress jackal and wild boar activity, in line with mesopredator release and landscape of fear theories10,55,56, our findings revealed no significant effect of wolf activity on these species. Instead, the interaction between culling pressure and land protection emerged as the strongest driver of both jackal and boar activity. Jackals responded to culling and land use similarly to wolves48, suggesting a shared attraction to livestock resources. Wild boars, as flexible generalist omnivores, also rely heavily on anthropogenic food subsidies in human-dominated landscapes40,41. In our models, these subsidies are proxied by the proximity-weighted land-use layers (croplands, orchards and towns; see Methods and Table 4), while the nature reserve gradient functions as an inverse index of subsidy exposure, capturing the joint absence of human resources. The spatial distribution of these subsidies likely creates foraging overlap among wolves, jackals and boars, potentially masking any suppressive top-down effects that wolves might otherwise exert. This suggests that in areas where anthropogenic food subsidies inflate mesopredator densities, apex predators may no longer regulate them effectively at the landscape scale. Despite high wolf density in the Golan Heights, jackals outnumber them by up to 100:170, making broad-scale suppression unlikely. Wolf influence thus appears restricted to protected areas, where they are safe from culling, face lower levels of human disturbance and can maintain stable pack territories. Outside these areas, culling and other disturbances disrupt wolf pack social structure in precisely those areas where livestock, trash and crops are most available. In these high-risk, high-resource zones, wolves are more likely to forage alone48, weakening their ability to exert top-down pressure on overabundant jackals and boars. This diminished regulatory role aligns with growing evidence that mesopredator activity is often shaped more by attraction to food resources than by apex predator avoidance, even in undisturbed landscapes42,43,71. In such human-dominated systems, apex predator influence becomes increasingly context-dependent, with stronger effects in protected areas where food subsidies and culling pressures are lower24,45. Ultimately, our findings reinforce the role of human activities as the dominant regulatory force of mesopredator dynamics, demonstrating the necessity of both limiting bottom-up attractants and preserving top-down regulatory forces for effective mesopredator management and ecosystem functionality72.
Gradient of interacting anthropogenic pressures
In human-dominated landscapes, anthropogenic pressures often shape species abundance and activity patterns more strongly than natural trophic interactions21,58. However, species-specific responses to these pressures manifest differently: in our system, jackals decreased their activity under high culling pressure in protected areas, whereas wild boars decreased their activity under high culling pressure in non-protected areas. These differing responses demonstrate that culling effects are species-specific and mediated by land management. Increased jackal activity in high-risk modified areas suggests that only where bottom-up subsidies were weakened—in the most protected and natural areas (> 26.7% land protection, the threshold value)—did intense culling suppress their activity. In contrast, wild boars’ avoidance of culling in agricultural areas may reflect their capacity for learned risk avoidance73, or the relatively low levels of boar culling in protected areas. While these findings support the primacy of humans as the dominant regulatory force for mesopredators in this system, trophic interactions among predators and prey remained a key driver for prey activity, both inside and outside protected areas. Gazelles responded more strongly to their natural predators, wolves (positively, β = 1.13) and jackals (negatively, β = -0.26), than they did to land management or predator culls. This species-specific variation highlights that, even amid strong anthropogenic influences, apex predators continue to play a crucial role in structuring trophic dynamics in fragmented systems14—though their influence depends on the surrounding human context and land use configuration.
Ecological and management implications
Our findings reveal critical thresholds at which apex predators and ecosystem processes continue to function as expected on one side but become severely disrupted on the other. The quantified land protection thresholds—11.4% for wolf–gazelle associations, 19.6% for wild boar response to culling and 26.7% for jackal response to culling—were low in absolute terms but high relative to the fragmented landscape of the Golan Heights (50th, 62nd and 65th quantiles, respectively). In this system, wolves fulfilled elements of their ecological role and trophic dynamics were remarkably resilient in natural patches but not in nearby disturbed ones, even across distances of just a few hundred meters, highlighting the synergistic potential between small-scale reserves and apex predator function. Although all four focal species were widespread across the landscape, their ecological functions diverged sharply with land use, supporting growing evidence that apex predator presence alone is insufficient to sustain ecosystem balance. This parallels recent findings from Yellowstone National Park, USA, where large carnivore populations were successfully restored, but full ecological restoration was contingent on additional conditions enabling broader processes, such as reduced ungulate browsing and beaver recolonization74. Similarly, our results indicate that both apex predator presence and land protection must align in order to sustain regulatory species interactions. Together with prior studies16,75, our findings support the broader applicability of apex predator theory in highly fragmented landscapes—though the extent of top-down regulation likely depends on system-specific thresholds and is increasingly modulated by cumulative human pressures.
Our study highlights both the ecological potential and limitations of apex predators in sustaining trophic dynamics in human-dominated landscapes. In a region where wolves and golden jackals occur at some of their highest global densities31,46,48,70, we provide new insights into the ongoing golden jackal expansion across Old World landscapes, where they increasingly overlap with recovering apex predators, vulnerable prey and anthropogenic pressures3. The resilience of predator–prey dynamics observed in our system—up to a breaking point of human disturbance—supports the view that coexistence with large carnivores in highly developed regions is feasible, provided that management policies are supportive76. However, they also point to the need for new strategies that account for ecosystem thresholds and address cumulative human impacts such as culling, food subsidies and habitat degradation that may override natural regulation and push resilient systems toward ecological imbalance. Effective conservation in fragmented landscapes thus requires an integrated approach beyond predator recovery, incorporating land-use management strategies that mitigate anthropogenic pressures and enable apex predator functionality. By leveraging apex predators’ top-down roles while controlling human-driven bottom-up effects, such approaches could reduce reliance on mesopredator culling and offer a more sustainable path for wildlife management and ecosystem resilience. Future research should aim to refine our understanding of apex-mesopredator dynamics and inform strategies that balance biodiversity conservation with human land use in increasingly multifunctional landscapes.
