Abstract
To investigate the protective effects of fullerenol applied before ischemia induction and desflurane anesthesia applied during ischemia–reperfusion (IR) induction in the lungs and kidneys of a lower-extremity IR injury rat model. After receiving ethical approval, we randomly divided 30 rats into five groups: sham (S), IR, IR with 100 mg/kg fullerenol (IR-FUL), IR with 6.7% desflurane (IR-DES), IR with 100 mg/kg fullerenol and 6.7% desflurane (IR-FUL-DES). Fullerenol was administered 30 min before the IR procedure in the IR-FUL and IR-FUL-DES groups, and desflurane was administered during the IR procedure in the IR-DES and IR-FUL-DES groups. During the procedure, an atraumatic microvascular clamp was placed in the aorta for 120 min. The clamp was then removed to achieve reperfusion for 120 min. Finally, at the end of reperfusion, we evaluated the extracted lung and kidney tissue samples and assessed them biochemically and histopathologically. The lung damage scores of the IR-FUL, IR-DES, and IR-FUL-DES groups were significantly lower than those of the IR group (p < .0001, p = .002, and p < .0001, respectively). The renal tubule injury scores of the IR, IR-FUL, IR-DES, and IR-FUL-DES groups were significantly higher than those of the S group (p < .0001). By contrast, the renal tubule injury scores of the IR-FUL and IR-FUL-DES groups were significantly lower than those of the IR group (p < .0001 and p = .001, respectively). Moreover, kidney intercellular adhesion molecule 1 (ICAM1) expression was significantly lower in all the treatment groups, particularly the IR-FUL group, than in the IR group, and lung ICAM1 expression was significantly lower in the IR-FUL and IR-FUL-DES groups than in the other treatment groups. In the lung and kidney tissues, thiobarbituric acid reactive substance levels, catalase activity, glutathione-S-transferase activity, and arylesterase activity were relatively high in the treatment groups. The application of fullerenol before and after desflurane anesthesia during IR has protective effects on rat lungs and kidneys. In particular, histopathology confirmed that the application of fullerenol 30 min before IR induction and desflurane anesthesia during IR induction reduced oxidative stress and alleviated IR-related damage in the lungs and kidneys. These findings may have important translational relevance, suggesting potential perioperative strategies for protecting organs from ischemia–reperfusion injury in clinical settings.
Similar content being viewed by others
Introduction
Ischemia–reperfusion (IR) injury is a serious process that can occur during various medical and surgical interventions. The basic pathophysiology of IR injury involves microvascular dysfunction after reperfusion (i.e., blood flow restoration) in ischemic tissues, which manifests as impaired endothelium-dependent dilatation of arterioles, increased fluid filtration and leukocyte occlusion in capillaries, and leukocyte compression and plasma protein extravasation in post-capillary venules. In the initial phase after reperfusion, activated endothelial cells in the microcirculation produce more superoxide radicals but less nitric oxide. This superoxide–nitric oxide imbalance in endothelial cells leads to the production and release of inflammatory mediators such as platelet-activating factor and tumor necrosis factor (TNF). This increases the biosynthesis of adhesion molecules and mediates leukocyte–endothelial cell adhesion. Inflammatory mediators released due to reperfusion may also activate endothelial cells in distant organs that were not initially exposed to IR injury. This distant response to IR can lead to leukocyte-dependent microvascular damage, a characteristic of multiple organ dysfunction syndrome1. Reperfusion may increase the risk of cell necrosis in ischemic organs, thereby limiting their return to function2,3.
Lower-extremity IR injury may cause lung damage and dysfunction characterized by interstitial edema, resulting in the need for prolonged ventilatory and inotropic support in some patients4. Lung damage following lower-extremity IR injury depends on neutrophil activation and sequestration in the lungs. Acute lower-extremity IR injury occurs because of cross-clamping of the aorta, particularly during aortic surgery. Ischemic damage can extend beyond the affected organs, affecting distant organs such as the lungs. Reperfusion in an ischemic limb may prevent the limb from developing but may also cause multisystem organ failure, which can be fatal. The severity of the post-ischemic inflammatory response in tissues may be the same as that in distant organs. In humans, aortic clamping and reperfusion of the lower extremities may be followed by pulmonary vasoconstriction and respiratory dysfunction, independent of capillary wedge pressure.
