Abstract
Dapagliflozin (DAPA), an SGLT-2 inhibitor, shows peritoneal protection and can alleviate high glucose-induced peritoneal fibrosis. Yet, its precise molecular mechanism is unknown. This study aims to explore DAPA’s protective effect on the peritoneum and its underlying mechanism. In vitro, human peritoneal mesothelial cells (HPMCs) were isolated from peritoneal dialysate and cultured. HMrSV5 cells were stimulated with 2.5% D-Glucose (high glucose, HG) for 48 h, then cultured in D-glucose DMEM medium with or without DAPA. To assess SGLT2i-induced ENKUR down-regulation, HMrSV5 cells were treated with DAPA for 24 h while overexpressing ENKUR. In vivo, six-week-old male Sprague-Dawley rats were treated with high-glucose dialysate via an intraperitoneal catheter, with or without addition of DAPA. Changes in SGLT2, ENKUR, PI3K/AKT pathways, and EMT markers were evaluated in HPMCs and the rat model. As dialysis duration increases the morphology of the cells transitioned from a cobblestone appearance to a spindle shape. Immunofluorescence analysis confirmed the mesothelial cell origin and revealed an upregulation of ENKUR and the PI3K/AKT signaling pathway, which are associated with the occurrence of EMT. DAPA was found to decrease the expression of ENKUR and inhibit the activation of the PI3K/AKT pathway induced by high glucose in HMrSV5 cells. In rats subjected to PD, we observed a reduction in ultrafiltration capacity, an increase in peritoneal thickness, and elevated levels of SGLT2, ENKUR, PI3K/AKT and EMT markers. Notably, these alterations were mitigated by intragastric administration of DAPA. DAPA effectively ameliorates high glucose-induced peritoneal fibrosis through downregulation of ENKUR/PI3K/AKT signaling pathway.
Similar content being viewed by others
Introduction
Peritoneal dialysis (PD) is widely used for the long-term treatment of patients with end-stage renal disease (ESRD)1, PD relies on the patient’s peritoneal membrane to remove toxins and maintain electrolyte balance. Compared to hemodialysis, PD has the advantage of efficient removal of middle and large molecules, as well as continuous elimination of toxins and water2. However, the development of peritoneal fibrosis (PF) mediated by high glucose solutions is a significant factor leading to dropout of PD among ESRD patients3,4. Although recent guidelines suggest certain treatments, such as steroids and tamoxifen for PF5, the available evidence and efficacy remain inadequate, prompting the need for further therapeutic discoveries.
PF is a recognized cause of ultrafiltration failure and the discontinuation of PD, mainly attributed to factors such as high glucose, hypoxia and peritonitis. The Epithelial-mesenchymal transition (EMT) of HPMCs plays a central role in the development of PF6. During EMT, HPMCs lose their characteristic features and undergo transformation into fibroblasts, leading to excessive production of extracellular matrix components such as collagen IV (COL-IV) and fibronectin (FN)7. Currently, the precise mechanism of peritoneal EMT induced by high glucose has not been fully clarified, and there is a lack of effective pharmacological interventions to solve this issue.
The SGLT2 inhibitor is a medication for lowering blood glucose levels, that targets glucose reabsorption by inhibiting SGLT2 in renal tubular cells8. Emerging researches have indicated the potential of SGLT2 inhibitors in mitigating the risks associated with kidney and cardiovascular disease9,10,11, regardless of diabetes status. Although there is currently limited evidence to support the use of SGLT2 inhibitors in dialysis patients, recent studies have demonstrated the presence of SGLT2 in HPMCs and its ability to ameliorate structural and functional alterations in the peritoneum induced by high-glucose peritoneal dialysis solutions12,13,14,15,16. However, the mechanism underlying this beneficial effect requires further investigation.
Enkurin (ENKUR) has been identified as a potential regulator or effector of TRPC ion channels, and its proline-rich N-terminal region allows it to directly bind and interact with the p85 regulatory subunit of PI3K17. PI3K/AKT pathway is an important mechanism of EMT/fibrosis in peritoneal mesothelial cells18. Recent studies have implicated ENKUR in EMT in various cancers, including lung cancer19,20, nasopharyngeal carcinoma21 and liver cancer22. It has been suggested that ENKUR may participate in EMT in lung cancer by regulating the PI3K/Akt pathway. However, the role of ENKUR in peritoneal EMT/fibrosis has not been reported. In addition, our research group previously conducted in vitro studies that revealed elevated expression of ENKUR, PI3K and AKT in a model of high glucose-induced transdifferentiation of HPMCs23. The silencing of ENKUR resulted in the alleviation of high glucose-induced EMT. Conversely, the use of AKT agonists reversed this effect (not yet published). Previous studies conducted at our center have confirmed that dapagliflozin (DAPA) inhibits podocyte EMT under diabetic conditions by downregulating the IGF1R/PI3K signal pathway24. Building upon these promising findings, our study aimed to investigate the potential therapeutic effects of DAPA as an SGLT2 inhibitor in addressing PF induced by high glucose and elucidate its underlying molecular mechanism of action in a PF model.