Methods
Study area
The study was conducted in the Golan Heights (33.0000° N 35.4500° E), a ~ 1200 km2 primarily basaltic plateau at the crossroads of Israel, Syria, Lebanon and Jordan48. The region has changed hands multiple times—from the Ottoman Empire to French and British mandates, and later to Syria. Since 1967, Israel has controlled and administered the western two-thirds of the Golan Heights. While largely stable, the area remains militarized, with active training zones and extensive minefields covering ~ 50% of the landscape. Despite its geopolitical tensions, the Golan Heights has emerged as a key biodiversity refuge in the region70, primarily due to Israeli wildlife protection laws, the absence of recreational hunting and increased food availability from agricultural. These factors have facilitated a significant rise in certain wildlife species, including the globally endangered mountain gazelle (Gazella gazella), a species of global conservation concern49,50. However, this wildlife recovery has also led to increased agricultural losses and sparked conflicts between farmers and conservation efforts46,77.
The Golan spans elevations of 150–1500 m and has a Mediterranean, with hot and dry summers and cold and wet winters (400–800 mm annual rainfall). The dominant habitat is grasslands interspersed with springs and seasonal streams and pockets of protected oak woodland. The human population of the Golan is approximately 52,000 people, split between 36 towns and agricultural settlements (data courtesy of Israel Central Bureau of Statistics 2021). The dominant land use is livestock grazing (~ 400–600 km2), surrounding smaller nature reserves (~ 295 km2), larger closed military firing zones (~ 380 km2) and fenced minefields (~ 160 km2). Cattle, numbering about 25,000 head78, are typically free-ranging, with limited use of livestock guardian dogs79, and protective enclosures remain costly to maintain and often ineffective80. Common smaller mammal species include red fox (Vulpes vulpes), European badger (Meles meles), Cape hare (Lepus capensis), Egyptian mongoose (Herpestes ichneumon) and Indian crested porcupine (Hystrix indica).
Focal species
The large wild mammal (> 10 kg mean adult body weight) community in the Golan Heights comprises four key, widespread species:
-
1.
Grey Wolf (Canis lupus): The Golan wolf population numbered just 8–15 individuals in a 1976 survey, but has since rebounded considerably46. Today, it represents one of the highest recorded wolf densities worldwide (~ 100 wolves/1000 km2), with expansion into adjacent areas81.
-
2.
Golden Jackal (Canis aureus): Jackals have also proliferated, reaching 12–24 individuals/km2—among the highest densities globally70,82. Despite heavy culling efforts to control their numbers, high jackal densities persist due to abundant anthropogenic food resources such as livestock carcasses and agricultural surplus31.
-
3.
Wild Boar (Sus scrofa): Boar occur at high densities, serving both prey for some wolf packs46 and a major agricultural pest47. As generalist omnivores with a highly flexible trophic role, boar also feed on carrion and are known to prey on ungulate neonates41.
-
4.
Mountain Gazelle (Gazella gazella): Prior to 1967, the Golan Heights had few gazelles. In the early 1970s, ~ 400 gazelles were translocated from a stable population in northern Israel, about 50 km away, leading to a population boom (~ 6000 individuals by the 1980s) and subsequent culling campaign due to crop damages50. Culling, disease, predation, poaching and vehicle collisions later led to drastic declines in the 1990s, but recent surveys show moderate recovery, with an estimated 750 gazelles in the southern and central Golan83.
Anthropogenic pressures on wildlife in the Golan
In the Golan heights, livestock losses to wolves and jackals have been a persistent issue since their recovery77,81,84, while wild boar also cause significant agricultural damage47. Until the mid-1990s, calf depredations were primarily attributed to jackals77, but as wolf densities peaked by the late 1990s, wolf attacks on cattle became more frequent, eventually surpassing those by jackals46,81 (Supplementary Fig. S1). To curb retaliatory killings and illegal poison baiting that could harm other wildlife, the Israel Nature and Parks Authority (INPA), the government agency responsible for managing nature reserves and wildlife populations, issued a predator culling program in the early 2000s84. This program aims to keep the wolf and jackal populations at densities tolerable for local cattle ranchers while also conserving the broader ecosystem51. Since its implementation, approximately 30 wolves (ca. 25% of the population) and 1000 jackals are culled annually in and around livestock grazing pastures51. Recent research indicates that lethal management did not deter wolves or jackals from grazing pastures31,48. Culling permits for wild boar are also issued by the INPA following crop raids, with ~ 1400 boar culled annually by INPA rangers and local farmers to prevent further damage. (A. Reichmann, personal communication). As the culling programs mandate thorough documentation of each event, the INPA is able to provide long-term culling datasets that are more detailed than most management or harvest policies from other parts of the world.