Studies on IR injury-related organ damage and desflurane anesthesia have reported conflicting results. One study observed that desflurane does not have a protective effect against lung damage despite the protective effects of inhaled anesthetics against lung damage5.
Lower-extremity IR injury has similar consequences in kidney tissues. Surgical procedures involving the infrarenal aorta and large arteries of the lower extremities can lead to rhabdomyolysis in skeletal muscles and remote kidney damage6.
Fullerenes, also called C60 molecules, are spherical molecules with 30 carbon double bonds, which are extremely effective free radical scavengers; as such, they are called radical sponges. Pure fullerenes are soluble in only a few types of solvents (e.g., toluene and dichlorobenzene), and their water-soluble derivatives, such as fullerenol, have been used in various biological systems and have demonstrated robust antioxidant properties7.
The development of nano-based methods for IR injury prevention, particularly during and after surgical procedures in the lower extremities, along with clarification of the underlying mechanisms and their effectiveness, is warranted. Therefore, we investigated the protective effects of fullerenol against lung and kidney tissue damage in rats with lower-extremity IR injury anesthetized using desflurane.
Based on the established role of oxidative stress and inflammation in ischemia–reperfusion injury, we hypothesized that both fullerenol and desflurane would reduce tissue damage by attenuating inflammatory responses and oxidative injury through modulation of cytokines such as TNF-α and adhesion molecules like ICAM1.
Materials and methods
Wistar albino rats were obtained from Gazi University Laboratory Animal Breeding and Experimental Research Center (GÜDAM, Ankara, Turkey). Ethical approval was obtained from the Gazi University Animal Experiments Local Ethics Committee (number: G.Ü.ET-21.049). The study was conducted at the same center in accordance with the ARRIVE guidelines. All procedures complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Wistar albino male rats weighing 225–275 g were randomly divided into five groups (each group; n = 6): sham (s), IR, IR-fullerenol C60 (IR-FUL), IR-desflurane (IR-DES), and IR-fullerenol C60-desflurane (IR-FUL-DES). Nonetheless, future studies will benefit from larger cohorts (n: 7–8) to increase robustness and reproducibility. The rats were anesthetized with intraperitoneal (ip) ketamine (100 mg/kg) and xylazine (10 mg/kg). Midline laparotomy without any additional surgical intervention was performed in the S group. In the IR group, midline laparotomy and a traumatic microvascular clamp were placed in the infrarenal abdominal aorta for 120 min and then reperfused for 120 min. Next, 500 IU/kg sodium heparin was given to maintain reperfusion after occlusion. Temperature drop and cyanosis (purple color) of the paw were evaluated as ischemia. Tourniquets were applied to all subjects by the same person, thus ensuring complete standardization. Following a 2-h ischemia period, tourniquets were opened and 2-h reperfusion was initiated8. 100 mg/kg Fullerenol was administered intravenously 30 min before the induction of ischemia. Desflurane was delivered via inhalation starting 10 min before reperfusion and continued for 30 min post-reperfusion. The paw color returning to normal and becoming pink was evaluated as reperfusion. The left leg color returning to normal and distal pulses being palpable were evaluated as reperfusion. In a study conducted by Gyurkovics et al., IR injury was demonstrated in the kidneys, lungs, and rectus femoris muscles of rats using modeling with clamping and revascularization of the infrarenal abdominal aorta9. The IR-FUL, IR-DES, and IR-FUL-DES groups underwent the same surgical procedures as the IR group. Fullerenol C60 (Fullerene-C60; 98%; 1 g, CAS no. 99685-96-8; Millipore Sigma) was administered (100 mg/kg, ip) 30 min before ischemia10,11,12. Rats were anesthetized in a transparent plastic box (40 × 40 × 70 cm), and desflurane was administered at a 6.7% inspiratory concentration at a rate of 4 L/min in 100% O2 for 4 h13,14.
Following the end of the reperfusion period, all rats were anesthetized using ketamine (100 mg/kg, ip) and xylazine (10 mg/kg, ip) injection, and were sacrificed by exsanguination during blood sample 5–10 ml collection from the heart. After heart rate and respiration ceased, monitoring was continued for a further 2 min to confirm death. Lung and kidney tissues were harvested 2 h after reperfusion for histological and immunohistochemical analyses.