Results
Expression of SGLT2, ENKUR and PI3K/AKT pathways in human peritoneal effluent HPMCs
In order to investigate the effect of a high glucose environment on the expression of SGLT2, ENKUR, and the PI3K/AKT pathways in HPMCs, we collected peritoneal effluent from patients undergoing PD with over 3 years. Mesothelial-cell cultures derived from effluents of patients undergoing continuous ambulatory PD exhibited markedly varied morphological features. The cells ranged from having a cobblestone-like appearance similar to mesothelium derived from omentum to being fibroblast-like cells or a mix of different cell populations (Fig. 1a). The prevalence of non-epithelioid cells appeared to be related to the duration of continuous ambulatory PD in each patient. Immunocytochemistry was used to identify the source of cells in the effluent fluid. The results showed that the cytoplasm of cultured outflow fluid cells exhibited positive staining for vimentin, as observed through red fluorescence in the regions of positive expression. After DAPI counterstaining, the nuclei appeared to be intact. No fluorescence signals were detected for Factor VIII or leukocyte marker CD45, indicating that these markers were not expressed in the cells (Fig. 1b).
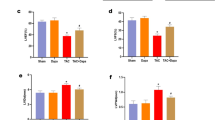
Expression of SGLT2, ENKUR and PI3K/AKT pathways in human peritoneal effluent HPMCs. (a) Morphological changes of HPMCs isolated and cultured from peritoneal dialysis effluents of PD patients of different ages (100x). (b) Immunofluorescence identification of isolated and cultured HPMCs from peritoneal dialysis effluents of PD patients. (c,d) Expression levels of SGLT2, ENKUR, COI-IV, a-SMA, E-cadherin and PI3K/AKT pathway in each group were detected by Western blot and qRT-PCR. HPMCs: human peritoneal mesothelial cells.**p < 0.01, ***p < 0.001 for ANOVA. Uncropped gels and blots were included in a Supplementary Information file.
Western blot (Fig. 1c) and qRT-PCR (Fig. 1d) indicated that the expression of E-cadherin, an epithelial cell marker, was down-regulated in peritoneal mesothelial cells of PD effluent. Conversely, the expression of mesenchymal cell markers, such as αlpha-Smooth muscle actin (a-SMA) and COL-IV was up-regulated. This finding suggest that EMT occurred in HPMCs with the prolongation of dialysis age. Furthermore, the expression of SGLT2, ENKUR and the phosphorylation levels of PI3K, AKT were up-regulated in the cells undergoing EMT.
DAPA attenuates peritoneal thickening and improves peritoneal function induced by high glucose dialysate
To investigate the effects of DAPA on the peritoneum in a high glucose dialysate environment, we conducted experiments using a rat model of uremic PD. These uremic rats (not-diabetic) received oral gavage administration of DAPA (1 mg/kg) while undergoing PD using high glucose dialysate. HE and Masson staining reveal that infusion of HG-PDF (4.25%D-glucose) result in peritoneal thickening and subperitoneal collagen deposition. However, treatment with DAPA reversed these changes (Fig. 2a, b). Compared with the sham operation group, the HG-PDF perfusion group showed a decreas in the ultrafiltration volume and D/D0 glucose ratio. However, the DAPA treatment group exhibited a lesser decrease in these parameters (Fig. 2c). Therefore, these findings indicate that DAPA treatment can reduce peritoneal thickening induced by HG-PDF and improve peritoneal ultrafiltration function. Therefore, DAPA may have a protective effect on the peritoneum in the context of high glucose exposure during PD.
Dapagliflozin attenuates peritoneal thickening and improves peritoneal function induced by high glucose dialysate. (a,b) Representative images of HE and Masson’s trichrome staining in the peritoneal tissues of rats from the sham operation group (sham), uremic peritoneal dialysis group (PD) and uremic peritoneal dialysis + DAPA group (PD + DAPA). (c) Peritoneal balance test was carried out with 2.5% peritoneal dialysate for 4 h at the end of the treatment. Ultrafiltration capacity was quantitatively analyzed by intraperitoneal content accumulation, and glucose transport was measured and analyzed by peritoneal equilibrium test at 0 h and 4 h, respectively. D and D0 denote glucose concentration of peritoneal effluents at 0 and 4 h, **p < 0.01, *** p < 0.001 for ANOVA.
DAPA attenuates PF induced by HG-PDF
To evaluate the impact of DAPA on PF induced by HG-PDF, various assessments were conducted. IHC staining showed that the expression of a-SMA and fibronectin (FN) in peritoneal tissue of rats in HG-PDF infusion group was higher than that in sham operation group, and the above changes could be alleviated by DAPA treatment(Fig. 3a). At the same time, the western blot analysis and qRT-PCR results also confirmed that DAPA treatment could reduce the PF induced by HG-PDF (Fig. 3b, c).