Camera trap design
Based on the typical patch sizes found in the Golan Heights—such as areas between primary roads and nature reserves (Fig. 1A)—we divided the study area into twelve ~ 30-km2 sampling sites. These sites were categorized into four types: ‘high culling’, ‘low culling’, ‘nature reserve’ and ‘minefield’ (Fig. 1B), reflecting the spatial distribution of wolf culling events and policy. Whereas ‘nature reserve’ and ‘minefield’ both represent areas with no culling, the latter is also devoid of human activity (and wildlife typically do not trigger the landmines). While nature reserves may include limited grazing to thin vegetation and prevent fires, they represent a baseline of minimal anthropogenic bottom-up food subsidies. In contrast, sites outside protected areas include croplands and orchards with irrigation systems, which provide plant-based food and water sources for wildlife such as wild boars and jackals. Additional anthropogenic food subsidies—including young livestock, carcasses and refuse—are available in pastures and around towns and army bases. Thus, sites located farther from nature reserves tend to have greater access to human-associated food resources.
We received INPA culling data, including the exact coordinates and dates of wolves and jackals shot in the ten years preceding this study, to designate high and low-culling area polygons. High-culling areas had an average of 2.27 (± 0.85 SD) wolf culling and 226.4 (± 25.31 SD) jackal culling events per year, whereas low-culling areas had an average of 0.27 (± 0.25 SD) wolf and 38.2 (± 7.66 SD) jackal culling events per year. These areas, defined based on the spatial distribution of past wolf and jackal culling events rather than predefined management zones, were primarily composed of grazing pastures and their surroundings. To account for extreme outliers of recent culling due to unusually concentrated culling at one site, we set the culling pressure values of camera stations above the 80th culling quantile (0.22) to a maximum value (1). We normalized the lower 80 quantiles by dividing their values by the original 80th culling quantile value.
We sub-divided each 30 km2 polygon into 1 × 1 km cells, of which we randomly selected five cells. We set a camera trap in each cell’s centroid (Browning Dark Ops HD Pro X, model BTC 6HDPX, Morgan, UT, USA). In total, we collected wildlife images from 60 camera locations across the twelve polygons, divided into the four culling categories. Thus, each culling category featured three polygons, sampled by five cameras each. This produced 15 cameras per culling category. We enforced a minimum distance of 1 km between locations to ensure independence between cameras. Cameras were active between August 30 and December 14, 2020 (i.e., twelve weeks), for 5997 camera nights in total. The average number of trap nights per camera was 100 with a standard deviation of 16.22.
For optimal coverage, we set cameras facing animal trails at a 45° angle, strapped to metal stakes 0.5 m off the ground and facing north to prevent false triggers due to sunrise. We set the camera traps to capture 8-image ‘rapid fire’ sequences at each trigger to record sequences of animals crossing the camera’s field of vision. At each camera’s installation and during biweekly visits, we recorded data on the dynamic environment surrounding each camera that may affect detectability. These included camera sensitivity setting, vegetation structure, path type, bottlenecks caused by fencing, ability to pass behind the camera, livestock presence and whether the camera was found in place, askew, or gone (Table 3). Vegetation structure and post-camera accessibility were re-scored at each visit and incorporated into the detection sub-model to account for seasonal variation (August-December) that could affect detection. Because the region experiences negligible snowfall, snow was excluded as a covariate. All cameras operated concurrently within the same date range, ensuring comparable temporal coverage across sites.
Image processing
We defined a single detection as the sighting of an animal (one or more individuals of the same group) by one camera within a 20-min interval. Since the animals in this study were unmarked, the number of animals per encounter was recorded as the maximum number of individuals counted within a single frame within that 20-min interval. We filtered false triggers using Microsoft’s MegaDetector v4.1 (https://github.com/microsoft/CameraTraps/blob/main/megadetector.md)85 and managed camera trap data and species identification using Camelot v1.6.16 (https://camelotproject.org/)86.
Spatial predictor generation
Given the availability of precise culling coordinates for wolf, jackal and boar, we applied the exponential decay function method developed by Shamoon et al.60 using a 7-km radius based on the average daily foraging distance of Golan wolves46, to create spatial gradients that incorporate both proximity to land use patches and patch size for each covariate. Thus, culling pressure was calculated for each camera location based on the number of culling events and each event’s distance from a given point. We sourced landcover and land use maps from Hamaarag, Israel’s National Ecosystem Assessment Program87, nature reserve maps from the Israel Nature and Parks Authority and firing zone and minefield maps from the Israel Defense Forces. Using the same decay function, we generated spatial gradients reflecting the influence of proximity and size of each land use variable (Table 4). Thus, the proximity-weighted Croplands, Orchards, and Infrastructure layers indexed access to anthropogenic food subsidies (e.g., crops, livestock feed, refuse). Conversely, the Nature Reserves layer serves as an inverse indicator of subsidy exposure because agriculture and built infrastructure are excluded from reserve boundaries; higher reserve values therefore reflect lower exposure to cropland/orchard/settlement resources in the surrounding vicinity. All culling and land use predictors were generated with identical decay and standardized to a 0–1 scale to ensure comparability across models.
We ran a pairwise Pearson’s correlation test between all state function covariates to guarantee that models would not contain highly correlated variables (r >|0.7|). We created count histories for our four focal species where each row represents a spatially independent camera location, each column represents the sampling occasion and the value in the cell is the sum of individuals detected per sampling location and sampling occasion. We used a two-week sampling occasion, meaning the period of twelve weeks that the cameras were operational was considered as six sampling occasions in the count history. If a camera was not operational for an entire two-week period or most of it, we removed it from the detection history.