Although one study showed that desflurane anesthesia after ketamine–xylazine anesthesia induction had positive effects on cardiac functions, there is insufficient data in the literature regarding the effects of this combination on mortality in rats15. In our study, cardiac functions were found to be normal, and no death was observed in rats administered desflurane after ketamine xylazine induction (data not shown).
Biochemical analysis
Biochemical analysis was performed using the methods described in our previous studies16,17. Measurements of thiobarbituric acid reactive substances (TBARS), malondialdehyde (MDA) levels, and thiobarbituric acid (TBA) reactive substances were performed by Van Ye et al.’s method18. Catalase (CAT) activity was measured using the Aebi’s method19. Glutathione S-transferase (GST) enzyme activity was measured using the methods described by Habig et al.20. The amount of sample protein was determined using the Lowry method with BSA used as the standard protein21. The results were expressed as IU/mg of protein for enzymes and nmol/mg of protein for TBARS. Arylesterase (ARE) activity was measured using Brites’ method22.
Histopathological evaluation of lung and kidney specimens
Lung and kidney specimens were immersed in 10% neutral buffered formalin and left fixation for 72 h. Subsequently, the fixed specimens were processed routinely for paraffin embedding. Tissue sections with a thickness of 5 µm were cut from paraffin blocks using a microtome (Leica RM2245, Germany). For histopathological analysis, lung sections were stained with hematoxylin and eosin (H&E), and kidney sections were stained with both H&E and Periodic acid–Schiff (PAS). All prepared slides were examined under a computer-aided light microscope (DM 4000 B, Germany), and images were captured using the Leica LAS V4.9 software.
Kidney specimens stained with either H&E or PAS were observed at 200× and 400× magnifications, and the injury was evaluated semi-quantitatively. For this target, 10 randomly chosen non-overlapping fields from the cortex of each kidney section were assessed for swelling and vacuolization of tubular epithelial cells, loss of brush borders, tubular epithelial cell sloughing, and hyaline cast formation. Given the above-mentioned parameters, a mean tubular injury score was assigned for each kidney sample such that 0 points indicated no tubular injury, whereas 1, 2, 3, 4, and 5 points indicated ≤ 10%, 10–25%, 25–45%, 45–75%, and > 75% tubular involvement in injury, respectively23,24.
Lung tissue sections were assessed according to the lung injury scoring system established by the American Thoracic Society. Accordingly, 20 randomly chosen non-overlapping fields in H&E-stained lung sections were examined at 200× and 400× magnifications. In each field, neutrophils within the alveolar spaces (parameter A; 0: none, 1: 1–5, 2: > 5), neutrophils in the interstitial space (parameter B; 0: none, 1: 1–5, 2: > 5), hyaline membranes (parameter C; 0: none, 1: 1, 2: > 1), proteinaceous debris filling the airspaces (parameter D; 0: none, 1: 1, 2: > 1), and alveolar septal thickening (parameter E; 0: < 2 × , 1: 2 × –4 × , 2: > 4 ×) were evaluated as described above. The sum of the injury scores of each animal was calculated according to the following formula25,26:
Immunohistochemical evaluation of lung and kidney specimens
Tissue sections were immunohistochemically analyzed for TNF alpha (TNF-α) and intercellular adhesion molecule 1 (ICAM1) expression. To that end, deparaffinization and rehydration of the tissue sections were followed by heat-induced antigen retrieval in citrate buffer (pH 6.0). Incubation with 3% H2O2, as well as protein blocking (Thermo Fisher Scientific, TA-125-UB, USA), was performed to block endogenous peroxidase activity and to prevent the nonspecific binding of antibodies, respectively. Tissue sections were then incubated with anti-TNF-α (1:100, Elabscience, E-AB-33121, USA; RRID:AB_2923019) and anti-ICAM1 (1:100, Bioss, bs-0608R, USA; RRID:AB_10856895) primary antibodies overnight at 4 °C. Next, incubation of the sections with biotinylated secondary antibody (Thermo Fisher Scientific, TP-125-BN, USA) was followed by streptavidin-peroxidase application. Finally, 3,3′-diaminobenzidine (DAB) chromogen-substrate complex (Thermo Fisher Scientific, TA-125-HDX, USA) was used to observe the immunoreactivity. Stained lung and kidney sections were observed under 400× magnification using a light microscope (Leica DM 4000 B, Germany), and 10 randomly chosen non-overlapping fields were captured. TNF-α and ICAM1 immunopositive staining intensity (optical density, OD) was quantified in these micrographs using ImageJ software (ImageJ Image Processing and Analysis in Java, 1.48v; National Institutes of Health, USA) (https://imagej.net/ij/)27.