Dapagliflozin attenuates peritoneal fibrosis induced by high glucose peritoneal dialysate. (a) Representative images of a-SMA and FN immunohistochemical staining in the peritoneal tissues of rats from the sham operation group (sham), uremic peritoneal dialysis group (PD) and uremic peritoneal dialysis + DAPA group (PD + DAPA). (b,c) Protein and gene expression levels of SGLT2, COI-IV, a-SMA and E-cadherin in peritoneal tissue of rats in each group were analyzed by Western blot and qRT-PCR.**p < 0.01, *** p < 0.001 for ANOVA. Uncropped gels and blots were included in a Supplementary Information file.
DAPA attenuates PF and is dependent on the ENKUR/PI3K/AKT pathway
Compared to the sham operation group, the expression of ENKUR (Fig. 4a) was significantly increased in the peritoneal tissues of the HG-PDF group, along with an increase in the phosphorylation levels of PI3K、AKT. However, treatment with DAPA reversed these increases in ENKUR and the phosphorylation levels of PI3K、AKT (Fig. 4b). These results are consistent with the results obtained from Western blot analysis. Therefore, these results strongly suggested that DAPA effectively attenuates PF and this protective effect is mediated through the ENKUR/PI3K/AKT pathway.
DAPA attenuates peritoneal fibrosis and is dependent on the ENKUR/PI3K/AKT pathway. (a) Expression of ENKUR in the peritoneal tissue of rats in each group was analyzed by Western blot and qRT-PCR. (b) Protein expression level of PI3K/AKT pathway in peritoneal tissue of rats in each group was analyzed by Western blot.**p < 0.01, *** p < 0.001 for ANOVA. Uncropped gels and blots were included in a Supplementary Information file.
DAPA attenuates EMT of HMrSV5 stimulated by high glucose by inhibiting ENKUR/PI3K/AKT pathway
Previous study conducted by our group revealed an increased expression of ENKUR, p-PI3K and p-Akt in the high glucose-induced human peritoneal mesothelial cell transdifferentiation model. Based on these findings, we selected the high glucose-induced human peritoneal mesothelial cell transdifferentiation model and treated cells with different concentrations of DAPA. To assess cell viability, we performed a CCK-8 assay. The results demonstrated that high glucose stimulation led to a decrease in cell viability, while DAPA treatment increased the cell viability in a certain concentration range (Fig. 5a). Based on these findings, we chose 0.5 μm, 1 μm and 5 μm as the intervention concentrations of DAPA.
DAPA attenuates EMT of HMrSV5 stimulated by high glucose by inhibiting ENKUR/PI3K/AKT pathway. (A) Cell viability of HMrSV5 treated with different concentrations of DAPA (0,0.1,1,5,10,20,30 µ M) under high glucose (HG) conditions was determined by CCK8 assay. (B,C) Treated with DAPA (0,0.1,1,5 µ M) and exposed to HG, the protein and gene expression levels of E-cadherian, α-SMA, COL-IV, ENKUR and PI3K/AKT pathway in HMrSV5 cells treated with DAPA (0,0.1,1,5 µ M) and exposed to HG were analyzed by Western blot and qRT-PCR.**p < 0.01, *** p < 0.001 for ANOVA. Uncropped gels and blots were included in a Supplementary Information file.
Furthermore, Western blot (Fig. 5b) and qRT-PCR analysis (Fig. 5c) indicated that DAPA treatment could reduce the occurrence of EMT in HMrSV5 cells, as evidenced by a decrease in the up-regulation of ENKUR and the activation of PI3K/AKT pathway induced by high glucose. To further investigate the binding interaction between SGLT2 and ENKUR, as well as its downstream molecular mechanisms, we examined the function of a lentiviral vector containing the full coding region of ENKUR in HMrSV5 cells, assessing its expression via Western blot analysis. The findings demonstrated the successful construction of the OE-ENKUR virus (Fig. 6b), and that OE-ENKUR significantly enhanced EMT induced by HG stimulation. Conversely, treatment with DAPA inhibited the OE-ENKUR-induced EMT enhancement (Fig. 6c). The anti-fibrotic effect of ENKUR-mediated SGLT2i on peritoneal fibrosis was confirmed through co-immunoprecipitation (Fig. 6a). As anticipated, DAPA markedly suppressed the expression of the HG-stimulated ENKUR/PI3K/AKT pathway during the EMT process, thereby exerting an antifibrotic effect.
Study on the Mechanism of Anti-Fibrosis of DAPA. (a) Cell lysates of the indicated groups were immunoprecipitated with anti-SGLT2 antibodies, followed by WB with an anti-ENKUR antibodies; (b) Evaluation of the expression level of ENKUR protein relative to b-ACTIN in ENKUR overexpressed HMrSV5 cells (OE-ENKUR) and control cells (OE-NC) by WB; (c) WIth the basis of OE-ENKUR, treated with DAPA (5µM) and exposed to HG, the protein expression levels of E-cadherian, α-SMA, COL-IV, ENKUR and PI3K/AKT pathway in HMrSV5 were analyzed by Western blot and qRT-PCR. **p < 0.01, *** p < 0.001 for ANOVA. Uncropped gels and blots were included in a Supplementary Information file.