Statistical analysis
Multi-species co-occurrence models have been developed to quantify interactions between unmarked species88, with sampling designs and species like our system. However, we chose not to implement this method due to the high site occupancy of all species across the study site and the difficulty of inferring ecological interactions from co-occurrence data89. Instead, we initially opted for Bayesian co-abundance models to examine how the abundance of a dominant species impacts the abundance of a subordinate species. While this approach may quantify predator–prey relationships when all appropriate confounding covariates are accounted for, this approach requires substantial (i.e., 100 + detections of both dominant and subordinate species) along a gradient of sites where one or both species remain present or locally extinct90, and our data failed to meet these requirements. All preliminary testing of co-abundance models failed to produce converging species interaction parameters (Rhat > 1.2; see Supplementary Table S4).
Site-level activity estimation
Rather than implementing co-abundance models when our data failed to meet its requirements, we generated site-level activity estimates (i.e., relative number of detections) to examine relationships between species and their environment. We modeled species’ activity in response to landscape, culling and predation pressures using N-mixture models91. While N-mixture models produce robust density estimates for species when all model assumptions are met, camera trap data often violates these assumptions due to non-independence between sampling locations for animals that travel large distances, such as cursorial wolves92. Therefore, we treat the output of the N-mixture models as site-level relative activity estimates rather than true density estimates. N-mixture models measures both imperfect detection of species and the spatial variation in latent abundance. The models’ hierarchy comprises abundance and detection functions. We used a zero-inflated Poisson distribution for all wolf abundance models and a Poisson distribution for all jackal, boar and gazelle abundance models. The detection function included eight possible factors that may affect animal detectability (Table 3). For each species, we first tested each detection covariate individually by implementing univariate models and ranked them by AIC to identify the most informative factors93. We then tested different combinations of these covariates, limiting them to four per function to avoid overparameterization, and ranked them by AIC (Supplementary Table S5). The best-performing detection function (Eq. 1) was consistent across all four species, allowing us to apply it in all subsequent N-mixture models.
Equation 1. The final detection function used in N-mixture models for all four species:
After the best detection formula was determined, we generated detection-corrected activity estimates of wolves, jackals and boars at each site. We started with an intercept-only N-mixture model containing an empty state function and the best detection formula. We estimated the posterior distribution of each species’ latent abundance using empirical Bayes methods and extracted the best unbiased predictor and confidence intervals across all sampling locations. We estimated all species’ abundance and ran N-mixture models using the ‘unmarked’ package (v1.5.0; https://cran.r-project.org/package=unmarked)94 in R Statistical Software (v4.3.1; https://www.R-project.org/)95.
Integrated N-mixture models
We used the site-level activity estimates of wolf, jackal and boar as fixed effect predictor variables alongside land use and culling variables to examine how they impact the activity of other species. We employed integrated N-mixture models91, incorporating state functions containing combinations of the site-level covariates listed in Table 4 and the activity of our focal species. We tested additive and interactive combinations of these predictors in accordance with our hypotheses for each species. Specifically, we assumed culling and sympatric predator pressures to be additive. In contrast, to test whether the effects of culling and predation pressure varied along a gradient of land use, we included interactions between these effects and land use variables in the models’ state functions. We ranked the resulting models by AIC to determine the top N-mixture models that best explained each species’ relative activity patterns (Supplementary Table S3).
Structural equation models (SEM)
We expanded our analysis by employing piecewise structural equation models (SEM) to assess community-wide effects of predation, culling and landscape pressures. While N-mixture models provided a species-specific approach, SEMs allow for a holistic analysis of the community as an interconnected system96. Our SEMs incorporated the detectability-corrected abundance measures detailed in the site-level activity estimation section, following the two-stage approach of Cunningham et al. (2020). Unlike classic SEM, which calculates parameter estimates globally, piecewise SEM uses individual regressions to estimate local pathways within a hypothesized causal network97. This approach allows each response variable to be modeled independently, accommodating a wide range of distributions and model types and making it particularly useful for ecological datasets that often violate the assumptions of classical SEM97. We implemented the models using the ‘piecewiseSEM’ R package (version 2.3.0.1; https://jslefche.github.io/piecewiseSEM/)59. To visualize our assumed relationships, we developed an a priori directed acyclic graph (Fig. 2), informed by previous research on trophic cascade theory, the mesopredator release hypothesis and the known effects of culling on each species. We then constructed the SEM by fitting individual regressions for each species using generalized linear models (GLMs) with the same culling, predator activity and land use covariates used in the N-mixture models (Table 4). To propagate error in each species GLM, we set site weights to the inverse of the standard errors (1/SE) of their site-level abundance estimate (derived from the N-mixture models), thereby accounting for uncertainties and ensuring that variability in detectability was appropriately incorporated into the SEM framework (Luskin and Mendes, in review).
Model selection
We explored six different variations of the above SEM to account for potential complexities in the relationships among culling, land use and species interactions. These differed in (i) whether wolf activity was modeled as an endogenous response (via a wolf GLM) or treated as an exogenous predictor; (ii) inclusion/exclusion of interactive effects between culling and land use for wolf, jackal and boar activity to test whether the effects of culling were dependent on the context of land use; (iii) inclusion/exclusion of a wolf activity × land-protection interaction in the gazelle GLM to investigate how predation risk and land use might interactively shape gazelle activity. We assessed global fit using Shipley’s test of d-separation (Fisher’s C)98,99, with p > 0.05 indicating acceptable fit59, and ranked models by AIC93, D-separation indicated residual associations between jackal and boar activity and between jackal culling and wolf activity; lacking a priori causal direction, we modeled these as partial correlations (conditional associations)59. SEMs that modeled wolf activity as a response lowered AIC but failed the Fisher’s C criterion, whereas models treating wolves as exogenous met fit criteria. The preferred SEM therefore included culling × land-protection for jackals and boar and wolf-activity × land-protection for gazelles. Full SEM rankings are provided in Supplementary Table S1.