Statistical analysis
SPSS for Windows (version 20.0; IBM, Chicago, IL, USA) was used for the statistical analysis. Each categorical variable was analyzed using the Shapiro–Wilk test. The results were analyzed using the Kruskal–Wallis test followed by Dunn’s test and One-way ANOVA followed by Tukey’s test. Statistical significance p-value of < 0.05. All values are expressed as means ± standard deviations.
Results
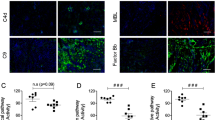
Histopathological analysis revealed the presence of normal alveolar structures in the S group. By contrast, the IR, IR-FUL, IR-DES, and IR-FUL-DES groups demonstrated a range of histopathological findings in the lung tissues, ranging from leukocyte infiltration into the alveolar spaces to alveolar wall thickening (Fig. 1). Compared to the S group, the IR, IR-FUL, IR-DES, and IR-FUL-DES groups had significantly higher lung damage scores (p < 0.0001, p = 0.002, p < 0.0001, and p < 0.0001, respectively). Moreover, compared to the IR group, the IR-FUL, IR-DES, and IR-FUL-DES groups had significantly lower lung damage scores (p < 0.0001, p = 0.002, and p < 0.0001, respectively; Table 1).
Micrographs representing the H&E stained lung sections. Alveolar spaces were infiltrated by neutrophils in the IR groups, whereas neutrophil infiltration in the interstitial area was noted in the treatment groups. Arrows: neutrophils infiltrating the alveolar spaces; waved arrows: neutrophils infiltrating the interstitial space; arrowhead: alveolar septal thickening. H&E; hematoxylin and eosin stain. 200× and 400× magnifications.
In the S group, renal tissue showed normal glomerular and tubular structures in the histopathological analysis; however, the IR group showed degenerative alterations, such as enlarged tubular structures accompanied by hyaline cast formation, loss of brush border and vacuolization of tubular epithelial cells. Some areas also showed epithelial cell detachment from the basement membrane (Fig. 2). The renal tubular injury score was significantly higher in the IR, IR-FUL, IR-DES, and IR-FUL-DES groups than in the S group (p < 0.0001). Moreover, the renal tubular damage score was significantly lower in the IR-FUL and IR-FUL-DES groups than in the IR group (p < 0.0001 and p = 0.001, respectively; Table 1).
Micrographs representing the kidney sections stained with either H&E and PAS. Asterisk: hyaline casts; arrow: vacuolization in tubular epithelial cells; curved arrow: brush border of the proximal tubular epithelial cells, which is better distinguishable with PAS staining; arrowhead: loss of brush border; waved arrow: detachment of tubular epithelial cells from the basement membrane. H&E, hematoxylin and eosin stain; PAS, Periodic acid–Schiff. 200× and 400× magnifications.
Lung TNF-α expression was significantly higher in the IR and IR-DES groups than in the S group (p = 0.002 and 0.038, respectively). However, TNF-α expression levels in lung specimens were lower in the IR-FUL and IR-FUL-DES groups than in the IR group (p = 0.008 and 0.026, respectively; Fig. 3; Table 2).
Kidney TNF-α expression was significantly higher in the IR, IR-DES, and IR-FUL-DES groups than in the S group (p < 0.0001, p = 0.001, and p = 0.004, respectively). Moreover, kidney TNF-α expression was significantly lower in the IR-FUL and IR-FUL-DES groups than in the IR group (p = 0.001 and 0.010, respectively; Fig. 3; Table 3).