Discussion
The results of our study demonstrate that DAPA, a SGLT2 inhibitor, has a significant impact on PF and EMT in both in vivo and in vitro models. Moreover, our study revealed that DAPA treatment modulated the ENKUR/PI3K/AKT pathway to exert its protective effects (Figure 7). Our study provides novel insights into the potential therapeutic role of DAPA in PF. The ENKUR/PI3K/AKT pathway appears to be a key mechanism through which DAPA exerts its protective effects, suggesting its potential as a targeted therapeutic approach.
DAPA ameliorates high glucose-induced peritoneal fibrosis through downregulation of ENKUR/PI3K/AKT Signaling pathway. Prolonged high-glucose peritoneal permeate induction transformed peritoneal mesothelial cells into fibroblasts, with peritoneal thickening and subperitoneal collagen deposition, whereas DAPA attenuated these changes by inhibiting the ENKUR/PI3K/AKT pathway.
DAPA is a highly potent, reversible and selective SGLT2 inhibitor used for the treatment of type 2 diabetes25. DAPA and other SGLT2 inhibitors inhibit gluose reabsorption in renal proximal tubual cells.At present, more and more researchers pay attention to the research on the pathogenesis of fibrosis in various organs, such as the SGLT2 inhibitor prevented renal fibrosis after renal ischemia/reperfusion injury through a VEGF-dependent pathway26,27 and suppressed heart fibrosis through inhibition of the transforming growth factor-β/Smad pathway and activation of Nrf2/ARE signaling28,29. In the study by Michael S Balzer et al.13, it was found that intraperitoneal perfusion of SGLT2 inhibitor DAPA improved the structural and functional changes induced by HG-PDF in peritoneum. However the potential molecular mechanism underlying the potential effects of DAPA on PF remains unclear. Therefore, our study aimed to investigate the effects of DAPA treatment on PF. Considering the limitations of daily intraperitoneal administration of DAPA in a clinical practice, we employed oral gavage to evaluate the potential preventive effect of DAPA on high-glucose-induced PF. Consistent with previous studies13, our experimental resluts demonstrated that oral gavage administration of DAPA effectively alleviate peritoneal thickness, reduce the degree of PF induced by HG-PDF, and improve peritoneal function in rats (Figure 2). Furthermore, in vitro experiments using high glucose-stimulated HMrSV5 also confirmed that DAPA treatment reduced EMT (Figure 3). These findings provide evidence for the potential therapeutic role of DAPA in high glucose-induced PF. In previous study, we observed that DAPA inhibits EMT in podocyte under diabetic conditions by down-regulating IGF1R/PI3K signaling pathway. Building upon these findings, we propose that DAPA may attenuate high glucose-induced PF by modulating the PI3K signaling pathway. Our in vivo and in vitro experiments have consistently shown that DAPA effectively inhibit the activation of PI3K/AKT signal pathway (Figs. 4 and 5).
ENKUR has been identified as a crutial adapter protein involved in the localization of Ca2 + osmotic ion channels in sperm17. Its proline-rich N-terminal region enables direct binding and interaction with the p85 regulatory subunit of PI3K. Emerging evidence has demonstrated the involvement of ENKUR in tumor EMT, such as lung cancer19,20, nasopharyngeal carcinoma21 and liver cancer22 etc., where it regulates the EMT process through the PI3K/AKT pathway. However, the role of ENKUR in peritoneal EMT/ fibrosis remains unexplored. In our study, we observed increased expression of a-SMA and COL-IV, decreased expression of E-cadherin, and upregulation of ENKUR in peritoneal mesothelial cells from patients with long dialysis duration (Figure 1). This suggests the involvement of ENKUR in HPMCs EMT. In our previous in vitro study, we demonstrated that the expression of ENKUR, p-PI3K and p-AKT increased in the transdifferentiation model of HPMCs induced by high glucose. Silencing ENKUR could reduce the EMT induced by high glucose, while AKT agonist could reverse the this effect (unpublished). These results indicate that ENKUR is involved in high glucose-induced EMT of HPMCs, and potentially through the PI3K/AKT pathway.
In the rat model of PF, we observed upregulation of ENKUR and activation of PI3K/AKT pathway, both of which were reversed by DAPA (Figure 4). Furthermore, DAPA could inhibit the upregulation of ENKUR and the activation of PI3K/AKT pathway in HMrSV5 cells induced by high glucose (Figure 6c). Mechanism studies have shown that DAPA can eliminate the occurrence of EMT caused by overexpression of ENKUR. In vivo and in vitro studies, we verified the anti-fibrotic effect of DAPA on PF, and potentially mediated through the ENKUR/PI3K/AKT pathway.