Ecological threshold estimation
To interpret the interactive effects of culling and land use on jackal and boar activity, we generated conditional predictions for jackal and boar responses to culling based on the 25th and 75th quantiles of the nature reserve variable (Table 4). Predicted activity was plotted across observed culling intensities, stratified by low and high land protection, to illustrate context-dependent effects and identify turning points in these effects. While thresholds are challenging to quantify100,101, we determined the land protection value at which the slope of the culling–activity relationship reached zero. These points represent the thresholds at which the direction of the culling effect on jackal and boar activity changed. For gazelles, we assessed their activity at the 25th and 75th quantiles of wolf activity across a continuous land protection gradient. We then identified the land protection value at which the response curves for high and low wolf activity intersected, marking the threshold where wolf activity appeared beneficial for gazelles. Under the exponential decay function used, protection values can decline by up to 25% over ~ 750 m of unprotected land, meaning that distances of a few hundred meters could measurably increase subsidy exposure enough to shift a site across the threshold.
Data availability
The camera trap data for all recorded species, along with the R code used to perform the analyses, are publicly available on GitHub at https://github.com/zacha46/golan_wolves_SEM. All relevant data and scripts necessary to reproduce the results are included in the repository.
References
Ripple, W. J. et al. Status and ecological effects of the world’s largest carnivores. Science 343 (2014).
Chapron, G. et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346, 1517–1519 (2014).
Bernardi, C. D. et al. Continuing recovery of wolves in Europe. PLOS Sustain. Transform. 4, e0000158 (2025).
Venter, O. et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7, 12558 (2016).
Callan, R., Nibbelink, N. P., Rooney, T. P., Wiedenhoeft, J. E. & Wydeven, A. P. Recolonizing wolves trigger a trophic cascade in Wisconsin (USA). J. Ecol. 101, 837–845 (2013).
Newsome, T. M. & Ripple, W. J. A continental scale trophic cascade from wolves through coyotes to foxes. J. Anim. Ecol. 84, 49–59 (2015).
Kuijper, D. P. J. et al. Wolves recolonize novel ecosystems leading to novel interactions. J. Appl. Ecol. 61, 906–921 (2024).
Laundré, J. W., Hernández, L. & Altendorf, K. B. Wolves, elk, and bison: Reestablishing the ‘landscape of fear’ in Yellowstone National Park, U.S.A. Can. J. Zool. 79, 1401–1409 (2001).
Suraci, J. P., Clinchy, M., Dill, L. M., Roberts, D. & Zanette, L. Y. Fear of large carnivores causes a trophic cascade. Nat. Commun. 7, 10698 (2016).
Gaynor, K. M., Brown, J. S., Middleton, A. D., Power, M. E. & Brashares, J. S. Landscapes of fear: Spatial patterns of risk perception and response. Trends Ecol. Evol. 34, 355–368 (2019).
Prugh, L. R. et al. The rise of the mesopredator. Bioscience 59, 779–791 (2009).
Ritchie, E. G. & Johnson, C. N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998 (2009).
Wegge, P., Odden, M., Pokharel, CPd. & Storaas, T. Predator–prey relationships and responses of ungulates and their predators to the establishment of protected areas: A case study of tigers, leopards and their prey in Bardia National Park, Nepal. Biol. Conserv. 142, 189–202 (2009).
Gerber, N. et al. Do recolonising wolves trigger non-consumptive effects in European ecosystems? A review of evidence. Wildl. Biol. https://doi.org/10.1002/wlb3.01229 (2024).
Magioli, M. et al. Human-modified landscapes alter mammal resource and habitat use and trophic structure. Proc. Natl. Acad. Sci. 116, 18466–18472 (2019).
Ausilio, G., Sand, H., Månsson, J., Mathisen, K. M. & Wikenros, C. Ecological effects of wolves in anthropogenic landscapes: The potential for trophic cascades is context-dependent. Front. Ecol. Evol. 8, 577963 (2021).
Hernández, L. & Laundré, J. W. Foraging in the ‘landscape of fear’ and its implications for habitat use and diet quality of elk Cervus elaphus and bison Bison bison. Wildl. Biol. 11, 215–220 (2005).
Gordon, C. E., Feit, A., Grüber, J. & Letnic, M. Mesopredator suppression by an apex predator alleviates the risk of predation perceived by small prey. Proc. R. Soc. B Biol. Sci. 282, 20142870 (2015).
Cunningham, C. X., Johnson, C. N. & Jones, M. E. A native apex predator limits an invasive mesopredator and protects native prey: Tasmanian devils protecting bandicoots from cats. Ecol. Lett. 23, 711–721 (2020).
Krofel, M., Giannatos, G., Ćirovič, D., Stoyanov, S. & Newsome, T. M. Golden jackal expansion in Europe: A case of mesopredator release triggered by continent-wide wolf persecution?. Hystrix Ital. J. Mammal. 28, 9–15 (2017).
Dorresteijn, I. et al. Incorporating anthropogenic effects into trophic ecology: Predator—prey interactions in a human-dominated landscape. Proc. R. Soc. B Biol. Sci. 282, 20151602 (2015).
Ordiz, A. et al. Effects of human disturbance on terrestrial apex predators. Diversity 13, 68 (2021).