Lung ICAM1 expression was significantly lower in all the treatment groups, particularly in the IR-FUL group, than in the IR group (p < 0.001; Fig. 4; Table 2). Similarly, ICAM1 expression in the kidney was significantly lower in the IR-FUL and IR-FUL-DES groups than in the IR group (p < 0.0001 and p = 0.006, respectively; Fig. 4; Table 3).
Biochemical findings
CAT, GST, and ARE activities and TBARS levels showed significant between-group differences in both the lung and kidney tissues (p < 0.0001; Tables 4 and 5).
Lung TBARS levels were significantly higher in the IR group than in the S group (p < 0.0001) but significantly lower in the IR-FUL, IR-DES, and IR-FUL-DES groups than in the IR group (p < 0.0001, p = 0.003, and p < 0.0001, respectively). Moreover, lung CAT enzyme activity was significantly lower in the IR and IR-DES groups than in the S group (p < 0.0001 and p = 0.006, respectively) but significantly higher in the IR-FUL and IR-FUL-DES groups than in the IR group (p < 0.0001 and p = 0.001, respectively). Furthermore, lung GST enzyme activity was significantly lower in the IR group than in the S group (p < 0.0001) but significantly higher in the IR-FUL and IR-FUL-DES groups than in the IR group (p < 0.0001 and p = 0.003, respectively). Finally, lung ARE enzyme activity was significantly lower in the IR, IR-DES, and IR-FUL-DES groups than in the S group (p < 0.0001, p = 0.001, and p = 0.025, respectively) but significantly higher in the IR-FUL, IR-DES, and IR-FUL-DES groups than in the IR group (p < 0.0001, p = 0.008, and p < 0.0001, respectively; Table 4).
Renal TBARS levels were significantly higher in the IR and IR-DES groups than in the S group (p < 0.0001 and p = 0.008, respectively) but were significantly lower in the IR-FUL, IR-DES, and IR-FUL-DES groups than in the IR group (p < 0.0001, p = 0.002, and p < 0.0001, respectively). Moreover, kidney CAT enzyme activity was significantly lower in the IR and IR-DES groups than in the S group (p = 0.002 and 0.022, respectively) but significantly higher in the IR-FUL and IR-FUL-DES groups than in the IR group (p = 0.015 and 0.026, respectively). Furthermore, renal GST enzyme activity was significantly lower in the IR and IR-DES groups than in the S group (both p < 0.0001) but significantly higher in the IR-FUL, IR-DES, and IR-FUL-DES groups than in the IR group (p < 0.0001, p = 0.006, and p < 0.0001, respectively). Finally, renal ARE enzyme activity was significantly lower in the IR, IR-DES, and IR-FUL-DES groups than in the S group (p < 0.0001, p < 0.0001, and p = 0.0001, respectively) but significantly higher in the IR-FUL and IR-FUL-DES groups than in the IR group (p < 0.0001 and p = 0.005, respectively; Table 4).
Discussion
This study demonstrated that both fullerenol and desflurane had protective effects against ischemia–reperfusion injury in lung and kidney tissues. Histological and immunohistochemical findings supported reduced inflammation and oxidative damage in the treated groups.
Our findings from this study may have significant translational significance by suggesting potential perioperative strategies for fullerenol to protect organs from ischemia–reperfusion injury in clinical settings.
Reperfusion of an acute ischemic extremity can lead to systemic complications, eventually resulting in considerable morbidity and mortality. For instance, lower-extremity IR injury can damage lung and kidney tissues28,29. Similarly, current findings indicate that oxidative damage due to ischemia in the lower extremity, followed by reperfusion, results in damage to lung and kidney tissues. Nevertheless, fullerenol administered 30 min before IR injury induction, along with anesthesia using desflurane (a frequently used inhalation anesthetic agent) during IR induction, may protect against lung and kidney damage.
In contrast to other volatile anesthetics, desflurane does not protect against lung IR damage5,30. In the present study, we obtained similar results to those of previous studies. The use of desflurane resulted in lower lung injury scores compared to IR. Moreover, compared with the IR group, the IR-FUL and IR-FUL-DES groups showed significantly lower lung injury scores.