There are several limitations in our study that should be acknowledged. Firstly, wile we observed the involvement of the ENKUR/PI3K/AKT pathway in the anti-fibrotic effects of DAPA, the specific mechanism by which SGLT2 inhibitors affect ENKUR remains unclear. Secondly, it is important to explore potential pathways and mechanisms through which DAPA may exert its anti-fibrotic effect on PF. Lastly, additional studies are warranted to explore the long-term effects, safety, and efficacy of DAPA in human subjects.
In conclusion, our study demonstrates that DAPA can effectively alleviate PF induced by high glucose. DAPA achieves this by targeting the ENKUR/PI3K/AKT pathway and inhibiting EMT in HPMCs. These findings suggest a potential therapeutic role for DAPA in the management of PF. Overall, our finding provides a strong foundation for future investigations and potentially offers new potential treatment options for the management of PF.
Materials and methods
Ethics statement
The experimental protocols involving both animal and human subjects were meticulously designed to adhere to the highest ethical standards. For human and animal experiments, the procedures strictly followed the institutional guidelines and conformed to the criteria stipulated in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2023-KY-1070). These protocols were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University, Zhengzhou University, China. Additionally, the animal study was reported in accordance with the ARRIVE guidelines to ensure transparency and reproducibility30.
In the context of human research, the study involving human samples was approved by the Ethics Committee for Human Research of the First Affiliated Hospital of Zhengzhou University (2023-KY-1344). All methodologies employed were in strict compliance with relevant guidelines and regulations. Furthermore, written informed consent was obtained from each participant prior to their involvement in the study. All methods were carried out in accordance with relevant guidelines and regulations including ARRIVE guidelines. The Clinical trial number: not applicable.
Primary cells and cell lines
PD effluent was obtained from PD patients after dwell times ranging from 8 to 10 h. All patients in this study used baite PD solution. The exclusion criteria were as follows: presence of systemic inflammatory disease, peritonitis, a history of malignant tumor, and the use of glucocorticoids or immunosuppressive agents in the past year. Based on the duration of dialysis, the patients subgroups were categorized as follows: PD < 7d (less than 7 days on dialysis), 1y < PD < 3y(1–3 years on dialysis), PD > 3y (more than 3 years) on dialysis. The samples were processed immediately upon collection. HPMCs were obtained by centrifugation (1000 rpm,10 min) from the collected PD effluent31, the HPMCs were then cultured in T25 culture flasks using DMEM-F12 medium (Gibco) containing 1% penicillin-streptomycin double antibiotic solution (Suolaibao, China) and 15% fetal bovine serum (FBS, Gibco). The cells underwent fusion within approximately 12–14 days of culture. During this time, the culture medium was refreshed every 2–3 days to maintain optimal cell growth and viability.
The human peritoneal mesothelial cell line HMrSV5 (Hua Tuo, China) was cultured in DMEM-F12 medium (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin-streptomycin double antibiotic solution (Suolaibao, China). The cell was incubated in a humidified air environment with 5% carbon dioxide(CO2) at 37℃. To investigate the protective mechanisms of DAPA, HMrSV5 cells were divided into into different experimental groups: HG (2.5% D-Glucose) groups, HG + 0.5 μm DAPA groups, HG + 1 μm DAPA groups and HG + 5 μm DAPA groups. The cells were stimulated with 2.5% D-Glucose as HG for 48 h, follows by treatment with different concentrations of DAPA (KKL MED; KM6324) for 24 h. ENKUR overexpression was achieved by constructing, packaging, and titrating lentivirus vectors that encoded the ENKUR gene (oe-ENKUR; HanHeng; LV88022711). A negative vector without meaningful expression served as a control (oe-NC; HanHeng; LV88022710). HMrSV5 were transfected with the constructed lentivirus (multiplicity of infection: 80) for 24 h, and the transducing efficiency was determined using western blotting 48 h after transfection.