Guiden, P. W., Bartel, S. L., Byer, N. W., Shipley, A. A. & Orrock, J. L. Predator-prey interactions in the anthropocene: Reconciling multiple aspects of novelty. Trends Ecol. Evol. 34, 616–627 (2019).
Oriol-Cotterill, A., Valeix, M., Frank, L. G., Riginos, C. & Macdonald, D. W. Landscapes of Coexistence for terrestrial carnivores: The ecological consequences of being downgraded from ultimate to penultimate predator by humans. Oikos 124, 1263–1273 (2015).
Layman, C. A., Quattrochi, J. P., Peyer, C. M. & Allgeier, J. E. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 10, 937–944 (2007).
Lee, S. X. T., Amir, Z., Moore, J. H., Gaynor, K. M. & Luskin, M. S. Effects of human disturbances on wildlife behaviour and consequences for predator-prey overlap in Southeast Asia. Nat. Commun. 15, 1521 (2024).
van Eeden, L. M. et al. Managing conflict between large carnivores and livestock. Conserv. Biol. 32, 26–34 (2018).
Garshelis, D. L., Noyce, K. V. & St-Louis, V. Population reduction by hunting helps control human-wildlife conflicts for a species that is a conservation success story. PLoS ONE 15, 1–20 (2020).
Herfindal, I. et al. Does recreational hunting of lynx reduce depredation losses of domestic sheep?. J. Wildl. Manag. 69, 1034–1042 (2005).
Musiani, M. et al. Seasonality and reoccurrence of depredation and wolf control in western North America. Wildl. Soc. Bull. 33, 876–887 (2005).
Dolev, A., Kapota, D., Talmon, I. & Reichmann, A. Jackal over population interface—from theory to reality. Isr. J. Ecol. Evol. 7, 137–144 (2016).
Treves, A., Krofel, M. & McManus, J. Predator control should not be a shot in the dark. Front. Ecol. Environ. 14, 380–388 (2016).
Creel, S. & Rotella, J. J. Meta-analysis of relationships between human offtake, total mortality and population dynamics of gray wolves (Canis lupus). PLoS ONE 5, e12918 (2010).
Estes, J. A. et al. Trophic downgrading of planet earth. Science 333, 301–306 (2011).
Faria, D. et al. The breakdown of ecosystem functionality driven by deforestation in a global biodiversity hotspot. Biol. Conserv. 283, 110126 (2023).
Samhouri, J. F., Levin, P. S. & Ainsworth, C. H. identifying thresholds for ecosystem-based management. PLoS ONE 5, e8907 (2010).
Banks-Leite, C. et al. Using ecological thresholds to evaluate the costs and benefits of set-asides in a biodiversity hotspot. Science 345, 1041–1045 (2014).
Johnson, C. N., Isaac, J. L. & Fisher, D. O. Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proc. R. Soc. B Biol. Sci. 274, 341–346 (2006).
Wolf, C. & Ripple, W. J. Prey depletion as a threat to the world’s large carnivores. R. Soc. Open Sci. 3, 160252 (2016).
Luskin, M. S. et al. Cross-boundary subsidy cascades from oil palm degrade distant tropical forests. Nat. Commun. 8, 2231 (2017).
Moore, J. H. et al. The rise of hyperabundant native generalists threatens both humans and nature. Biol. Rev. 98, 1829–1844 (2023).
Newsome, T. M. et al. The ecological effects of providing resource subsidies to predators. Glob. Ecol. Biogeogr. 24, 1–11 (2015).
Ciucci, P., Mancinelli, S., Boitani, L., Gallo, O. & Grottoli, L. Anthropogenic food subsidies hinder the ecological role of wolves: Insights for conservation of apex predators in human-modified landscapes. Glob. Ecol. Conserv. 21, e00841 (2020).
Ethier, C. A., Barnas, A. F., Boucher, N. P., Baillie-David, K. & Fisher, J. T. Lethal wolf control elicits change in moose habitat selection in unexpected ways. J. Wildl. Manag. https://doi.org/10.1002/jwmg.22620 (2024).
Kuijper, D. P. J. et al. Paws without claws? Ecological effects of large carnivores in anthropogenic landscapes. Proc. R. Soc. B Biol. Sci. 283, 20161625 (2016).
Reichmann, A. & Saltz, D. The Golan wolves: The dynamics, behavioral ecology, and management of an endangered pest. Isr. J. Zool. 51, 87–133 (2005).
Davidson, A., Malkinson, D. & Shanas, U. Wild boar foraging and risk perception-variation among urban, natural, and agricultural areas. J. Mammal. 103, 945–955 (2022).
Preiss-Bloom, S., Shamon, H., Ben-Ami, D. & Dayan, T. Landscape of risk: Responses of grey wolves to lethal control in a mosaic landscape. Eur. J. Wildl. Res. 71, 24 (2025).
IUCN SSC Antelope Specialist Group. Gazella gazella, Mountain Gazelle. IUCN Red List Threat. Species 2017 e.T8989A50 (2017).
Yom-Tov, Y., Balaban, A., Hadad, E., Weil, G. & Roll, U. The plight of the Endangered mountain gazelle Gazella gazella. Oryx 55, 771–778 (2021).
Reichmann, A. & Dolev, A. Wolf Management in Northern Israel—2020 1–7 (Israel Nature and Parks Authority, 2020).