In the present study, fullerenol, novel nanoparticle with potent antioxidant activity and protective effects31,32,33,34,35,36, protected against lung and renal tubule damage in groups administered fullerenol alone or in combination with desflurane. Similar to our results previous studies have shown that fullerenol reduces TNF-α levels with its antioxidant effect in cases in which TNF-α levels are elevated, such as sepsis37.
Specifically, renal tubule injury score, kidney ICAM1 expression, and kidney TBARS levels were lower in the group treated with fullerenol and desflurane than in the group treated with desflurane alone. Schuhmann and colleagues also demonstrated that the anti-inflammatory activity of fullerenol reduces increased molecules such as TNF-α and ICAM1, preserving transendothelial electrical resistance in inflamed tissue38. Our study supports these results. Therefore, we speculate that the combination of desflurane and fullerenol may enhance the antioxidant response. In our 2024 study with sevoflurane, we also noted that the combination of desflurane and fullerenol protects against distant organ damage caused by lower extremity ischemia–reperfusion, likely through antioxidant mechanisms. This study also yielded results supporting our previous hypothesis12.
The literature has demonstrated the protective effects of fullerenol against IR injury in various organ tissues, including the brain39, liver40, intestines38,41, kidneys42, and lungs43. However, few studies have reported the effects of fullerenol combined with desflurane anesthesia in rats with lower extremity IR injury. Although our findings differ from some previous studies reporting limited antioxidant effects of fullerenol, these discrepancies may be due to differences in dosage, model type, or timing of administration. Further research is needed to clarify these differences.
Conclusion
It is known that lower extremity IR injury can cause damage to vital organs such as the lungs44 and kidneys45. Desflurane anesthesia with fullerenol C60 may help reduce the damage caused by lower extremity IR injury and the associated mortality and morbidity. In our study, we observed that the administration of fullerenol C60 protected the lung and kidney tissues against damage in rats with lower extremity IR injury under desflurane anesthesia. Therefore, we believe that the administration of fullerenol C60 before the induction of IR injury and desflurane anesthesia during the induction of IR injury may protect against damage to the rat lungs and kidneys. Furthermore, it is recommended that future studies evaluate IR injury and drug effects using methods such as microalbuminuria and arterial blood gas (PaO2), arterial partial pressure of carbon dioxide (PaCO2) analysis.
Limitations
-
1.
Duration of the study: We sacrificed the rats in which we induced lower extremity IR injury immediately at the end of the experiment. Therefore, we were unable to observe the long-term protective effects of administering fullerenol and desflurane anesthesia in these rats.
-
2.
Sex differences: Because we used only male rats in our experiments, we were unable to report sex differences among our study samples. Therefore, long-term studies including both male and female rats are needed.
-
3.
Budget: Due to budget insufficiency, IR damage and drug effects could not be demonstrated with methods such as microalbuminuria and arterial blood granulation analysis accompanied by H&E staining.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Kalogeris, T., Baines, C. P., Krenz, M. & Korthuis, R. J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298, 229–317 (2012).
Zhao, T. et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact. Mater. 20(7), 47–72 (2021).
Linfert, D., Chowdhry, T. & Rabb, H. Lymphocytes and ischemia-reperfusion injury. Transplant. Rev. 23(1), 1–10 (2009).
Welbourn, R. et al. Role for tumor necrosis factor as mediator of lung injury following lower torso ischemia. J. Appl. Physiol. 70(6), 2645–2649 (1991).
Strosing, K. M. et al. Inhaled anesthetics exert different protective properties in a mouse model of ventilator-induced lung injury. Anesth. Analg. 123(1), 143–151 (2016).
Gyurkovics, E. et al. Postconditioning of the lower limb–protection against the reperfusion syndrome. J. Surg. Res. 169(1), 139–147 (2011).
David, W. I. F., Ibberson, R. M., Dennis, T. J. S., Hare, J. P. & Prassides, K. Structural phase transitions in the fullerene C60. Europhys. Lett. 18(3), 219–225 (1992).
Kirisci, M., Guneri, B., Seyithanoglu, M. & Kazanci, U. Lycopene hampers lung injury due to skeletal muscle ischemia-reperfusion in rat model. Int. J. Vitam. Nutr. Res. (2020).