Animals and experimental protocols
Male Sprague-Dawley rat weighing 208 + 0.2 g and aged 4–6 weeks were obtained from The Animal Center of Zhengzhou University. The experimental environment was maintained as follows: the rats had access to adequate water and food, the temperature was set at 23 ± 2 ◦C, a light/ dark cycle of 12 h were used, and the relative humidity was maintained at 50–60%. The rats were acclimatized for seven days prior to the commencement of the experimental procedures. The rats were then randomly divided into three groups: the sham operation group, the High glucose peritoneal dialysate (HG-PDF) infusion group, and the HG-PDF + DAPA group (n = 6 in each group). The model of sham operation group was established by bilateral renal capsulotomy, bilateral renal decapsulation was performed in two stages with a 7-day interoperative interval. Establishment of a 5/6 Nephrectomy Uremic Rat Model, following successful anesthesia(1% Sodium Pentobarbital (40 mg/kg)), the rat was positioned in the right lateral decubitus position. A surgical incision was made 0.5 cm lateral to the vertebral column and 1 cm inferior to the left costal margin. Sequential dissection was performed through the skin, muscle, and fascial layers to expose the left kidney. After mobilizing the left kidney, renal decapsulation was performed. The predetermined renal tissue (upper and lower poles, each comprising 1/3 of the kidney) was ligated and excised. The excised tissue was weighed, with the resection volume calculated based on the standard ratio of total kidney weight to body weight (0.74%). Hemostasis was achieved using gelatin sponge, and the incision was closed after confirming absence of active bleeding. One week post-left nephrectomy, right total nephrectomy was performed using identical anesthetic protocol and positioning. The right renal pedicle was ligated at the hilum, followed by complete renal excision. Following successful induction of the uremic model, a peritoneal dialysis (PD) catheter was surgically implanted. Under anesthesia, rats were placed in the supine position and secured. A sterile, self-made PD catheter (autoclaved 3 mm ID silicone catheter) was inserted 2 cm below the costal margin and 0.2 cm lateral to the abdominal midline. The perforated end of the catheter was positioned intraperitoneally, and the entry site was secured with a purse-string suture followed by muscle layer closure. A subcutaneous tunnel was created, exiting approximately 1 cm caudal to the midpoint between the ears32. Catheter patency was confirmed by successful infusion and drainage of 20 mL heparinized saline (100 U/mL). The catheter was then sealed with a heparin cap and flushed daily with 50 U heparin. After a 7-day recovery period, standardized PD was initiated using 4.25% peritoneal dialysis solution (3 ml/kg ). Dialysate was instilled daily, retained intraperitoneally for 2 h, and drained within the home cage without anesthesia. In the HG-PDF + DAPA group, rats received intraperitoneal infusion of HG-PDF along with intragastric administration of DAPA at dose of 1 mg/kg. All rats in the three groups underwent the respective infused for a duration of four weeks.) All animals were administered prophylactic penicillin G therapy (400,000 IU daily via intraperitoneal injection) for 3 days following each surgical procedure to prevent postoperative infection.After recording the relevant data, an overdose of anesthetic (1% sodium pentobarbital) was administered via intraperitoneal injection to euthanize the rat painlessly. Death was confirmed by assessing pupil dilation and cessation of heartbeat before proceeding with peritoneal tissue collection.
Histology and immunohistochemistry staining
The paraffin-embedded tissue blocks were sectioned into 4 μm thick slices and subsequently deparaffinized. The slices underwent hematoxylin and eosin (H&E) and Masson’s trichrome staining to assess tissue morphology and fibrosis. The thickness of the peritoneum and the degree of fibrosis were observed under a light microscope and analyzed semi-quantitatively using Image-Pro Plus version 6.0 software. For immunohistochemical (IHC) staining, the protocol provided by the Zhongshan Jinqiao immunohistochemical assay kit (sp-9001, Beijing) was utilized. Primary antibodies applied in the staining included α-SMA (1:200, Proteintech, China) and Fibronectin (1:200, Proteintech, China). Positive immunostaining was also assessed semi-quantitatively using Image-Pro Plus version 6.0 software.
Western blot analysis
Total cellular proteins were extracted with RIPA lysate enriched with protease and phosphatase inhibitors (Suolaibao, China). The concentration of the extracted proteins was determined using a BCA kit (Suolaibao, China). The proteins were then denatured and subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before being transferred onto a PVDF membrane. This membrane was blocked with 5% skimmed milk powder for one hour and subsequently incubated overnight at 4 °C with primary antibodies, including PI3K (Proteintech, 60225-1-Ig, 1:5000), p-PI3K (CST, 4228T, 1:1000), AKT (Proteintech, 60203-2-Ig, 1:5000), p-AKT (Proteintech, 66444-1-Ig, 1:5000), E-cadherin (Proteintech, 20874-1-AP, 1:5000), α-SMA (Proteintech, 14395-1-AP, 1:5000), Collagen IV (Proteintech, 19674-1-AP, 1:5000), ENKUR (Origene, TA804173S, 1:3000; Proteintech, 26400-1-AP, 1:5000),SGLT2 (Santa, sc-393350, 1:1000) and β-Actin (Proteintech, 20536-1-AP, 1:5000). After incubation, the membrane was washed with Tris-buffered saline with 0.1% Tween-20 (TBST) and exposed to HRP-labeled secondary antibodies for one hour at room temperature. Imaging was carried out using an ECL kit (Epizyme, China) and Amersham Imager l680 (GE). The grayscale bands were analyzed via ImageJ software. The relative protein expression was calculated as the ratio of the target protein to the gray value of the internal reference protein β-actin. The level of protein phosphorylation was calculated as the ratio of phosphorylated protein to total protein.
Quantitative real-time PCR
Total RNA was extracted from HPMC using the Trizol method (Invitrogen). The concentration and purity of the extracted RNA’s were evaluated. The RNA was then reverse transcribed into cDNA using a reverse transcription kit (Takara, Japan). PCR reactions were carried out using a SYBR Green PCR kit (Vazyme, China) in a real-time quantitative PCR instrument (Thermo Fisher Scientific, USA). Primers, obtained from Shangya Bio (China), were used under the following amplification conditions: pre-denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. The relative expression of mRNAs was quantified by the 2-ΔΔCT method with GAPDH serving as the internal reference gene. The concentration and purity of extracted RNAs were quantitatively assessed using a NanoDrop™ 2000 spectrophotometer (Thermo Scientific), with acceptance criteria set at A260/A280 ratios ≥ 1.8 (indicating protein-free RNA) and A260/A230 ratios ≥ 2.0 (confirming minimal organic/phenol contamination).