QGIS Development Team. QGIS Geographic Information System (QGIS Association). at <https://www.qgis.org>
Takimoto, G. & Nishijima, S. A simple theory for the mesopredator release effect: When does an apex predator protect their shared prey from a mesopredator?. Oikos 2022, 1–8 (2022).
Berger, K. M. & Gese, E. M. Does interference competition with wolves limit the distribution and abundance of coyotes?. J. Anim. Ecol. 76, 1075–1085 (2007).
Berger, K. M. & Conner, M. M. Recolonizing wolves and mesopredator suppression of coyotes: Impacts on pronghorn population dynamics. Ecol. Appl. 18, 599–612 (2008).
Ripple, W. J., Wirsing, A. J., Wilmers, C. C. & Letnic, M. Widespread mesopredator effects after wolf extirpation. Biol. Conserv. 160, 70–79 (2013).
Mori, E., Benatti, L., Lovari, S. & Ferretti, F. What does the wild boar mean to the wolf?. Eur. J. Wildl. Res. 63, 1–5 (2017).
van Beeck Calkoen, S. T. S. et al. Numerical top-down effects on red deer (Cervus elaphus) are mainly shaped by humans rather than large carnivores across Europe. J. Appl. Ecol. 60, 2625–2635 (2023).
Lefcheck, J. S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579 (2016).
Shamoon, H., Saltz, D. & Dayan, T. Fine-scale temporal and spatial population fluctuations of medium sized carnivores in a Mediterranean agricultural matrix. Landsc. Ecol. 32, 1243–1256 (2017).
Brogi, R. et al. Intra-guild competition and ecosystem services of mammal scavengers in a new colonized wolf landscape. Behav. Ecol. Sociobiol. 79, 20 (2025).
Arnon, A., Wronski, T., Malkinson, D., Izhaki, I. & Davis, M. Cascading effects of anthropogenic excess food for predators on a Peri-urban population of an endangered ungulate. Anim. Conserv. https://doi.org/10.1111/acv.13014 (2025).
Jiménez, J. et al. Restoring apex predators can reduce mesopredator abundances. Biol. Conserv. 238, 108234 (2019).
Schneider, M. F. Habitat loss, fragmentation and predator impact: spatial implications for prey conservation. J. Appl. Ecol. 38, 720–735 (2001).
Johnson, C. J., Ray, J. C. & St-Laurent, M.-H. Efficacy and ethics of intensive predator management to save endangered caribou. Conserv. Sci. Pract. 4, e12729 (2022).
Gerling, L. The impact of wolf predation on western Canada boreal woodland caribou populations: A critical review of the evidence. Can. Wildl. Biol. Manag. (2017). https://cwbm.ca/the-impact-of-wolf-predation-on-western-canada-boreal-woodland-caribou-populations-a-critical-review-of-the-evidence/.
Lamb, C. T. et al. Effectiveness of population-based recovery actions for threatened southern mountain caribou. Ecol. Appl. 34, e2965 (2024).
Brown, D. E. & Conover, M. R. Effects of large-scale removal of coyotes on pronghorn and mule deer productivity and abundance. J. Wildl. Manag. 75, 876–882 (2011).
Keech, M. A. et al. Effects of predator treatments, individual traits, and environment on moose survival in Alaska. J. Wildl. Manag. 75, 1361–1380 (2011).
Barash, A. et al. Possible origins and implications of atypical morphologies and domestication-like traits in wild golden jackals (Canis aureus). Sci. Rep. 13, 1–15 (2023).
Bassing, S. B. et al. Mammalian predator co-occurrence affected by prey and habitat more than competitor presence at multiple time scales. Ecol. Monogr. 95, e1648 (2025).
Elmhagen, B. & Rushton, S. P. Trophic control of mesopredators in terrestrial ecosystems: Top-down or bottom-up?. Ecol. Lett. 10, 197–206 (2007).
Brogi, R., Apollonio, M., Brivio, F., Merli, E. & Grignolio, S. Behavioural syndromes going wild: Individual risk-taking behaviours of free-ranging wild boar. Anim. Behav. 194, 79–88 (2022).
Hobbs, N. T., Johnston, D. B., Marshall, K. N., Wolf, E. C. & Cooper, D. J. Does restoring apex predators to food webs restore ecosystems? Large carnivores in Yellowstone as a model system. Ecol. Monogr. 94, e1598 (2024).
Stier, A. C. et al. Ecosystem context and historical contingency in apex predator recoveries. Sci. Adv. 2, e1501769 (2016).
Linnell, J. D. C., Swenson, J. E. & Andersen, R. Predators and people: Conservation of large carnivores is possible at high human densities if management policy is favourable. Anim. Conserv. 4, 345–349 (2001).
Yom-Tov, Y., Ashkenazi, S. & Viner, O. Cattle predation by the golden jackal Canis aureus in the Golan Heights. Israel. Biol. Conserv. 73, 19–22 (1995).
Israel Central Bureau of Statistics. National Agricultural Census 2017—Livestock. (2021). https://www.cbs.gov.il/he/publications/doclib/2021/%D7%9E%D7%A4%D7%95%D7%AA-%D7%A9%D7%A0%D7%AA%D7%95%D7%9F/cattle_census2017.xlshttps://www.cbs.gov.il/he/publications/doclib/2021/%D7%9E%D7%A4%D7%95%D7%AA-%D7%A9%D7%A0%D7%AA%D7%95%D7%9F/sheep_census2017.xls
Gavagnach, C. & Ben-Ami, D. Management and behavior of livestock guarding dogs in a multiuse rural landscape in Northern Israel. Rangel. Ecol. Manag. 88, 85–99 (2023).