Gyurkovics, E. et al. Postconditioning of the lower limb—protection against the reperfusion syndrome. J. Surg. Res. 169(1), 139–147 (2011).
Vapa, I. et al. Effect of fullerenol C60(OH)24 on lipid peroxidation of kidneys, testes and lungs in rats treated with doxorubicine. Eur. J. Drug Metab. Pharmacokinet. 37(4), 301–307. https://doi.org/10.1007/s13318-012-0092-y (2012).
Şengel, N. et al. The effect of sevoflurane and fullerenol C60 on the liver and kidney in lower extremity ischemia-reperfusion injury in mice with streptozocin-induced diabetes. Int. J. Nanomed. 12(18), 7543–7557. https://doi.org/10.2147/IJN.S432924 (2023).
Arpaci, A. H. et al. Effect of fullerenol C60 on lung and renal tissue in lower extremity ischemia-reperfusion injury in sevoflurane-treated rats. Mol. Med. Rep. 29(3), 1–13 (2024).
Gobut, H. et al. Effects of cerium oxide (CeO2) on liver tissue in liver ischemia-reperfusion injury in rats undergoing desflurane anesthesia. BMC Anesthesiol. 23(1), 40. https://doi.org/10.1186/s12871-023-01999-0 (2023).
Slingo, J. M. & Slingo, M. E. The science of climate change and the effect of anaesthetic gas emissions. Anaesthesia 79(3), 252–260. https://doi.org/10.1111/anae.16189 (2024).
Clarke, K. W., Song, D. Y., Alibhai, H. I. K. & Lee, Y. H. Cardiopulmonary effects of desflurane in ponies, after induction of anaesthesia with xylazine and ketamine. Vet. Rec. 139(8), 180–185 (1996).
Ozdemirkan, A. et al. Effect of cerium oxide on kidney and lung tissue in rats with testicular torsion/detorsion. Biomed. Res. Int. 22(2022), 3176455. https://doi.org/10.1155/2022/3176455 (2022).
Kartal, S. et al. The efficacy of dexmedetomidine on lung injury induced by renal ischemia/reperfusion in diabetic rats. Anaesth. Pain Intensive Care 24, 272–278 (2020).
Van Ye, T. M. et al. Inhibition of intestinal lipid peroxidation does not minimize morphological damage. J. Surg. Res. 55, 553–558 (1993).
Catalase, A. H. In Methods of Enzymatic Analysis (ed. Bergmeyer, H. U.) 673–677 (Academic Press, 1974).
Habig, W. H., Pabst, M. J. & Jakoby, W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139 (1974).
Lowry, O. et al. Protein measurement with folin phenol reagent. J. Biol. Chem 182, 265–275 (1951).
Brites, F. D. et al. Paraoxonase 1 and platelet-activating factor acetylhydrolase activities in patients with low HDL-cholesterol levels with or without primary hypertriglyceridemia. Arch. Med. Res. 35, 235–240 (2014).
Garbaisz, D. et al. Attenuation of skeletal muscle and renal injury to the lower limb following ischemia-reperfusion using mPTP inhibitor NIM-811. PLoS ONE 9(6), e101067 (2014).
Shih, J. M. et al. Effects of fish oil-based lipid emulsion on inflammation and kidney injury in mice subjected to unilateral hind limb ischemia/reperfusion. Cytokine 111, 49–57 (2018).
Huwae, T. E. et al. Reperfusion interval as a prevention of lung injury due to limb ischemia–Reperfusion after application of tourniquet in murine experimental study. Indian J. Orthop. 54, 704–710 (2020).
Matute-Bello, G. et al. An Official American Thoracic Society Workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 44(5), 725–738 (2011).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9(7), 671–675 (2012).
Yassin, M. M., Harkin, D. W., Barros D’Sa, A. A., Halliday, M. I. & Rowlands, B. J. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J. Surg. 26(1), 115–121 (2002).
Apichartpiyakul, P., Shinlapawittayatorn, K., Rerkasem, K., Chattipakorn, S. C. & Chattipakorn, N. Mechanisms and interventions on acute lower limb ischemia/reperfusion injury: A review and insights from cell to clinical investigations. Ann. Vasc. Surg. 86, 452–481 (2022).