Primer sequences are shown in Table 1:
Immunofluorescence
To prepare HPMC cell crawls for Immunofluorescence staining, the cells were fixied with 4% paraformaldehyde for 30 min after attachment to the culture vessel. This was followed by permeabilization with 2% Triton X-100 for 15 min and blocking with 10% goat serum for one hour at room temperature. Subsequently, the cells were incubated overnight at 4℃with primary antibodies, including Vimentin (SANTA, sc-6260,1:50), CD45 (SANTA, sc-1178,1:50) and Factor VIII (SANTA,1;50,sc-17832). After washing the cells with PBS, they were incubated in the dark for one hour with FITC-labeled goat anti-rabbit secondary antibody (Servicebio, GB 22303, 1:100). Another wash with PBS was performed, and the cell nuclei were stained with DAPI (Beyotime Biotechnology). Fluorescence microscopy was performed using a Thermo Scientific instrument (USA) to visualize and capture the immunofluorescent signals from the stained cells.
Co-immunoprecipitation
After lysing cells with lysis buffer combined with protease inhibitor cocktail tablets, lysates were denatured. The protein complexes were denatured in 1× Loading buffer (Kangwei Century, CW0027S) supplemented with 5% β-mercaptoethanol at 95 °C for 5 min, followed by immediate cooling on ice. Samples were then diluted 1:5 in modified RIPA buffer (Beyotime, P0013) containing protease/phosphatase inhibitor cocktail (solarbio, P1260-1 ml). Immunoprecipitation was performed after centrifugation of the supernatant fluids. A pre-clearing step with immunoglobulin G and protein A/G-agarose beads was followed by incubation with SGLT2 (1:50 dilution, sc-393350; santa) antibody and protein A/G-agarose beads. WB analysis was performed after collecting the immunocomplex, washing it five times, and boiling it with sodium dodecyl sulfate loading buffer. Anti-ENKUR (1:5000 dilution, 26400-1-AP; Proteintech, ) was used as the primary antibody of WB.
Statistical analysis
All experiments were carried out at least three times to ensure reproducibility. Data are presented as mean ± standard error of the mean. Statistical differences between groups were analyzed using one-way analysis of variance (ANOVA) followed by the Least Significant Difference (LSD) test, utilizing IBM SPSS Statistics 25.0. A p < 0.05 was considered statistically significant.
Data availability
All data generated or analysed during this study are included in this published article and further queries can be directed to corresponding author.
References
Li, P. K. et al. Changes in the worldwide epidemiology of peritoneal Dialysis. Nat. Rev. Nephrol. 13(2), 90–103 (2017).
Teitelbaum, I. Peritoneal Dialysis. N Engl. J. Med. 385(19), 1786–1795 (2021).
Davies, S. J. et al. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal Dialysis. J. Am. Soc. Nephrol. 12(5), 1046–1051 (2001).
Williams, J. D. et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 13(2), 470–479 (2002).
Brown, E. A. et al. Length of time on peritoneal Dialysis and encapsulating peritoneal Sclerosis - Position paper for ISPD: 2017 update. Perit. Dial Int. 37(4), 362–374 (2017).
Aroeira, L. S. et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal Dialysis patients: pathologic significance and potential therapeutic interventions. J. Am. Soc. Nephrol. 18(7), 2004–2013 (2007).
Yanez-Mo, M. et al. Peritoneal Dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl. J. Med. 348(5), 403–413 (2003).
Nair, S. & Wilding, J. P. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J. Clin. Endocrinol. Metab. 95(1), 34–42 (2010).
Talha, K. M., Anker, S. D. & Butler, J. SGLT-2 inhibitors in heart failure: A review of current evidence. Int. J. Heart Fail. 5(2), 82–90 (2023).
McGuire, D. K. et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A Meta-analysis. JAMA Cardiol. 6(2), 148–158 (2021).
Keener, A. B. SGLT2 inhibitors breathe life into kidney-disease care. Nature 615(7951), S2–S4 (2023).
Schroppel, B. et al. Expression of glucose transporters in human peritoneal mesothelial cells. Kidney Int. 53(5), 1278–1287 (1998).
Balzer, M. S. et al. SGLT2 Inhibition by intraperitoneal Dapagliflozin mitigates peritoneal fibrosis and ultrafiltration failure in a mouse model of chronic peritoneal exposure to High-Glucose dialysate. Biomolecules 10(11) (2020).
Zhou, Y. et al. SGLT-2 inhibitors reduce glucose absorption from peritoneal Dialysis solution by suppressing the activity of SGLT-2. Biomed. Pharmacother. 109, 1327–1338 (2019).