Kopler, I. & Malkinson, D. Differential response of mammals to agricultural fences—The effects of species vagility and body size. Basic Appl. Ecol. 33, 79–88 (2018).
Reichmann, A. & Dolev, A. Wolf Monitoring and Management in Northern Israel—2021 Summary (Israel Nature and Parks Authority, 2022).
Singh, A., Mukherjee, A., Dookia, S. & Kumara, H. N. High resource availability and lack of competition have increased population of a meso-carnivore—A case study of Golden Jackal in Keoladeo National Park, India. Mammal Res. 61, 209–219 (2016).
Dolev, A., Sinai, I., Federman, R., Goldstein, C. & Shapira, O. Gazelle Counts in Northern Israel—2024 Summary. (Israel Nature and Parks Authority, 2024). https://naturescience.parks.org.il/article.asp?num=29
Nemtzov, S. C. & King, R. Management of wild canids (fox, jackal and wolf) in Israel, with respect to their damage to agriculture, and to the spread of rabies. Adv. Vertebr. Pest Manag. Ii 219–230 (2001).
Beery, S., Morris, D. & Yang, S. Efficient pipeline for camera trap image review. (2019). Preprint at http://arxiv.org/abs/1907.06772
Hendry, H. & Mann, C. Camelot—Intuitive software for camera-trap data management. Oryx 52, 15–15 (2018).
Ben-Moshe, N. & Renan, I. State of Nature Report 2022—Trends and Threats. (Hamaarag—Israel’s National Ecosystem Assessment Program, Steinhardt Museum of Natural History, Tel Aviv University, 2022). https://hamaarag.org.il/en/report/the-state-of-nature-report-2022-trends-and-threats-volume-2/.
Richmond, O. M. W., Hines, J. E. & Beissinger, S. R. Two-species occupancy models: A new parameterization applied to co-occurrence of secretive rails. Ecol. Appl. 20, 2036–2046 (2010).
Blanchet, F. G., Cazelles, K. & Gravel, D. Co-occurrence is not evidence of ecological interactions. Ecol. Lett. 23, 1050–1063 (2020).
Amir, Z., Sovie, A. & Luskin, M. S. Inferring predator–prey interactions from camera traps: A Bayesian co-abundance modeling approach. Ecol. Evol. 12, 1–15 (2022).
Royle, J. A. N-mixture models for estimating population size from spatially replicated counts. Biometrics 60, 108–115 (2004).
Link, W. A., Schofield, M. R., Barker, R. J. & Sauer, J. R. On the robustness of N-mixture models. Ecology 99, 1547–1551 (2018).
Burnham, K. P. & Anderson, D. R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304 (2004).
Fiske, I. J. & Chandler, R. B. Unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 43, 1–23 (2011).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2025). https://www.R-project.org/.
Fan, Y. et al. Applications of structural equation modeling (SEM) in ecological studies: an updated review. Ecol. Process. 5, 19 (2016).
Grace, J. B. et al. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 3, art73 (2012).
Shipley, B. A new inferential test for path models based on directed acyclic graphs. Struct. Equ. Model. Multidiscip. J. 7, 206–218 (2000).
Shipley, B. Confirmatory path analysis in a generalized multilevel context. Ecology 90, 363–368 (2009).
Banks-Leite, C., Larrosa, C., Carrasco, L. R., Tambosi, L. R. & Milner-Gulland, E. J. The suggestion that landscapes should contain 40% of forest cover lacks evidence and is problematic. Ecol. Lett. 24, 1112–1113 (2021).
Spake, R. et al. Detecting thresholds of ecological change in the anthropocene. Annu. Rev. Environ. Resour. 47, 797–821 (2022).
Acknowledgements
We thank Yehuda Samuel, Itamar Yairi and Gilad Guy for their field assistance and Ori Ismach, Yaara Kenet and Yehuda Samuel for their tireless attention to detail while identifying animals. We are grateful to the Israel Nature and Parks Authority rangers and ecologists for their assistance and facilitation of this study. Thanks to Arian Wallach for support in conceptual development. We would like to thank Matthew Luskin and the Ecological Cascades Lab at the University of Queensland for providing feedback that improved the analysis. Thanks to the Smaller-Winnikow Fellowship and JNF-KKL for granting SPB with an award for excellency in environmental studies.
Funding
Funding was provided by The Sherman Foundation. S.P.B. was supported by a graduate fellowship from Tel Aviv University and by a Smaller-Winnikow Fellowship for Excellency in Environmental Studies from JNF-KKL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization of this study. S.P.B. and H.S. designed the experiment. S.P.B. collected and analyzed the data, produced the visualizations, and wrote the manuscript. Z.A. co-developed the analytical approach, contributed to the manuscript’s structure and writing, and prepared the project code for public release. H.S., D.B.A. and T.D. provided critical feedback during manuscript revision. Funding was jointly provided by D.B.A. and T.D. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study involved only non-invasive camera trapping and analysis of archival management records. No animals were captured, handled, or experimentally manipulated. Field activities were authorized by the Israel Nature and Parks Authority under research permit no. 42882. All methods were carried out in accordance with relevant guidelines and regulations, and reporting follows the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Preiss-Bloom, S., Shamon, H., Amir, Z. et al. Human disturbance thresholds determine the ecological role of an apex predator. Sci Rep 15, 37604 (2025). https://doi.org/10.1038/s41598-025-21402-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-21402-x