Oshima, Y. et al. Desflurane inhalation before ischemia increases ischemia-reperfusion-induced vascular leakage in isolated rabbit lungs. Springerplus 5(1), 2031 (2016).
Nozdrenko, D. et al. Protective effect of water-soluble C60 fullerene nanoparticles on the ischemia-reperfusion injury of the muscle soleus in rats. Int. J. Mol. Sci. 22(13), 6812 (2021).
Kartal, H. et al. The effect of fullerenol C60 on skeletal muscle after lower limb ischemia reperfusion injury in streptozotocin-induced diabetic rats. J. Surg. Med. 4(6), 451–455 (2020).
Nozdrenko, D. M. et al. Impact of C 60 fullerene on the dynamics of force-speed changes in soleus muscle of rat at ischemia-reperfusion injury. Fiziol Zh 61(2), 48–59 (2015).
Yin, J. J. et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 30(4), 611–621 (2009).
Tsai, M. C., Chen, Y. H. & Chiang, L. Y. Polyhydroxylated C60, fullerenol, a novel free-radical trapper, prevented hydrogen peroxide- and cumene hydroperoxide-elicited changes in rat hippocampus in-vitro. J. Pharm. Pharmacol. 49(4), 438–445 (1997).
Lai, H. S., Chen, W. J. & Chiang, L. Y. Free radical scavenging activity of fullerenol on the ischemia-reperfusion intestine in dogs. World J. Surg. 24(4), 450–454 (2000).
Zhang, T. et al. Fullerenol as a novel therapeutic agent for sepsis-induced cardiomyocytes damage. Appl. Phys. A 130(3), 194 (2024).
Schuhmann, M. K. & Fluri, F. Effects of fullerenols on mouse brain microvascular endothelial cells. Int. J. Mol. Sci. 18(8), 1783 (2017).
Vani, J. R., Mohammadi, M. T., Foroshani, M. S. & Jafari, M. Polyhydroxylated fullerene nanoparticles attenuate brain infarction and oxidative stress in rat model of ischemic stroke. EXCLI J. 20(15), 378–390 (2016).
Guan, Y. et al. Nanotheranostics for the Management of Hepatic Ischemia-Reperfusion Injury. Small 17(23), e2007727 (2021).
Amani, H. et al. Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries. J. Mater. Chem. B 5(48), 9452–9476 (2017).
Chien, C. T., Chen, C. F., Chiang, L. Y. & Lai, M. K. Novel water-soluble hexa (sulfobutyl) fullerene attenuates apoptosis formation after ischemic renal failure. Fullerene Sci. Technol. 7(4), 529–540 (1999).
Lai, Y. L., Murugan, P. & Hwang, K. C. Fullerene derivative attenuates ischemia-reperfusion-induced lung injury. Life Sci. 72(11), 1271–1278 (2003).
Seekamp, A., Warren, J. S., Remick, D. G., Till, G. O. & Ward, P. A. Requirements for tumor necrosis factor-alpha and interleukin-1 in limb ischemia/reperfusion injury and associated lung injury. Am. J. Pathol. 143(2), 453–463 (1993).
Wever, K. E. et al. Remote ischaemic preconditioning by brief hind limb ischaemia protects against renal ischaemia-reperfusion injury: the role of adenosine. Nephrol. Dial. Transplant. 26(10), 3108–3117 (2011).
Author information
Authors and Affiliations
Contributions
M.A., G.K., Z.K., and A.K. designed the study, analyzed, and interpreted the data. V.Ş., M.A. and I.G. performed the experiments. M.A., G.K., and A.K. confirmed the authenticity of the raw data. A.K., M.A., and Z.K. provided scientific and technical assistance and critically revised the manuscript for important intellectual content. Z.K. and G.K. collected samples. Z.Y., S.Ö.A.D., and A.D.D. performed the cellular and molecular experiments. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Animal Research Committee of Gazi University (Ankara, Turkey; approval no. G.Ü.ET-21.049).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kip, G., Köksal, Z., Yığman, Z. et al. Organ-protective effects of fullerenol and desflurane in a rat model of ischemia–reperfusion injury. Sci Rep 15, 40138 (2025). https://doi.org/10.1038/s41598-025-23812-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-23812-3