Shentu, Y. et al. Empagliflozin, a sodium glucose cotransporter-2 inhibitor, ameliorates peritoneal fibrosis via suppressing TGF-beta/Smad signaling. Int. Immunopharmacol. 93, 107374 (2021).
Martus, G., Bergling, K. & Oberg, C. M. Dual SGLT1/SGLT2 inhibitor Phlorizin reduces glucose transport in experimental peritoneal Dialysis. Perit. Dial Int. 43(2), 145–150 (2023).
Sutton, K. A. et al. Enkurin is a novel calmodulin and TRPC channel binding protein in sperm. Dev. Biol. 274(2), 426–435 (2004).
Zhang, J. et al. Role of CIP4 in high glucose induced epithelial–mesenchymal transition of rat peritoneal mesothelial cells. Ren. Fail. 35(7), 989–995 (2013).
Ma, Q. et al. ENKUR acts as a tumor suppressor in lung adenocarcinoma cells through PI3K/Akt and MAPK/ERK signaling pathways. J. Cancer. 10(17), 3975–3984 (2019).
Liu, J. H. et al. The small molecule chemical compound Cinobufotalin attenuates resistance to DDP by inducing ENKUR expression to suppress MYH9-mediated c-Myc deubiquitination in lung adenocarcinoma. Acta Pharmacol. Sin. 43(10), 2687–2695 (2022).
Hou, R. et al. Chemically synthesized Cinobufagin suppresses nasopharyngeal carcinoma metastasis by inducing ENKUR to stabilize p53 expression. Cancer Lett. 531, 57–70 (2022).
Hou, R. et al. ENKUR expression induced by chemically synthesized Cinobufotalin suppresses malignant activities of hepatocellular carcinoma by modulating beta-catenin/c-Jun/MYH9/USP7/c-Myc axis. Int. J. Biol. Sci. 18(6), 2553–2567 (2022).
Huang, Q. et al. Endoglin aggravates peritoneal fibrosis by regulating the activation of TGF-beta/ALK/Smads signaling. Front. Pharmacol. 13, 973182 (2022).
Guo, R. et al. SGLT2 inhibitors suppress epithelial-mesenchymal transition in podocytes under diabetic conditions via downregulating the IGF1R/PI3K pathway. Front. Pharmacol. 13, 897167 (2022).
Dhillon, S. Correction to: Dapagliflozin: A review in type 2 diabetes. Drugs 79(18), 2013 (2019).
Zhang, Y. et al. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int. 94(3), 524–535 (2018).
Pirklbauer, M. et al. Unraveling reno-protective effects of SGLT2 Inhibition in human proximal tubular cells. Am. J. Physiol. Ren. Physiol. 316(3), F449–F462 (2019).
Li, C. et al. SGLT2 Inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 18(1), 15 (2019).
Lee, S. G. et al. Dapagliflozin attenuates diabetes-induced diastolic dysfunction and cardiac fibrosis by regulating SGK1 signaling. BMC Med. 20(1), 309 (2022).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18(7), e3000410 (2020).
He, L. et al. Twist contributes to proliferation and epithelial-to-mesenchymal transition-induced fibrosis by regulating YB-1 in human peritoneal mesothelial cells. Am. J. Pathol. 185(8), 2181–2193 (2015).
Gonzalez-Mateo, G. T. et al. Surgical techniques for catheter placement and 5/6 nephrectomy in murine models of peritoneal Dialysis. J. Vis. Exp.(2018).
Acknowledgements
Jiahan Liu and Xinxin Xu receives careful guidance from Lijie Zhang, Zhanzheng Zhao and Jing Xiao, as well as grant support from the Department of Nephrology, The First Affiliated Hospital of Zhengzhou University and the Renal Research Institution of Zhengzhou University. Jiahan Liu, Lijie Zhang, Xinxin Xu, Tianxin Jiang, Zhanzheng Zhao and Jing Xiao receive grant support from the Department of Nephrology, The First Affiliated Hospital of Zhengzhou University and the Renal Research Institution of Zhengzhou University.We would like to acknowledge all members of the research group for valuable discussion. Credit to https://www.biorender.com/ for their drawing assistance.
Funding
This research was supported by the Henan Provincial Key Scientific Research Project Plan for Higher Education Institutions (Grant No. 23A320019), the Henan Provincial Medical Science and Technology Research Joint Venture Project (Grant No. SBGJ202402040).
Author information
Authors and Affiliations
Contributions
Jiahan Liu and Xinxin Xu: Data curation, Visualization, Writing-Original draft preparation.Jiahan Liu and Xinxin Xu contributed equally to this work. Lijie Zhang: Conceptualization, Methodology, Writing-Reviewing and Editing, Tianxin Jiang: Visualization, Investigation. Zhanzheng Zhao: Reviewed. Jing Xiao: Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study is reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, J., Xu, X., Zhang, L. et al. Dapagliflozin ameliorates high glucose-induced peritoneal fibrosis through downregulation of ENKUR/PI3K/AKT signaling pathway. Sci Rep 15, 38334 (2025). https://doi.org/10.1038/s41598-025-22195-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-22195-9